Linköping University | Department of Physics, Chemistry and Biology Bachelor thesis, 16 hp | Biology programme: Physics, Chemistry and Biology Spring term 2018 | LITH-IFM-x-EX-- 18/3528--SE
The Effects of Abiotic Stress on
Alternative Splicing in
Non-specific Lipid Transfer Proteins
in Marchantia polymorpha
Hanna Ahlsén
Examinator, Jordi Altimiras, IFM Biologi, Linköpings universitet Tutor, Johan Edqvist, IFM Biologi, Linköpings universitet
2
1Denna rapport är ett examensarbete på kandidatnivå (16 hp) som har genomförts i samarbete med en
studentkollega, Linnéa Fredén. Samarbetet har omfattat projektetplanering samt insamling och bearbetning av data, medan studenterna var för sig har författat och strukturerat rapporten i alla dess delar Datum Date 2018-06-21 Avdelning, institution Division, Department
Department of Physics, Chemistry and Biology Linköping University
URL för elektronisk version
ISBN
ISRN: LITH-IFM-x-EX--18/3528--SE
_________________________________________________________________ Serietitel och serienummer ISSN
Title of series, numbering ______________________________
Språk Language Svenska/Swedish Engelska/English ________________ Rapporttyp1 Report category Licentiatavhandling Examensarbete C-uppsats D-uppsats Övrig rapport _____________ Titel
Title The Effects of Abiotic Stress on Alternative Splicing in Non-specific Lipid Transfer Proteins in Marchantia polymorpha
Författare
Author Hanna Ahlsén
Nyckelord
Keyword
abiotic stress, alternative splicing, cold, drought, Marchantia polymorpha, non-specific lipid transfer proteins
Sammanfattning
Abstract
Due to global warming, our planet will experience more extreme weather conditions. Plants can protect themselves against these abiotic stress conditions with their stress response, which includes alternative splicing of certain genes. Alternative splicing is a post-transcriptional process where a single gene gives rise to different mRNAs, which in turn produces different proteins. In plants, this is usually done by intron retention. One type of protein that may be involved in this stress response are the non-specific lipid transfer proteins (LTPs). Indeed, evidence of intron retention has been found in the LTP genes in the liverwort Marchantia polymorpha, called MpLTPd. To investigate whether this alternative splicing is caused by abiotic stress or not, I subjected the moss to two different types of stress trials, drought and cold, and compared the general expression of the intron in MpLTPd2 and MpLTPd3 from the stressed samples to samples from a moss grown under normal conditions. I found that the expression of the intron did change in the stressed moss, but none of the differences were significant. This suggests that alterative splicing in MpLTPd2 and MpLTPd3 is not caused by cold and drought and that the intron-containing protein plays no role in the protection of M. polymorpha against abiotic stress.
3 Table of contents
1 Abstract ... 4
2 Introduction ... 4
3 Material & methods... 6
3.1 Database search ... 6
3.2 Growth of Marchantia polymorpha ... 7
3.3 Stress trials ... 7
3.4 RNA isolation and cDNA synthesis ... 7
3.5 Primer design ... 8
3.6 Gene expression analysis ... 8
3.7 Normalization of gene expression ... 8
3.8 Statistical analysis ... 9
4 Results ... 9
4.1 Intron and exon presence in mRNA sequencing experiments ... 9
4.2 Alternative splicing in MpLTPd2 and MpLTPd3 ... 10
4.3 Gene expression ... 10
4.4 Statistical analysis ... 13
5 Discussion ... 13
5.1 Social & ethical aspects ... 15
5.2 Conclusion ... 15
6 Acknowledgements ... 16
7 References ... 16
4 1 Abstract
Due to global warming, our planet will experience more extreme weather conditions. Plants can protect themselves against these abiotic stress conditions with their stress response, which includes alternative splicing of certain genes. Alternative splicing is a post-transcriptional process where a single gene gives rise to different mRNAs, which in turn produces different proteins. In plants, this is usually done by intron
retention. One type of protein that may be involved in this stress response are the non-specific lipid transfer proteins (LTPs). Indeed, evidence of intron retention has been found in the LTP genes in the liverwort
Marchantia polymorpha, called MpLTPd. To investigate whether this
alternative splicing is caused by abiotic stress or not, I subjected the moss to two different types of stress trials, drought and cold, and compared the general expression of the intron in MpLTPd2 and MpLTPd3 from the stressed samples to samples from a moss grown under normal conditions. I found that the expression of the intron did change in the stressed moss, but none of the differences were significant. This suggests that alterative splicing in MpLTPd2 and MpLTPd3 is not caused by cold and drought and that the intron-containing protein plays no role in the protection of M.
polymorpha against abiotic stress.
2 Introduction
It is no secret that our planet is currently experiencing global warming. Because of this we will, among other things, experience more extreme weather conditions. This can be very damaging to plants, which are essentially sessile in nature. The plants will need to protect themselves from different types of abiotic stress, including but not limited to higher temperatures and dry conditions (Prasad et al., 2011). To achieve this, they make use of their stress response that can be triggered by both biotic and abiotic stimuli. Apart from heat and drought, abiotic stress moments also include cold and high salinity. If we want to understand how plants protect themselves, we need to understand the stress response. We know that an example of the stress response against heat is to invoke the highly conserved heat shock response which results in the production of heat shock proteins (Nover et al., 1996). Interestingly, heat shock proteins sometimes undergo a procedure that appears to be very important for stress protection in plants – alternative splicing (Kannan et al., 2017).
