wastewater
A TI O N O F N ITR O G EN R EC O V ER Y F R O M C O N C ENTR A TE D W A STE W A TE R 202 1 ISBN 978-91-7485-496-1 ISSN 1651-9256Address: P.O. Box 883, SE-721 23 Västerås. Sweden Address: P.O. Box 325, SE-631 05 Eskilstuna. Sweden E-mail: info@mdh.se Web: www.mdh.se
Mälardalen University Press Licentiate Theses No. 303
INVESTIGATION OF NITROGEN RECOVERY
FROM CONCENTRATED WASTEWATER
Aubrey Shenk 2021
School of Business, Society and Engineering
Mälardalen University Press Licentiate Theses No. 303
INVESTIGATION OF NITROGEN RECOVERY
FROM CONCENTRATED WASTEWATER
Aubrey Shenk 2021
Copyright © Aubrey Shenk, 2021 ISBN 978-91-7485-496-1
ISSN 1651-9256
Printed by E-Print AB, Stockholm, Sweden
Copyright © Aubrey Shenk, 2021 ISBN 978-91-7485-496-1
ISSN 1651-9256
Acknowledgements
“Anything is possible when you have the right people there to support you” Misty Copeland
I would like to express my deep appreciation for my family and friends in the United States for their encouragement through the past two years. Leaving the country was a decision that surprised many of them, but I could not have achieved what I have today without their support. I’d like to thank Dr. Berat Haznedaroglu and Dr. David Blersch for their roles in the start of my academic career and their contagious enthusiasm for research.
I’d like to thank Prof. Monica Odlare and Prof. Eva Thorin for their continuing guidance throughout the process of my licentiate, allowing me freedom to choose my own path, and Dr. Sebastian Schwede for his passion and excitement that always inspires me to think bigger than my original plans. To all of the friends and colleagues I have met at MDH and beyond I thank you for your words of wisdom and assistance in adjusting to a new university and a new culture.
Finally, but most importantly, I would like to thank my husband Mike for his never wavering love and support. You have allowed me to dream bigger and accomplish more than I ever thought possible.
Västerås, Sweden, March 2021
Acknowledgements
“Anything is possible when you have the right people there to support you” Misty Copeland
I would like to express my deep appreciation for my family and friends in the United States for their encouragement through the past two years. Leaving the country was a decision that surprised many of them, but I could not have achieved what I have today without their support. I’d like to thank Dr. Berat Haznedaroglu and Dr. David Blersch for their roles in the start of my academic career and their contagious enthusiasm for research.
I’d like to thank Prof. Monica Odlare and Prof. Eva Thorin for their continuing guidance throughout the process of my licentiate, allowing me freedom to choose my own path, and Dr. Sebastian Schwede for his passion and excitement that always inspires me to think bigger than my original plans. To all of the friends and colleagues I have met at MDH and beyond I thank you for your words of wisdom and assistance in adjusting to a new university and a new culture.
Finally, but most importantly, I would like to thank my husband Mike for his never wavering love and support. You have allowed me to dream bigger and accomplish more than I ever thought possible.
Summary
Nitrogen recovery from wastewater treatment for fertilizers is a research topic that exists at the intersection of multiple topics important to the future of sustainable society. First, nitrogen recovery from wastewater implies a departure from the current methods of nitrogen mitigation, which involve nitrogen removal by conversion of various aqueous species to inert nitrogen gas. Secondly, by recovering nitrogen from wastewater specifically, there is the opportunity to begin a circular economy where value added products can be obtained from material that has historically been seen as a “waste”. Current wastewater treatment involves nitrogen removal through the biological transformation of aqueous nitrogen species to inert nitrogen gas. This process
is energy intensive and risks the production of air pollutants such as N2O as
intermediates in the biological transformation. If this nitrogen can be captured in a form that can be reused, a valuable product can be achieved with the potential reduction of both the energy required at the wastewater treatment plant as well as the carbon footprint. Finally, by recovering nitrogen in a form that can be used in agriculture as a fertilizer, additional environmental benefits can be realized by reducing reliance on Haber-Bosch based ammonia production, which is also energy intensive and contributes harmful emissions to the atmosphere.
The work described in the following licentiate aims to consider the current status of nitrogen recovery from wastewater for fertilizers as a research topic. Literature was analytically examined to compare different techniques in terms of energy requirements, cost for fertilizer production, market for final fertilizer product, and technological readiness. The most interesting findings from this review were that there seems to be a disconnect between the fertilizer product produced by nitrogen recovery techniques and the market, which will become a challenge if these techniques are implemented at a large scale. The attitude of the farmers with regards to fertilizers from waste was overall positive, with their concerns mainly focused on the performance
Summary
Nitrogen recovery from wastewater treatment for fertilizers is a research topic that exists at the intersection of multiple topics important to the future of sustainable society. First, nitrogen recovery from wastewater implies a departure from the current methods of nitrogen mitigation, which involve nitrogen removal by conversion of various aqueous species to inert nitrogen gas. Secondly, by recovering nitrogen from wastewater specifically, there is the opportunity to begin a circular economy where value added products can be obtained from material that has historically been seen as a “waste”. Current wastewater treatment involves nitrogen removal through the biological transformation of aqueous nitrogen species to inert nitrogen gas. This process
is energy intensive and risks the production of air pollutants such as N2O as
intermediates in the biological transformation. If this nitrogen can be captured in a form that can be reused, a valuable product can be achieved with the potential reduction of both the energy required at the wastewater treatment plant as well as the carbon footprint. Finally, by recovering nitrogen in a form that can be used in agriculture as a fertilizer, additional environmental benefits can be realized by reducing reliance on Haber-Bosch based ammonia production, which is also energy intensive and contributes harmful emissions to the atmosphere.
The work described in the following licentiate aims to consider the current status of nitrogen recovery from wastewater for fertilizers as a research topic. Literature was analytically examined to compare different techniques in terms of energy requirements, cost for fertilizer production, market for final fertilizer product, and technological readiness. The most interesting findings from this review were that there seems to be a disconnect between the fertilizer product produced by nitrogen recovery techniques and the market, which will become a challenge if these techniques are implemented at a large scale. The attitude of the farmers with regards to fertilizers from waste was overall positive, with their concerns mainly focused on the performance
ability and cost of the product. Additionally, many techniques such as microbial fuel cells and microbial electrolysis cells have been unable to move past the laboratory phase despite being researched for many years. This indicates there are cost and technological barriers that are preventing the further scale up and implementation of these techniques. Energy and cost analyses will be crucial to motivate investment into these processes, and these are missing for many of the techniques found around this topic.
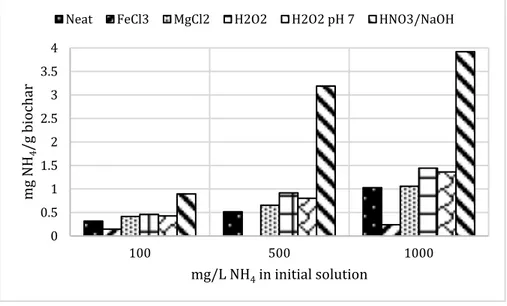
To contribute to this field, experimental work was also included to assess the potential for ammonium adsorption from concentrated wastewater for fertilizer production. The experimental work focused on the utilization of the solid product of pyrolysis of sewage sludge (biochar) for adsorption and explored the potential enhancement of the char with various chemical treatments. The char with the best ammonium adsorption performance was
found to be using a treatment of HNO3 followed by and NaOH, with an
adsorption capacity of 4 mg NH4/g biochar. This char was compared with
commercially activated carbon and clinoptilolite for full scale applications. It was found that even with this increased adsorption capacity, the use of chemically enhanced sewage sludge biochar for full scale applications is not realistic. The amount of raw material required for the complete recovery of ammonium from reject water at the municipal wastewater treatment plants exceeds the total amount of sewage sludge generated. Therefore it is recommended that the goal of incorporating sewage sludge biochar with wastewater treatment is to produce a solid fertilizer product loaded with ammonium (which would provide ammonium-N and phosphorus for plant growth, as well as carbon and other minerals for soil amendment) rather than having the goal be complete ammonium recovery from the wastewater stream.
ability and cost of the product. Additionally, many techniques such as microbial fuel cells and microbial electrolysis cells have been unable to move past the laboratory phase despite being researched for many years. This indicates there are cost and technological barriers that are preventing the further scale up and implementation of these techniques. Energy and cost analyses will be crucial to motivate investment into these processes, and these are missing for many of the techniques found around this topic.
