Faculty of Veterinary Medicine and Animal Sciences
The body language of dairy calves
– Investigating emotions through ear posture,
tail posture, and stepping behaviour
Kirste McCrea
Department of Animal Environment and Health Degree project 30 credits
The body language of dairy calves: Investigating emotions through ear posture, tail posture, and stepping behaviour
Kirste McCrea
Supervisor: Linda Keeling
Department: Animal Environment and Health Assistant Supervisor: Daiana De Oliveira Department: Animal Environment and Health Examiner: Else Verbeek
Department: Animal Environment and Health
Credits: 30 credits Level: A2E
Course title: Degree project in Animal Science Course code: EX0567
Programme: Independent project Place of publication: Uppsala Year of publication: 2019 Cover picture: Kirste McCrea
Online publication: http://stud.epsilon.slu.se
Keywords: Emotions, body language, body posture, valence, arousal, ear posture, tail posture, stepping, dimensional model of core affect, behaviour
Sveriges lantbruksuniversitet
Swedish University of Agricultural Sciences Faculty of Veterinary Medicine and Animal Sciences Department of Animal Environment and Health
Table of Contents
ABSTRACT ... 1
1. INTRODUCTION ... 1
1.1. DEFINING EMOTION ... 2
1.2. POSITIVE AND NEGATIVE EMOTIONS ... 2
1.3. ASSESSING EMOTIONS ... 2
1.4. BODY LANGUAGE AND EMOTIONS ... 3
1.5. DIMENSIONAL MODEL OF CORE AFFECT ... 4
1.6. EXPERIMENTAL STIMULI ... 5
2. MATERIALS AND METHODS ... 6
2.1. ETHICS ... 6
2.2. ANIMALS AND HOUSING ... 6
2.3. EXPERIMENTAL SET-‐UP ... 6 2.4. HABITUATION ... 7 2.5. TREATMENT ... 7 2.6. EXPERIMENTAL PROCEDURE ... 9 2.7. BEHAVIOURAL OBSERVATIONS ... 10 2.8. HEART RATE ... 12 2.9. STATISTICAL ANALYSIS ... 12 3. RESULTS ... 13 3.1. EAR POSTURES ... 13 3.1.1. Forward ears ... 14 3.1.2. Backward ears ... 16
3.1.3. Asymmetric right ear ... 17
3.1.4. Asymmetric left ear ... 19
3.1.5. Axial ears ... 19
3.2. STEPPING BEHAVIOUR ... 19
3.3. TAIL POSTURES ... 20
3.3.1. Tucked tail posture ... 21
3.3.2. Other tail postures ... 21
3.4. OTHER BEHAVIOURAL OBSERVATIONS ... 22
4. DISCUSSION ... 22
4.1. EAR POSTURE ... 22
4.2. STEPPING BEHAVIOUR ... 26
4.3. TAIL POSTURES ... 27
4.4. LYING BEHAVIOUR ... 28
4.5. IMPLICATIONS FOR THE DIMENSIONAL MODEL OF CORE AFFECT ... 29
4.6. PRACTICAL APPLICATIONS ... 30
4.7. LIMITATIONS AND FUTURE RESEARCH ... 31
5. CONCLUSION ... 32
6. POPULAR SCIENTIFIC SUMMARY ... 33
7. ACKNOWLEDGMENTS ... 34
Abstract
While improving animal welfare has become an important task in both the agricultural and scientific community, increased knowledge on positive emotions in animals and on methods of assessing welfare are needed in order to continue to move forward in this endeavour. Through understanding the connection between the body language of animals and their emotional state, further progress can be made. This study assessed the ear postures, tail postures, and stepping behaviour of 16 dairy calves when exposed to four experimental stimuli expected to elicit emotional states of varying valence and arousal levels, and a control. The stimuli included feeding of concentrates, gentle stroking, the absence of social interactions/stimuli, and spraying with water. Results were then compared to body posture predictions based on previous research where ear and tail postures of dairy cows during different activities were analyzed and plotted onto an arousal/valence framework i.e. the dimensional model of core affect. Predictions for the ear postures backward, asymmetric right, and asymmetric left during a positive, low arousal emotional state showed a trend of being supported. The backward and forward ear postures of calves may be more influenced by changes in arousal, while the asymmetric right ear indicated an emotional state of positive, high arousal. The highest occurrence of stepping behaviour was displayed during the boredom stimulus, indicating that this treatment was perceived as being of high arousal by the calves, rather than low arousal as intended. Overall tail activity of the calves was low. No tail predictions were confirmed, however, a new posture of a tucked tail seemed to be representative of a negative, high arousal emotional state. Further research is needed to determine the role that age, experience, and stimuli play in regards to an animal’s emotional state and to determine the validity of using the dimensional model of core affect to predict the body language and emotional state of animals in varying situations.
