This is an author produced version of a paper published in Journal of Oral
Pathology and Medicine. This paper has been peer-reviewed but does not
include the final publisher proof-corrections or journal pagination.
Citation for the published paper:
Chrcanovic, Bruno; Cavalieri Gomes, Carolina; Santiago Gomez, Ricardo.
(2018). Central giant cell lesion of the jaws : an updated analysis of 2270
cases reported in the literature. Journal of Oral Pathology and Medicine, vol.
47, issue 8, p. null
URL: https://doi.org/10.1111/jop.12730
Publisher: Wiley
This document has been downloaded from MUEP (https://muep.mah.se) /
DIVA (https://mau.diva-portal.org).
Accepted
Article
This article has been accepted for publication and undergone full peer review but has not
DR BRUNO CHRCANOVIC (Orcid ID : 0000-0002-3460-3374)DR CAROLINA CAVALIÉRI GOMES (Orcid ID : 0000-0003-1580-4995)
PROFESSOR RICARDO SANTIAGO GOMEZ (Orcid ID : 0000-0001-8770-8009) Article type : Review
Central giant cell lesion of the jaws: an updated analysis of 2270 cases reported in the literature
Bruno Ramos Chrcanovic 1* Carolina Cavalieri Gomes 2 Ricardo Santiago Gomez 3*
1
Department of Prosthodontics, Faculty of Odontology, Malmö University, Malmö, Sweden. bruno.chrcanovic@mau.se; brunochrcanovic@hotmail.com
2
Department of Pathology, Biological Sciences Institute, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil. carolinacgomes@ufmg.br
3
Department of Oral Surgery and Pathology, School of Dentistry, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil. rsgomez@ufmg.br
DEPARTMENT OF PROSTHODONTICS, FACULTY OF ODONTOLOGY, MALMÖ UNIVERSITY, MALMÖ, SWEDEN; DEPARTMENT OF ORAL SURGERY AND PATHOLOGY, SCHOOL OF DENTISTRY, UNIVERSIDADE FEDERAL DE MINAS GERAIS, BELO HORIZONTE, BRAZIL
* Corresponding authors
Ricardo Santiago Gomez, Department of Oral Surgery and Pathology, School of Dentistry, Universidade Federal de Minas Gerais, Belo Horizonte, Brazil. rsgomez@ufmg.br
and
Bruno Ramos Chrcanovic. Department of Prosthodontics, Faculty of Odontology, Malmö University, Carl Gustafs väg 34, SE-214 21, Malmö, Sweden. bruno.chrcanovic@mau.se; brunochrcanovic@hotmail.com Mobile: +46 725 541 545 Fax: +46 40 6658503
Accepted
Article
KEYWORDS
Central giant cell lesion; central giant cell granuloma; aggressiveness classification; bone disease; clinical features; treatment; recurrence rate
ABSTRACT
Purpose. To review all available data published on central giant cell lesion (CGCL) of the jaws into a comprehensive analysis of its clinical/radiologic features, with emphasis on the predictive factors associated with its recurrence.
Methods. An electronic search was undertaken in 5 databases (February/2018), looking for reporting cases of CGCLs.
Results. 365 publications were included, comprising 2270 lesions. CGCLs were more prevalent in women and the mandible. Cortical bone perforation occurred in 50% of the cases. Marginal/segmental resection was more often performed in larger lesions, and drug therapy was more frequent in small lesions. Recurrence was reported in 232/1316 cases (17.6%). The recurrence rate of the aggressive lesions (22.8%) after surgical treatment was higher than non-aggressive lesions (7.8%). Four out of five CGCLs showed partial/total regression with pharmacological treatment. Aggressive lesions showed a worse response to corticosteroids than non-aggressive lesions. For the lesions submitted to surgery as the first treatment, curettage, enucleation or marginal resection in relation to segmental resection, aggressive lesions, cortical bone perforation, and tooth root resorption were associated with increased recurrence rate. Recurrence related to a combination of surgical/pharmacological treatment could not be evaluated due to the variety of protocols.
Conclusions. Aggressive CGCLs recur more often than the non-aggressive ones. Despite sometimes showing poor response to corticosteroid injection or surgical curettage, a combination of both treatment strategies should be considered in aggressive cases to reduce morbidities associated with radical surgery. The best protocol to manage aggressive and non-aggressive lesions remains to be determined.
KEYWORDS
Central giant cell lesion; central giant cell granuloma; aggressiveness classification; bone lesions; clinical features; treatment; recurrence rate
Accepted
Article
INTRODUCTION
Central giant cell lesion (CGCL) of the jaws is a localized, benign but sometimes aggressive osteolytic lesion of the jaws histopathologically characterized by multinucleated osteoclast-like giant cells intermingled with oval to spindle-shaped mononuclear cells.1 Although benign, the reported recurrence rate has been relatively high in some clinical series,2,3 and some lesions show an “aggressive” behavior.4,5 The relatively high recurrence rate and the variation in aggressiveness have encouraged the surgeons to search for new therapeutic options for CGCL. The standard therapies are surgical curettage or enucleation but more recently other therapeutical options using drugs have also been performed. These drugs include corticoid, calcitonin, interferon, monoclonal antibody, and bisphosphonates. In light of this, the aim of the present systematic review was to review all available data published on CGCL into a comprehensive analysis of its clinical/radiologic features with emphasis on the predictive factors associated with its recurrence.
MATERIALS AND METHODS
This study followed the PRISMA Statement guidelines.6
Search strategies, Study selection, Data extraction
See Supplemental Appendix.