To contribute to this field, experimental work was also included to assess the potential for ammonium adsorption from concentrated wastewater for fertilizer production. The experimental work focused on the utilization of the solid product of pyrolysis of sewage sludge (biochar) for adsorption and explored the potential enhancement of the char with various chemical treatments. The char with the best ammonium adsorption performance was
found to be using a treatment of HNO3 followed by and NaOH, with an
adsorption capacity of 4 mg NH4/g biochar. This char was compared with
commercially activated carbon and clinoptilolite for full scale applications. It was found that even with this increased adsorption capacity, the use of chemically enhanced sewage sludge biochar for full scale applications is not realistic. The amount of raw material required for the complete recovery of ammonium from reject water at the municipal wastewater treatment plants exceeds the total amount of sewage sludge generated. Therefore it is recommended that the goal of incorporating sewage sludge biochar with wastewater treatment is to produce a solid fertilizer product loaded with ammonium (which would provide ammonium-N and phosphorus for plant growth, as well as carbon and other minerals for soil amendment) rather than having the goal be complete ammonium recovery from the wastewater stream.
Swedish summary
Arbetet som beskrivs i denna licentiatavhandling syftar till att undersöka möjligheterna att utnyttja kväveåtervinning från avloppsvatten för produktion av gödselmedel. Genom att analysera litteratur inom området så jämfördes olika tekniker avseende energibehov, kostnad för gödselproduktion, marknaden för slutprodukten, samt hur färdig själva tekniken är. De mest intressanta resultaten från denna granskning var att det verkar saknas en
koppling mellan det gödselmedel som produceras med
kväveåtervinningstekniker och marknadsbehovet, vilket kommer att bli en utmaning om dessa tekniker ska implementeras i stor skala. Jordbrukarnas attityd gentemot gödselmedel från avfall var överlag positiv, och deras eventuella tveksamhet handlade främst om slutproduktens effektivitet och kostnad. Dessutom har många tekniker, som till exempel mikrobiella bränsleceller och mikrobiella elektrolysceller inte kunnat ta sig förbi laboratoriefasen trots att det har forskats på detta i många år. Detta tyder på att det finns kostnadsrelaterade och tekniska hinder som förhindrar att dessa tekniker skalas upp implementeras. Energi- och kostnadsanalyser är avgörande för att motivera investeringar i dessa processer, och dessa saknas för många av de tekniker som finns inom detta ämne.
För att bidra till kunskapsluckan inom området så omfattar avhandlingen även experimentella studier för att utvärdera om ammoniumadsorption från koncentrerat avloppsvatten kan användas för gödselproduktion. De experimentella studierna var fokuserade på möjligheterna att använda restprodukten från pyrolys av avloppsslam (biokol) för adsorption, samt om effektiviteten kan förbättras om restprodukten genomgår olika kemiska behandlingar. Resultaten visade att det biokol som hade bäst förmåga att adsorbera ammonium hade genomgått en behandling av HNO3 följt av och NaOH, och uppnådde därmed en adsorptionskapacitet på 4 mg NH4 / g biokol. Detta biokol jämfördes med kommersiellt aktivt kol och klinoptilolit för applikationer i stor skala. Det visade sig att trots den ökade
Swedish summary
Arbetet som beskrivs i denna licentiatavhandling syftar till att undersöka möjligheterna att utnyttja kväveåtervinning från avloppsvatten för produktion av gödselmedel. Genom att analysera litteratur inom området så jämfördes olika tekniker avseende energibehov, kostnad för gödselproduktion, marknaden för slutprodukten, samt hur färdig själva tekniken är. De mest intressanta resultaten från denna granskning var att det verkar saknas en
koppling mellan det gödselmedel som produceras med
kväveåtervinningstekniker och marknadsbehovet, vilket kommer att bli en utmaning om dessa tekniker ska implementeras i stor skala. Jordbrukarnas attityd gentemot gödselmedel från avfall var överlag positiv, och deras eventuella tveksamhet handlade främst om slutproduktens effektivitet och kostnad. Dessutom har många tekniker, som till exempel mikrobiella bränsleceller och mikrobiella elektrolysceller inte kunnat ta sig förbi laboratoriefasen trots att det har forskats på detta i många år. Detta tyder på att det finns kostnadsrelaterade och tekniska hinder som förhindrar att dessa tekniker skalas upp implementeras. Energi- och kostnadsanalyser är avgörande för att motivera investeringar i dessa processer, och dessa saknas för många av de tekniker som finns inom detta ämne.
För att bidra till kunskapsluckan inom området så omfattar avhandlingen även experimentella studier för att utvärdera om ammoniumadsorption från koncentrerat avloppsvatten kan användas för gödselproduktion. De experimentella studierna var fokuserade på möjligheterna att använda restprodukten från pyrolys av avloppsslam (biokol) för adsorption, samt om effektiviteten kan förbättras om restprodukten genomgår olika kemiska behandlingar. Resultaten visade att det biokol som hade bäst förmåga att adsorbera ammonium hade genomgått en behandling av HNO3 följt av och NaOH, och uppnådde därmed en adsorptionskapacitet på 4 mg NH4 / g biokol. Detta biokol jämfördes med kommersiellt aktivt kol och klinoptilolit för applikationer i stor skala. Det visade sig att trots den ökade
adsorptionskapacitet så är användningen av kemiskt förbättrad biokol från avloppsslam inte realistiskt i fullskalatillämpningar. Den mängd råvara som skulle krävas för fullständig återvinning av ammonium från avloppsvattnet vid kommunala avloppsreningsverk överstiger den totala mängden avloppsslam som genereras. Rekommendationen är därför att biokol från avloppsslam bör användas inom avloppsvattenrening för att producera en fast gödselprodukt laddat med ammonium (som skulle ge ammonium-N och fosfor för växttillväxt, liksom kol och andra mineraler för jordförändring) snarare än försöka uppnå fullständig ammoniumåtervinning från avloppsvattnet.
adsorptionskapacitet så är användningen av kemiskt förbättrad biokol från avloppsslam inte realistiskt i fullskalatillämpningar. Den mängd råvara som skulle krävas för fullständig återvinning av ammonium från avloppsvattnet vid kommunala avloppsreningsverk överstiger den totala mängden avloppsslam som genereras. Rekommendationen är därför att biokol från avloppsslam bör användas inom avloppsvattenrening för att producera en fast gödselprodukt laddat med ammonium (som skulle ge ammonium-N och fosfor för växttillväxt, liksom kol och andra mineraler för jordförändring) snarare än försöka uppnå fullständig ammoniumåtervinning från avloppsvattnet.
List of papers
Publications included in the thesis
This thesis is based on the following papers, which are referred to in the text by their roman numerals:
I. Beckinghausen, A., Odlare, M., Thorin, E., Schwede, S. 2020. “From Removal to Recovery: An Evaluation of Nitrogen Recovery Techniques from Wastewater.” Applied Energy 263: 114616. https://doi.org/10.1016/j.apenergy.2020.114616.
II. Beckinghausen, A., Reynders, J., Merckel, R., Wu, Y.W., Marais, H., Schwede, S. 2020. “Post-Pyrolysis Treatments of Biochars from Sewage Sludge and A. Mearnsii for Ammonia (NH4-n) Recovery.”
Applied Energy 271 (August): 115212.
https://doi.org/10.1016/j.apenergy.2020.115212.
Publications not included in the thesis
I. Beckinghausen, A., Dahlquist, E., Schwede, S., Lindroos, N., Retkin, R., Laatikainen, R. 2019. “Downstream Processing of Bioredined Lactate from Lake Bottom Zero Fiber Deposit - a Techno-Eocnomic
Study on Energy Efficient Production of Green Chemicals.” 11th
International Conference on Applied Energy, Västerås, Sweden. II. Beckinghausen, A., Reynders, J., Merckel, R., Wu, Y.W., Schwede,
S. 2019. “Comparison of Biochar Enhancements for Ammonia (NH4–
N) Sorption from Concentrated Wastewater.” 11th International
Conference on Applied Energy, Västerås, Sweden.