1. Introduction
The importance of providing a high level of welfare for animals is clear, particularly for those animals within production systems. In recent years, animal welfare has become a driving incentive for consumers on a global scale when purchasing animal products (Markova-Nenova and Wätzold, 2018; Sonoda et al., 2018; Spain et al., 2018). While this has presented some farming challenges, producers also stand to benefit from this growing trend, as animal welfare has been linked to positive benefits in supporting a variety of production traits, e.g. through reduced stress and disease occurrence (von Keyserlingk et al., 2009). However, the desire for consumers to have access to meat, egg, and dairy products that are produced ethically and responsibly has created an increasing need to not only improve welfare standards, but also to identify what good animal welfare looks like for specific production animals. While there are various aspects of this issue to consider, studying emotional states in animals is an important factor towards continued progress in this area. In order for this to be achieved successfully, the animal’s experience of both positive and negative emotions needs to be addressed, as well as methods of how these emotions can be reliably measured and interpreted.
1.1. Defining emotion
In order to begin studying emotions, it is essential to define what is meant by the term “emotion” or “emotional state”. Paul and Mendl (2018) outline the importance of using objective prescriptive definitions rather than descriptive definitions when researching animal emotion. In this way, cultural constructs that can arise through the use of descriptive definitions of emotions can be set aside and focus can be directed to understanding the biological structure and processing of subjective emotions in animals (Barrett, 2006). For this reason, the word emotion within this report is used in the same way as Anderson and Adolphs (2014), who define emotion as “…an internal, central (as in central nervous system) state, which is triggered by specific stimuli (extrinsic or intrinsic to the organism)” and argue that “…this state is encoded by the activity of particular neural circuits that give rise, in a causal sense, to externally observable behaviours, as well as to associated cognitive, somatic, and physiological responses”.
1.2. Positive and negative emotions
When assessing welfare, it is important to consider both the positive and the negative emotions that an animal might experience, as animal welfare is not only the absence of negative experiences, but also the presence of positive ones (Boissy et al., 2007; Mellor, 2012; Keeling, 2019). While much research has been done investigating negative emotions in production animals, particularly in regards to fear and anxiety (Powell et al., 2016; de Boyer des Roches et al., 2018; Muri et al., 2019), the study of positive emotions has only recently begun to receive more attention. This may be due, in part, to the added complexity that studying positive emotions entails. Boissy et al. (2011) suggests that positive emotions are more variable and versatile, as they tend to arise in “opportunity situations” where the fitness of the animal may be improved but is not necessarily essential for its survival (Boissy et al., 2007). Even so, many researchers have focused their attention on the study of positive emotions despite the challenges it entails, and as a result, more information and knowledge within this area is beginning to emerge for a variety of production animal species (Reimert et al., 2013; Proctor and Carder, 2015; Tamioso et al., 2017).
1.3. Assessing emotions
There are many different methods available to aid in effectively studying emotions in animals. Observing physiological changes within in an animal, such as heart rate (Katayama et al., 2016) and hormone levels (Möstl and Palme, 2002), is one such way to analyze emotional states and is a technique often used within research. Through observing the changes of nasal temperatures in dairy cows, Proctor and Carder (2015) determined that a drop in nasal temperature occurred as a result of the cows
experiencing a positive, low arousal stimuli. However, limitations of the
implementation of this method were apparent, such as periods of fluctuations in body temperature due to oestrous cycles and environmental temperatures. This suggests that
physiological data alone may not be enough to determine what an animal is experiencing and while important, is often not a practical tool of assessment in on-farm situations. Thus, other techniques such as behavioural observations become increasingly important as non-invasive and efficient methods of assessing animal welfare and emotions. Numerous behavioural tests have been developed for this specific purpose, such as the open-field test (Perals et al., 2017) and the judgement bias test (Horback and Parsons, 2019), among many others, with their implementation in research providing insights into animal emotion (Boissy and Erhard, 2014). Other behavioural indicators that are useful to assess when investigating animal welfare and positive emotions include play behaviour, exploration, facial expressions and body posture (Keeling, 2019).
1.4. Body language and emotions
Recently, interpreting the body language of production animals has been used as a behavioural method to assess emotions in farm animals. The analysis of ear postures in connection to emotional states seems to be of particular interest, though other types of body language have been explored as well. de Oliveira and Keeling (2018) assessed ear, neck and tail postures of dairy cows during different barn activities that were thought to provoke varying emotional valences and arousal levels. They observed specific overall body postures of the cows based on the activity being performed, and generated predictions of expected body postures of cows in other situations eliciting different valences and arousal levels of emotion (de Oliveira and Keeling, 2018). In another study, Boissy et al. (2011) associated four pre-determined ear postures with neutral and negative emotions in sheep. Similarly, Proctor and Carder (2014) identified ear postures in dairy cows that were observed more frequently with exposure to a positive, low arousal stimuli. Several of the postures identified also resembled ear postures observed in sheep that were present during the experience of a positive emotional state (Reefmann et al., 2009). While it is important to consider species-specific behavioural responses during the expression of emotion, these studies also suggest that there may be strong similarities between some production animals, such as dairy cows and sheep.