Inclusion and Exclusion Criteria
Eligibility criteria included publications reporting cases of CGCL. The studies needed to contain enough clinical, radiological and histological information to confirm the diagnosis. Clinical trials, cohort studies, case-control studies, cross-sectional studies, case series, and case reports were included. Exclusion criteria were immunohistochemical studies, histomorphometric studies, radiological studies, genetic studies, histopathological or cytopathological studies, cell proliferation/apoptosis studies, in vitro studies, and review papers, unless any of these publication categories had reported any cases with enough clinical, radiological and histological information. Hybrid tumors containing parts of CGCL were not considered for this study, as they may behave differently from non-hybrid CGCL.
The definitions and criteria of the WHO1 were used to define a lesion as CGCL: an unencapsulated proliferation of mononuclear spindle-shaped and polygonal cells with osteoclast-type multinucleated giant cells in a vascular background.
Accepted
Article
A number of conditions that can present with lesions that histologically are indistinguishable from the CGCL of bone were excluded, including brown tumors of hyperparathyroidism (or cases with altered levels of parathyroid hormone, calcium, and phosphorus), cherubism, and, less commonly, some inherited syndromes. These include Ramon syndrome,7 Schimmelpenning syndrome,8 Noonan syndrome,9 “Noonan-like syndrome, cherubism, and polyarticular pigmented villonodular synovitis”, “ocular-ectodermal syndrome”,10 neurofibromatosis type 1,11 Jaffe Campanacci syndrome,12 tumor-induced osteomalacia/rickets,13 and patients with multiple lesions.
Analyses
The lesions were classified as aggressive or non-aggressive based on a modified criteria proposed by Chuong et al.4 and Kaban et al.5 The tumors that showed at least 3 of the following features were classified as aggressive CGCL: (a) size greater than 5 cm, (b) rapid growth, (c) root resorption, (d) tooth displacement, (e) cortical bone thinning, and (f) cortical bone perforation. It is important to stress here that we did not take into consideration the criterion ‘recurrence after curettage’ because it cannot be used as a predictive marker. The tumors with less than 3 of the above clinical-radiological findings were classified as non-aggressive lesions. Tumors equal or greater than 5 cm were considered aggressive tumors based on this characteristic alone.
The mean, standard deviation (SD), and percentages were presented as descriptive statistics. Kolmogorov–Smirnov test was performed to evaluate the normal distribution of the variables, and Levene’s test evaluated homoscedasticity. The performed tests for two independent groups were Student’s t-test or Mann-Whitney test, depending on the normality. Pearson’s chi-squared or Fisher’s exact tests were used for categorical variables. The probability of recurrence was calculated for some variables, whenever possible, in odds ratio (95% confidence interval). The degree of statistical significance was considered p<0.05. All data were statistically analyzed using SPSS version 25 software (SPSS Inc., Chicago, IL, USA).
RESULTS
Literature search
The study selection process is summarized in Figure S1 (see Supplemental Appendix). The search strategy in the databases resulted in 2567 papers. Search in Google Scholar, and Crossref resulted in, respectively, 16 and 51 eligible papers not found in the five main databases. A number of 869 articles were cited in more than one database (duplicates). The reviewers independently screened the abstracts for those articles related to the aim of the review. Of the resulted 1765 studies, 1238 were excluded for not being related to the topic or not presenting clinical cases.
Accepted
Article
Additional hand-searching of journals and the reference lists of selected studies did not yield additional papers. The full-text reports of the remaining 527 articles led to the exclusion of 145 because they did not meet the inclusion criteria. Moreover, 17 studies were excluded because their cases from the same service or University of the cases from other publications, presenting the possibility of duplicated cases. Thus, a total of 365 publications were included in the review (see Supplemental Appendix).
Description of the Studies and Analyses
We included in the present review 365 publications reporting 2270 CGCL cases. Table 1 presents demographic and clinical features of all cases. The lesion was more prevalent in women than in men, at a 1.56:1 proportion. The mean age of the patients was 25.8±15.3 years (range 0-85), being higher in women. Figure 1 shows the distribution of the lesions according to age, with the highest prevalence in the second and then third decade of life. The lesions were more prevalent in the mandible in comparison to the maxilla, but there was no clear prevalence concerning the different regions of the jaws (Figure 2). The lesions had a mean size of 3.9±2.1 cm (min-max, 0.5-15.0; n=789).
Time of follow-up was informed for 852 lesions, with a mean±SD of 53.2±41.0 months (min-max, 1-324). The follow-up period was up to 1 year for 15.5% of the lesions year, 33.4% for up to 2 years, 44.2% for up to 3 years, 59.9% for up to 4 years, and 74.2% for up to 5 years. Treatment of the lesions was known in 1206 (out of 2270, 53.1%) cases, of which 73.3% consisted of curettage or enucleation. There were 232 recurrences (17.6%) in 1316 lesions (110 of these 1316 cases had information on recurrence, but the authors did not provide any information on which specific therapy was performed. That is why the specific treatment was known for only 1206 lesions). The interval from initial treatment to the first recurrence (information available for 95 out of the 232 recurrences) ranged from 0.5 to 276 months after treatment, with a mean interval of 19.6±31.9 months. Almost 80% of the recurrent cases were diagnosed within the first 2 years after treatment. Considering cases with available information, 1130 CGCLs were submitted to only one therapy, 182 to 2 therapies, 47 lesions to 3, 18 lesions to 4, 4 lesions to 5 steps, and one lesion each to 6, 9 and 12 therapeutic steps.