5
Alternative splicing is a common post-transcriptional process in
eukaryotes where several proteins can be expressed from the same gene. This can be performed in several different ways. In animals, the most common process is exon skipping, where an exon (coding sequence) in the transcript is skipped over and is absent in the mature mRNA (Pan et
al., 2008). In plants, the most common form of alternative splicing is
intron retention, where an intron (non-coding sequence) is not spliced away, which is the normal process, and becomes part of the mature
mRNA (Reddy et al., 2013). Thanks to alternative splicing, the number of genes can remain the same, but the number of mRNA transcripts can increase, resulting in a higher diversity of proteins. The more complex the organism, the more genes display alternative splicing tends to be (Iñiguez
et al., 2017). This can be seen in humans, where more than 90% of genes
containing several exons can undergo alternative splicing (Wang et al., 2008). This number is lower in plants, but alternative splicing is still involved in several important processes, such as plant growth and of course the stress response (Yuchen et al., 2017). For example, in genes containing introns in the model plant Arabidopsis thaliana, the level of alternative splicing is about 61% (Marquez et al., 2012).
Several plants have shown an increase in alternative splicing in genes involved in the abiotic stress response. Salt stress in cotton (Gossypium
davidsonii) and arabidopsis (Arabidopsis thaliana) invokes an increase in
alternative splicing in several salt response genes (Zhu et al., 2018; Feng
et al, 2015), while heat stress causes more alternative splicing in grape
(Vitis vinifera), wheat (Triticum aestivum), several mangrove species (Sonneratia) and arabidopsis, among others (Jiang et al., 2017; Liu et al., 2018; Yamg et al., 2017; Kannan et al., 2017). Arabidopsis also responds to cold and drought with increased alternative splicing (Iida et al., 2004; Liu et al., 2018).
One family of proteins that might be involved in the stress response are the non-specific lipid transfer proteins, or LTPs (Guo et al., 2013; Edstam
et al., 2014). LTPs are found in abundance in all examined land plants
(Edstam et al., 2011). Apart from the possible stress protection, LTP functions may involve the transport of lipids which forms the cuticula in plants (Edstam et al., 2013) and contribution to plant growth (Nieuwland
et al., 2005). Though the function of these proteins is unclear, their
structure has been documented; all the proteins are made up of four conserved disulfide bridges consisting of eight cysteines (Shin et al., 1995) and their size is normally smaller than 10 kDa (Kader, 1996; Salminen et al., 2016) Depending on the distance between the cysteines,
6
the proteins can be split into different types (Shin et al., 1995). The five major types are the LTP1, LTP2, LTPc, LTPd and LTPg (Edstam et al., 2011). It is possible that the earliest form of the LTPs are the LTPd and LTPg types (Edstam et al., 2011; Salminen et al., 2016). It is fitting, then, that the LTPd type can be found in the liverwort Marchantia polymorpha, one of the earliest forms of land plants (Kenrick and Crane, 1997). In M.
polymorpha, the LTPd type consists of eight different proteins,
MpLTPd1-MpLTPd8 (Salminen et al., 2016). In relation to other vascular plants, this is a rather low amount of LTP genes which can facilitate the study of the different LTP types (Bowman et al., 2017). Evidence of alternative splicing has been found in all MpLTPd genes (except for MpLTPd7) in M. polymorpha in the form of intron retention, but the cause is unknown. However, since a possible function of LTPd is to increase the plant’s tolerance against abiotic stress (Guo et al., 2013; Edstam et al., 2014), and since genes involved in the stress response are often alternatively spliced, one can assume that an increase of the intron-containing mRNA will be found in a stressed sample of M. polymorpha. Thus, the aim of this study was to investigate whether alternative splicing truly exists in the MpLTPd genes in the liverwort Marchantia
polymorpha, and to examine whether the intron-containing mRNA codes
for a protein with a function relating to abiotic stress protection or not. This study will examine two of the genes with the highest expression in
M. polymorpha, MpLTPd2 and MpLTPd3 (Fig. S1).
3 Material & methods 3.1 Database search
To verify the presence of the intron and thus alternative splicing in the mRNA sequence of LTPd genes in M. polymorpha, a database search was conducted using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi). First, experiments that had sequenced the mRNA genome of M. polymorpha were collected from the SRA database
(https://www.ncbi.nlm.nih.gov/sra). Then, those sequences were compared to the sequences for the intron and the exon of MpLTPd1-MpLTPd8. When a match was found, the accession codes for the relevant database sequences were noted.
7 3.2 Growth of Marchantia polymorpha
M. polymorpha was grown asexually from gemmae on agar plates sealed
with micropore tape. The plates were placed in a tissue culture chamber (CLF Plant Climatics, CU-36L/5) and the moss was grown for four weeks at 22 °C. There were six plants on each agar plate and four plates in total (Fig. 1).
