http://www.diva-portal.org
This is the published version of a paper published in Lindbergia.
Citation for the original published paper (version of record): Jägerbrand, A., During, H. (2005)
Effects of simulated shade on growth, number of branches and biomass in Hylocomium splendens and Racomitrium lanuginosum.
Lindbergia, 30(3): 117-124
Access to the published version may require subscription. N.B. When citing this work, cite the original published paper.
Permanent link to this version:
Accepted 6 May 2006 Copyright © LINDBERGIA 2006
Lindbergia 30: 117–124. Lund 2006
Effects of simulated shade on growth, number of branches and
biomass in Hylocomium splendens and Racomitrium
lanuginosum
Annika K. Jägerbrand and Heinjo J. During
Jägerbrand, A. K. and During, H. J. 2006. Effects of simulated shade on growth, number of branches and biomass in Hylocomium splendens and Racomitrium
lanuginosum. – Lindbergia 30: 117–124.
The effects of simulated shade in terms of reduced light quantity (PPFD) and changed red:far-red ratio (R:FR ratio) on the growth in length, in number of branches and in biomass, were examined in a greenhouse experiment with
Hylocomium splendens and Racomitrium lanuginosum, two species from
ha-bitats with different light conditions (with H. splendens often in more shaded microsites). Using ten intact moss turfs per species which had been collected on Iceland at 4 m distance between replicate turfs, we tested, whether light quantity affected growth and biomass, whether changes in light quantity and red:far-red ratio affected the number of branches, and whether the two species differed in these responses. Reduced light quantity (i.e. PPFD level) caused a greater length increase, decreased biomass, and biomass:length ratio in both species, but the magnitude of response varied greatly between species. Fur-thermore, in R. lanuginosum spectral shade (i.e. reduced PPFD and a lower R:FR ratio) generally caused stronger responses than neutral shade, with only a reduction in PPFD. H. splendens (from the shaded habitat) responded less strongly to the shade treatments than R. lanuginosum (from the open habitat) did. In addition to these effects of shading, there were strong effects of the turf of origin in both species, and in many cases the interaction between turf of origin and shading treatment was significant as well.
A. K. Jägerbrand (annika.jagerbrand@botany.gu.se), Dept of Plant and En-vironmental Sciences, Göteborg Univ., Box 461, SE-405 30 Göteborg, Swe-den. – H. J. During, Dept. of Plant Ecology, P.O. Box 800.84, NL-3508 TB Utrecht, the Netherlands.
The light intensity rapidly declines with depth in natural moss patches (van der Hoeven et al. 1993) and in dense moss cushions the light extinction in the upper few centimetres is nearly complete (Clymo and Hayward 1982, Rydin and McDonald 1985, Skre et al. 1983). Owing to the shortage of light, bryo-phyte shoots and leaves may turn brown (van der Hoeven et al. 1993), and moss parts situated deeper
in the canopy become brown and apparently dead (Skre et al. 1983, Rydin 1995). Thus, even with the rather low light compensation points (Kallio and Hei-nonen 1973, Skre and Oechel 1981) and low light saturation points (Skre and Oechel 1981) displayed by many bryophytes, light may become limiting for photosynthesis. Light limitation is further enhanced when bryophytes grow beneath a dense cover of can-opy plants (Pedersen et al. 2001). Light limitation combined with burial by vascular plant litter brought about by the increase in vascular plants has been sug-gested to be the cause of the decline of bryophytes
and lichens in experiments simulating global climate change in Alaska and northern Sweden (Cornelissen et al. 2001) and increased nitrogen deposition in
Sphagnum bogs (Berendse et al. 2001), although
shade experiments in situ have failed to show respons-es in the same direction (van Wijk et al. 2003).
Canopy shade implies both a decrease in light quan-tity (photosynthetic photon flux density, PPFD) and a change in quality (e.g. decreased red:far-red (R:FR) ratio). A reduction in PPFD will primarily affect rates of photosynthesis and thus, traits related to growth, such as biomass production, but spectrally neutral shading may also affect morphogenesis and charac-ters such as internode or petiole length and branching (Huber and Stuefer 1997). In many species, a change in R:FR ratio will affect morphogenesis even strong-er (Corré 1983, Hubstrong-er and Stuefstrong-er 1997).