A number of 818 cases had clinical and radiological information enough to classify the lesions in aggressive or non-aggressive according to established criteria,4,5 although the criterion ‘recurrence after curettage’ was not considered. Because the clinical-radiological aggressiveness of the tumor is an important confusion factor in the evaluation of the recurrence rate and patient management, each treatment modality was analyzed according to this parameter. Table 2 shows the recurrence
Accepted
Article
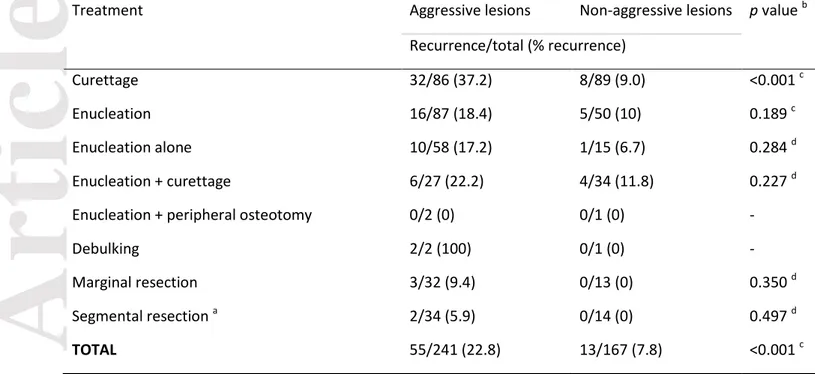
rate according to the first treatment performed (only surgeries considered) in both aggressive and non-aggressive groups of CGCLs, for when the information about both treatment and recurrence was available. In general, 55/241 (22.8%) aggressive lesions recurred after surgical treatment, which is significantly higher compared to 13/167 (7.8%) in non-aggressive lesions. More than one-third of the aggressive tumors treated by curettage recurred, in sharp contrast with the 9% recurrence rate in the non-aggressive group. For enucleation, debulking, marginal or segmental resection no significant difference was found when the groups were compared.
Table S1 (see Supplemental Appendix) shows the type of therapy performed in lesions of different size ranges. Apparently, the choice for curettage was not influenced by the size of the lesion. More invasive surgical approaches (marginal and segmental resection) were more often performed in larger lesions, and the choice for therapy with a drug decreased as the lesion increased in size.
Table 3 shows the recurrence rate for CGCLs according to different factors – for the lesions submitted to surgery (curettage, enucleation, marginal/segmental resection) as the first treatment. The following factors showed a statistically significant increase in the recurrence rate: curettage, enucleation or marginal resection in relation to segmental resection, aggressive lesions, cortical bone perforation, and tooth root resorption.
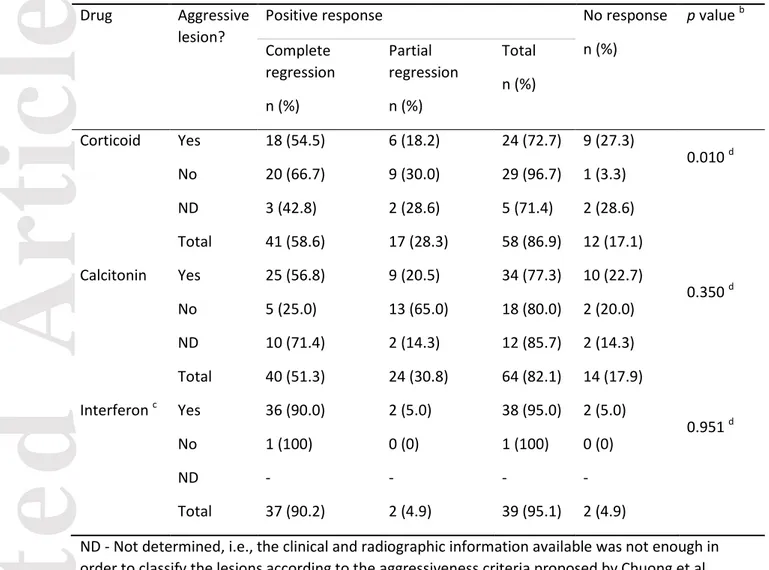
Concerning drug therapies, 109 cases were treated at least once with calcitonin, 100 with a corticosteroid, 70 with interferon, 9 with a monoclonal antibody, 8 with bisphosphonates, and 4 with chemotherapy. These cases were not always exclusively treated by only one of these drugs; they were treated, for example, first with corticoid and later with calcitonin. Moreover, the drug therapy was preceded by surgery in some cases, or surgery was later performed in case of no or partial regression of the lesion when first treated with a drug. Table 4 shows a comparison of recurrence between aggressive and non-aggressive lesions after drug treatment with corticosteroids, calcitonin, or interferon, for the lesions with available information about aggressiveness, drug treatment, and recurrence. Those cases in which patients were previously treated with chemotherapy, radiotherapy or by simultaneous or consecutive use of two different drugs were excluded from the analysis. In general, four out of five patients with CGCL showed partial or total regression with pharmacological treatment. Aggressive lesions presented a less intense response to corticosteroid therapy than non-aggressive lesions. Most of the lesions treated with interferon presented a positive response, but all cases except for one (interferon alone, with complete regression) were preceded by enucleation.