Figure 1. One of the agar plates with growing plants of M. polymorpha, this one with the lid removed. Each of the four plates contained six plants grown asexually from gemmae. The moss pictured had been growing for four weeks.
3.3 Stress trials
The stress trials used in this study were cold and drought, which were performed separately. For cold, one agar plate with moss was placed on ice for 24h. For drought, the lid from one agar plate was removed for 40h. 3.4 RNA isolation and cDNA synthesis
Moss tissue from the control plants, the cold trial plants and the drought trial plants was collected. From each group, two samples were used, resulting in a total of six samples. To facilitate the extraction of RNA, the moss tissue was ground in separate microtubes. From the ground moss samples, RNA was extracted with an RNEasy Plant Mini Kit (Qiagen,
8
Hilden, Germany) according to the supplied protocol. The buffer used was RLT mixed with β-mercaptoethanol. Then, to check the purity of the samples, a quality control was performed using a NanoDrop ND-1000 Spectrophotometer (Saveen & Werner AB). Afterwards, possible DNA contaminations were eliminated using a DNA-free Kit (Ambion, Vilnius, Lithuania). Lastly, cDNA for all samples was synthesized with the help of a Revert Aid First Strand cDNA Synthesis Kit (Thermo Scientific, Vilnius, Lithuania).
3.5 Primer design
In order to verify alternative splicing in the moss tissue samples, both the intron and the exon had to be present. To identify these sequences,
primers that bind to the stop codon of the intron respectively the exon were used (Fig. S1). These were called the reverse primers, while the primers binding to the common start codon were called the forward primers (Table S1). For each gene, one test tube containing the intron reverse primers + the forward primer and one tube containing the exon reverse primers + the forward primer were used to measure the different expressions of the intron and exon. These samples were then used for a PCR analysis, where the expected results were the expressions of the intron respectively the exon for MpLTPd2 and MpLTPd3.
3.6 Gene expression analysis
To ensure the presence of both the intron and the exon in the control samples, several 25 µl PCR reactions were performed using a KAPA Taq PCR Kit (Kapa Biosystems, Kape Town, South Africa). To obtain the best results, a PCR program was optimized (95 °C for 3 min, 95 °C for 30 s, 55 °C for 30 s, 72 °C for 45 s, 4 °C for infinity, 35 cycles) by using a gradient PCR. The gradient was run from 55 to 62 °C for MpLTPd2 and from 53 to 60 °C for MpLTPd3.
3.7 Normalization of gene expression
A real time quantitative PCR (qPCR) was used in order to measure the quantity of the introns and the possible differences in their expression levels. Two 25 µl reactions each for MpLTPd2 and MpLTPd3 were executed, and Ct values for all samples were obtained. The normalized expression values (ΔCt) were then calculated as in equation 1.
ΔCt = Ct(target sample) − Ct(reference sample) (1)
The intron expression values from the cold and drought samples were then compared to the ones from the two types of control samples. These
9
were the exon expression value of MpLTPd2 and MpLTPd3 from the untreated moss, and a reference gene, MpACT (Saint-Marcoux et al., 2015). The higher the ΔCt value, the less the intron was expressed in the stressed moss compared to the references. Lastly, to examine the
difference in the level of intron transcription after the stress trials, the expression fold (2-ΔΔCt) was calculated. An expression fold value above
one indicated an upregulation of the intron, and the higher the value the larger the upregulation. A value below one indicated a downregulation. The ΔΔCt value was calculated according to equation 2.
ΔΔCt = ΔCt(target sample) − ΔCt(reference sample) (2)
3.8 Statistical analysis
In order to detect possible significant differences, a Mann-Whitney U test was performed on the ΔCt values. The significance level was set to 0.05.
4 Results
4.1 Intron and exon presence in mRNA sequencing experiments The sequences for both the intron and the exon were found in several database mRNA sequences (Table 1). However, the intron was not always present (Table 1B), supporting the existence of alternative splicing in M. polymorpha.
Table 1. Accession codes for database sequences where mRNA for M.
polymorpha has been sequenced. A) shows experiments where the intron and
exon were present, indicating alternative splicing, while in B) only the exon was found. Website used was https://blast.ncbi.nlm.nih.gov/Blast.cgi
A MpLTPd1 MpLTPd2 MpLTPd3 MpLTPd4 MpLTPd5 MpLTPd6 MpLTPd8 SRX1162423 SRX3661970 SRX3661970 SRX3661970 SRX3661970 SRX3661970 SRX3661975 SRX682815 SRX3661969 SRX3661969 SRX3661971 SRX3661969 SRX3661969 SRX3661972 SRX114615 SRX3661975 SRX1162423 SRX114615 SRX3661975 SRX114614 SRX447355 SRX114614 SRX114615 SRX114614 B MpLTPd1 MpLTPd2 MpLTPd3 MpLTPd4 MpLTPd5 MpLTPd6 MpLTPd8 SRX3661970 SRX3661970 SRX1162423 SRX3661970 SRX682815 SRX3661970 SRX3661975 SRX3661969 SRX682815 SRX447355 SRX447355
10
4.2 Alternative splicing in MpLTPd2 and MpLTPd3 The intron was expressed in both the MpLTPd2 and MpLTPd3
transcripts for all tested temperatures in the endpoint PCR (Fig. 2). The exon was also expressed in both genes and only failed to show up at 53 °C for MpLTPd3 (Fig. 2B). The higher the annealing temperature, the stronger the bands became, indicating a higher expression.