For bryophytes, a reduction in PPFD has been shown in laboratory, greenhouse or garden experi-ments to cause decreased growth rates in terms of absolute biomass increment (Rincon 1993, van der Hoeven et al. 1998, van der Wal et al. 2005) as well as biomass-based relative growth rates (Rincon 1993). Other studies found little or no effect of shading on biomass increment rates, but an increase in length increment rates (Bakken 1995, Rincon and Grime 1989). Similarly, Bergamini and Peintinger (2002) removed the cover of vascular plants in a field expe-riment, and found that this did not significantly af-fect biomass of the bryophyte shoots, but led to a lower length increment and a higher branch density of the main axis. These results are consistent with the set of responses known as the “shade avoidance syn-drome” (Smith and Whitelam 1997) in phanerogams, which is a response to a reduction in R:FR ratio in plant-induced shade or dense canopies. Phytochromes play an important role in this respect (Morgan and Smith 1979, Smith 1982). Phytochromes occur widely in bryophytes (Schneider-Poetsch et al. 1994, 1998, Lamparter and Bruecker 2004). They play a role in diagravitropic responses (Rethy et al. 1990), and have been assumed to be involved in bryophyte responses to shade as well (Bates 1998). Yet, effects of a reduc-tion in R:FR ratio of the light falling on bryophytes have received little attention.
Hoddinott and Bain (1979) showed that in the bryo-phytes studied such a decreased R:FR ratio led to a decreased mean height, which is in the opposite di-rection to the observations made on vascular plants. However, van der Hoeven et al. (1998) found no sig-nificant effects of a decreased R:FR ratio on the length increase, biomass or the morphology in two species. Interpretations of the light reduction experiments in bryophytes are complicated by the interacting nature of radiation and moisture, as a higher radiation level will inevitably lead to faster desiccation of
bryophy-tes and consequently a reduced growth (van der Ho-even et al. 1998).
The plastic response to shade differs between spe-cies, depending on their ecology and tolerance to low light levels. For vascular plants, evidence suggests that species from open habitats (e.g. grassland) should have a stronger shade avoidance response than spe-cies from shaded habitats, e.g. understorey vegeta-tion (Corré 1983, Morgan and Smith 1979, Smith 1982). For bryophytes, the study of Rincon and Grime (1989) points in the same direction. They found that bryophytes of more productive habitats (mesic grass-land) showed stronger plastic responses to different irradiances (although without any change in R:FR ratio) than bryophytes of unproductive (shaded wood-land floor) habitats.
In this paper, we compared the responses to simu-lated shade (both neutral shade, only affecting the PPFD, and spectral shade with changed PPFD and R:FR ratio) on the growth in terms of biomass, number of branches and the shoot length of two cir-cumpolar bryophytes, Hylocomium splendens (Hedw.) B.S.G. and Racomitrium lanuginosum (Hedw.) Brid. The experiment lasted seven months and was con-ducted in an unheated greenhouse in Utrecht, the Netherlands.
Both species have a wide distribution. H.
splen-dens is probably the most common moss in the
North-ern Hemisphere (Persson and Viereck 1983), where-as R. lanuginosum is cosmopolitan (Tallis 1958). Both species are ecologically important as they can be very dominant and locally reach high abundance. Despite the fact that they can be found within the same area, they have very different habitat preferences, H.
splen-dens prefers mesic habitats, and can be very
domi-nant in understorey vegetation (Tamm 1953), whereas
R. lanuginosum prefers more exposed and open
ha-bitats (Vitt and Marsh 1988, Ellis and Tallis 2003), and is especially favoured when the presence of vas-cular plants is reduced (Tallis 1964). Their ecologi-cal differences are also reflected in their different ecophysiology, H. splendens having a lower light compensation point for photosynthesis (ca 20 µmol m–2 s–1 at 5°C as compared to ca 60 µmol m–2 s–1 at
5°C for R. lanuginosum) and a higher temperature optimum for growth than R. lanuginosum has (20°C vs 5–10°C; Kallio and Heinonen 1973, Furness and Grime 1982). In fact, the light saturation level of R.
lanuginosum (some 1750 µmol m–2 s–1) is one of the
highest found among mosses (Kallio and Heinonen 1973).