Accepted
Article
DISCUSSION
The present study aimed to integrate the available data published in the literature on CGCL into an updated comprehensive comparative analysis of their clinical and radiologic features, as well as the frequency of recurrence. A review of pathological lesions is important because it provides information that can improve diagnostic accuracy, allowing pathologists and surgeons to make informed decisions and refine treatment plans to optimize clinical outcomes.14-16
Most reported cases of multiple concurrent CGCLs are associated with some form of inherited syndrome or systemic disease, hence the possibility that these lesions simply represent multiple CGCLs is considered unlikely.17 Taking into consideration the data from the study of Teixeira et al.,18 van den Berg et al.19 concluded that in almost all patients, multiple CGCLs are found mainly in those with germline mutations involving the RAS/MAPK pathway. As a consequence, patients with multiple CGCLs should be checked for underlying conditions. Since severe concomitant anomalies such as heart disease, cardiomyopathy, mental retardation, and skeletal anomalies are common in RASopathies, an extensive medical examination is warranted. With regard to the pathogenesis, multifocal and solitary CGCLs should be considered separate entities with different pathogenic pathways. For that reason, we excluded cases of multiple CGCLs from the present review. For a reason already mentioned somewhere in the text, a number of conditions that can present with lesions that histologically are indistinguishable from the CGCL of bone were also excluded from the review.
Patients with CGCLs showed a broad spectrum in respect to both tumor size and aggressiveness, varying from relatively small indolent lesions to rapidly growing lesions with aggressive signs and symptoms.20 Results are frequently difficult to interpret because of multiple protocols used within a particular study and lack of uniformity in reporting patient and tumor characteristics.21
CGCL is a bizarre condition of unpredictable course. While some lesions show a very good response to drugs or conservative surgical therapy, others recur even after more radical surgical treatment. This diverse biological behavior could be associated with a molecular heterogeneity of the lesions, but further studies are necessary to prove this assumption. It has been stated that the main drawback of surgical therapy is the reportedly high recurrence rate.22 The results of the present study show that this is not entirely true, at least for non-aggressive lesions. Regarding aggressive lesions, more conservative surgical approaches may need additional therapy, especially when it comes to curettage. Our results showed that more radical surgical approaches decrease the recurrence rate of aggressive lesions. On the other hand, radical surgical approaches are associated
Accepted
Article
with morbidities and can increase the risk of damage to vital structures, the risk of facial disfigurement, and the need for possibly complex reconstructive procedures in advanced cases.22
Thus, the distinction between aggressive and non-aggressive behavior seems to be critical in the establishment of the correct treatment plan.22 Using modified clinical-radiological criteria previously published,4,5 we classified all the lesions where enough information was available. We could extract the necessary clinical information for this classification in 818 cases. This classification of clinical aggressiveness has not been previously tested in such large number of cases, and we observed some very interesting findings. More than one-third of the aggressive giant cell lesions treated by curettage recurred, compared to 9% of the non-aggressive ones. We did not take into consideration the criterion ‘recurrence after curettage’ to classify the lesions as aggressive or not, as results of recurrence are, logically, not predictive of recurrence itself. Recurrence is a consequence, rather than a criterion for the definition of aggressiveness, and is not useful for doing future prospective analysis. Although some surgeons usually perform adjunct procedures after curettage, such as peripheral ostectomy, this information was not available in the majority of the studies. Therefore, we could not evaluate the possible contribution of peripheral ostectomy after curettage to prevent recurrence of aggressive giant cell lesions. Despite the limitations of our study, this information can be useful to guide surgeons in the treatment plan of patients with CGCL. The performance of adjunct procedures was, however, available for most of the cases treated by enucleation, although curettage after enucleation does not seem to decrease the recurrence rate in comparison to enucleation alone. It should be mentioned that the number of cases treated by enucleation followed by peripheral osteotomy was too low to draw any reliable conclusion.
Pharmacologic agents have been used as alternatives to surgical management. Although cases treated by drugs may need additional surgery, this surgery could be less aggressive/destructive in result to at least partial response and formation of surrounding bone with prior pharmacotherapy. The disadvantages of drug therapy for CGCL are the long duration of treatment, discomfort related to the excipients, and need for additional pharmacological treatment or surgery in nonresponsive or minimally responsive cases,22 besides the possible adverse effects.
Calcitonin therapy for CGCL was introduced by Harris23 in 1993. The therapeutic concept for the administration of calcitonin in the treatment of CGCLs is based on the study of Flanagan et al.24 that demonstrated that giant cells in CGCLs are osteoclasts. They showed that the giant cells were responsive to calcitonin, resulting in cytoplasmic quiescence and inhibition of bone resorption. Approximately 4 out of 5 aggressive and non-aggressive lesions show partial or total regression to calcitonin therapy, which gives support for additional studies using this therapy combined with surgery.
Accepted
Article
The use of corticosteroids for the treatment of CGCL was first proposed in 198825 and the most frequently adopted protocol was established by Terry and Jacoway26 in 1994. The protocol has been used by many authors in the treatment of CGCLs, although the mode of action is still not fully understood.27 It was shown in an in vitro study that dexamethasone has a direct effect on osteoclast formation and activity, stimulating the proliferation and differentiation of human osteoclast precursors and inhibiting the bone-resorbing activity of mature osteoclasts.28 We observed that non-aggressive lesions presented a better response to corticosteroid therapy than aggressive lesions. Even though aggressive lesions present a less intense response to corticosteroids, its use alone or in combination with curettage should be more investigated, considering the low morbidity associated with this therapy.