Figure 2. Results from the endpoint PCR reactions with a 1kb DNA ladder (Fermentas, #SM1163) as template. A) MpLTPd2, where the first four bands (well 1-4) all depict the intron, and the last four (well 5-8) represent the exon. The gradient was run from 55 to 62 °C. B) MpLTPd3, where the first four bands (well 1-4) all depict the intron, and the last three (well 6-8) represent the exon. The gradient was run from 53 to 60 °C.
4.3 Gene expression
The general expression values for the MpLTPd2 and MpLTPd3 intron transcripts from the stress trials are illustrated in Fig. 3 and 4,
respectively. The calculated ΔCt-values used for the graphs can be found in Table S2. A lower ΔCt-value indicates a higher expression of the intron, while a higher value means a lower intron expression. For the MpLTPd2 cold sample, there was a lower expression of the intron compared to both the reference gene MpACT (Fig. 3A) and the exon (Fig. 3B). For drought, not much of a difference was seen in comparison to MpACT (Fig. 3A), but the intron during drought stress appear to have been expressed slightly more than in the MpACT reference gene. A larger difference was seen when the reference gene was the exon (Fig. 3B), but it was still rather small. Here, however, the expression of the intron during drought stress conditions appeared to be higher than in the exon reference gene. No significant differences were found (Table 2).
11
Figure 3. The average general expression value (ΔCt) of MpLTPd2 after the stress trials. The lower the value, the higher the general expression. The bars represent the standard deviations. The intron values are compared to A) the reference gene MpACT and B) the MpLTPd2 exon.
For MpLTPd3, the differences were not as prominent as for MpLTPd2. The most noticeable difference for intron expression was seen in the drought sample compared to both MpACT (Fig. 4A) and the exon (Fig. 4B), where the intron was found in lower quantities. In the cold sample, the intron appeared to be expressed less when compared to the MpACT reference gene (Fig. 4A) than to the exon reference (Fig. 4B), where the difference was almost non-existent. No significant differences were found (Table 2).
Figure 4. The average general expression value (ΔCt) of MpLTPd3 after the stress trials. The lower the value, the higher the general expression. The bars represent the standard deviations. The intron values are compared to A) the reference gene MpACT and B) the MpLTPd3 exon.
0 2 4 6 8 10
Control Drought Cold
Δ Ct A 0 1 2 3
Control Drought Cold
Δ Ct B 0 2 4 6 8 10 12
Control Drought Cold
ΔC t A 0 2 4 6 8 10
Control Drought Cold
ΔC
t
12
The assessment of the transcriptional regulations during the stress trials are illustrated in Fig. 5 in the form of the expression fold (2-ΔΔCt). The most prominent changes are seen in MpLTPd2 (Fig. 5A), where the intron has been upregulated the most in the drought sample. The intron in the cold sample appears to have been both upregulated (reference
MpACT) and downregulated (reference exon). Since the values are similar, but opposite, this most likely indicates no change in expression. The changes are somewhat less dramatic for MpLTPd3 (Fig. 5B). The expression of the intron under drought conditions has been slightly upregulated compared to both reference genes, though the one regarding the exon reference is very small (2-ΔΔCt = 1,002), indicating no change in
expression. As for MpLTPd2, the intron under cold stress in MpLTPd3 has been upregulated or downregulated depending on which reference gene work as a comparison.
Figure 5. The level of transcriptional regulation after the stress trials, with the
values shown here as the expression fold (2-ΔΔCt). The control samples are set
to one. Values above one represent an upregulation, while values below one represent a downregulation. The expression fold of the introns are tested against both the reference gene MpACT (black) and the exon for the relevant MpLTPd gene (grey), and A) shows the results for MpLTPd2, while B) shows the results for MpLTPd3.
0 5 10
Drought Cold
A MpACT as reference MpLTPd2 exon as reference
0,5 1 1,5
Drought Cold
13 4.4 Statistical analysis
The Mann-Whitney U-test showed no significant differences for any of the samples tested, neither for MpLTPd2 nor MpLTPd3 (Table 2). For a result to be considered significant, a p-value below 0.05 was needed, and no such thing was found.
Table 2. Results from the Mann-Whitney U-test for MpLTPd2 and MpLTPd3 stress trials. A p-value below 0.05 is considered significant.
Gene Sample Reference U p
MpLTPd2 Drought MpACT 6 0.6857 MpLTPd2 Cold MpACT 1 0.1143 MpLTPd3 Drought MpACT 6 >0.999 MpLTPd3 Cold MpACT 5 0.8571 MpLTPd2 Drought MpLTPd2 exon 6 0.6857 MpLTPd2 Cold MpLTPd2 exon 6 0.6857 MpLTPd3 Drought MpLTPd3 exon 4 0.3429 MpLTPd3 Cold MpLTPd3 exon 7 0.8857 5 Discussion
I measured the expression levels of the introns in MpLTPd2 and MpLTP3 in Marchantia polymorpha after exposing them to stress conditions in the form of cold and drought. The results were then compared to samples from a moss grown under normal conditions.