We hypothesized, that a reduction in PPFD will lead to less growth in biomass, while a reduction in R:FR ratio will result in shoot elongation and reduced branching. We further assumed, that especially the morphogenetic responses to a reduced R:FR ratio will
be less pronounced in the understorey species (H.
splendens) than in the species from an open habitat
(R. lanuginosum).
Material and methods
Site of collections
Moss samples were collected in Thingvellir National Park (64°17′N 21°05′W), in SW Iceland. The site is situated at 120 m a.s.l. on a post-glacial lava-field, and has a maritime subarctic climate. Annual mean temperature at Thingvellir was 2.8°C during the pe-riod 1974–1988, and the mean annual precipitation was 1438 mm (Einarsson 1992). The area has been protected from sheep grazing since 1928 (Jónasson 1992), and one of the most extensive vegetation types is the well-developed R. lanuginosum moss heath (Thorsteinsson and Arnalds 1992). The moss heath vegetation is low-productive with few vascular plants and few trees (Thorsteinsson and Arnalds 1992), re-sulting in direct exposure of the moss during sun-shine and clear days. In the same area, but with less cover, there are scattered Betula pubescens Ehrh. trees and dwarf-shrub heath vegetation dominated by dif-ferent Salix spp. (Thorsteinsson and Arnalds 1992). The dwarf-shrub heath has a more species-rich vege-tation, and common bryophytes are H. splendens and
Pleurozium schreberi (Brid.) Mitt (Jägerbrand 2004).
The highest amounts of global radiation in Thing-vellir is found during the summer period (Einarsson 1992). Mosses in the subcanopy shade of the dwarf shrub heath are protected from large amounts of the insulation.
The species
Hylocomium splendens and R. lanuginosum are
long-lived perennial species with clonal growth (During 1979). The species have quite different morphology,
H. splendens has primary, secondary and tertiary
branching and is a weft-forming pleurocarpous bryo-phyte with sympodial or monopodial growth (La Farge-England 1996). Hylocomium splendens may initiate a new segment every year by sympodial bran-ching (Tamm 1953), with distinct annual markers of growth (Callaghan et al. 1978, 1997, Økland 1995).
Racomitrium lanuginosum has a main stem with a
variable number of lateral primary branches (Tallis 1959a, 1959b), and grows monopodially (La Farge-England 1996).
Simulated shade experiment
On 8 September 1998, 10 samples (20×20 cm) were collected along a transect at every 4 m for each spe-cies in Thingvellir National Park. The samples were transported to Utrecht, the Netherlands, under dark and relatively dry conditions. The 10 samples for each species were cut to a depth of ca 7 cm (which includ-ed all green parts plus some of the brown lower parts of the shoots) and carefully divided into 3 containers (9×9 cm, depth 7 cm), and the containers were syste-matically assigned to one of the treatments: hyaline cage, green shade cage or black shade cage. For each species, two containers were placed in a treatment cage (five of each treatment) in a greenhouse in Utrecht. The cages consisted of a bottomless thin wooden frame, which was covered with either one layer of colourless plastic film (i.e. hyaline cage, or controls), alone or together with black gauze (to re-duce light quantity), or a green plastic film (Lee Colortran International, Andover, Hants, UK; film no.122,Ferngreen)simulatingcanopyshade.The cag-escould be opened from above and measured 20×43 cm, with a height of 20 cm.
The hyaline cage functioned as control because this foil had hardly any effect on light quantity and qual-ity (Fig. 1), and it produced the same microclimatic conditions (increased moisture levels) as both types of shade cage (Huber 1997). Light spectra of cages were measured with a Li-Cor 1800 spectroradiome-ter (Fig. 1). The green and black shade cages reduced the PPFD with approximately 60% (Fig. 1). PPFD above the cages varied continuously, but was usually in the range of 50–200 µmol m–2 s–1. Additionally, in
the green cages the red:far-red ratio was also reduced, from 1.12 for the controls and black cages to 0.20, thereby simulating a canopy shade (Fig. 1). The ex-periment was started 24 September 1998. The moss-es were watered every day with demineralized water,
Fig. 1. Light spectra in cages measured with a Li-Cor 1800 spectroradiometer. Continuous line: control cages, broken line: green shade cages (reducing PPFD and R:FR ratio), stippled line: black shade cages (reducing PPFD only). R, FR: red (655–665 nm) and far-red (725–735 nm) bands used to calculate the R:FR ratio.
and three times during the experiment with a strong-ly diluted Hoagland nutrient solution. The experi-ment was terminated 1 May 1999, as the apex of many
H. splendens shoots for unknown reasons turned dark
brown.