The use of interferon comes from the hypothesis that CGCLs are proliferative lesions that would, therefore, respond to angiogenic therapy.29 The present review observed that the use of interferon alone did not lead to complete remission in any case described in the literature, and additional pharmacotherapy or surgery was indicated in all cases.22,30,31 The use of interferon remains limited to aggressive lesions given the toxicity of the treatment.32,33 Moreover, interferon therapy is not indicated in infants due to the risk of spastic diplegia.34
Molecular studies of CGCL may help to understand the pathogenesis of the aggressive and non-aggressive forms of the disease, and eventually may reveal markers to guide the personalized treatment of the patients. The finding that aggressive and non-aggressive tumors respond differently to surgical and pharmacological treatment suggests that more individualized therapy based on clinical, microscopic and molecular markers is necessary to establish effective protocols for this condition.
The results of our study have to be interpreted with caution because of its limitations. First, all included studies were retrospective reports, which inherently result in errors, with incomplete records. Secondly, many of the published cases had a short follow-up, which could have led to an underestimation of the actual recurrence rate. However, it is hard to define what it would be considered a short follow-up period to evaluate the recurrence of these lesions. Thirdly, many of the cases described were published as isolated case reports or small case series.
The heterogeneity concerning the applied treatment could be considered a drawback. However, these changes reflect the current clinical practice influenced by the search for a less toxic treatment with minimal morbidity and recurrences, while at the same time the clinician is challenged by new developments in literature based on mainly single case reports.22 Therefore, further studies including more cases with more detailed information of the combined protocol therapy used is still necessary for more evidence-based decision.
Accepted
Article
CONCLUSIONS
Aggressive CGCLs recur more often than the non-aggressive ones. Despite sometimes showing poor response to corticosteroid injection or surgical curettage, a combination of both treatment strategies should be considered in these aggressive cases in order to reduce morbidities associated with radical surgery. The best protocol to manage aggressive and non-aggressive lesions remains to be determined and may benefit from future molecular understanding of these lesions.
Funding/grant support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of conflicting interests
There are no conflicts of interest to declare.
Acknowledgments
We would like to thank the following people who provided us some articles: Dr. Douglas D. Damm, Dr. Andrea Ciorba, Dr. Orlando Cavezzi Jr., Dr. John K. Brooks, Dr. Atila Fernando Visinoni, Dr. Braz Campos Durso, Dr. Antonio Scarano, Mrs. Jill Runyan and Mrs. Jessica Lauria (Director of Communications and Communications and Media Coordinator, respectively, of the Florida Dental Association), Mrs. Sabrina Avendaño and Mrs. Claudia Rossi (librarians of the Asociación Odontológica Argentina), Mrs. Loraine Sedor (Director of Communications of the New Jersey Dental Association), Mrs. Ilia Silva Marambio (Procesos Técnicos y Referencia, Library of the Faculty of Odontology of the Universidad de Chile), Mrs. Mercedes Uribe Pérez (Blibliotecaria ADM), Mrs. Cinthya Tapia Ponce (Editora Ejecutiva of the Acta Pediátrica de México), Dr. Jorge Enrique Delgado (Editor-in-Chief of the journal Universitas Odontologica), Mrs. Denilza Lima Torres (Bibliotecária da ABO-GO), Mrs. Amelia Williamson DeStefano (Associate Editor, PennWell Dental Group), Mr. Noko Reagan Mojela (Editorial Assistant, South African Dental Journal). Last but not least, we would like to thank the librarians of Malmö University (with a special thanks to Ms. Anneli Svensson), who helped us to obtain some articles.
RSG is a research fellow at CAPES, Brazil, Proc. 88881.119257/2016-0. CCG is a research fellow at CAPES, Brazil, Proc. 88881.118879/2016-01.
Accepted
Article
REFERENCES
1. WHO. World Health Organization Classification of Head and Neck Tumours. 4th edition ed. Lyon: IARC Press; 2017.
2. Bezak B, Lehrke H, Elvin J, Gay L, Schembri-Wismayer D, Viozzi C. Comprehensive Genomic Profiling of Central Giant Cell Lesions Identifies Clinically Relevant Genomic Alterations. J Oral Maxillofac Surg. 2017;75(5):955-961.
3. De Lange J, Van den Akker HP. Clinical and radiological features of central giant-cell lesions of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99(4):464-470.
4. Chuong R, Kaban LB, Kozakewich H, Perez-Atayde A. Central giant cell lesions of the jaws: a clinicopathologic study. J Oral Maxillofac Surg. 1986;44(9):708-713.
5. Kaban LB, Troulis MJ, Ebb D, August M, Hornicek FJ, Dodson TB. Antiangiogenic therapy with interferon alpha for giant cell lesions of the jaws. J Oral Maxillofac Surg. 2002;60(10):1103-1111; discussion 1111-1103.
6. Moher D, Liberati A, Tetzlaff J, Altman DG, Grp P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med. 2009;151(4):264-269, W264.
7. Pina-Neto JM, Moreno AF, Silva LR, Velludo MA, Petean EB, Ribeiro MV, et al. Cherubism, gingival fibromatosis, epilepsy, and mental deficiency (Ramon syndrome) with juvenile rheumatoid arthritis. Am J Med Genet. 1986;25(3):433-441.
8. Ernst LM, Quinn PD, Alawi F. Novel oral findings in Schimmelpenning syndrome. Am J Med Genet A. 2007;143a(8):881-883.
9. Cohen MM, Jr., Gorlin RJ. Noonan-like/multiple giant cell lesion syndrome. Am J Med Genet. 1991;40(2):159-166.
10. Toriello HV, Bultman R, Panek RW, Hammers Y, Kohut G, Droste P, et al. Non-ossifying fibromas and giant cell reparative granulomas in a child with ocular-ectodermal syndrome. Clin Dysmorphol. 1999;8(4):265-268.