Though the study of tail posture in production animals may not be as advanced as in other species, such as dogs (Beerda et al., 1998; Stellato et al., 2017), information in connection to tail posture and emotion is beginning to emerge. de Oliveira and Keeling (2018) found that the predominate tail posture of dairy cows in three on-farm situations was hanging stationary, and that vigorous tail wagging of the cows was almost exclusively shown during the activity of brushing, indicating a connection to the experience of a positive emotional state. Another study analysed tail postures in sheep in response to a variety of situations thought to elicit different emotional states and found that a raised tail was observed most when sheep were separated from group members, indicating a negative emotional state (Reefmann et al., 2009). Recently, a study on pigs found a clear connection between the increased duration of tail movement and play behaviour, suggesting that this could be useful when assessing pig welfare (Marcet Rius et al., 2018).
Stepping behaviour has been used in a variety of behavioural tests as an indication of the arousal or activity level of the animal. Breuer et al. (2000) found that cows exposed to stockpersons who used loud vocalizations and moved quickly during handling procedures were correlated to increased stepping and kicking responses
during milking, indicating restlessness due to fear or stress. Another study found that cows with a higher rate of stepping during milking also faced the herd and vocalized more during periods of isolation, suggesting increased restlessness and nervousness, which in turn was correlated to lower milk yields (Hedlund and Løvlie, 2015). Regardless of the valence of the emotion, from these results it can be expected that higher frequencies of stepping is correlated to high arousal, while a decrease in stepping rate is associated with low arousal.
1.5. Dimensional model of core affect
As research investigating the emotions of animals has progressed, so too have
approaches of classifying and explaining them. One such approach avoids the use of discrete emotions i.e. anger, happiness, etc. and instead assesses the arousal level (excited vs. calm) and valence (positive vs. negative) of the emotion being
experienced (Russell, 2009; Mendl et al., 2010). An example of this is can be seen when considering the emotions of fear and depression. While both of these emotions are classified as being negative, fear elicits a higher level of arousal than when compared to depression, an intrinsically low arousal emotion. Through the use of the valence-arousal approach of classifying emotions, biases placed on the emotional states of animals based on human interpretation can be minimized. Any subjective state that can be classified based on its valence and arousal is therefore referred to as core affect (Russell, 2003). The dimensional model of core affect outlined by Mendl et al. (2010) proposes a framework in which this concept can be visualized. The two dimensional axes of the model represent the valence and arousal level of emotion. The axes intersect to create four distinct quadrants, each corresponding to a particular valence and arousal level of an emotion; positive high arousal (Q1), positive low arousal (Q2), negative low arousal (Q3), and negative high arousal (Q4). Emotional behavioural responses can then be plotted in order to achieve a more objective view of an animal’s emotional state.
The quadrant Q1 of the core affect model, has generally been suggested to correspond with motivational states, particularly those regarding feed or obtaining a reward, while Q2 is associated with the perception of a low level of threat (Mendl et al., 2010). These two quadrants make up the positively valenced quadrants of the model. Q3 of the model is associated with loss or lack of reward, while Q4
corresponds to situations where a high level of threat or danger is perceived (Mendl et al., 2010). These two quadrants make up the negatively valenced quadrants of the model.
The dimensional model of core affect has been practically implemented within research with promising results. After assessing the body postures of dairy cows performing various activities within their loose housing system, de Oliveira and Keeling (2018) plotted the behavioural responses observed from the cows into the dimensional model of core affect. From this, the authors were able to achieve a more comprehensive idea of how body posture relates to cow emotions, and generated predictions of body postures that might be expressed in other situations (de Oliveira and Keeling, 2018). While these predictions need further investigation as to their validity, the potential of creating behavioural predictions based on varying levels of valence and arousal, as opposed to specific emotions, could have implications for welfare assessments in the future.
1.6. Experimental stimuli
One approach to assess the emotions of an animal in an experimental study is to attempt to stimulate a particular emotion through the use of stimuli within the animal’s environment and analyse the subsequent behavioural response. The stimuli used can be selected to elicit a specific valence or arousal level of emotion. When experimental stimuli are being chosen, factors such as animal species and
experimental design should be taken into account, as such things can largely impact an animal’s response to a stimulus, and must be interpreted accordingly. A study comparing various novel stimuli and their effects on the emotional responses of dairy cows found that the use of a tactile water spray test, where three squirts of water were sprayed on the hind quarters of the cow, produced the greatest reactivity response seen in the form of startle behaviours when compared to the other two stimuli (Gibbons et al., 2009). While exploring the use of positive stimuli, Westerath et al. (2014) investigated if consuming special feed or being brushed was considered as positive by young cattle. The study concluded that the animals preferred concentrates to their ordinary feed and preferred or showed a tendency to prefer brushing from a habituated person to an empty compartment (Westerath et al., 2014). These results suggest that special feed and brushing may be useful in stimulating positive emotions in cows, while spraying cows with water seems to elicit a negative, high arousal emotional state.
