Tl särtryck
Nr 225 ' 1994
est and Evaluation of Salt/CMA Mixtures
ent Gustafson
aper presented at conference Anti-Icing Technology
eview , Minneapolis, USA, August 30 31, 1994
Väg- och
transport-forskningsinstitutet ,
VTI särtryck
Nr 225 0 1994
Test and Evaluation of Salt/CMA Mixtures
Kent Gustafson
Paper presented at conference Anti-Icing Technology
Review , Minneapolis, USA, August 30 31, 1994
cm)
Väg- och
transport-farskningsinstitutet
,
TEST AND EVALUATION OF SALT/CMA MIXTURES
Kent Gustafson, Research Director
Swedish Road and Transport Research Institute S-581 95 Linköping, Sweden
Introduction
The Swedish Road and Transport Research Institute (VTI), in cooperation with the
Swedish National Road Administration, has during many years conducted research projects of testing and evaluating salting methods and alternative deicing agents. During 1985 1990 the Swedish MINSALT programme, minimizing the adverse effects of salt , was carried out with the aim of finding more effective ways of improving skid resistance without having the negative effects of salt. The results from the MINSALT projects have been reported in different reports and
summarized in a nal report (1,2).
In regard to chemical de-icing - i.e. salting spreading methods have progressed from earlier dry salting to the spreading of prewetted salt and saline solutions. The results from the MINSALT- projects have led to a proposed strategy that will re-duce the salt consumption and make the salting more effective. This can be ac complished by working more with anti-icing measures, before the icy conditions occur, and less with de icing. Prewetted salt or brine should be used.
Experiments aimed at using NaCl more efficiently included studies of optimum spreading rates under different weather and road surface conditions. The impor-tance of the road structure, the wearing course and the salt (origins and gradations) were studied.
A number of different chemical alternatives to NaCl have been tested. In parti-cular, calcium magnesium acetate (CMA) has been studied more closely in regard to its ice melting capacity, corrosiveness and effect on concrete. Studies of alter-natives have also included chemicals suitable for runway purposes. Potassium
acetate, a liquid deicer, has been tested and has come to use on some Swedish
air-ports.
In 1993 the Swedish National Road Administration requested VTI to start a research project of testing and evaluating salt/CMA mixtures. The evaluation in-clude a eld experiment of the operational effects under varying weather and road surface conditions and laboratory testing of deicing properties, corrosive effect and the effect on cement concrete. The eld testing the first winter 1993/94 was carried out with a mixture of 80/20 by weight of NaCl/CMA. Some preliminary results from this rst winter and some laboratory testing will be given in this paper. A rst report is being written and will be published later this year.
Earlier test results with alternative deicing agents
CalciumMagnesium Acetate (CMA)
Studies of CMA were carried out in Sweden in the l980ies. To begin with, a small quantity of CMA was manufactured on a laboratory scale but when it was marketed commercially tests were conducted with the proprietary products. The studies that were carried out mainly at the VTI were chie y concerned with CMA's melting properties, corrosiveness and effect on cement concrete.
CMA's freezing point reduction, the lowest temperature at which melting can occur, varies according to the Ca/Mg ratio between about -lO°C and 28°C (NaCl about -21°C). The lowest and optimum freezing point is obtained with a Ca/Mg ratio of about 3/7-2/8 (in mol). The two CMA products "ICE-B-GON" and "Clearway CMA" have a ratio of 3/7.