11. Chrcanovic BR, Gomez RS, Freire-Maia B. Neurofibromatosis type 1 associated with bilateral central giant cell granuloma of the mandible. J Craniomaxillofac Surg. 2011;39(7):538-543. 12. Campanacci M, Laus M, Boriani S. Multiple non-ossifying fibromata with extraskeletal
anomalies: a new syndrome? J Bone Joint Surg Br. 1983;65(5):627-632.
13. Fernandez-Cooke E, Cruz-Rojo J, Gallego C, Romance AI, Mosqueda-Pena R, Almaden Y, et al. Tumor-induced rickets in a child with a central giant cell granuloma: a case report. Pediatrics. 2015;135(6):e1518-1523.
Accepted
Article
14. Chrcanovic BR, Brennan PA, Rahimi S, Gomez RS. Ameloblastic fibroma and ameloblastic fibrosarcoma: A systematic review. J Oral Pathol Med. 2018;47(4):315-325.
15. Chrcanovic BR, Gomes CC, Gomez RS. Peripheral giant cell granuloma: an updated analysis of 2824 cases reported in the literature. J Oral Pathol Med. 2018.
16. Chrcanovic BR, Gomez RS. Cementoblastoma: An updated analysis of 258 cases reported in the literature. J Craniomaxillofac Surg. 2017;45(10):1759-1766.
17. Edwards PC, Fox J, Fantasia JE, Goldberg J, Kelsch RD. Bilateral central giant cell granulomas of the mandible in an 8-year-old girl with Noonan syndrome (Noonan-like/multiple giant cell lesion syndrome). Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99(3):334-340. 18. Teixeira RC, Horz HP, Damante JH, Garlet GP, Santos CF, Nogueira RL, et al. SH3BP2-encoding
exons involved in cherubism are not associated with central giant cell granuloma. Int J Oral Maxillofac Surg. 2011;40(8):851-855.
19. van den Berg H, Schreuder WH, de Lange J. Multiple central giant cell tumour lesions are exclusively linked to syndromes related to RAS/MAPK pathway anomalies. Int J Oral Maxillofac Surg. 2017;46(10):1354-1355.
20. de Lange J, van den Akker HP, Veldhuijzen van Zanten GO, Engelshove HA, van den Berg H, Klip H. Calcitonin therapy in central giant cell granuloma of the jaw: a randomized double-blind placebo-controlled study. Int J Oral Maxillofac Surg. 2006;35(9):791-795.
21. Schreuder WH, Peacock ZS, Ebb D, Chuang SK, Kaban LB. Adjuvant Antiangiogenic Treatment for Aggressive Giant Cell Lesions of the Jaw: A 20-Year Experience at Massachusetts General Hospital. J Oral Maxillofac Surg. 2017;75(1):105-118.
22. Schreuder WH, van den Berg H, Westermann AM, Peacock ZS, de Lange J. Pharmacological and surgical therapy for the central giant cell granuloma: A long-term retrospective cohort study. J Craniomaxillofac Surg. 2017;45(2):232-243.
23. Harris M. Central giant cell granulomas of the jaws regress with calcitonin therapy. Br J Oral Maxillofac Surg. 1993;31(2):89-94.
24. Flanagan AM, Nui B, Tinkler SM, Horton MA, Williams DM, Chambers TJ. The multinucleate cells in giant cell granulomas of the jaw are osteoclasts. Cancer. 1988;62(6):1139-1145. 25. Jacoway JR, Howell FV, Terry BC. Central giant cell granuloma — an alternative to surgical
therapy. Oral Surg Oral Med Oral Pathol. 1988;66:572.
26. Terry BC, Jacoway JR. Management of central giant cell lesions: An alternative to surgical therapy. Oral Maxillofac Surg Clin N Am. 1994;6:579-601.
Accepted
Article
27. de Lange J, van den Akker HP, van den Berg H. Central giant cell granuloma of the jaw: a review of the literature with emphasis on therapy options. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(5):603-615.
28. Hirayama T, Sabokbar A, Athanasou NA. Effect of corticosteroids on human osteoclast formation and activity. J Endocrinol. 2002;175(1):155-163.
29. Kaban LB, Mulliken JB, Ezekowitz RA, Ebb D, Smith PS, Folkman J. Antiangiogenic therapy of a recurrent giant cell tumor of the mandible with interferon alfa-2a. Pediatrics. 1999;103(6 Pt 1):1145-1149.
30. Collins A. Experience with anti-angiogenic therapy of giant cell granuloma of the facial bones. Ann R Australas Coll Dent Surg. 2000;15:170-175.
31. de Lange J, van den Akker HP, van den Berg H, Richel DJ, Gortzak RA. Limited regression of central giant cell granuloma by interferon alpha after failed calcitonin therapy: a report of 2 cases. Int J Oral Maxillofac Surg. 2006;35(9):865-869.
32. Baldo BA. Side effects of cytokines approved for therapy. Drug Saf. 2014;37(11):921-943. 33. Goldman KE, Marshall MK, Alessandrini E, Bernstein ML. Complications of alpha-interferon
therapy for aggressive central giant cell lesion of the maxilla. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100(3):285-291.
34. Barlow CF, Priebe CJ, Mulliken JB, Barnes PD, Mac Donald D, Folkman J, et al. Spastic diplegia as a complication of interferon Alfa-2a treatment of hemangiomas of infancy. J Pediatr. 1998;132(3 Pt 1):527-530.