Measurements were taken on: length increase of the shoots; number of branches (on the new shoot part); biomass increase (dry weight of the new shoot parts, including the branches); and branch density (number of new branches divided by length increase). Furthermore, the biomass per unit shoot length of the newly formed parts was calculated. Measurements were performed on six shoots from each container, chosen in a systematical way. In some cases, the frag-ile shoots were destroyed, leaving 176 shoots for H.
splendens (59 from controls, 59 from green cages,
and 58 from black cages), and 179 shoots for R.
lanu-ginosum (59 from green cages). As the climate in the
greenhouse in Utrecht contrasted with the Icelandic climate, the point of new growth (i.e. from the start of the experiment) was often clearly visible on both species. For R. lanuginosum the new parts were more
green, probably because of the documented ability of the the hair points and awn teeth to change lengths and forms (Tallis 1959a, Vitt and Marsh 1998). For
H. splendens it was also possible to use markers of
annual growth (the sympodial branching pattern; see Tamm 1953, Økland 1995), the change in its growth direction towards light, or, as in some cases, leaf char-acters. Biomass was measured after drying to con-stant dry weight in 72 h at 70°C.
Statistical analyses
Prior to analyses all variables were transformed to zero skewness and expressed on a 0 to 1 scale to meet the assumptions of homogeneity of variances and normality (Økland et al. 2003). To test for differenc-es between specidifferenc-es and treatments, separate two-way ANOVAs were used for each response variable. Treat-ment effects and differences between the ten turfs of origin per species were tested by two-way mixed-model ANOVAs, followed by Tukey-Kramer post-hoc-tests for the treatment effects, suitable when
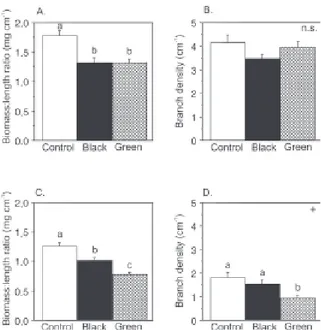
sam-Fig. 2. Effects of a simulated shade on growth in length (A, D), in number of branches (B, E) and in biomass (C, F) of
Hylocomium splendens (A-C) and Racomitrium lanuginosum (D-F). Bars indicate ± 1 SE. Bars with different letters
indicate significant differences analysed by Tukey-Kramer post-hoc-tests (p<0.05, and + = p£0.10, see Table 2 for the ANOVA,). ns=non-significant.
ratio (Table 2, Fig. 2, 3). In R. lanuginosum, bio-mass:length ratio was also reduced by a decrease in PPFD, but there was an additional effect of a decrease in R:FR ratio (Fig. 3c). Branch density was not sig-nificantly affected by a reduction of the PPFD alone, but significantly responded to a reduced R:FR ratio in this species (Fig. 3d). Biomass production and length increase differed strongly among the replicate moss turfs in both species (biomass marginally sig-nificant in H. splendens), and the interaction term was clearly significant for both species for almost all variables (Table 2), indicating markedly different re-sponses of the turfs to the shade treatments (see Fig. 4 for an example).
Discussion
Although the differences between the different orig-inal turf sections obscured some of the patterns in the results for both species, the general hypotheses were confirmed. Absolute biomass increase was lit-tle affected by the shade treatments when analysed per species (for unknown reasons), but when analys-ing responses of both species simultaneously, there were significant treatment effects on biomass produc-tion, thereby confirming our first hypothesis. Under
Fig. 3. Effects of a simulated shade on biomass:length ra-tio (A, C) and branch density (B, D) in Hylocomium
splen-dens (A, B) and Racomitrium lanuginosum (C, D). Bars
indicate ± 1 SE. Bars with different letters indicate signif-icant differences analysed by Tukey-Kramer post-hoc-tests (p<0.05 and + = p<0.10, see Table 2 for the ANOVA). ns=non-significant.