The melting effect of CMA does not vary so widely, however, because of the
Ca/Mg ratio, but depends more on the shape, size and density of the particles. Melting ability has been tested on blocks of ice at different temperatures. In
figure 1 the result from one melting test at -2°C is shown. The CMA pellets were
2-3 mm in size. CMA has lower solubility than CaC12 and NaCl and the melting activity is therefore slower. CMA has a poorer melting effect than CaC12 and NaCl but better than urea. It should be noted that CMA has a very slow initial melting reaction while NaCl and especially CaC12 have a very rapid melting effect. The same relations have been determined at lower temperatures, tests at -6°C and -10O C, but the slower melting effect compared to CaC12 and NaCl are even more pro-minent.Perhaps the greatest positive effect of CMA as compared with NaCl is reduced
corrosion. Several different corrosion studies have been carried out with CMA. Corrosion tests with CMA on car body steel showed that CMA is much lesscorro-sive than. NaCl and CaClz. The weight loss on steel plates, which were covered
with a mix of mud and the deicer for 100 days where after the test period consider-ably less for CMA compared to the other deicing chemicals in the test. Immersion test with aluminium plates also showed promising results for CMA, which were less corroding than NaCl, CaC12 and urea. The difference in speed of corrosion was however not so marked as with steel.
Several different studies concerning the freeze/thaw and chemical effect of CMA on cement concrete have been carried out. As shown by freeze/thaw tests, the chloride salts clearly peak at a concentration of 3 4%. After declining with slightly rising concentration, the degree of damage increases dramatically for high con-centrations of CaC12 and MgClz. Here the chemical effect manifests itself. For NaCl, on the other hand, the degree of damage clearly diminishes with a rising concentration and is extremely small for a saturated solution. According to freeze/thaw tests, CMA's incidence of damage rises in direct proportion to its con-centration up to the same level as NaCl's maximum (which occurs at about 3 %). See figure 2.
Tests at the Lund Institute of Technology in1985 86 were aimed at studying the chemical effect of CMA and other substances on concrete. The specimens in
NaCl-solution were little damaged, while the effect on the cement concrete in
CMA was considerable. Samples in CaC12 solution were also heavily damaged
and perhaps even faster than with CMA. The tests were carried out with CMA of dubious composition and the relevance of the results is therefore open to question, especially as present-day (1994) CMA for road maintenance purposes is a diffe-rent product altogether and comprises a stable compound without an excess of acid. These tests have therefore been duplicated during 1991/92. The results showed that there was some effect of CMA on cement concrete but this effect wasmuch less than in the rst test (4, 5).
To study the effect of de-icing agents, and CMA in particular, on concrete under more realistic and varying conditions, eld experiments were carried out during the years 1986-1990. After the period of exposure the concrete specimens were tested and analysed with respect to compressive strength, tensile splitting strength, carbonation depth, frost resistance, chloride content and acetate content. A thin grinding analysis was also carried out. The results of the tests show that all exposure damage with respect to CMA was less than for NaCl and other chloride
salts.
Sodium formate and potassium acetate
Besides the more detailed examination of CMA, a number of other alternatives have been considered. In some cases, certain small-scale studies have been
con-ducted. The substances which have been tested are among others sodium formate and potassium acetate (proprietary name Clearway-l).
Limited laboratory tests concerning melting effect and freezing point depression
were made with sodium formate as small pellets (<l mm). The result showed that
sodium formate is comparable to NaCl as a deicer. The same result was shown in a minor field test. Sodium formate as a saturated brine was spread on hoar frost and as anti-icing measure. In both cases comparison was made to NaCl brine, and no difference between the two deicers could be seen.
Another chemical investigated was non-corrosive potassium acetate solution (Clearway-l) for primarily air eld use. This is a 50% by weight aqueous solution with addition of a small amount of corrosion inhibitor to fullfill the rigorous de-mands for use on airfields.
Potassium acetate has been tested in the laboratory and on airfields during some winters. Potassium acetate has a very low freezing point, appr. -40°C with a 50 % by weight solution. As the acetate is a liquid it has a very rapid melting effect. The eld tests showed that compared to Urea the acetate had a more rapid and better melting effect but there was a question about its long term effect. Potassium acetate is hygroscopic, (draws moisture out of the air), which can lead to longer wet periods, dilution and perhaps refreezing.
The positive effects of potassium acetate compared to NaCl and Urea are reduced corrosion and environmental damage. The major drawback is the price which is even higher than for CMA.