The aim of this study is to investigate the body language of dairy calves through the analysis of ear posture, tail posture and stepping behaviour when exposed to stimuli of both positive and negative valences, and high and low levels of arousal. Specifically, it is hypothesized that based on the previous research of de Oliveira & Keeling (2018) using the dimensional model of core affect, calves will exhibit different ear and tail postures coinciding to various combinations of valences and arousal levels that make up the four quadrants of core affect space. Predictions of calf stepping behaviour within each of these four quadrants were also made based on the findings of Hedlund and Løvlie (2015) and Breuer et al. (2000). Some quadrants contain multiple predictions for ear and tail postures and no tail posture was plotted in the quadrant Q1. This is due to how the components loaded during the principal component analysis in the study conducted by de Oliveira and Keeling (2018) and therefore, no tail posture hypothesis for Q1 was made. The predicted responses are as follows:
Positive valence, high arousal (Q1): ears backward, and a high occurrence of
stepping.
Positive valence, low arousal (Q2): ears asymmetric right/ears asymmetric left/ears
backward, tail vigorously wagging, and a low occurrence of stepping.
Negative valence, low arousal (Q3): ears forward, tail bent sideward, and a low
occurrence of stepping.
Negative valence, high arousal (Q4): ears axial, tail hanging stationary/directed tail
2. Materials and Methods
2.1. Ethics
This study was conducted in compliance with the animal ethics application C 58/13 for Lövsta.
2.2. Animals and housing
This study used 16 female dairy calves housed at the Swedish Livestock Research Centre, Lövsta, in Uppsala, Sweden. All data used in the study was collected between October and November 2018. A mixture of Swedish Red (n=6) and Holstein (n=10) calves were used, ranging from 5.5-8 months old, and weighing 170-280 kg, based on the availability of the research facility. The calves were group housed and fed ad libitum roughage twice daily by the farm staff. All calves that participated in the study appeared to be healthy and in good body condition.
2.3. Experimental set-up
Directly outside the home pen of the calves, an alleyway was gated off on both sides in order to create a temporary experimental pen 2.2m x 1.25m in size (Fig. 1), to be used during treatments. One length of the experimental pen was the outer wall of the home pen, while the other side was a gate, which was fastened upright and acted as a wall. Two video cameras were set up at the front and back of the experimental pen in order to best capture the ear postures, tail postures, and stepping behaviour of the focal calf throughout the treatment. Two experimenters were present throughout each experimental session; one outside the front of the gated experimental pen in order to administer the stimuli and operate the camera, while the other was positioned at the back of the experimental pen to operate both the camera and timer used during treatment.
Fig 1. Schematic diagram of experimental pen used to test experimental stimuli on
2.4. Habituation
The calves included within the study were habituated to the experimenters, the experimental pen and the equipment that was used (i.e. cameras, heart rate monitor and plastic feed shovel). Habituation was carried out over a period of four days. Of the calves available to participate in the study, only those that allowed the experimenters to approach them and handle them were chosen. This was done, as calves with a generally fearful disposition would be less likely to experience the positive treatments as positive. During the first two days of habituation the experimenters entered the home pen of the calves and gently stroked them while talking in a soft voice for a period of 10 minutes. Once the calves allowed the experimenters to approach and handle them from all sides without moving away, the stroking habituation was considered to be complete. On the third day of habituation each calf was individually led into the experimental pen where the experimenters introduced the calf to a small plastic shovel that was used to present the desirable feed of concentrates that would be used within the experiment. The calf was then fed a small amount of the concentrate from the feed shovel to ensure that the calf would be interested in the feed. Once this was confirmed, the experimenters stood by the calf, only interacting with them briefly if the calf became stressed, through speaking gently and allowing the calf to lick or smell them. The calf remained in the experimental pen for a total period of 10 minutes, after which they were led back into the home pen. On the final day of habituation each calf was again led into the experimental pen and fitted with a heart rate monitor. The calf then underwent the same habituation procedure in the pen as the previous day and was led back into the home pen after a period of 10 minutes.
2.5. Treatment
Four different stimuli were selected to correspond to each quadrant of the core affect space. These quadrants are as follows: high arousal/ positive valence (Q1), low arousal/ positive valence (Q2), low arousal/ negative valence (Q3), and high arousal/ negative valence (Q4). In the order of Q1-Q4 the experimental stimuli included novel feed given to the focal calf (feed), stroking of and gentle speaking to the focal calf by the experimenter (stroke), a period of no stimuli (boredom) and the focal calf being sprayed by water (spray)(Fig. 2, Fig. 3). A control treatment, where the experimenter stood by the calf but did not interact with her, was also added in order to account for the presence of the experimenter who administered the stimuli during the feed, stroke and spray treatments. The control treatment occurred directly before the calf was exposed to the boredom stimulus. The order that each calf was exposed to the stimuli was chosen using a balanced Latin Square design in order to account for any possible effects of treatment order. Three separate blocks of calves were tested over three weeks, in which case all 16 animals had participated in the study. All calves experienced each stimulus once, with one stimulus treatment per day, over the course of four days.