List of papers
Publications included in the thesis
This thesis is based on the following papers, which are referred to in the text by their roman numerals:
I. Beckinghausen, A., Odlare, M., Thorin, E., Schwede, S. 2020. “From Removal to Recovery: An Evaluation of Nitrogen Recovery Techniques from Wastewater.” Applied Energy 263: 114616. https://doi.org/10.1016/j.apenergy.2020.114616.
II. Beckinghausen, A., Reynders, J., Merckel, R., Wu, Y.W., Marais, H., Schwede, S. 2020. “Post-Pyrolysis Treatments of Biochars from Sewage Sludge and A. Mearnsii for Ammonia (NH4-n) Recovery.”
Applied Energy 271 (August): 115212.
https://doi.org/10.1016/j.apenergy.2020.115212.
Publications not included in the thesis
I. Beckinghausen, A., Dahlquist, E., Schwede, S., Lindroos, N., Retkin, R., Laatikainen, R. 2019. “Downstream Processing of Bioredined Lactate from Lake Bottom Zero Fiber Deposit - a Techno-Eocnomic
Study on Energy Efficient Production of Green Chemicals.” 11th
International Conference on Applied Energy, Västerås, Sweden. II. Beckinghausen, A., Reynders, J., Merckel, R., Wu, Y.W., Schwede,
S. 2019. “Comparison of Biochar Enhancements for Ammonia (NH4–
N) Sorption from Concentrated Wastewater.” 11th International
Contents
Acknowledgements ... iv
Summary ...v
Swedish summary ... vii
List of papers ... ix
List of figures ... xiii
List of tables ... xv
Nomenclature ... xvi
1 INTRODUCTION ...1
1.1 Background and motivation ...2
1.2 Objectives and research questions ...3
1.3 Contributions to knowledge ...4
2 METHODOLOGY ...5
2.1 Literature analysis methodology ...7
2.1.1 Selection of appropriate studies ...7
2.1.2 Calculation of important technological parameters ...7
2.1.3 Comparison and analysis of data ...8
2.2 Experimental methodology ...8
2.2.1 Kinetics and isotherm tests ...8
2.2.2 Packed column tests ...9
2.2.3 Chemical treatments of adsorbents ...9
2.2.4 Benchmark Simulation Model ... 10
3 RESULTS AND DISCUSSION ... 12
3.1 Literature review results ... 12
3.1.1 Nitrogen recovery and energy efficiency of different techniques .. 12
3.1.2 Cost/benefit of different techniques ... 15
3.1.3 Additional findings ... 16 3.2 Experimental results ... 16 3.2.1 Kinetic test ... 16
Contents
Acknowledgements ... iv Summary ...vSwedish summary ... vii
List of papers ... ix
List of figures ... xiii
List of tables ... xv
Nomenclature ... xvi
1 INTRODUCTION ...1
1.1 Background and motivation ...2
1.2 Objectives and research questions ...3
1.3 Contributions to knowledge ...4
2 METHODOLOGY ...5
2.1 Literature analysis methodology ...7
2.1.1 Selection of appropriate studies ...7
2.1.2 Calculation of important technological parameters ...7
2.1.3 Comparison and analysis of data ...8
2.2 Experimental methodology ...8
2.2.1 Kinetics and isotherm tests ...8
2.2.2 Packed column tests ...9
2.2.3 Chemical treatments of adsorbents ...9
2.2.4 Benchmark Simulation Model ... 10
3 RESULTS AND DISCUSSION ... 12
3.1 Literature review results ... 12
3.1.1 Nitrogen recovery and energy efficiency of different techniques .. 12
3.1.2 Cost/benefit of different techniques ... 15
3.1.3 Additional findings ... 16
3.2 Experimental results ... 16
3.2.2 Isotherm test ... 17
3.2.3 Lab-scale column experiments ... 20
3.3 Results from BSM2 simulation ... 22
3.4 Discussion ... 23
3.4.1 Sewage sludge as a valorised material ... 23
3.4.2 The future of nitrogen recovery ... 24
4 CONCLUSION ... 25
5 FUTURE WORK ... 29
5.1 Hydrothermal carbonization of sewage sludge ... 29
5.2 Gas permeable membrane technology ... 30
REFERENCES ... 31
PAPERS ... 39
3.2.2 Isotherm test ... 17
3.2.3 Lab-scale column experiments ... 20
3.3 Results from BSM2 simulation ... 22
3.4 Discussion ... 23
3.4.1 Sewage sludge as a valorised material ... 23
3.4.2 The future of nitrogen recovery ... 24
4 CONCLUSION ... 25
5 FUTURE WORK ... 29
5.1 Hydrothermal carbonization of sewage sludge ... 29
5.2 Gas permeable membrane technology ... 30
REFERENCES ... 31
List of figures
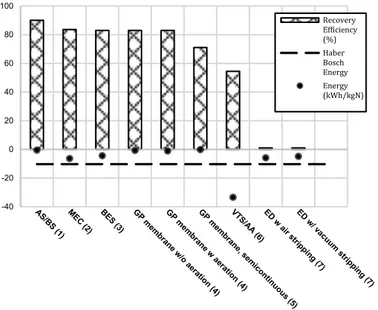
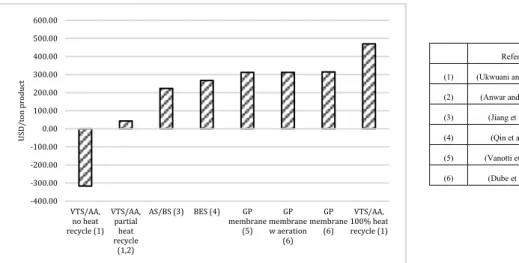
Figure 1. Schematic of wastewater treatment plant for BSM2 model ... 11 Figure 2. Recovery and energy balance details for reject water and digestate
data ... 14 Figure 3. Cost/benefit analysis of reject water and digestate data ... 15 Figure 4. Ammonium adsorption capacities of different materials ... 17 Figure 5. Effluent concentration of ammonium from upflow column testing
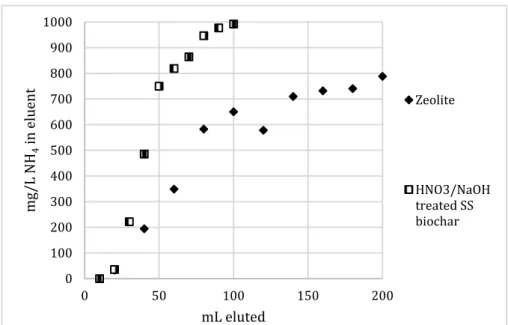
using untreated materials ... 20 Figure 6. Effluent concentration of upflow column using zeolite and best
treated biochar ... 21
List of figures
Figure 1. Schematic of wastewater treatment plant for BSM2 model ... 11 Figure 2. Recovery and energy balance details for reject water and digestate
data ... 14 Figure 3. Cost/benefit analysis of reject water and digestate data ... 15 Figure 4. Ammonium adsorption capacities of different materials ... 17 Figure 5. Effluent concentration of ammonium from upflow column testing
using untreated materials ... 20 Figure 6. Effluent concentration of upflow column using zeolite and best
List of tables
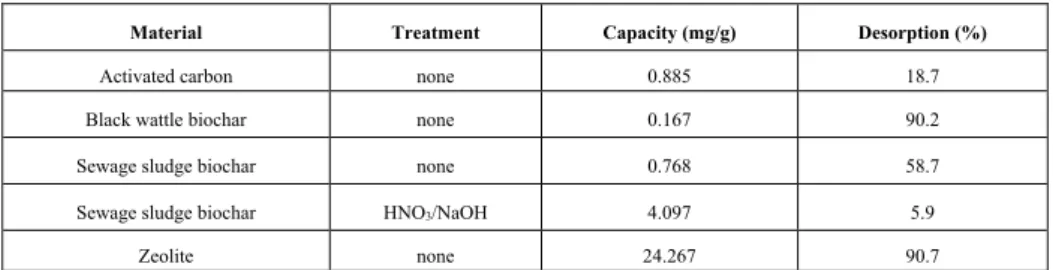
Table 1. Model results, RME and surface characteristics ... 18 Table 2. Capacities for adsorption and desorption of ammonium ... 22
List of tables
Table 1. Model results, RME and surface characteristics ... 18 Table 2. Capacities for adsorption and desorption of ammonium ... 22
Nomenclature
Abbreviations
VTS/AA Vacuum Thermal Stripping/Acid Absorption technique AS/BS Ammonia Stripping/Biogas Scrubbing technique BES BioElectrochemical System
GP Gas Permeable
MEC Microbial Electrolysis Cell ED ElectroDialysis
Symbols
NH4+ Ammonium ion
Nomenclature
Abbreviations
VTS/AA Vacuum Thermal Stripping/Acid Absorption technique AS/BS Ammonia Stripping/Biogas Scrubbing technique BES BioElectrochemical System
GP Gas Permeable
MEC Microbial Electrolysis Cell ED ElectroDialysis
Symbols
Mälardalen University Press Licentiate Theses 1
1 Introduction
“The world will soon enter a decade that will be decisive for both current and future generations and for all life on this planet. It is the world’s responsibility and within its power to make it a decade of action and delivery for sustainable development” – Antonio Guterres, Secretary-General of the United Nations
Sustainable development is the current buzzword sweeping the globe, as countries attempt to increase the standard of living worldwide through initiatives inspired by the UN Sustainable Development Goals for 2030. In the developed world, many of the goals such as access to basic sanitation and drinking water have been met, therefore more lofty goals like increasing biodiversity and protecting the environment can be set and focused on. Rockström et al (2009) suggested planetary boundaries as a concept that various environmental indicators should be kept within a certain range to prevent environmental change on a global scale. One boundary that they report is the biogeochemical nitrogen cycle, in which the industrial and agricultural fixation of nitrogen should be limited to 35 Tg N/year. Since the recent nitrogen fixation was estimated in 2013 as 121 Tg N/year (Nykvist, Sverige, and Naturvårdsverket 2013), this represents a key target for sustainable development to reduce human impact on the environment. Some environmental impacts of the imbalance of the nitrogen cycle include changes in tropospheric ozone, increased atmospheric particulate matter and degradation of air quality, as well as eutrophication of waterways (Fowler et al. 2013). Some researchers have even posited that reactive nitrogen may have a similar magnitude of impact that carbon dioxide currently has on the global environmental system, once we are able to determine its effects over a longer time scale (Battye, Aneja, and Schlesinger 2017).