Table 1. Two-way ANOVAs testing differences between species and the shade treatments in separately analyses on the increase in length, number of new branches, new biomass produced, dry weight:length ratio of new mass, and branch density per cm new shoot in Hylocomium
splen-dens and Racomitrium lanuginosum from a greenhouse
experiment in Utrecht. Variable DF F P Length increase Species 1 0.31 .5800 Treatments 2 13.50 <0001 Species × treatments 2 1.00 .3690 Residual 349 No.of branches Species 1 4.93 .0270 Treatments 2 0.50 .6063 Species × treatments 2 5.95 .0029 Residual 349
Dry weight new shoot
Species 1 0.70 .4038 Treatments 2 3.15 .0440 Species × treatments 2 1.39 .2512 Residual 349 Biornass:length ratio Species 1 7.70 .0058 Treatments 2 30.94 <.0001 Species × treatments 2 3.51 .0310 Residual 349 Branch density Species 1 39.89 <.0001 Treatments 2 3.79 .0235 Species × treatments 2 4.45 .0124 Residual 349
ple sizes are uneven. Statistical analyses were per-formed using the Statview ® 5.0.1 and SPSS 10.0 software packages.
Results
Shading led to significantly greater length increase, lower biomass production, and lower biomass:length ratio of new parts in both species (Table 1, Fig. 2, 3). Significant differences between the species were found in the number of branches, biomass:length ratio and branch density (Table 1), with higher values for H.
splendens in all cases. Significant interaction effects
between species and treatments were found for bio-mass production, biobio-mass:length ratio and branch density (Table 1). For H. splendens, both shade treat-ments (spectral and neutral shade) caused similar in-creases in length and dein-creases in biomass:length
shade conditions, the reduced PPFD may cause a low-er photosynthetic rate, directly affecting length growth and biomass production. In this study a decreased PPFD did indeed cause a length growth increase, a reduced biomass increment and a lower biomass: length ratio, which is in accordance with previous studies (Rincon and Grime 1989, Bakken 1995).
The spectral shade treatment caused a greater re-duction in biomass, biomass:length ratio, and branch density in R. lanuginosum than the black-gauze shade treatment, but the same responses were not found in
H. splendens. Thus, there is also support for the two
hypotheses on R:FR ratio effects: that a reduced R:FR ratio causes a stronger length increase and reduces
the number of branches, and that a change in R:FR ratio has a stronger morphogenetic effect on the spe-cies from open habitats (R. lanuginosum) than on the understorey species (H. splendens). In addition, our results for R. lanuginosum also show (for the first time) that a reduced R:FR ratio caused an additive shade effect in bryophytes, compared with a shade treatment reducing the PPFD level per se (esp. bio-mass:length ratio).
There were large differences in the magnitude of response between the species. For example, H.
splen-dens had a mean length increment of 2.48 cm,
where-as that of R. lanuginosum wwhere-as only 1.19 cm. These differences probably reflect their contrasting
ecolo-Table 2. Results of mixed-model ANOVAs for effects of shade treatments (fixed factor) and moss turf of origin (random factor) on the increase in length, number of new branches, new biomass produced, dry weight:length ratio of new mass, and branch density per cm new shoot in Hylocomium splendens and Racomitrium lanuginosum from a greenhouse experiment in Utrecht. The ANOVAs were followed by Tukey-Kramer post-hoc test (Fig. 2, 3).
variable Treatment Moss turf Trmt × Turf
F p F p F p
DF 2 9 18
Hylocomium splendens
Length increase 3.77 0.04 4.11 0.005 4.72 0.000
No. of branches 2.09 0.15 1.96 0.15 1.56 0.08
Dry weight new shoot 0.14 0.87 2.25 0.07 4.50 0.000 Biomass:length ratio 7.17 0.005 0.95 0.51 1.90 0.02 Branch density 1.43 0.27 1.64 0.18 0.78 0.73 Residual DF: 146 Racomitrium lanuginosum Length increase 2.63 0.10 3.24 0.02 6.73 0.000 No. of branches 1.41 0.27 0.82 0.61 3.02 0.000
Dry weight new shoot 2.97 0.08 3.39 0.01 2.28 0.004 Biomass:length ratio 20.9 0.000 1.44 0.24 1.11 0.35
Branch density 2.90 0.08 0.74 0.70 2.21 0.005
Residual DF: 149
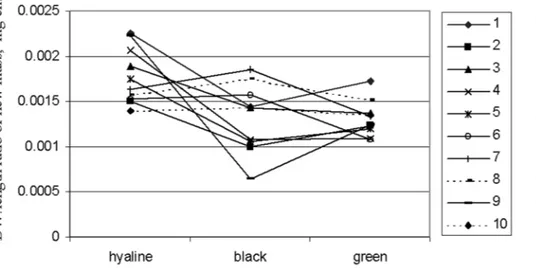
Fig. 4.