Administration of the stimuli is described as follows:
Fig. 2. Representative diagram of the dimensional model of core affect and the
correspondence of the experimental stimuli with Q1-Q4.
Q4:
Spray
Arousal ValenceQ3:
Boredom
Q1:
Feed
Q2:
Stroke
Fig. 3. Photographs of the four experimental stimuli (a) spray, (b) feed, (c) boredom,
and (d) stroke.
(a) (b)
1. Feed: the calf was given a small feed shovel full of concentrate at whichever height she placed her head and was allowed to eat ad libitum for a total period of three minutes (out of six minutes).
2. Stroke: the calf was spoken to gently by the experimenter and was scratched or stroked on the face and neck, with focus on areas that appeared to be preferred by the calf for a total period of three minutes (out of six minutes). 3. Boredom: the calf was left in the experimental pen by itself for a period of 40
minutes.
4. Spray: the calf was sprayed with a small spray bottle containing water on whichever body part was facing the experimenter, once approximately every five seconds, for a total period of three minutes (out of six minutes).
5. Control: the experimenter stood by the calf but did not interact with her for a total period of six minutes.
2.6. Experimental procedure
All experimental sessions were carried out between 13:00 and 17:00 each day, with each calf undergoing treatment at approximately the same time each day. Before treatment began, the calf was led into the experimental pen and fitted with a heart rate monitor. The calf then stood in the experimental pen for a pre-treatment phase of one minute to allow for pre-treatment behavioural observations and a baseline heart rate to be recorded. During this time, the experimenter at the front of the experimental pen stood by the camera, approximately two meters away, with their back to the calf. The calf was then subjected to a pre-determined, randomly chosen stimulus during a stimulus-treatment phase of six minutes. At the start of the stimulus-treatment phase, the experimenter positioned themselves on the left side of the experimental pen, facing the calf, and administered the stimulus for 15 seconds. The stimulus was then removed for 15 seconds while the experimenter remained in their position, and continued to face the calf. For the following 30 seconds the experimenter repeated this procedure on the right side of the experimental pen. These 15 seconds “stimulus on” and “stimulus off” periods continued as described, with the experimenter alternating between left and right sides of the experimental pen, until all six minutes of the stimulus-treatment phase had concluded. This resulted in a total period of three minutes “stimulus on” and three minutes “stimulus off”. This experimental design of administering the stimulus for 15 seconds on and 15 seconds off was chosen due to the age of the calves. During a brief pilot study, it was apparent that some calves might not be interested in a stimulus for the entire six minutes the treatment occurred, or conversely, may become over stimulated and react negatively. Through allowing a brief 15 seconds break from the stimuli, this could be avoided. Additionally, it is possible that a specific behaviour could be expressed as a reaction to the stimulus itself, and not necessary elicited from an emotional response. Having a period of 15 seconds without a stimulus present allows for later analysis of the two periods (stimulus on and stimulus off) to determine if any differences between them were present.
When the stimulus-treatment phase was completed the experimenter returned to their position by the camera, with their back to the calf. The calf remained in the
experimental pen for a post-treatment phase of one minute. After this time the heart rate monitor was removed and the calf was allowed back into the home pen.
The experimental procedure described was followed for all treatments with the exception of boredom. The boredom treatment was combined with the control treatment session due to time restraints and began directly after the control was completed (Fig. 4). At this time, the experimenter (who was standing with their back to the calf at the end of the post-treatment phase of control) sat down by the camera on a stool and did not move or interact with the calf for a period of 40 minutes. The final six minutes of these 40 minutes was considered to be the boredom treatment. Combining the boredom with the control made it difficult for any pre-treatment or post-treatment phase of boredom to be truly comparable to the other experimental sessions, as this was the only treatment that occurred directly after another stimulus. Additionally, in all other treatments, the calf could differentiate between all three phases of the treatment sessions. During the boredom stimulus, this was not possible and made the design of the
pre-treatment phase, stimulus-treatment phase and post-treatment phase very similar to each other. As a result, no pre-treatment or post-pre-treatment phase was included during the boredom treatment. After the boredom treatment was concluded, the heart rate monitor was removed and the calf was allowed back into the home pen.