Mälardalen University Press Licentiate Theses 1
1 Introduction
“The world will soon enter a decade that will be decisive for both current and future generations and for all life on this planet. It is the world’s responsibility and within its power to make it a decade of action and delivery for sustainable development” – Antonio Guterres, Secretary-General of the United Nations
Sustainable development is the current buzzword sweeping the globe, as countries attempt to increase the standard of living worldwide through initiatives inspired by the UN Sustainable Development Goals for 2030. In the developed world, many of the goals such as access to basic sanitation and drinking water have been met, therefore more lofty goals like increasing biodiversity and protecting the environment can be set and focused on. Rockström et al (2009) suggested planetary boundaries as a concept that various environmental indicators should be kept within a certain range to prevent environmental change on a global scale. One boundary that they report is the biogeochemical nitrogen cycle, in which the industrial and agricultural fixation of nitrogen should be limited to 35 Tg N/year. Since the recent nitrogen fixation was estimated in 2013 as 121 Tg N/year (Nykvist, Sverige, and Naturvårdsverket 2013), this represents a key target for sustainable development to reduce human impact on the environment. Some environmental impacts of the imbalance of the nitrogen cycle include changes in tropospheric ozone, increased atmospheric particulate matter and degradation of air quality, as well as eutrophication of waterways (Fowler et al. 2013). Some researchers have even posited that reactive nitrogen may have a similar magnitude of impact that carbon dioxide currently has on the global environmental system, once we are able to determine its effects over a longer time scale (Battye, Aneja, and Schlesinger 2017).
2
1.1
Background and motivation
Nitrogen is an essential element to all life, as it a component for chlorophyll and amino acids (which are the building blocks of proteins) as well as nucleic acids that make up DNA. Humans have capitalized on this important nutrient by concentrating it with other nutrients and applying it to agriculture in the form of fertilizers, to increase food production. Currently about one half of the global population is supported by food that would not be available without the productivity increase that fertilizers provide (Fowler et al. 2013). However, when these nutrients leave the agricultural system, they cause increases in plant productivity that that can have negative consequences. Eutrophication is defined as the increase in growth of algae or other plants in aquatic systems due to an overabundance of minerals and nutrients. Nitrogen has been cited as a key nutrient that can cause eutrophication, which can lead to decreased dissolved inorganic carbon and dissolved oxygen levels, increased pH and reduced water quality (Chislock et al. n.d.). Eutrophication has also been tied to increased methane emissions from these water bodies as organic substrates increase and methanogenesis rates rise (Beaulieu, DelSontro, and Downing 2019). Nitrogen introduction to waterways must therefore be carefully monitored and controlled to prevent eutrophication. In addition to eutrophication of waterways, reactive nitrogen in the atmosphere creates secondary pollutants including ozone, other photochemical oxidants, aerosols and atmospheric particulate matter which degrades air quality
(Fowler et al. 2013). Nitrous oxide (N2O) also has a long atmospheric lifetime
of 100 years and has a global warming potential of 298, therefore its contribution to climate change is almost 300 times more than the same mass of carbon dioxide.
One potential path for nitrogen to be introduced into natural waterways is through wastewater treatment plant effluent. Wastewater treatment plants have the responsibility to treat water that has been used for private or public use, to remove contamination and return the water to the natural system. The Urban Waste Water Directive (92/271/EEC) calls for a discharge limit of 15 mgN/L for European wastewater treatment plants that discharge to sensitive areas, and further that 70-80% of the influent nitrogen should be removed (Hauck et al. 2016). In 2018, the degree of treatment in municipal wastewater treatment plants in Sweden only reduced the influent load by 64% (Myhr and Dimberg 2020). Nitrogen removal techniques transition ammonium ions from wastewater to nitrogen gas that is ultimately lost to the atmosphere. Through biological nitrogen removal (nitrification-denitrification) there is also increased potential for NOx emissions due to the reactive intermediates in the process, the impact of which can reach up to 80% of the total operational carbon footprint of the treatment plant (Desloover et al. 2012).
2
1.1
Background and motivation
Nitrogen is an essential element to all life, as it a component for chlorophyll and amino acids (which are the building blocks of proteins) as well as nucleic acids that make up DNA. Humans have capitalized on this important nutrient by concentrating it with other nutrients and applying it to agriculture in the form of fertilizers, to increase food production. Currently about one half of the global population is supported by food that would not be available without the productivity increase that fertilizers provide (Fowler et al. 2013). However, when these nutrients leave the agricultural system, they cause increases in plant productivity that that can have negative consequences. Eutrophication is defined as the increase in growth of algae or other plants in aquatic systems due to an overabundance of minerals and nutrients. Nitrogen has been cited as a key nutrient that can cause eutrophication, which can lead to decreased dissolved inorganic carbon and dissolved oxygen levels, increased pH and reduced water quality (Chislock et al. n.d.). Eutrophication has also been tied to increased methane emissions from these water bodies as organic substrates increase and methanogenesis rates rise (Beaulieu, DelSontro, and Downing 2019). Nitrogen introduction to waterways must therefore be carefully monitored and controlled to prevent eutrophication. In addition to eutrophication of waterways, reactive nitrogen in the atmosphere creates secondary pollutants including ozone, other photochemical oxidants, aerosols and atmospheric particulate matter which degrades air quality
(Fowler et al. 2013). Nitrous oxide (N2O) also has a long atmospheric lifetime
of 100 years and has a global warming potential of 298, therefore its contribution to climate change is almost 300 times more than the same mass of carbon dioxide.