Some contradictory results regarding the transcriptional regulation of the introns after the stress trials were found. According to Fig. 3 and 4, in both MpLTPd2 and MpLTPd3 the intron under drought stress conditions was seemingly more or less upregulated compared to both controls
(MpACT and the relevant gene’s exon). The intron during cold stress, on the other hand, appeared to be either downregulated (compared to
MpACT) or upregulated (compared to the exon), probably indicating a no change scenario. Nevertheless, these results suggests that the number of introns being transcribed does increase during abiotic stress in the form of drought, more so in MpLTPd2 than MpLTPd3. However, when the ΔCt-values were analyzed using a Mann-Whitney U-test, no significant
differences were found between the drought and cold stress trials and the references. This could mean that the LTPd genes increase during abiotic stress, but the intron does not. Thus, my results indicate that alternative splicing in LTPd genes in M. polymorpha is not caused by abiotic stress, at least not in the form of cold and drought. Most of the previously done
14
experiments regarding abiotic stress and alternative splicing have used heat as a stress inductor, likely because it is one of the most harmful stress situations for plants (Laloum et al.,2018), and because it is one of the effects caused by global warming. For example, Physcomitrella
patens, another moss with a similar LTP content as in M. polymorpha
(Edstam et al., 2011) has been shown to respond to heat stress with an increase in alternative splicing (Chang et al., 2014). However, this was not done primarily by intron retention, the most common way for plants to perform alternative splicing. Instead, it was done mainly by exon skipping, which is most common in animals, and by using alternative donor/acceptor sites (Chang et al., 2014). This could possibly be the case for M. polymorpha too. If so, then alternative splicing could exist as a response to abiotic stress, but not in the form of intron retention.
Another reason I might not have seen any differences in the amount of intron transcripts is because I performed the stress trials separately. In an experiment with wheat by Liu et al. (2018), they discovered that the combination of drought and heat stress resulted in different kinds of alternative splicing than when the stress was simply drought or heat. Perhaps the introns in the MpLTPd genes in M. polymorpha have a similar function and will only be transcribed into mRNA when several stress moments occur at the same time. Also, the type of stress trials chosen in this study could be another reason for me not seeing an increase in alternative splicing. It is possible that alternative splicing of MpLTPd genes is caused by a single abiotic stress situation, but not drought or cold. Instead, it could perhaps be heat or high salinity.
Lastly, it is possible that my sample size (two samples for each gene in all trials) was simply too small for me to obtain a result that accurately
portrays the situation of M. polymorpha in reality. For a more realistic result, a larger sample size is desirable. Thus, from my results, it is impossible to say with any certainty if alternative slicing in MpLTPd2 and MpLTPd3 is affected by cold and drought, or if the intron-containing mRNA produces a protein which helps increase M. polymorpha’s
15 5.1 Social & ethical aspects
It is likely that LTPs affect plant growth and help protect plants against abiotic stress (Guo et al, 2013; Edstam et al, 2014). A deeper
understanding of these proteins can give us knowledge about how plants protect themselves and how they are affected by stress. This would
enable us to develop more resilient plants and make it easier to give them an optimal environment for growth.
Some ethical aspects could become relevant if a significant upregulation of the intron had been found in the stressed moss. This result would indicate a protective function, meaning triggering this type of alternative splicing in plants could be beneficial. In that case, we would be dealing with genetic modification, a somewhat controversial topic. But as long as the hypothetical procedure and the resulting plants are examined and tested properly, I would encourage the use of genetic modification for this cause. Then, we could all benefit from the increased resilience of valuable plants against the effects of global warming.
5.2 Conclusion
In summary, this study have focused on the possible increase in
alternative splicing caused by abiotic stress in two of the LTPd genes in
Marchantia polymorpha, MpLTPd2 and MpLTPd3. The moss was
subjected to stress trials in the form of drought and cold. The different samples were then analyzed in order to discover if the intron of the two genes had an increased expression compared to a moss grown under normal conditions. This would indicate an increase in alternative splicing, meaning the intron could play a role in the protection against abiotic stress. Some differences in the intron expression between the stressed moss and the control moss were indeed found, with drought being the one with the highest upregulation of the intron’s transcription. However, no significant differences were found, implying that the cause of alternative splicing is not abiotic stress. This could mean that the introns in
MpLTPd2 and MpLTPd3 have another function, or, possibly, none at all. Due to a small sample size, it is difficult to draw any certain conclusions.
16 6 Acknowledgements
I would like to thank my tutor Johan Edqvist, who patiently answered all my questions and guided me through these experiments. I would also like to thank Linnéa Fredén, who was my partner during this project and helped completed this study with me.