2.7. Behavioural observations
All behavioural observations of the calves during the experimental procedure were recorded using two video cameras. One was situated at the front of the experimental pen, while the other was situated at the back. A detailed ethogram adapted from de Oliveira and Keeling (2018) was used to observe ear postures, stepping behaviour and tail postures (Fig. 5). All relevant video data was then analysed using the Mangold Interact program (version 18.0.2.13). Duration and occurrences of ear and tail postures, and frequency of stepping were recorded using continuous observations. Due to the large amount of data, only pre-treatment, post-treatment and minutes 0-1, 2-3, and 4-5 of the stimulus-treatment phase were analysed for ear postures. These three specific minutes within the stimulus-treatment phase were chosen for analysis as they represented the beginning, middle and end of the stimulus-treatment phase. In order to verify that these three chosen minutes gave an accurate representation of the six minutes of the stimulus-treatment phase, preliminary data was gathered over the full six minutes, from three different calves. Once this was analysed and the duration of time spent in each ear posture per minute was found, it was then compared to the analysis of the same data, using only the aforementioned three minutes. These preliminary calculations determined that the chosen three minutes were adequately representative of the entire six minutes of the stimulus-treatment phase. When
1
minute Pre-treatment phase of control 6
minutes Stimulus-treatment phase of control 1
minute Post-treatment phase of control 34
minutes No stimulus 6
minutes Stimulus-treatment phase of boredom
Fig. 4. Diagram of control and boredom treatment
recording tail postures, if none of the five tail postures included in the behavioural ethogram were occurring, the tail was hanging down and was stationary. All video data for stepping and tail postures was used in the final analysis. Other behavioural observations recorded included lying, urination, and defecation.
Fig. 5. Representative diagrams of ear and tail postures observed in calves during various phases
(pre-treatment, stimulus-treatment, post-treatment) of treatments (feed, stroke, boredom, spray, and control) adapted from de Oliveira and Keeling (2018) and description of stepping behaviour.
Ears are directed forward, with the tip of the ear at an angle of more than 30% from the perpendicular Ears are straight out to the side, perpendicular to the head-rump axis
Axial Asymmetric Asymmetric Backward Forward Left Right
Ears are oriented in opposite directions; left ear is backwards and right ear is axial or forward
Ears are oriented in opposite directions; right ear is backwards and left ear is axial or forward
Ears are directed backwards, with the tip of the ear at an angle of more than 30% from the perpendicular
Tucked Small Directed Vigorous Bent wagging wagging wagging sideward
Relaxed movement from side to side; the tail tag must not
pass the imaginary vertical line between pin bones Tail is directed deliberately to a certain area of the body Tail is moving vigorously and in any direction Tail is bent to one side (left or right) and remains there Tail is hanging downwards and actively held by the calf underneath the body Stepping Front right leg
Front left leg Back right leg Back left leg
Front right leg is lifted off the ground and set down again Front left leg is lifted off the ground and set down again Back right leg is lifted off the ground and set down again Back left leg is lifted off the ground and set down again
2.8. Heart rate
During the pre-treatment phase, stimulus-treatment phase, and post-treatment phase, the heart rate of the focal calf was recorded using a Polar H10 heart rate sensor and the Polar Beat heart rate monitoring application. Due to the scope of the project, the analysis of heart rate was not included in this report.
2.9. Statistical analysis
Statistical analysis of all behavioural data was completed using Minitab® 18.1. The duration and occurrence of the ear postures forward, backward and asymmetric right were analysed using a general linear mixed model. Individual behavioural postures were considered as the response variable in the model, while treatment and phase of treatment were considered as fixed factors. Individual calf was considered to be a random effect. Breed was not included in the model as each calf acted as its own control. All variables within the analysis were first tested for normality using the Anderson-Darling normality test. Variables that were not normally distributed (all variables apart from the total mean duration of backward ears) were transformed for analysis using the square root transformation method and were later back transformed to obtain final results. For all ear variables analysed using the general linear mixed model, 95% confidence intervals were used in place of standard deviation or standard error when reporting means, as these could not be reliably back transformed. The ear postures of asymmetric left and axial could not be transformed into a normal distribution, and as such, analysis of these ear postures was completed using the non-parametric Friedman test. During this test each ear posture was considered as the response variable in the model, while the four stimuli and the control were considered as treatments. Individual calves were considered as a block and each phase of treatment was analyzed individually. The Mann-Whitney test was then used to determine which medians were significantly different from one another. Pairwise comparisons were only made between treatments within each phase and within treatment between phases.
The frequency of total stepping was analysed using a general linear mixed model. In the model, frequency of total stepping was considered as the response variable, while treatment and phase of treatment were considered as fixed factors, as in the previous analysis. Individual calf was considered to be a random effect. Breed was not included in the model as each calf acted as its own control. As the stepping data was not normally distributed, it was transformed for analysis using the square root transformation method and was later back transformed to obtain final results. 95% confidence intervals were used in place of standard deviation or standard error when reporting means, as these could not be reliably back transformed. Stepping behavioural observations were analysed considering treatment as the main effect, as well as when considering interactions between treatment and phase. If a calf chose to lie down at any point during the treatment, the stepping data for whichever phase this occurred in was considered as missing.
Tail posture data was found to be non-normally distributed and as this could not be corrected with any standard transformation method, tail postures were therefore analzyed using the non-parametric Friedman test. Each individual tail posture was considered as the response variable within the model, while the four stimuli and control were considered as treatments. Individual calves were considered as a block. As interactions of phase could not be considered using the Friedman test, each phase of treatment was analyzed indivudally. The Mann-Whitney test was then used to determine which medians were significantly different from one another. Whenever a calf laid down during a treatment session, this tail data was considered as missing. Due to the restrictions of the Friedman test, all tail data from calves with any missing data was then ommitted in order to run the final analysis, leaving data from a total of twelve calves.