Biomass(DW):length ratio of the new shoot parts of
Hylocomium splendens shoots,
average values of 5-6 replicate shoots from ten moss turfs collected at distances of 4 m along a line transect in Iceland, cultivated in hyaline, black and green shade cages.
gy, H. splendens exists in a more productive habitat (the dwarf shrub heath), whereas the R.
lanugino-sum moss heath is nutrient-poor and low-productive.
This is supported by measurements of nitrogen and chlorophyll content, with concentrations in H.
splen-dens often twice as high as those in R. lanuginosum
(Jägerbrand et al. 2006).
In vascular plants, shade effects may cause a re-duction in branching density (Huber and Stuefer 1997). The shade responses in branching densities reported by previous studies on mosses did not con-form to this pattern, however: higher branching den-sities in response to decreased light quantity (Ber-gamini and Peintinger 2002) or to decreased R:FR ratio (Hoddinott and Bain 1979), or no effects at all (van der Hoeven et al. 1998). The length growth in-crease shown by both species under shade conditions and the morphogenetic responses to a reduced R:FR ratio shown by R. lanuginosum suggest, that at least some elements of the shade avoidance syndrome may also be found among bryophytes. The effects of the green-shade treatment suggest, that phytochromes may be involved in bryophytes as they do in phaner-ogams, but a role for blue-light-sensitive crypto-chromes (Liscum et al. 2003) as a cause of the in-creased elongation in the treatment of reduced PPFD shown by both species cannot be excluded, since such photoreceptors have been found in bryophytes as well (Suetsugu and Wada 2003).
A rather unexpected result of this study was the large difference in almost all respects between the shoots extracted from the ten replicate moss turf patches of both species, collected along a transect in a fairly homogeneous area at distances of only 4 m. Although genetically based differences between the patches cannot be excluded, other influences seem more likely. The patches differed visually in shoot density and turf height, and perhaps, interactions between their canopy structure and the conditions in the shade cages caused the differences found (Peder-sen et al. 2001). The strong interaction effects show-ing the large differences in even the direction of the responses to shade among the patches (Fig. 4) re-main enigmatic, however.
Acknowledgements – We would like to thank the National
park of Thingvellir, Iceland who allowed us to collect sam-ples to carry out this study, and the staff in the greenhous-es of the Utrecht Botanical Garden, the Netherlands. AKJ would also like to thank Magnus Popp and Gudjon Helgi Thorvaldsson for practical help with the experiments. We thank Håkan Rydin for constructive comments and sug-gestions. This study was supported by fundings from Helge Ax:son Johnsons fond, the Swedish Royal Forestry and Agricultural Academy (Grant No. SF-339), Göteborgs Uni-versitets överskottsfond and NorFa to AKJ.
References
Bates, J. W. 1998. Is ‘life-form’ a useful concept in bryo-phyte ecology? – Oikos 82: 223–237.
Bakken, S. 1995. Effects of nitrogen supply and irradi-ance on growth and nitrogen status in the moss
Dicra-num majus from differently polluted areas. – J. Bryol.
18: 707–721.
Berendse F., van Breemen, N., Rydin, H. et al. 2001. Raised atmospheric CO2 levels and increased N deposition cause shifts in plant species composition and produc-tion in Sphagnum bogs. – Global Change Biol. 7: 591– 598.
Bergamini, A. and Peintinger, M. 2002. Effects of light and nitrogen on morphological plasticity of the moss
Calliergonella cuspidata. – Oikos 96: 355–363.
Callaghan, T. V., Collins, N. J. and Callaghan, C. H. 1978. Photosynthesis, growth and reproduction of
Hylocomi-um splendens and PolytrichHylocomi-um commune in Swedish
Lapland. Strategies of growth and population dynam-ics of tundra plants 4. – Oikos 31: 73–88.