Accepted
Article
FIGURE LEGENDS
Figure 1. Distribution of central giant cell lesions according to age, for the cases which the patients’ age were informed (n=1701).
Figure 2. Topographical distribution of the known precise locations (n=721) of central giant cell lesions, with 218 cases affecting the maxilla and 503 the mandible. Cases involving multiple regions (or an entire quadrant) are indicated by arrows. Numbers at the top and bottom of the lines indicate cases involving both adjoining regions: anterior/premolar, premolar/molar. The asterisk (*) indicates the number of lesions from the mandibular body that reached the angle and/or ramus. For the rest of the lesions (n=1549), the location was the ‘maxilla’ (n=260), ‘mandible’ (n=534), ‘anterior maxilla’ (n=89), ‘posterior maxilla’ (n=55), ‘anterior mandible’ (n=158), ‘posterior mandible’ (n=236), ‘maxillary sinus’ (n=31), ‘mandibular condyle’ (n=9), ‘mandibular ramus’ (n=20), ‘mandibular body’ (n=17), ‘mandibular coronoid’ (n=2), ‘mandibular angle’ (n=4), ‘mandibular angle/ramus’ (n=6), ‘palate’ (n=10), ‘nasolabial groove’ (n=3), ‘almost entire mandible’ (n=4), ‘mandibular body/parasymphysis’ (n=3), ‘mandibular ramus/condyle’ (n=2), ‘mandibular ramus/condyle/coronoid’ (n=1), ‘mandibular angle/ramus/condyle’ (n=1), ‘mandibular body/symphysis’ (n=2), ‘mandibular bilateral body/symphysis’ (n=1), ‘mandibular retromolar area’ (n=1), and not available (n=100). For those cases with available information, 152 out of 1104 lesions (13.8%) crossed the midline of the jaws, and the maxillary sinus was significantly affected in 54 out 168 lesions (32.1%) in the maxilla. Eleven lesions reached the coronoid process and 20 the condylar process of the mandible.
Table Legends
Table 1. Demographic and clinical features of central giant cell lesions described in the literature. Table 2. Comparison of recurrence between aggressive and non-aggressive central giant cell lesions after first treatment (being a surgery) – for the lesions with available information about treatment and recurrence.
Table 3. Recurrence rate for central giant cell lesions according to different factors – for the lesions submitted to surgery (curettage, enucleation, marginal or segmental resection) as the first
treatment, and for the lesions with available information about both recurrence and the factors here included.
Table 4. Comparison of recurrence between aggressive and non-aggressive central giant cell lesionsa after drug treatment – for the lesions with available information about aggressiveness, drug
Accepted
Article
Table 1. Demographic and clinical features of central giant cell lesions described in the literature.
Variables
n 2270
Age (years), mean±SD (min-max) 25.8±15.3 (0-85; n=2216) Men 22.3±16.9 (0-85; n=663) Women 25.8±16.3 (1-85; n=1031) p value a <0.001 Gender, n (%) Men 876 (39.2) Women 1357 (60.8) Unknown 37 Jaw, n (%) Maxilla 668 (30.8) Mandible 1503 (69.2) Unknown 99 Bone expansion, n (%) Yes 745 (91.7) No 67 (8.3) Unknown 1458 Symptomatic, n (%) Yes 183 (20.6) No 706 (79.4) Unknown 1381
Cortical bone perforation, n (%)
Yes 329 (50.9) No 317 (49.1) Unknown 1624 Cortical thinning, n (%) Yes 328 (84.3) No 61 (15.7) Unknown 1881 Locularity, n (%) Unilocular 509 (61.8) Multilocular 314 (38.2) Unknown 1447 Tooth displacement/unerupted, n (%) Yes 300 (38.3) No 483 (61.7) Unknown 1487
Tooth root resorption, n (%)
Yes 183 (22.8)
No 620 (77.2)
Unknown 1467
Accepted
Article
Curettage 407 (33.8) Enucleation 476 (39.5) Enucleation alone 165 Enucleation + curettage 304 Enucleation + peripheral osteotomy 7 Debulking 5 (0.4) Marginal resection 63 (5.2) Segmental resection c 82 (6.8) Corticoid 75 (6.2) Calcitonin 80 (6.6) Interferon 2 (0.2) Monoclonal antibody 5 (0.4) Radiotherapy 4 (0.3) Unknown 1064Recurrence after first treatment, n (%)
Yes 232 (17.6)
No 1084 (82.4)
Unknown 954
Follow-up time (months), mean±SD (min-max)
53.2±41.0 (1-324; n=852) Lesion size (cm), mean±SD
(min-max)
3.9±2.1 (0.5-15.0; n=789) SD – standard deviation
a
Comparison of the mean age between men and women (Mann-Whitney test) c
Accepted
Article
Table 2. Comparison of recurrence between aggressive and non-aggressive central giant cell lesions after first treatment (being a surgery) – for the lesions with available information about treatment and recurrence.
Treatment Aggressive lesions Non-aggressive lesions p value b
Recurrence/total (% recurrence)
Curettage 32/86 (37.2) 8/89 (9.0) <0.001 c
Enucleation 16/87 (18.4) 5/50 (10) 0.189 c
Enucleation alone 10/58 (17.2) 1/15 (6.7) 0.284 d
Enucleation + curettage 6/27 (22.2) 4/34 (11.8) 0.227 d
Enucleation + peripheral osteotomy 0/2 (0) 0/1 (0) -
Debulking 2/2 (100) 0/1 (0) -
Marginal resection 3/32 (9.4) 0/13 (0) 0.350 d
Segmental resection a 2/34 (5.9) 0/14 (0) 0.497 d
TOTAL 55/241 (22.8) 13/167 (7.8) <0.001 c
a
Resection with continuity defect b
Comparison of the recurrence rates between aggressive and non-aggressive lesions c
Pearson’s chi-squared test d
Accepted
Article
This article is protected by copyright. All rights reserved.