Lying behaviour of calves was analyzed using the non-parametric Kruskal-Wallis test. As the majority of lying was observed during the boredom stimulus, which did not include a pre-treatment or post-treatment phase, only the stimulus-treatment phase of the various stimuli was analyzed. Within the model, occurrence of lying was considered as the response variable, while treatment was considered as the factor.
3. Results
Data were analyzed from observations recorded during the different treatments (feed, stroke, boredom, spray, and control) for each of the 16 calves, resulting in a total of 80 observation sessions. Due to the number of ear postures and behavioural observations within the study, an overview of the results are first presented, followed by the analysis of each individual ear posture, including both duration and occurrences, in regard to treatment effects during the stimulus-treatment phase. The pre-treatment and post-treatment phases are then discussed. Finally, results are presented from the analysis of stepping behaviour, tail postures, and lying behaviour, using the same structure as described.
3.1. Ear postures
The backwards ear posture was the predominant posture overall, typically occurring for 43-64% of the stimulus treatment time, apart from during the feed stimulus (Fig. 6). The ear posture asymmetric right was expressed for the longest total mean duration during the feed stimulus (35%), and along with the forward ear posture, was the second most common posture observed throughout the stimuli. The ear postures asymmetric left and axial were observed the least of all postures, occurring around 2-8% of the stimulus treatment time.
3.1.1. Forward ears
Significant differences were found between stimuli treatments and the total mean duration spent in the forward ear posture (F=4.02, p<0.001)(Fig. 7a). During the stimulus-treatment phase the total mean duration of time spent in the forward ear posture was significantly higher during the feed stimulus (mean: 15.82, 95% CI: 11.08 to 21.41) when compared to the stroke (mean: 5.86, 95% CI: 3.14 to 9.42) (p=0.022), boredom (mean: 5.98, 95% CI: 3.23 to 9.58) (p=0.027), and control (mean: 5.92, 95% CI: 3.19 to 9.50) (p=0.025) stimuli. When considering the pre-treatment phase and the post-treatment phase, the total mean duration of forward ears showed a trend towards being significantly higher in the post-treatment phase of feed when compared to the pre-treatment phase of feed (p=0.051). For all other treatments, the total mean duration spent in the forward ear posture stayed relatively constant throughout the different phases.
Significant differences were found between stimuli treatments and the total mean occurrences of the forward ear posture (F=6.23, p<0.001)(Fig. 7b). During the stimulus-treatment phase, total mean occurrences of the forward ear posture during the feed stimulus (mean: 6.15, 95% CI: 4.64 to 7.88) were significantly higher than
Fig. 6. Diagrams of the percentage of time spent in each ear posture during the
various stimuli of the stimulus-treatment phase.
Spray
Feed
Stroke
Boredom
the stroke (mean: 3.10, 95% CI: 2.05 to 4.36)(p=0.044), boredom (mean: 1.45, 95% CI: 0.77 to 2.34)(p<0.001), and control (mean: 2.45, 95% CI: 1.53 to 3.57)(p=0.002) stimuli. The total mean occurrence of the forward ear posture during the spray stimulus (mean: 4.83, 95% CI: 3.50 to 6.38) was also significantly higher than during the boredom stimulus (p<0.001). When considering the pre-treatment phase and the post-treatment phase the total mean occurrences of the forward ear posture significantly decreased during the post-treatment phase of spray (mean: 1.95, 95% CI: 1.14 to 2.97) when compared to the stimulus-treatment phase of spray (p=0.013).
0 5 10 15 20 25 30 35 40 45
Pre-treatment Stimulus-treatment Post-treatment
T otal me an d u rati on (s ) of for w ar d e ar s p er mi n u te Treatment phase
Forward Ears
Boredom Control Feed Spray Stroke A B B B (a) 0 2 4 6 8 10 12 14 16Pre-treatment Stimulus-treatment Post-treatment
T otal me an oc cu rr en ce s of for w ar d e ar s p er mi n u te Treatment phase
Forward Ears
Boredom Control Feed Spray Stroke A CD D ABC BCD D CD AB (b)Fig. 7. (a) Mean and upper 95% confidence interval of total duration (s) of time spent in the
forward ear posture per minute and (b) mean and upper 95% confidence interval of total occurrences of forward ears per minute, by calves during the pre-treatment, stimulus-treatment and post-stimulus-treatment phases of boredom, control, feed, spray and stroke. Significantly different means do not share a letter (A, B, C, D) and are considered to be significant when p≤0.05. Pairwise comparisons were only made between treatments within each phase and within treatment between phases.