Callaghan, T. V., Carlsson, B. Å., Sonesson, M. et al. 1997. Between-year variation in climate-related growth of cir-cumarctic populations of the moss Hylocomium
splen-dens. – Funct. Ecol. 11: 157-165.
Clymo, R. S. and Hayward, P. M. 1982. The ecology of
Sphagnum. – In: Smith, A. J. E (ed.), Bryophyte
ecolo-gy. Chapman & Hall, pp. 229–289.
Cornelissen, J. H. C., Callaghan, T. V., Alatalo, J. M. et al. 2001. Global change and arctic ecosystems: is li-chen decline a function of increases in vascular plant biomass? – J. Ecol. 89: 984–994.
Corré, W.J. 1983. Growth and morphogenesis of sun and shade plants. II. The influence of light quality. – Acta Bot. Neerl. 32: 185–202.
During, H. J. 1979. Life strategies of bryophytes: a pre-liminary review. – Lindbergia 5: 2–18.
Einarsson, M. Á. 1992. Climatic conditions of the Thingvallavatn area. – Oikos 64: 96–104.
Ellis C. J. and Tallis J. H. 2003. Ecology of Racomitrium
lanuginosum in British blanket mire – evidence from
the palaeoecological record. – J. Bryol. 25: 7–15. Furness, S. B. and Grime, J. P. 1982. Growth rate and
temperature responses in bryophytes. II. A comparative study of species of contrasted ecology. – J. Ecol. 70: 525-536.
Hoddinott, J. and Bain, J. 1979. The influence of simulat-ed canopy light on the growth of six acrocarpous moss species. – Can. J. Bot. 57: 1236–1242.
Huber, H. 1997. Architectural plasticity of stoloniferous and erect herbs in response to light climate. – Thesis, Utrecht Univ.
Huber, H. and Stuefer, J. F. 1997. Shade-induced changes in the branching pattern of a stoloniferous herb: func-tional response or allometric effect? – Oecologia 110: 478–486.
Jónasson, P.M. 1992. Exploitation and conservation of the Thingvallavatn catchment area. – Oikos 64: 32–39. Jägerbrand, A. K. 2004. Patterns of species richness and
vegetative performance in heath ecosystems at Þing-vellir, Southwest Iceland. – Icel. Agric. Sci. 16–17: 29– 38.
Jägerbrand, A. K., Jonsdottir, I. S. and Økland, R. H. 2006. Phenotypic variation at different spatial scales in
rela-tion to environment in two circumpolar bryophyte spe-cies. – Lindbergia 30: 125–142.
Kallio, P. and Heinonen, S. 1973. Ecology of
Rhacomitri-um lanuginosRhacomitri-um (Hedw.) Brid. – Rep. Kevo Subarctic
Res. Stat. 10: 43–54.
La Farge-England, C. 1996. Growth form, branching pat-tern, and perichaetial position in mosses: cladocarpy and pleurocarpy redefined. – Bryologist 99: 170–186. Lamparter, T. and Bruecker, G. 2004. Phytochrome in
mosses. – In: Wood, A. J., Oliver, M. J. and Cove, D. J. (eds), New frontiers in bryology: physiology, molecu-lar biology and functional genomics. Kluwer, pp. 157– 175.
Liscum, E., Hodgson, D. W. and Campbell, T. J. 2003. Blue light signaling through the cryptochromes and pho-totropins. So that’s what the blue is all about. – Plant Physiol. 133: 1429–1436.
Morgan, D. C. and Smith, H. 1979. A systematic relation-ship between phytochrome-controlled development and species habitat for plants grown in simulated natural radiation. – Planta 45: 253–258.
Økland, R. H. 1995. Population ecology of the clonal moss
Hylocomium splendens in Norwegian boreal spruce
for-ests. I. Demography. – J. Ecol. 83: 697-712.
Økland R. H., Rydgren K. and Økland T. 2003. Plant spe-cies composition of boreal spruce swamp forests: closed doors and windows of opportunity. – Ecology 84: 1909– 1919.
Pedersen B., Hanslin H. M. and Bakken S. 2001. Testing for positive density-dependent performance in four bryo-phyte species. – Ecology 82: 70–88.
Persson, H. and Viereck, L. A. 1983. Collections and dis-cussions of some bryophytes from Alaska. – Lindber-gia 9: 5–20.