One potential path for nitrogen to be introduced into natural waterways is through wastewater treatment plant effluent. Wastewater treatment plants have the responsibility to treat water that has been used for private or public use, to remove contamination and return the water to the natural system. The Urban Waste Water Directive (92/271/EEC) calls for a discharge limit of 15 mgN/L for European wastewater treatment plants that discharge to sensitive areas, and further that 70-80% of the influent nitrogen should be removed (Hauck et al. 2016). In 2018, the degree of treatment in municipal wastewater treatment plants in Sweden only reduced the influent load by 64% (Myhr and Dimberg 2020). Nitrogen removal techniques transition ammonium ions from wastewater to nitrogen gas that is ultimately lost to the atmosphere. Through biological nitrogen removal (nitrification-denitrification) there is also increased potential for NOx emissions due to the reactive intermediates in the process, the impact of which can reach up to 80% of the total operational carbon footprint of the treatment plant (Desloover et al. 2012).
Mälardalen University Press Licentiate Theses 3
This energy intensive step is important in returning the water quality to an appropriate level but also represents a lost opportunity for resource recovery by allowing the removed nitrogen to escape to the atmosphere. The aeration energy required for biological treatment (biological oxygen demand and nitrogen removal) can contribute up to 50% of the total energy requirements of the wastewater treatment plant (Nowak 2003).
Though nitrogen is required to be removed at wastewater treatment plants, nitrogen is also required for the production of mineral fertilizers. The Haber-Bosch process is an industrial process that takes atmospheric nitrogen and reacts with natural gas over a catalyst to form ammonia. Both the denitrification at treatment plants and the Haber-Bosch process consume 45 MJ/kg N and are completely independent (Maurer, Schwegler, and Larsen 2003). As the world moves forward into a more circular-economy mindset, the idea of tying these two processes to save energy and natural resources can be considered. Rather than transforming the ammonia to nitrogen gas and then transforming it back to ammonia, nitrogen recovery for fertilizer reuse suggests finding a way to capture the ammonia to use directly as a nitrogen fertilizer. The work contained in this licentiate aims to explore nitrogen recovery techniques for transforming ammonium from wastewater into a fertilizer substitute.
This topic has been increasing steadily as a topic of importance in research for the past twenty years, as the sustainability of current nitrogen removal techniques from wastewater is questioned, and as the attitude towards wastewater shifts from viewing it as a nuisance to a resource. Phosphorus recovery has also been an issue of interest since phosphorus is a limited natural resource that will likely be depleted in the next 100 years (Cooper et al. 2011). Natural gas, which is used for the production of ammonia from the Haber Bosch process, is actually more limited than phosphorus reserves, with an estimated longevity of 55 years, which provides another justification for the recovery of nitrogen (Fixen and Johnston 2012).
1.2
Objectives and research questions
The purpose of this work is to explore and evaluate the current state of nitrogen recovery techniques using wastewater as a nitrogen source, that produce fertilizers as the end product. This literature research was coupled with experimental work to solve the following three research questions:
1. What is the current status and potential of nitrogen recovery techniques for wastewater applications? (Paper I)
Mälardalen University Press Licentiate Theses 3
This energy intensive step is important in returning the water quality to an appropriate level but also represents a lost opportunity for resource recovery by allowing the removed nitrogen to escape to the atmosphere. The aeration energy required for biological treatment (biological oxygen demand and nitrogen removal) can contribute up to 50% of the total energy requirements of the wastewater treatment plant (Nowak 2003).
Though nitrogen is required to be removed at wastewater treatment plants, nitrogen is also required for the production of mineral fertilizers. The Haber-Bosch process is an industrial process that takes atmospheric nitrogen and reacts with natural gas over a catalyst to form ammonia. Both the denitrification at treatment plants and the Haber-Bosch process consume 45 MJ/kg N and are completely independent (Maurer, Schwegler, and Larsen 2003). As the world moves forward into a more circular-economy mindset, the idea of tying these two processes to save energy and natural resources can be considered. Rather than transforming the ammonia to nitrogen gas and then transforming it back to ammonia, nitrogen recovery for fertilizer reuse suggests finding a way to capture the ammonia to use directly as a nitrogen fertilizer. The work contained in this licentiate aims to explore nitrogen recovery techniques for transforming ammonium from wastewater into a fertilizer substitute.
This topic has been increasing steadily as a topic of importance in research for the past twenty years, as the sustainability of current nitrogen removal techniques from wastewater is questioned, and as the attitude towards wastewater shifts from viewing it as a nuisance to a resource. Phosphorus recovery has also been an issue of interest since phosphorus is a limited natural resource that will likely be depleted in the next 100 years (Cooper et al. 2011). Natural gas, which is used for the production of ammonia from the Haber Bosch process, is actually more limited than phosphorus reserves, with an estimated longevity of 55 years, which provides another justification for the recovery of nitrogen (Fixen and Johnston 2012).
1.2
Objectives and research questions
The purpose of this work is to explore and evaluate the current state of nitrogen recovery techniques using wastewater as a nitrogen source, that produce fertilizers as the end product. This literature research was coupled with experimental work to solve the following three research questions:
1. What is the current status and potential of nitrogen recovery techniques for wastewater applications? (Paper I)
4
2. What is the ammonium capacity potential of adsorbent materials using chemical or physical enhancements post-pyrolysis? (Paper II)
3. What are the full-scale material requirements for implementing adsorption of ammonium from reject water using these materials? (Paper II)
1.3
Contributions to knowledge
The two journal papers included in this thesis contributed to the field in the following ways:
• Paper I consisted of a review of technologies for nitrogen recovery from wastewater sources for the production of fertilizers. It included nitrogen recovery efficiencies, energy analyses, and economic analyses to give a more comprehensive review of the current status of these techniques. Additionally, the review covered the gap that exists between research and the real world, in the lack of communication between researchers and end users of the fertilizer product which would be important for full scale implementation of these techniques.
• Paper II was based on experimental work to determine ammonium recovery efficiency of different biochar materials from synthetic concentrated wastewater. This article aimed to put these values into context through extrapolating the results from laboratory scale to full scale and determining how realistic adsorption for nitrogen removal could be with these materials.
4
2. What is the ammonium capacity potential of adsorbent materials using chemical or physical enhancements post-pyrolysis? (Paper II)
3. What are the full-scale material requirements for implementing adsorption of ammonium from reject water using these materials? (Paper II)
1.3
Contributions to knowledge
The two journal papers included in this thesis contributed to the field in the following ways:
• Paper I consisted of a review of technologies for nitrogen recovery from wastewater sources for the production of fertilizers. It included nitrogen recovery efficiencies, energy analyses, and economic analyses to give a more comprehensive review of the current status of these techniques. Additionally, the review covered the gap that exists between research and the real world, in the lack of communication between researchers and end users of the fertilizer product which would be important for full scale implementation of these techniques.
• Paper II was based on experimental work to determine ammonium recovery efficiency of different biochar materials from synthetic concentrated wastewater. This article aimed to put these values into context through extrapolating the results from laboratory scale to full scale and determining how realistic adsorption for nitrogen removal could be with these materials.
Mälardalen University Press Licentiate Theses 5
2 Methodology
In order to understand the current state of nitrogen recovery from wastewater, analysis was performed including energetic and economic estimations from values found in literature. Recovery techniques of interest should be easy to implement with proven scalability and low energy requirements. Additionally, the target wastewater stream for the experimental work was reject water (recirculated water available after dewatering of anaerobic
digestate, average ammonium concentration of 1000 mg/L NH4+), so the
technique should be able to handle high concentrations of ammonium. Reject water was chosen as the target stream due to its high concentration and low volume. Because the reject water stream is recycled back to the inlet of the plant after the dewatering of anaerobic digestate or sewage sludge, it represents a constant nitrogen load on the system that can account for up to 30% of the total N load on the wastewater treatment plant (Guo, Stabnikov, and Ivanov 2010). This stream also is less complex than incoming wastewater since it has undergone numerous treatment steps before recirculating. Since the stream has a high concentration with low volume, no costly additional treatment steps like nano-filtration would be necessary before recovery. Ultimately the process chosen for the experimental testing was adsorption.