7 References
Bowman JL, Kohchi T, Yamato KT, Jenkins J, Shu S, Ishizaki K, Yamaoka S, Nishihama R, Nakamura Y, Berger F, et al. (2017). Insights into land plant evolution garnered from the Marchantia
polymorpha genome. Cell, 171: 287–304
Chang CY, Lin WD, and Tu SL. (2014). Genome-Wide Analysis of Heat-Sensitive Alternative Splicing in Physcomitrella patens. Plant
Physiology, 165: 826–840
Edstam MM, Blomqvist K, Eklöf A, Wennergren U and Edqvist J. (2013). Coexpression patterns indicate that GPI-anchored non-specific lipid transfer proteins are involved in accumulation of cuticular wax, suberin and sporopollenin. Plant Molecular Biology, 83: 625-649 Edstam MM, Laurila M, Höflund A, Raman A, Dahlström KM, Salinen TA, Edqvist J and Blomqvist K. (2014). Characterization of the GPI anchored lipid transfer proteins in the moss Physcomitrella patens.
Plant Physiolgy and Biochemistry, 75: 55-68
Edstam MM, Viitanen L, Salminen TA and Edqvist J. (2011).
Evolutionary history of the non-specific lipid transfer proteins. Molecular
Plant, 4: 947-964
Feng J, Li J, Gao Z, Lu Y, Yu J, Zheng Q, Yan S, Zhang W, He H, Ma L and Zhu Z. (2015). SKIP Confers Osmotic Tolerance during Salt Stress by Controlling Alternative Gene Splicing in Arabidopsis.
Molecular Plant, 8: 1038-1052
Guo C, Ge X and Ma H. (2013). The rice OsDIL gene plays a role in drought tolerance at vegetative and reproductive stages. Plant Mol. Biol., 82: 239-253
17
Iida K, Seki M, Sakurai T, Satou M, Akiyama K, Toyoda T, Konagaya A and Shinozaki K. (2004). Genome-wide analysis of alternative pre-mRNA splicing in Arabidopsis thaliana based on full-length cDNA sequences. Nucleic Acids Research, 17: 5096-5103 Iñiguez LP, Ramírez M, Barbazuk WB and Hernández G. (2017) Identification and analysis of alternative splicing events in Phaseolus
vulgaris and Glycine max. BMC Genomics, 18: 650
Jiang J, Liu X, Liu G, Liu C, Li S and Wang L. (2017). Integrating omics and alternative splicing reveals insights into grape response to high temperature. Plant Physiology, 173: 1502-1518
Kader JC. (1996). Lipid-transfer proteins in plants. Annual review of
plant physiology and plant molecular biology, 47: 627-654
Kannan S, Halter G, Renner T and Waters ER. (2017). Patterns of alternative splicing vary between species during heat stress. AoB Plants, 10: 1-11
Kenrick P and Crane PR. (1997). The origin and early evolution of plants on land. Nature, 389: 33-39
Laloum T, Martín G and Duque P. (2018). Alternative Splicing Control of Abiotic Stress Responses. Trends in Plant Science, 23: 140-150
Liu Z, Qin J, Tian X, Xu S, Wang Y, Li H, Wang X, Peng H, Yao Y, Hu Z, Ni Z, Xin M and Sun Q. (2018). Global profiling of alternative splicing landscape responsive to drought, heat and their combination in wheat (Triticum aestivum L.). Plant Biotechnology Journal, 16: 714-726 Marquez Y, Brown JW, Simpson C, Barta A and Kalyna M. (2012). Transcriptome survey reveals increased complexity of the alternative splicing landscape in Arabidopsis. Genome Research, 6: 1184-1195 Nieuwland J, Feron R, Huisman BA, Fasolino A, Hilbers CW, Derksen J and Mariani C. (2005). Lipid transfer proteins enhance cell wall extension in tobacco. Plant Cell, 17: 2009-2019
Nover L, Scharf KD, Gagliardi D, Vergne P, Czarnecka-Verner E and Gurley WB. (1996). The HSF world: classification and properties of plant heat stress transcription factors. Cell Stress & Chaperones, 1: 215-223
18
Pan Q, Shai O, Lee LJ, Frey BJ and Blencowe BJ. (2008). Deep surveying of alternative splicing complexity in the human transcriptome by high-throughput sequencing. Nature Genetics, 40: 1413–1415
Prasad PVV, Pisipati SR, Momčilović I and Ristic Z. (2011).
Independent and combined effects of high temperature and drought stress during grain filling on plant yield and chloroplast EF-Tu expression in spring wheat. Journal of Agronomy and Crop Science, 197: 430-441 Reddy ASN, Marquez Y, Kalyna M and Barta A. (2013). Complexity of the alternative splicing landscape in plants. Plant Cell, 25: 3657–83 Saint-Marcoux D, Proust H, Dolan L and Langdale JA. (2015). Identification of reference genes for real-time quantitative PCR
experiments in the liverwort Marchantia polymorpha. PLoS One, 10: 3 (e0118678)
Salminen TA, Blomqvist K and Edqvist J. (2016). Lipid transfer proteins: classification, nomenclature, structure, and function. Planta, 244: 971-997
Shin DH, Lee JY, Hwang KY, Kim KK and Suh SW. (1995). High-resolution crystal structure of the non-specific lipid-transfer protein from maize seedlings. Structure, 3: 189-199
Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP and Burge CB. (2008). Alternative isoform regulation in human tissue transcriptomes. Nature, 456: 470-476
Yang Y, Guo W, Shen X, Li J, Yang S, Chen S, He Z, Zhou R and Shi S. (2017). Identification and characterization of evolutionarily conserved alternative splicing events in a mangrove genus Sonneratia.