Rethy, R., Fredericq, H., De Greef, J. et al. 1990. Light and the diagravitropic growth of Marchantia
polymor-pha L. thalli: phytochrome-controlled epinasty. – Mem.
Soc. R. Bot. Belg. 12: 77–88.
Rincon, E. 1993. Growth responses of six bryophyte spe-cies to different light intensities. – Can. J. Bot. 71: 661– 665.
Rincon, E. and Grime, J. P. 1989. Plasticity and light in-terception by six bryophytes of contrasted ecology. – J. Ecol. 77: 439–446.
Rydin, H. 1995. Effects of density and water level on re-cruitment, mortality and shoot size in Sphagnum popu-lations. – J. Bryol. 18: 439–453.
Rydin, H. and McDonald, A. J. S. 1985. Tolerance of
Sphagnum to water level. – J. Bryol. 13: 571–578.
Schneider-Poetsch, H. A. W., Marx, S., Kolukisaoglu, H. U. et al. 1994. Phytochrome evolution: phytochrome genes in ferns and mosses. – Physiol. Plant. 91: 241– 250.
Schneider-Poetsch, H. A. W., Kolukisaoglu, U., Clapham, D. H. et al. 1998. Non-angiosperm phytochromes and the evolution of vascular plants. – Physiol. Plant. 102: 612–622.
Skre, O. and Oechel, W. C. 1981. Moss functioning in different taiga ecosystems in interior Alaska. I. Sea-sonal, phenotypic and drought effects on photosynthe-sis and response pattern. – Oecologia 48: 50–59. Skre, O., Oechel, W. C. and Miller, P. M. 1983. Moss leaf
water content and solar radiation at the moss surface in a mature black spruce forest in central Alaska. – Can. J. For. Res. 13: 860–868.
Smith, H. 1982. Light quality, photoperception and plant strategy. – Annu. Rev. Plant Physiol. 33: 481–518. Smith, H. and Whitelam G. C. 1997. The shade avoidance
syndrome: multiple responses mediated by multiple phy-tochromes. – Plant Cell Environ. 20: 840–844. Suetsugu, N. and Wada, M. 2003. Cryptogam blue-light
photoreceptors. – Curr. Op. Plant Biol. 6: 91–96. Tallis, J. H. 1958. Studies in the biology and ecology of
Rhacomitrium lanuginosum Brid. I. Distribution and
ecology. – J. Ecol. 46: 271–288.
Tallis, J. H. 1959a. Studies in the biology and ecology of
Rhacomitrium lanuginosum Brid. II. Growth,
reproduc-tion and physiology. – J. Ecol. 47: 325–350.
Tallis, J. H. 1959b. Periodicity of growth in
Rhacomitri-um lanuginosRhacomitri-um. – J. Linn. Soc. Lond. 56: 212–217.
Tallis, J. H. 1964. Growth studies on Rhacomitrium
lanu-ginosum. - Bryologist 67: 417–422.
Tamm, C. O. 1953. Growth, yield and nutrition in carpets of a forest moss (Hylocomium splendens). – Meddel. Statens Skogsforskningsinst. 43: 1–140.
Thorsteinsson, I. and Arnalds, Ó. 1992. The vegetation and soils of the Thingvallavatn area. – Oikos 64: 105– 116.
Van der Hoeven, E. C., Huynen, C. I. J. and During, H. J. 1993. Vertical profiles of biomass, light intercepting area and light intensity in chalk grassland mosses. – J. Hatt. Bot. Lab. 74: 261–270.
Van der Hoeven, E. C., Korporaal, M. and van Gestel, E. 1998. Effects of simulated shade on growth, morpholo-gy and competitive interactions in two pleurocarpous mosses. – J. Bryol. 20: 301–310.
Van der Wal, R., Pearce, I. S. K. and Brooker, R. W. 2005. Mosses and the struggle for light in a nitrogen-polluted world. – Oecologia 142: 159–168.
Van Wijk, M. T., Clemmensen, K. E., Shaver, G. R. et al. 2003. Long-term ecosystem level experiments at Too-lik Lake, Alaska, and at Abisko, northern Sweden: gen-eralizations and differences in ecosystem and plant type responses to global change. – Global Change Biol. 10: 105–123.
Vitt, D. H. and Marsh, C. 1988. Population variation and phytogeography of Racomitrium lanuginosum and R.