Adsorption is a simple process by which a substance is bound to the surface of an adsorbent by chemical or physical means. Inorganics like ammonium can be bound to adsorbents by stoichiometric ion exchange, electrostatic attraction, surface precipitation, or physical entrapment (Sizmur et al. 2017). Ion exchange is the process of chemical sorption between surface functional groups and ions in the liquid phase, where cations leave the solid surface and are replaced by other ions from the liquid. The ability of a material to interact in this way can be measured using cation exchange capacity and is pH dependent. Electrostatic attraction is a form of physical sorption that does not require the stoichiometric release of cations from the surface of the material, but rather the surface charge is able to adhere the ions
Mälardalen University Press Licentiate Theses 5
2 Methodology
In order to understand the current state of nitrogen recovery from wastewater, analysis was performed including energetic and economic estimations from values found in literature. Recovery techniques of interest should be easy to implement with proven scalability and low energy requirements. Additionally, the target wastewater stream for the experimental work was reject water (recirculated water available after dewatering of anaerobic
digestate, average ammonium concentration of 1000 mg/L NH4+), so the
technique should be able to handle high concentrations of ammonium. Reject water was chosen as the target stream due to its high concentration and low volume. Because the reject water stream is recycled back to the inlet of the plant after the dewatering of anaerobic digestate or sewage sludge, it represents a constant nitrogen load on the system that can account for up to 30% of the total N load on the wastewater treatment plant (Guo, Stabnikov, and Ivanov 2010). This stream also is less complex than incoming wastewater since it has undergone numerous treatment steps before recirculating. Since the stream has a high concentration with low volume, no costly additional treatment steps like nano-filtration would be necessary before recovery. Ultimately the process chosen for the experimental testing was adsorption.
Adsorption is a simple process by which a substance is bound to the surface of an adsorbent by chemical or physical means. Inorganics like ammonium can be bound to adsorbents by stoichiometric ion exchange, electrostatic attraction, surface precipitation, or physical entrapment (Sizmur et al. 2017). Ion exchange is the process of chemical sorption between surface functional groups and ions in the liquid phase, where cations leave the solid surface and are replaced by other ions from the liquid. The ability of a material to interact in this way can be measured using cation exchange capacity and is pH dependent. Electrostatic attraction is a form of physical sorption that does not require the stoichiometric release of cations from the surface of the material, but rather the surface charge is able to adhere the ions
6
directly. Surface precipitation is most common for metal contaminants where an insoluble salt is formed due to interactions with other minerals on the surface of the adsorbent. Finally, physical entrapment can occur if the ions are small enough to enter the pores of the adsorbent.
Pyrolysis of waste materials like garden waste, sewage sludge, etc. produces a solid product called biochar, which has been shown to also have potential to capture ammonia while including another circular economy path by reusing waste products to make adsorbents. The main biochar of interest for this study was sewage sludge, since it is an important topic of interest in Sweden. The use of sewage sludge on agricultural land has been an ongoing debate in Sweden for many years (Bengtsson and Tillman 2004). Since landfill use in Sweden is extremely low (which is the third most common method for disposing of sewage sludge in the EU behind incineration (“Eurostat - Data Explorer” n.d.)), alternatives must be researched to prepare for this eventuality. The digested sewage sludge was obtained from Linz am Rhein – Unkel Municipal Wastewater Treatment Plant and then pyrolyzed at 550-600°C for 16-30 min (PYREG GmbH, Germany). In addition to sewage sludge-based biochar, biochar from the invasive species A. mearnsii (black wattle) was tested (produced by Pretoria University). Black wattle is one of the worst invasive species in South Africa, denoted as a category 2 invasive species, but also has an invasive presence in other countries like USA, Ethiopia, Kenya, Jamaica, Brazil and New Zealand (“Acacia Mearnsii (Black Wattle)” n.d.). Due to material availability issues limited testing was done with this material compared to the sewage-sludge materials. Additionally, mineral based materials that were tested included sepiolite (Gemina International, Turkey), LECA clay (LECA International, Norway) and clinoptilolite (Zeocem, Slovakia). The best material from the initial testing was clinoptilolite, which was then used to compare against the results of the sewage sludge biochar. Despite showing promising results in Balci and Dinçel (2002) sepiolite performed poorly in the high ammonium solutions of this experiment. LECA clay performed similarly to results by Sharifnia et al. (2016) with very low ammonium removal. Due to the low capacities in initial tests, the results with these materials are not reported.
6
directly. Surface precipitation is most common for metal contaminants where an insoluble salt is formed due to interactions with other minerals on the surface of the adsorbent. Finally, physical entrapment can occur if the ions are small enough to enter the pores of the adsorbent.
Pyrolysis of waste materials like garden waste, sewage sludge, etc. produces a solid product called biochar, which has been shown to also have potential to capture ammonia while including another circular economy path by reusing waste products to make adsorbents. The main biochar of interest for this study was sewage sludge, since it is an important topic of interest in Sweden. The use of sewage sludge on agricultural land has been an ongoing debate in Sweden for many years (Bengtsson and Tillman 2004). Since landfill use in Sweden is extremely low (which is the third most common method for disposing of sewage sludge in the EU behind incineration (“Eurostat - Data Explorer” n.d.)), alternatives must be researched to prepare for this eventuality. The digested sewage sludge was obtained from Linz am Rhein – Unkel Municipal Wastewater Treatment Plant and then pyrolyzed at 550-600°C for 16-30 min (PYREG GmbH, Germany). In addition to sewage sludge-based biochar, biochar from the invasive species A. mearnsii (black wattle) was tested (produced by Pretoria University). Black wattle is one of the worst invasive species in South Africa, denoted as a category 2 invasive species, but also has an invasive presence in other countries like USA, Ethiopia, Kenya, Jamaica, Brazil and New Zealand (“Acacia Mearnsii (Black Wattle)” n.d.). Due to material availability issues limited testing was done with this material compared to the sewage-sludge materials. Additionally, mineral based materials that were tested included sepiolite (Gemina International, Turkey), LECA clay (LECA International, Norway) and clinoptilolite (Zeocem, Slovakia). The best material from the initial testing was clinoptilolite, which was then used to compare against the results of the sewage sludge biochar. Despite showing promising results in Balci and Dinçel (2002) sepiolite performed poorly in the high ammonium solutions of this experiment. LECA clay performed similarly to results by Sharifnia et al. (2016) with very low ammonium removal. Due to the low capacities in initial tests, the results with these materials are not reported.
Mälardalen University Press Licentiate Theses 7
2.1
Literature analysis methodology
2.1.1 Selection of appropriate studies
In order to understand the current status of nitrogen recovery techniques from wastewater, an analysis of literature was performed. To narrow down the field, research was chosen that used recovery techniques to produce a fertilizer substitute as the final product. Studies also must use a wastewater stream as the nitrogen source and had to give enough data to allow for the comparison of different technological parameters. Some research recovered nitrogen in a form that may or may not be suitable for use as a fertilizer, such as recovery by assimilation into algae. These articles were not included since there is risk that algae can also uptake heavy metals and other contaminants that could be harmful to plant growth. The studies were organized for comparison by wastewater type in order to group together technologies that target similar sources in terms of nitrogen concentration and complexity.
2.1.2 Calculation of important technological parameters
The main technological parameter that was compared for all of the studies was nitrogen recovery efficiency, the percent of incoming nitrogen that was successfully recovered into the final product. If recovery efficiencies were not reported, they were calculated as the mass of nitrogen in the final product divided by the mass in the feedstock. The next set of parameters focused on the energy analysis of the technology, which in some cases were reported based on experimental results, and other cases were reported as general energy requirements for a class of technology. Energy return on investment (EROI) was calculated for all studies that included both an energy requirement and energy recovery value, and it represented how close the technology was to energy neutrality. Finally using the energy balances, cost of chemicals and electricity, and revenue from the sale of the final fertilizer product an economic analysis was performed. This economic analysis could be useful for industrial partners in order to choose practical technologies for their specific wastewater stream to implement for nitrogen recovery.