Scientific Reports, 8: 4425
Zhu G, Li W, Zhang F and Guo W. (2018). RNA-seq analysis reveals alternative splicing under salt stress in cotton, Gossypium davidsonii.
19 8 Appendix A CCCTGTGAGCTGTGGGAGACTATCCGATCGTAGCCAACAACTTGCTTGGAGGGAAACGGCCTGAAGCTC CTGGGAGAGATTTTCCTCATGGCATTCGTTAAGGTTGTCGTCGTGTGCTTCATTGCGGTTTTGATCGCG GATCTTGTGGCGGCACAGAGCTGTCCCCAGACCATCACATTCAGCCCCTGCCTCACGAACGTCGGAAAC ACCGGCACGCCTCCAGCTTACGACAGTCAGTGTTGTAAACTGGTGAGGGATCCGAAATGGACGGACCAG TGCCTGTGCGCCGTCGTCTCCTCGCACTTTCCCGGTGTGGACGAGACTAAGGCCCTTAATCTGGCACAC GATTGCGGCAGGCGTGTACCGAAGGGCATTTACTGCGATGGTACGTCTCCCACCTGTGTTTTTTCTCAT TTTGATTTTTCTCATTTTCGTCCTGCTCTGCGCACCGATTCCTGAAAGGACTTCGCCTCAATAATATGG GACCTCATTGCTCGCTCATTTCCTCTTGTTTTATGTCGTCACGAGATCGTGGGTTTCTTGCGCTAGCGC CAAGTAGTGAAACGTGATTGTTGTCGTAGTTCCACCTTATTCCTCGAGTTTGACACGAGTCACTGAGCT GAAGTCTTGTCGTCTATGTCAGTGTCTGAATTTGACTGTGTTTTTGTCGATGCATCTGCAGGCAGGCCA GTGCCTCCGTAAAGACTCTCTTTACCTCCCGCGTTCGAAACACTTGAGGTCGAAGACGTTTGCATCGGT CGTTCTACGTAGGAACTAGGAGACATCGAATGTAGAGGGGAGTTATTCGCTGGGTTTTCTCTGGCAAAA CTGTAAATTTTGGCCATACACGGCGTGTTAGTTGTAATCAAGGTCACTCTTTTGCTCTACC B CTGCTCTCCAGTGCCTCTGCCTCAACGAAATGAGTAGTGGCTGTGATTGTCAAACAATCACAGCACACC TGCTTACGGCTCGAGCACTAGACGTGGCAGGGGAGGAAACTCAGGCAGCGGGTAGGGAAAAGGGATGGA GGGAGCCGAGGCAGGGAACTTTGGTATAAATGTGCGAAGCCTCACCTTTTATTGTCATCCTTCTTCACC GTTTGCATTGTCTGTCCAGGCTGCTTCTCACTAAGGCTTCGCGTCTGCACGGATACATCTCACGAGCAT CACTCATTCGAGTCTCTCTGTCTCACAATGGATCCCAGAAATGCACAGCTCGCTGCATTTGTCTTCCTT GCGATGGTCGTCGTGGCCAACGCTCAGGCGTGCCCCGGTTTGACGAGCTACGCATCCTGCCTTCAGTAC GGGAAGAAAGGCAACGCCTTTCCACCCGCCAACAGCCCATGCTGCCAGAAAATCAGAACCACTTCCGAG AAGTGCTTGTGCGACACTGCTTCCAACAACAACGGACTCGCTGACTTCGACCAACTTATCCAGCTGCCT CAGAAGTGTGGTCGCACCGTTCCTAAGGGCACCTACTGCCGAGGTAAGATTCTCGTTCCCATTGCGATG CTCTCCGCATACCTCAGCAATCCCGCTTTCTGCACTTTAGCCCTTCGACAAAGTTATGTCATGCTGTCA AACACAAACGTCTTCCCTTGAAACTTCTCCCACAGATATTTCGATCGTACCTATATATACATATCTAGC ACGGACACCCATCTCAGTCTCGCAGCCCAGGTTCTGTTTGATTGTACAGCTTAGATGACACACGCACTT TTTTCTAAAGTACTAGGACCCATTATACTGCCAGAATTTTATTCTGTCATCTCCTTTCTAGTTAATGGT AGTGGAGCTGGAATGATTCGAATTCAGCTGAATTCATCGAATAACATATGGTGGAACTTTCTCAGAATC TCTAGAATGCATTCGTTTTTTTGTTACTTTCTATTTTACTATTCTAGACTATTATAGAATTGCCCAGAG TTGTGAACTCGTCTCGTCACGCCCAAAGCTCTCCTTGATTTTCGAACATATTCTAACCCGATCATGGTC TGGTCCTTGTGCAGGCAAGAAGGTTCCCGGATGGTAGAGCTCCGGCTCTTCATTGCGGCACTTGGATGA GCATGAGGTATACAAAGGTCGCCGCCAAAGTTTGTCTGTGAGACCCCTTGTCGTTGACGACGGGGAATT GAGCTGTAGGTCCCTGATAGCAAGCTGTTCATAGCAGCCTCGCAGTGACTTGAGATTTCAACACTATCA TCGTGAAGCTTTGAGGATACTTAATCACACGAAGCTGCCAGTAGTGACTAGTTGAGACATCTCTAGCTT CTCTCTTCCCTTTTCTTCGTTACATCATCATCCTCCTCCGTTACCCCACAGCAGCCCAGGTACAGGCTC AGATGCATATGGTGCCCCAGATGCAAGCTGAGTGTAGCTGGCTGCAATTTGTGTCCCTCGTTCCAGATC TTGTGTCACCTCAAGAGTGCCGAAAATACTCTTTAATATGAACTGCCATCCGCAGCAAGCGATGTTATT CGTCTTCAGATTAATCTGTGTCTACATTATCTCTG
Figure S1. Genomic sequences of Marchantia polymorpha where the intron is highlighted in grey. The sequences marked in black represent the positions where the forward and the reveres primers are designed to bind. Two different genes are shown, A) MpLTPd2 and B) MpLTPd3.