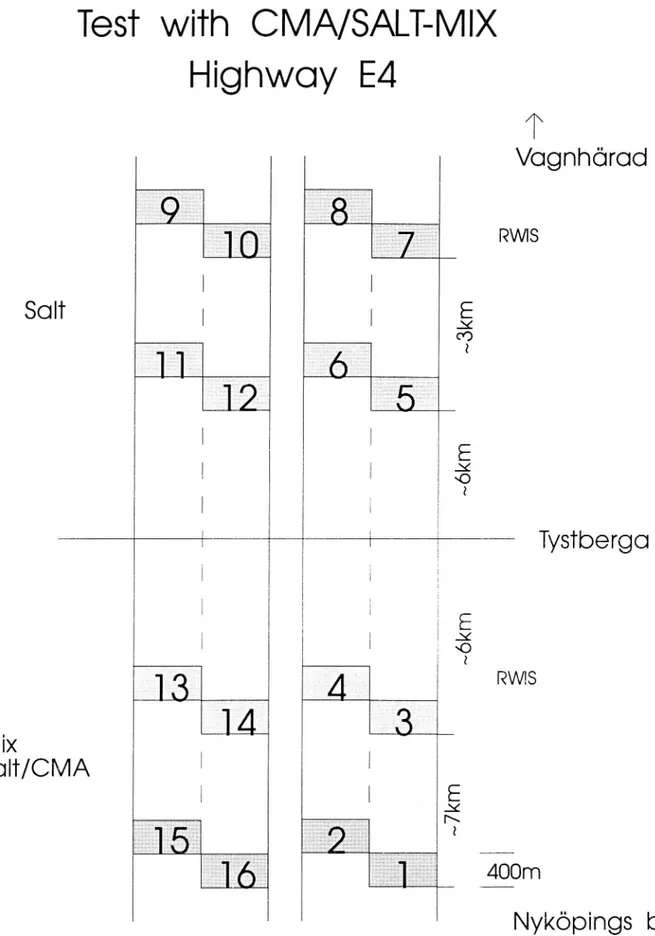
Field test of salt/CMA mix on Highway E4 at Nyköping
In the light of very promising results regarding the corrosive effect of a mixture of salt and CMA it was decided to evaluate this under Swedish conditions. Tests in Minnesota (6) had shown that a mixture of 80% salt and 20% CMA could have a much lower corrosion rate on steel plates in laboratory experiment than plain salt. The testing and evaluating of salt/CMA mixes started in the winter 93/94 and in-clude both laboratory and eld experiments regarding deicing, corrosive and ope-rational effect in eld. Laboratory tests include deicing and corrosion properties and the chemical effect on cement concrete.
To study the operational effects of the mixture of salt/CMA under realistic and varying conditions, eld experiments are being carried out last winter and planned to be continued the next on Highway E4 near Tystberga just north of Nyköping. Highway E4 is a four (4) lane divided highway with bituminous surfacing. The
test section has a SMA, stone mastic asphalt wearing surface and the control
section has a dense asphalt concrete surface. The test section is both the north-bound and southnorth-bound lanes of E4 located south of the Tystberga intersection. The control section is also both the northbound and southbound lanes of the same highway located north of the Tystberga intersection. Within each test and control section are a number of sampling sections consisting of 400 meter long sub-sections in the driving and passing lanes (see gure 3). The sampling sub-sections were selected to be uniform with regard to pavement cross section, atness, traffic and other conditions. The beginning and ending of each sampling section is marked along the side of the highway. This segment of Highway E4 will not ex-perience the effect of rush hour or commuting traf c.
Friction measurements and pavement surface observations are made at each of the sampling sections during a number of situations with slippery conditions. The measurements are made using a Saab Friction Tester. The friction measurements and observations were usually made before spreading operation, 10 to 30 minutes after spreading, and then at intervals of every 45 to 60 minutes until a stable condition or bare pavement was reached.