Mälardalen University Press Licentiate Theses 7
2.1
Literature analysis methodology
2.1.1 Selection of appropriate studies
In order to understand the current status of nitrogen recovery techniques from wastewater, an analysis of literature was performed. To narrow down the field, research was chosen that used recovery techniques to produce a fertilizer substitute as the final product. Studies also must use a wastewater stream as the nitrogen source and had to give enough data to allow for the comparison of different technological parameters. Some research recovered nitrogen in a form that may or may not be suitable for use as a fertilizer, such as recovery by assimilation into algae. These articles were not included since there is risk that algae can also uptake heavy metals and other contaminants that could be harmful to plant growth. The studies were organized for comparison by wastewater type in order to group together technologies that target similar sources in terms of nitrogen concentration and complexity.
2.1.2 Calculation of important technological parameters
The main technological parameter that was compared for all of the studies was nitrogen recovery efficiency, the percent of incoming nitrogen that was successfully recovered into the final product. If recovery efficiencies were not reported, they were calculated as the mass of nitrogen in the final product divided by the mass in the feedstock. The next set of parameters focused on the energy analysis of the technology, which in some cases were reported based on experimental results, and other cases were reported as general energy requirements for a class of technology. Energy return on investment (EROI) was calculated for all studies that included both an energy requirement and energy recovery value, and it represented how close the technology was to energy neutrality. Finally using the energy balances, cost of chemicals and electricity, and revenue from the sale of the final fertilizer product an economic analysis was performed. This economic analysis could be useful for industrial partners in order to choose practical technologies for their specific wastewater stream to implement for nitrogen recovery.
8
2.1.3 Comparison and analysis of data
By organizing the studies based around the wastewater stream used for the nitrogen source, the comparison of techniques could be performed with a common baseline. The studies available for analysis decreased as the analysis got more involved, since many studies did not report energy or economic costs associated with the production of the final product. Another overall analysis was performed to compare the final fertilizer produced with the actual fertilizer market composition that is used worldwide currently. This was an interesting comparison to determine whether the market of the final product is being considered for these techniques, or if there exists some disconnect. In addition to the fertilizer market, the attitudes of those that will purchase the fertilizer is important to consider. As part of this analysis, we interviewed a local farmer on his thoughts regarding fertilizers derived from wastewater sources, to provide a voice to an important player in this circular economy scenario. If the buyers are not willing to purchase fertilizers from waste, then pursuing these technologies further would not make sense.
2.2
Experimental methodology
2.2.1 Kinetics and isotherm tests
After the literature review, experiments were designed to test the chosen technique of adsorption on wastewater with a high concentration of ammonium. First, the material capacities were tested using batch isotherm tests. Each test consisted of 1 g material to 10 mL of solution, and the
solutions ranged from 1-1000 mg/L NH4+ adjusted to pH 8 to be consistent
with reject water pH. With a pKa of 9.3, this pH value allowed nitrogen to
stay in the NH4+ form in the liquid, and no volatilization would occur. The
solutions were shaken for 168 hours, and samples were taken at regular intervals to determine the proper equilibrium time. After determining the equilibrium time for the untreated materials, different chemical pretreatments were implemented to try to increase the ammonium capacity by changing the surface characteristics of the materials. Isotherm tests were performed to compare the effects on ammonium capacity of the materials.
To compare the adsorption capabilities of the various materials, Langmuir and Freundlich isotherms were utilized according to Equations 1 and 2 respectively:
8
2.1.3 Comparison and analysis of data
By organizing the studies based around the wastewater stream used for the nitrogen source, the comparison of techniques could be performed with a common baseline. The studies available for analysis decreased as the analysis got more involved, since many studies did not report energy or economic costs associated with the production of the final product. Another overall analysis was performed to compare the final fertilizer produced with the actual fertilizer market composition that is used worldwide currently. This was an interesting comparison to determine whether the market of the final product is being considered for these techniques, or if there exists some disconnect. In addition to the fertilizer market, the attitudes of those that will purchase the fertilizer is important to consider. As part of this analysis, we interviewed a local farmer on his thoughts regarding fertilizers derived from wastewater sources, to provide a voice to an important player in this circular economy scenario. If the buyers are not willing to purchase fertilizers from waste, then pursuing these technologies further would not make sense.
2.2
Experimental methodology
2.2.1 Kinetics and isotherm tests
After the literature review, experiments were designed to test the chosen technique of adsorption on wastewater with a high concentration of ammonium. First, the material capacities were tested using batch isotherm tests. Each test consisted of 1 g material to 10 mL of solution, and the
solutions ranged from 1-1000 mg/L NH4+ adjusted to pH 8 to be consistent
with reject water pH. With a pKa of 9.3, this pH value allowed nitrogen to
stay in the NH4+ form in the liquid, and no volatilization would occur. The
solutions were shaken for 168 hours, and samples were taken at regular intervals to determine the proper equilibrium time. After determining the equilibrium time for the untreated materials, different chemical pretreatments were implemented to try to increase the ammonium capacity by changing the surface characteristics of the materials. Isotherm tests were performed to compare the effects on ammonium capacity of the materials.
To compare the adsorption capabilities of the various materials, Langmuir and Freundlich isotherms were utilized according to Equations 1 and 2 respectively:
Mälardalen University Press Licentiate Theses 9
qs =1+aKLCLCss (1)
qs= aFCsbF (2)
where qs represents the solid phase solute equilibrium concentration in
mg/g and Cs is the fluid phase solute equilibrium concentration in mg/L. The
Langmuir isotherm is relevant for homogenous adsorption and assumes
monolayer capacity. The constant KL reflects the solute adsorptivity and aL
is related to the energy of adsorption. The Freundlich isotherm is better suited for heterogeneous surfaces and does not assume monolayer adsorption. The
constants in the Freundlich isotherm represent adsorbent capacity (aF) and a
heterogeneity factor (bF). This factor ranges between zero and 1, increasing
heterogeneity as the value approaches zero (Mckay 1995).
2.2.2 Packed column tests
The main purpose for the column tests were to determine experimental capacities of the materials based on a system that would be a scaled-down version of a proper installation. The setup was based on a continuous up-flow column, where artificial wastewater was pumped upwards through the bottom of a plastic 30 mL column (at 1 mL/min), through 25 mL of adsorbent, and the effluent was collected out of the top. The ammonium concentration was recorded using a HACH ISENH4 probe and quantified using flow injection analysis (FIA5000). Batch experiments are important for determining an experimental maximum with no limits on contact time, but for practical implementation a continuous system is more realistic. The effect of limited contact time on the actual adsorption can then be determined. For comparison, commercially available activated carbon and clinoptilolite (zeolite) were also tested with the column set up.
2.2.3 Chemical treatments of adsorbents
To enhance the ammonium capacity of the sewage sludge biochar, five different chemical treatments were chosen from literature. Before chemical enhancement, the sewage sludge biochar was ground and sieved to between 0.5-1 mm particle size.
Mälardalen University Press Licentiate Theses 9
qs=1+aKLCLCss (1)
qs= aFCsbF (2)
where qs represents the solid phase solute equilibrium concentration in
mg/g and Cs is the fluid phase solute equilibrium concentration in mg/L. The
Langmuir isotherm is relevant for homogenous adsorption and assumes
monolayer capacity. The constant KL reflects the solute adsorptivity and aL
is related to the energy of adsorption. The Freundlich isotherm is better suited for heterogeneous surfaces and does not assume monolayer adsorption. The
constants in the Freundlich isotherm represent adsorbent capacity (aF) and a
heterogeneity factor (bF). This factor ranges between zero and 1, increasing
heterogeneity as the value approaches zero (Mckay 1995).
2.2.2 Packed column tests
The main purpose for the column tests were to determine experimental capacities of the materials based on a system that would be a scaled-down version of a proper installation. The setup was based on a continuous up-flow column, where artificial wastewater was pumped upwards through the bottom of a plastic 30 mL column (at 1 mL/min), through 25 mL of adsorbent, and the effluent was collected out of the top. The ammonium concentration was recorded using a HACH ISENH4 probe and quantified using flow injection analysis (FIA5000). Batch experiments are important for determining an experimental maximum with no limits on contact time, but for practical implementation a continuous system is more realistic. The effect of limited contact time on the actual adsorption can then be determined. For comparison, commercially available activated carbon and clinoptilolite (zeolite) were also tested with the column set up.
2.2.3 Chemical treatments of adsorbents
To enhance the ammonium capacity of the sewage sludge biochar, five different chemical treatments were chosen from literature. Before chemical enhancement, the sewage sludge biochar was ground and sieved to between 0.5-1 mm particle size.