20
Table S1. Primers used for PCR and qPCR reactions for MpLTPd2 and MpLTPd3 genes of M. polymorpha.
Name Amino acids Sequence (5’ to 3’) Tm (°C) GC-content
D2ORFF 24 CACCTCATGGCATTCGTTAAGGTT 61.0 45.8 % D2ORFR 21 TCAGGAATCGGTGCGCAGAGC 63.7 61.9 % 2D2ORFR 24 AGAGAGTCTTTACGGAGGCACTGG 64.4 54.2 % D3ORFF 24 CACCATGGATCCCAGAAATGCACA 62.7 50 % D3ORFR 23 TCAAGGGAAGACGTTTGTGTTTG 58.9 43.5 % 2D3ORFR 23 TGAAGAGCCGGAGCTCTACCATC 64.2 56.5 %
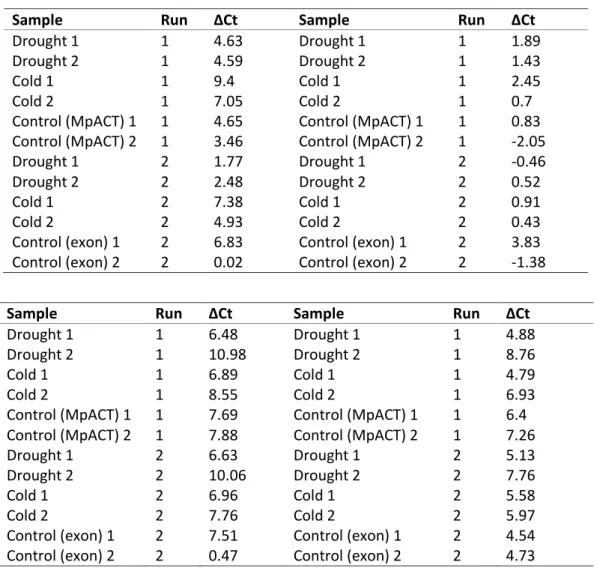
Table S2. Expression values (ΔCt) for control, drought and cold sample from the qPCR reactions; A) shows the MpLTPd2 intron compared to the reference gene MpACT (left) and the MpLTPd2 intron compared to the MpLTPd2 exon (right,) and B) shows the MpLTPd3 intron compared to the reference gene MpACT (left) and the MpLTPd3 intron compared to the MpLTPd3 exon (right).
A
Sample Run ΔCt Sample Run ΔCt
Drought 1 1 4.63 Drought 1 1 1.89
Drought 2 1 4.59 Drought 2 1 1.43
Cold 1 1 9.4 Cold 1 1 2.45
Cold 2 1 7.05 Cold 2 1 0.7
Control (MpACT) 1 1 4.65 Control (MpACT) 1 1 0.83
Control (MpACT) 2 1 3.46 Control (MpACT) 2 1 -2.05
Drought 1 2 1.77 Drought 1 2 -0.46
Drought 2 2 2.48 Drought 2 2 0.52
Cold 1 2 7.38 Cold 1 2 0.91
Cold 2 2 4.93 Cold 2 2 0.43
Control (exon) 1 2 6.83 Control (exon) 1 2 3.83
Control (exon) 2 2 0.02 Control (exon) 2 2 -1.38
B
Sample Run ΔCt Sample Run ΔCt
Drought 1 1 6.48 Drought 1 1 4.88
Drought 2 1 10.98 Drought 2 1 8.76
Cold 1 1 6.89 Cold 1 1 4.79
Cold 2 1 8.55 Cold 2 1 6.93
Control (MpACT) 1 1 7.69 Control (MpACT) 1 1 6.4
Control (MpACT) 2 1 7.88 Control (MpACT) 2 1 7.26
Drought 1 2 6.63 Drought 1 2 5.13
Drought 2 2 10.06 Drought 2 2 7.76
Cold 1 2 6.96 Cold 1 2 5.58
Cold 2 2 7.76 Cold 2 2 5.97
Control (exon) 1 2 7.51 Control (exon) 1 2 4.54