In addition to the friction measurements and pavement surface observations, the atmospheric conditions and pavement temperatures are monitored using the two (2) RWIS stations which are located in the test and control sections respectively. The moisture content of the snow is not being monitored. The operators of the spreading vehicles report on the times of application and application rates.
The ratio of the salt/CMA mixture used in the field testing is 80/20 by weight. This mixture was prepared by using a small double hopper with a conveyor belt (see gure 4). The accuracy of the mixture was checked by separating the salt and CMA compounds after the mixing.
The chemical applied to the control section was dry salt or salt pre-wetted with salt brine. However in all the situations that was followed this rst winter only dry salt was used. The reason for this was that these situations were all during snow
storms. The mixture of salt/CMA for the test section was also spread either dry or
pre-wetted with salt brine. In most situations, but not all, dry salt/CMA mix was
used. All salt brine is saturated solution of 23 % by weight. The rate of pre-wetting is 30 % by weight. The spreader vehicle for the salt/CMA mixture has a 8 m3 hopper and 2000 liter liquid storage tank. The pre-wetting take place at the spinner.
Corrosion
In the laboratory, corrosion tests are being conducted using steel plates which are covered with a mixture of mud and the chemical for 100 days. The corrosion tests are being conducted in accordance with Svensk Standard 88186039. The chemical
is being tested in two series, the rst with two (2) different mixture ratios of
salt/CMA; 90/10 and 80/20 and the second serie with ve (5) mixture ratios
90/10, 80/20, 70/30, 60/40 and 50/50. The experiments also included tap water, NaCl, CMA, NaAc (Sodium Acetate), CaC12 and MgClz. The reason for making two series of tests was the fact that some of the results in the first test were a little
surprising and not in accordance with earlier experience.
Another laboratory test will be conducted at the Swedish National Testing and Research Institute in Borås. This is a spray test performed in a climate chamber under simulated eld conditions. Earlier tests in the climate chamber have corre-lated very well to actual conditions on the road and the corrosive effect from these. This test will be carried out during the fall of 1994.
To study the effect of corrosion of the salt/CMA mixture under more realistic and varying conditions, eld experiments are being conducted by placing steel plates
in the median of the road, between the northbound and southbound lanes. Each
grouping of coupons consisted of ve (5) unpainted car body steel plates and five (5) painted coupons. There were one grouping facing the southbound and one the northbound on test and control sections. The painted coupons had a cali scratch in the painted surface. The atmospheric corrosion rate is monitored with ve (5) un painted plates at the same locations but at a far distance and uneffected from the road. By subtracting the atmospheric corrosion from the specimens at the road side the corrosive in uence from the road environment can be evaluated. See photos in gure 5.
Cement concrete
The cement concrete testing done by Lund Institute of Technology is planned to
be duplicated to evaluate the mixtures of salt/CMA. However, these tests are now
Preliminary results
The results from the rst corrosion test is shown in gure 6. The acetate salts, CMA and NaAc both have very low corrosion rate while CaC12 and MgC12 have very high. NaCl has higher than water but the difference is not very big. The rea-son for this could be the use of tap water. The difference between water and the mixes (10 and 20 %) with CMA are very small and the 10/90 mix has even higher rate than the plain salt, which is surprising. This rst serie didn t show any major decrease in corrosion effect by mixing CMA to salt.
The result of the second test is shown in gure 7. Also some of these results are a
little bit surprising. As in the rst test CMA and NaAc have the lowest and CaC12
and MgC12 the highest corrosion rate. NaCl has higher corrosion rate than tap water but the difference is not very big. Compared to NaCl there is no reduction in corrosive effect from 20 and 30 % mix with CMA, while 10 and 50 % give a small reduction. The biggest reduction is with the 40/60 salt/CMA mix.The second test show there could be a reduction in corrosion by adding CMA to the salt. However, this effect seems to be limited, especially for mixes with less than 30 % CMA. One should also point out that these results are not conclusive and that further testing hopefully will give more signi cant results.