Table 3. Recurrence rate for central giant cell lesions according to different factors – for the lesions submitted to surgery (curettage, enucleation, marginal or segmental resection) as the first treatment, and for the lesions with available information about both recurrence and the factors here included.
Factor Recurrence/total
(% recurrence)
p value Odds ratio (95% CI) p value
Surgical treatment
Curettage 146/928 (15.7) 0.558 b 0.909 (0.661, 1.250) 0.558 (Cur vs Enuc)
Enucleation 64/441 (14.5) 0.804 b 1.093 (0.541, 2.207) 0.804 (Cur vs Marg)
Marginal resection 10/59 (16.9) 0.010 b 0.240 (0.074, 0.773) 0.017 (Cur vs Seg)
Segmental resection a 3/70 (4.3) 0.621 b 1.202 (0.579, 2.494) 0.621 (Enuc vs Marg)
0.019 b 0.264 (0.081, 0.864) 0.028 (Enuc vs Seg) 0.017 b 0.219 (0.057, 0.839) 0.027 (Marg vs Seg) Aggressive lesion No 13/166 (7.8) <0.001 b 1 Yes 53/239 (22.2) 3.354 (1.763, 6.381) <0.001 Jaw Maxilla 45/300 (15.0) 0.658 b 1 Mandible 101/926 (16.1) 1.090 (0.744, 1.597) 0.658 Bone expansion No 1/14 (7.1) 0.233 c 1 Yes 81/429 (18.9) 3.026 (0.390, 23.464) 0.289
Cortical bone perforation
No 22/197 (11.2) <0.001 b 1 Yes 61/202 (30.2) 3.441 (2.014, 5.879) <0.001 Locularity Unilocular 55/264 (20.8) 0.666 b 1 Multilocular 28/147 (19.0) 0.894 (0.538, 1.485) 0.666
Tooth root resorption
No 52/307 (16.9)
<0.001 b 1
Yes 34/95 (35.8) 2.733 (1.634, 4.573) <0.001
Accepted
Article
This article is protected by copyright. All rights reserved.
0.1-2.0 9/63 (14.3) 0.500 b,d 0.750 (0.324, 1.735) d 0.502 2.1-4.0 21/189 (11.1) 0.268 b,e 1.596 (0.694, 3.667) e 0.271 4.1-6.0 25/119 (21.0) 0.327 b,f 1.592 (0.626, 4.050) f 0.329 >6.0 13/62 (21.0) 0.018 b,g 2.128 (1.130, 4.006) g 0.019 0.049 b,h 2.122 (0.991, 4.545) h 0.053 0.995 b,i 0.998 (0.469, 2.120) i 0.995 CI - confidence interval a
Resection with continuity defect b
Pearson’s chi-squared test c
Fisher’s exact test
Comparison of recurrence rates (or probability of recurrence) between lesions of size range: d 0.1-2.0 vs 2.1-4.0 e 0.1-2.0 vs 4.1-6.0 f 0.1-2.0 vs >6.0 g 2.1-4.0 vs 4.1-6.0 h 2.1-4.0 vs >6.0 i 4.1-6.0 vs >6.0
Accepted
Article
Table 4. Comparison of recurrence between aggressive and non-aggressive central giant cell lesionsa after drug treatment – for the lesions with available information about aggressiveness, drug
treatment, and recurrence. Drug Aggressive
lesion?
Positive response No response
n (%) p value b Complete regression n (%) Partial regression n (%) Total n (%) Corticoid Yes 18 (54.5) 6 (18.2) 24 (72.7) 9 (27.3) 0.010 d No 20 (66.7) 9 (30.0) 29 (96.7) 1 (3.3) ND 3 (42.8) 2 (28.6) 5 (71.4) 2 (28.6) Total 41 (58.6) 17 (28.3) 58 (86.9) 12 (17.1) Calcitonin Yes 25 (56.8) 9 (20.5) 34 (77.3) 10 (22.7) 0.350 d No 5 (25.0) 13 (65.0) 18 (80.0) 2 (20.0) ND 10 (71.4) 2 (14.3) 12 (85.7) 2 (14.3) Total 40 (51.3) 24 (30.8) 64 (82.1) 14 (17.9) Interferon c Yes 36 (90.0) 2 (5.0) 38 (95.0) 2 (5.0) 0.951 d No 1 (100) 0 (0) 1 (100) 0 (0) ND - - - - Total 37 (90.2) 2 (4.9) 39 (95.1) 2 (4.9)
ND - Not determined, i.e., the clinical and radiographic information available was not enough in order to classify the lesions according to the aggressiveness criteria proposed by Chuong et al. (1986) and Kaban et al. (2002)
a
Cases in patients who were previously treated by chemotherapy, radiotherapy or by simultaneous or consecutive use of two different drugs were excluded from the analysis.
b
Comparison of the recurrence rates between ‘positive response’ (complete/partial regression) and ‘no response’ in aggressive and non-aggressive lesions.
c
All cases except for one (drug alone, with complete regression) were preceded by enucleation d