10
2.2.3.1 FeCl3 and MgCl2 treatments
These two treatments were successful in increasing the adsorption capacity of woody biochar for metals adsorption, and so were considered as possible treatments for ammonium application as well (Chemerys and Baltrėnaitė
2017). The chemical solutions were 0.37M FeCl3*6H2O and 1M MgCl2*6
H2O, with a solid to liquid ratio of 30% w/w. The mixture was mixed for 2
hours at 100 RPM, then washed with three volumes of deionized water. Finally, the solid was dried in an oven at 105°C for 12 hours.
2.2.3.2 H2O2 treatments
Huff and Lee demonstrated the oxygenation effects of hydrogen peroxide on woody biochars using five concentrations for methyl blue adsorption (Huff
and Lee 2016). From these results, it was decided that 10% H2O2 should be
tried for its effect on ammonium adsorption capacity of biochar materials. The biochar was added to the hydrogen peroxide mixture at 1g:20mL. The mixture was mixed for 2 hours at 100 RPM, then washed with three volumes of deionized water and dried in an oven at 105°C for 12 hours. An additional treatment step was proposed by Wang et al (2015) to neutralize the pH after oxygenation for better ammonium adsorption. Therefore, an additional treatment was considered by treating the biochar with 1M HCl or 1M NaOH to adjust the pH value to 7 after the initial washing. Once the pH of the biochar was determined to be 7, the material was dried at 105°C for 12 hours.
2.2.3.3 HNO3/NaOH treatment
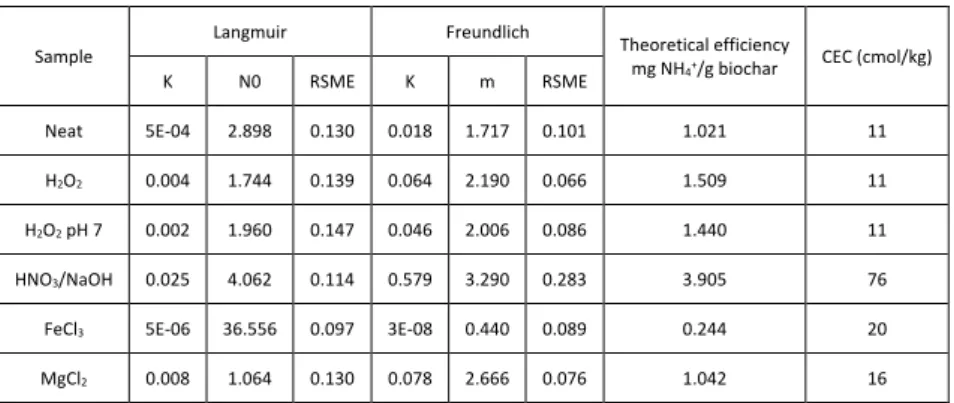
Another post-pyrolysis chemical treatment that was used to increase
ammonium adsorption capacity was a combination of HNO3 and NaOH
treatments (Vu et al. 2017). This treatment involved mixing the biochar in a
solution of 6M HNO3 for 8 hours at a ratio of 1g:5mL at 100 RPM. Then the
material was washed with three volumes of deionized water, and then added to 0.3M NaOH (at a ratio of 1g:20mL) for 24 hours at 100 RPM. Finally, the material was washed and dried in accordance with the previously mentioned methods.
2.2.4 Benchmark Simulation Model
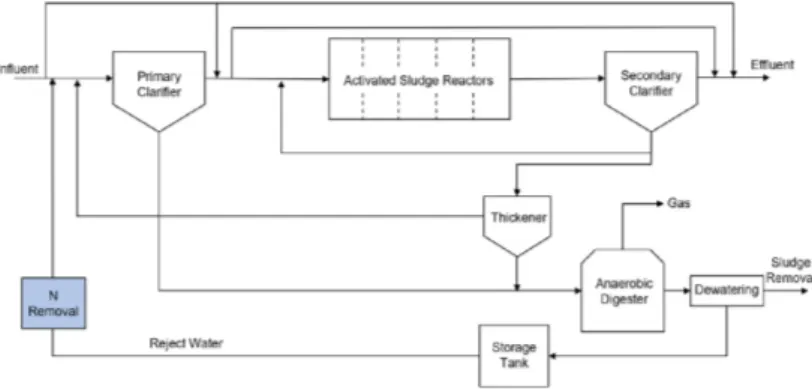
To draw conclusions from the experimental data for a full-scale implementation of this technique, the Benchmark Simulation Model (BSM2) was used (Gernaey et al. 2014). A basic sketch of the wastewater treatment plant setup for the model is shown below in Figure 1.
10
2.2.3.1 FeCl3 and MgCl2 treatments
These two treatments were successful in increasing the adsorption capacity of woody biochar for metals adsorption, and so were considered as possible treatments for ammonium application as well (Chemerys and Baltrėnaitė
2017). The chemical solutions were 0.37M FeCl3*6H2O and 1M MgCl2*6
H2O, with a solid to liquid ratio of 30% w/w. The mixture was mixed for 2
hours at 100 RPM, then washed with three volumes of deionized water. Finally, the solid was dried in an oven at 105°C for 12 hours.
2.2.3.2 H2O2 treatments
Huff and Lee demonstrated the oxygenation effects of hydrogen peroxide on woody biochars using five concentrations for methyl blue adsorption (Huff
and Lee 2016). From these results, it was decided that 10% H2O2 should be
tried for its effect on ammonium adsorption capacity of biochar materials. The biochar was added to the hydrogen peroxide mixture at 1g:20mL. The mixture was mixed for 2 hours at 100 RPM, then washed with three volumes of deionized water and dried in an oven at 105°C for 12 hours. An additional treatment step was proposed by Wang et al (2015) to neutralize the pH after oxygenation for better ammonium adsorption. Therefore, an additional treatment was considered by treating the biochar with 1M HCl or 1M NaOH to adjust the pH value to 7 after the initial washing. Once the pH of the biochar was determined to be 7, the material was dried at 105°C for 12 hours.
2.2.3.3 HNO3/NaOH treatment
Another post-pyrolysis chemical treatment that was used to increase
ammonium adsorption capacity was a combination of HNO3 and NaOH
treatments (Vu et al. 2017). This treatment involved mixing the biochar in a
solution of 6M HNO3 for 8 hours at a ratio of 1g:5mL at 100 RPM. Then the
material was washed with three volumes of deionized water, and then added to 0.3M NaOH (at a ratio of 1g:20mL) for 24 hours at 100 RPM. Finally, the material was washed and dried in accordance with the previously mentioned methods.
2.2.4 Benchmark Simulation Model
To draw conclusions from the experimental data for a full-scale implementation of this technique, the Benchmark Simulation Model (BSM2) was used (Gernaey et al. 2014). A basic sketch of the wastewater treatment plant setup for the model is shown below in Figure 1.
Mälardalen University Press Licentiate Theses 11 Figure 1. Schematic of wastewater treatment plant for BSM2 model
The basic wastewater treatment plant included two clarifiers, an activated sludge section, and anaerobic digestion. The nitrogen recovery was included by introducing a nitrogen removal function to the reject water line before it is recycled back to the incoming water stream. In this way the effects of recovering nitrogen from this stream on the overall electrical consumption and nitrogen balance of the plant could be determined. The main purpose in including the model was to determine both the overall effect of removing nitrogen from the reject water stream, and also to draw conclusions about the material balance since the sewage sludge for production of the biochar adsorbent would come directly from this process as well.
Mälardalen University Press Licentiate Theses 11 Figure 1. Schematic of wastewater treatment plant for BSM2 model
The basic wastewater treatment plant included two clarifiers, an activated sludge section, and anaerobic digestion. The nitrogen recovery was included by introducing a nitrogen removal function to the reject water line before it is recycled back to the incoming water stream. In this way the effects of recovering nitrogen from this stream on the overall electrical consumption and nitrogen balance of the plant could be determined. The main purpose in including the model was to determine both the overall effect of removing nitrogen from the reject water stream, and also to draw conclusions about the material balance since the sewage sludge for production of the biochar adsorbent would come directly from this process as well.