Preliminary results of the field test point in the same direction. The specimens have been analysed and a report is being written. The result showed a reduction but it was not very large. There was also a difference between the two directions. The reduction in corrosion rate for the CMA/salt mix was appr 50 % on plates facing southbound lanes and appr 20 % on plates facing the northbound lanes. Only the rst result is statistically signi cant. The difference in the two directions can be due to wind directions or traf c in uence. The difference in corrosion rate between salt and the mix is not only explained by the materials, but depend also to a large extent on the difference in number of applications. CMA/salt was spread
131 times while salt was spread 174 times, i.e. 30 % more times.
During the winter 1993/94 appr 30% more applications of deicing agent were made on the control section with salt which corresponded roughly to the same amount of more material spread. According to the maintenance personel this difference is due to the fact that they sometimes judged they had a longer lasting effect of the salt/CMA mix and therefore didn't have to apply chemical on the test section when they had to go out on the control section.
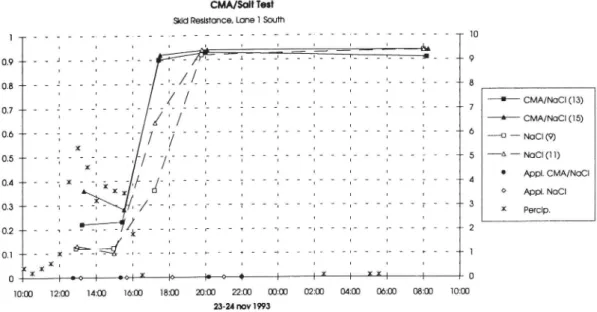
Nine snow storms, and appr. three times as many applications of chemicals, were followed closely by monitoring the skid resistance etc. during this winter. The result from some of these situations are shown in gure 8-11. The graphs show the variation in skid resistance on four monitoring sections during some slippery situations. Time of deicing measure and amount of precipitation have been marked in the graphs.
Figure 8 shows the difference between test (mix) and control (salt) sections in lane 1 (slow) south during a snow storm on Nov 23. In this situation there is a better
effect from the CMA/salt and it takes a longer time before the control sections
have bare pavement levels. A similar situation is shown in gure 9 which
compares test and control sections in lane 1 north during a snow fall on Dec 15-16. In this situation there is also a better effect, especially on one of the testsections (1), of the salt/CMA mix.
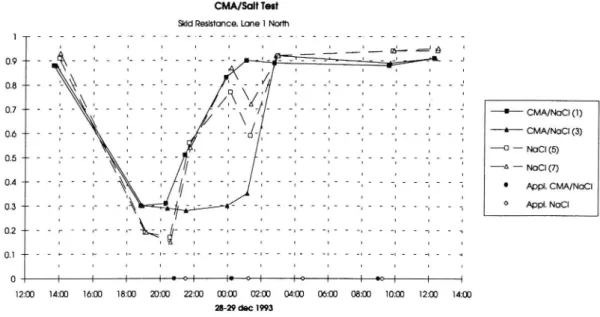
The deicing effect of the chemicals have differed between the two lanes in each direction. Usually there have been better surface conditions in the slow lane because mainly on more traf c. Especially during nighttime slippery conditions there have been worse conditions in the fast lane because the lack of traf c in this lane. Figure 10 and 11 show the skid resistance in the same situation, a snow
storm on Dec 28-29, but in different lanes. As described above the surface
condition is worse in the fast lane ( gure 10) compared to the slow lane ( gure 11). Comparison between test and control sections show somewhat different deicing effect. In gure 10 it can be seen that one of CMA/salt sections has an effect that is slower than on the other sections. In figure 11 there is a greater variation in skid resistance. During the rst part of the situation there is a better effect on CMA/salt sections, while the opposite is prevailing later on in this situation.
As could be seen in the above situations there is a variation in skid resistance between test and control sections, lanes, directions etc. To evaluate the effect of
the CMA/salt mix on road surface conditions compared to salt is therefore not very easy. However, some preliminary findings can be given even if these are not signi cant. The mix of 20/80 CMA/salt has worked as well or better than salt in
most situations. In some situations salt worked better but these were not as
frequent as the opposite.
Comments
This rst winter the testing of CMA/salt compared to salt gave the following preliminary results:
0 CMA/salt mix have in most situations worked as well or better than salt
0 maintenance personel feel there is sometimes a longer lasting effect with the mix
' laboratory and field tests showed less corrosion rate from the mix, but the effect was limited
The research of using the salt/CMA mixture will be conducted over two (2) winters. It is expected that an interim report will be published late 1994 and the
References
1. Öberg, G, Gustafson, K and Axelson, L: Effektivare halkbekämpning med
mindre salt. MINSALT-projektets huvudrapport. Statens väg- och
tra kinstitut. VTI Rapport 369, 1991, 236 p, 95 ref. (In Swedish)
Öberg, G, Gustafson, K and Axelson, L: More effective de-icing with less salt. Final report of the MINSALT-project. Summary. Swedish Road and Traffic Research Institute, VTI Rapport 369 SA, 1991, 58 p, 0 ref. (In English).
Verbeck, GJ and Klieger, P: Studies of "salt" scaling of concrete. Highway Research Board, Bulletin 1957 no 150, p 1 13
Peterson, O: The chemical effects on cement mortar of solutions of calcium magnesium acetate and other deicing salts. Report TVBM 3045, 1991. Divi-sion of building materials, Lund institute of technology, University of Lund. Peterson, 0: The chemical effects on cement mortar of solutions (two different concentrations) of calcium magnesium acetate and sodium chloride. Report TVBM 3049, 1992. Division of building materials, Lund institute of technology, University of Lund.
Fleege, E: Road Salt Additive Study. Minnesota Department of Transportation, 1991 .
List of figures Figure 1. Figure 2. Figure 3. Figure 4. Figure 5. Figure 6. Figure 7. Figure 8. Figure 9. Figure 10.
Ice melting effect of som deicers in laboratory testing at -2°C. Application rate: 20 g on 114 cm .
Concrete-Frost Testing acc. To Swedish Standard 137236. Tests with 3 % solutions of NaCl, CaClz, MgC12 and 3-25 % solutions of CMA. Weight loss after 56 cycles. Trends for NaCl, CaC12 and MgC12 according to Verbeck & Klieger, 1957 (3).
Layout of salt/CMA testing on Highway E4 at Nyköping.
Mixing of salt/CMA by using a small double hopper with a conveyor belt.
a) Field experiment of corrosion effect. 3 photos.
b) Steel plates (5 painted and 5 unpainted) for corrosion test. c) Steel plates for atmospheric corrosion.
Corrosion on car body steel. Test 94-1. Corrosion on car body steel. Test 94-2.
Skid Resistance during snow storm Nov 23 24 1993. Skid Resistance during snow storm Dec 15 16 1993. Skid Resistance during snow storm Dec 28 29 1993. Skid Resistance during snow storm Dec 28 29 1993.
10 Men A water 150 * (9 ' 4 NaCl Cat)!z CMA 100 < Urea m '4 15 3B 65 tån 240 Time min
Figure 1. Ice melting effect of some deicers in laboratory testing at -2°C. Application rate: 20 g on 114 cm2. Sca ng kg/m2
1
1,0 . 'I 'I / 0,8 l N Cl 'I IAX a .'l CaClz 0,6+ / \ / I \ I \ ! I C&Clz | &044 ' 'A
' I / X_t ///-'
CMA
I '/
././
I/ \ _ _/ ' I. x -- - 0/ 0,2* I \ / & \ MgCIz /" Xx o/'. xxå
&
110
15
2b
is?
Gone. %Figure 2. Concrete Frost Testing acc. To Swedish Standard 137236. Tests with
3 % solutions of NaCl, CaClz, MgC12 and 3-25 % solutions of CMA.
Weight loss after 56 cycles. Trends for NaCl, CaC12 and MgC12 according to Verbeck & Klieger, 1957 (3).
11
TesI with CIVIA/SALT-IVIIX
Highway E4
/]\
Vognhörod
RWIS
SOIT
I
I
E
I
I
%
| ZI
I
Tysfbergo
iI
5
I E
I
I
å
Z-| 3
4 .
RWIS
.
1.4
3 _
I\/I|x
SoH/CMA
I
I
_ÄÖ Om
Nyköpings bro
12
Figure 4. Mixing of salt/CMA b belt.
14
*.51:53a.;åaaÄCorrratem/da
HgoNaCI ' "CMA 20/80 10/90 NaAc MgCI2 CaCI2
Figure 6.
Corrosmn on car body steel
-
Test 94-2
ICorrratem /da
H20"
50/50 _" 30/70
10/90 '
NaAc
CaCI2
CMA . - 40/60
20/80
NaCl
MgCI2
15
CMA/Sali Test Skid Resistance, Lone] South
A A Ö ( O I ! ) 0 C C 2 _ _ _ \ _ o o A < o 0 0 A ~ § o 2 2 9 , 3 0 2
-&
%
%
(
5
5
6
9
0 0 2 2 2 2 2 I I T T , -o x I N I f ) ? l I 0.4 + 0.3 4 o.1 x -10:00 18m 20:03 22:00 00:00 02:03 04:03 06:00 081m 12:00 14:0) 16:0) 10:0) 24 nov 1993 23Figure 8. Skid Resistance during snow storm Nov 23-24 1993.
CMA/Salt Test
Skid Resistance, Lane 1 North
_l CMA/NGC! (1) + CMA/NaCl (3) _D __ NOCI (5) A ' NoCI (7) Appl. CMA/NGCI Appl. NGC! Perclp. O X lå-lédec 1993
16
CMA/Sali Test
Skid Resistance. Lone ] Nonh
l CMA/NaCl (1) _... CMA/NaCl (3) D NOCI(5) Appl. CMA/NaCl Appl. NOCI O 0.3 -12:03 1411) 10:00 1811) 20:00 22:00 00:00 02:00 04:03 00:00 08:03 14:00 MID 12:00 28-29 dec 1993
Figure 10. Skid Resistance during snow storm Dec 28 29 1993.
CMA/Soll Test Skid Resistance, Lone 2 North
m m 0 0 C 0 m m A A M M C C _D N00 (6) A _ NOCI (8) App!. CMA/NaCl App!. NoCI O _ _ . _ _ . _ . _ . _ . / / _ _ . _ --. --. --. --. ./ . /. _. /. _-,. /. . -. _ . _ _ . / A f . / D _ Av . _ . _ _ . K . = _ | I . : |. .. .. .| .. .| ,. .. | . u | . | _ . . / H l l l _ _ _ _ . . . . . / . _ _ _ . . . _ . k . h . . | _ | | _ | |. .. | _ t | . | | . | .. o _ | H K M . . . _ . . . _ _ ( = . -. -. -_ -_ -_ -_ -. . -. _ . _ _ . . _ _ K . _ _ . _ . _ . .. \ _ . -. . . -_ -_ -_ -_ -. X w -_ . -_ -_ . . . _ . \ . . . _ _ . _ . . _ _ . . . n | _ | | _ | n _ \ | | . | i . u .. .| | ... | # l l ! _ . _ _ . _ . . . . . _ \ _ . . . . _ _ _ I Ä | _ l | . . . I . ! | _ l l . | l _ | I P | _. | | . . . . . _ . . . . . _ _ _ _ . . . . . . . T . . . . . . . . . ... 9. B 7. b. 5. A 3. 7. .... 0 0 0 0 0 0 0 0 0 0 12:00 14:00 10:03 18:00 200) 22:00 (DID 02:(D 04:00 0611) 08110 14:00 16120 12:00 28-29 dec 1993