3.1.2. Backward ears
Significant differences were found between stimuli treatments and the total mean duration of the backward ear posture (F=3.98, p<0.001)(Fig. 8a). During the stimulus-treatment phase, the total mean duration spent in the backward ear posture was significantly higher in the stroke (mean: 29.54, 95% CI: 22.85 to 36.23)(p=0.042), boredom (mean: 32.39, 95% CI: 25.7 to 39.08)(p=0.004), and control (mean: 33.21, 95% CI: 26.52 to 39.90)(p=0.002) stimuli when compared to the feed stimulus (mean: 15.90, 95% CI: 9.21 to 22.59), where the duration of backward ears was the lowest. When considering the pre-treatment phase and the post-treatment phase, the total mean duration of backward ears was significantly higher in the pre-treatment phase of feed (mean: 32.69, 95% CI: 25.99 to 39.38) when compared to the stimulus-treatment phase of feed (p=0.003).
Significant differences were found between stimuli treatments and the total mean occurrences of the backward ear posture (F=3.79, p<0.001)(Fig. 8b). During the stimulus-treatment phase, the total mean occurrence of the backward ear posture was significantly lower in the boredom stimulus (mean: 3.58, 95% CI: 2.77 to 4.51) than during the stroke stimulus (mean: 6.47, 95% CI: 5.35 to 7.69)(p=0.003). When considering comparisons between the different phases, the total mean occurrence of backward ears was significantly lower in the post-treatment phase of spray (mean: 3.16, 95% CI: 2.40 to 4.04) when compared to the stimulus-treatment phase of spray (mean: 5.43, 95% CI: 4.41 to 7.69)(p=0.028). For all other treatments, the total mean occurrence of the backward ear posture stayed relatively constant throughout the different phases.
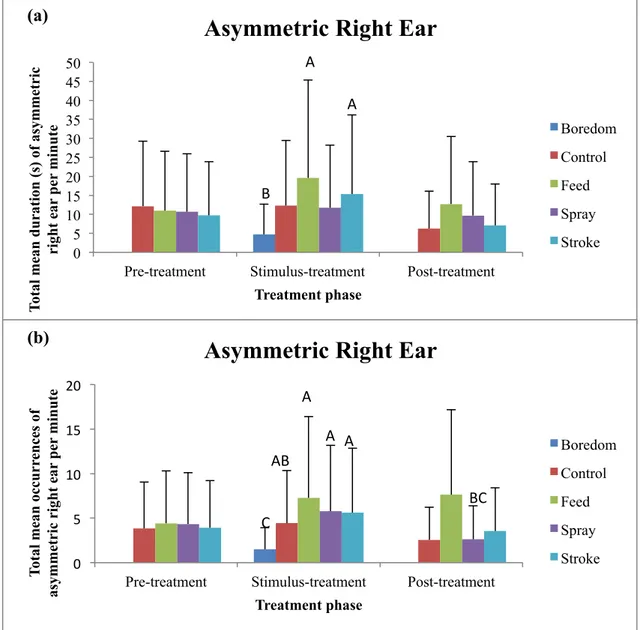
3.1.3. Asymmetric right ear
Significant differences were found between stimuli treatments and the total mean duration of the asymmetric right ear posture (F=4.01, p<0.001)(Fig. 9a). During the stimulus-treatment phase the total mean duration of asymmetric right ear during the feed (mean: 19.60, 95% CI: 14.27 to 25.77)(p<0.001) and stroke (mean: 15.32, 95% CI: 10.66 to 20.81)(p=0.003) stimuli was significantly higher than during the boredom (mean: 4.72, 95% CI: 2.33 to 7.96) stimulus. No significant differences were observed when considering the pre-treatment phase and the post-treatment phase, and the total
0 10 20 30 40 50 60 70 80
Pre-treatment Stimulus-treatment Post-treatment
T otal me an d u rati on (s ) of b ac k w ar d e ar s p er mi n u te Treatment phase
Backward Ears
Boredom Control Feed Spray Stroke B A A A A (a)Fig. 8. (a) Mean and upper 95% confidence interval of total duration (s) of time spent in
the backward ear posture per minute and (b) mean and upper 95% confidence interval of total occurrences of backward ears per minute, by calves during the pre-treatment,
stimulus-treatment and post-treatment phases of boredom, control, feed, spray, and stroke. Significantly different means do not share a letter (A, B, C) and are considered to be significant when p≤0.05. Pairwise comparisons were only made between treatments within each phase and within treatment between phases.
0 1 2 3 4 5 6 7 8 9 10
Pre-treatment Stimulus-treatment Post-treatment
T otal me an oc cu rr en ce s of b ac k w ar d ear s p er mi n u te Treatment phase
Backward Ears
Boredom Control Feed Spray Stroke AB A BC C (b)mean duration of the asymmetric right ear posture remained relatively constant throughout all other treatments.
Significant differences were found between stimuli treatments and the total mean occurrences of the asymmetric right ear posture (F=7.85, p<0.001)(Fig. 9b). During the stimulus-treatment phase total mean occurrences of the asymmetric right ear posture in the boredom stimulus (mean: 1.51, 95% CI: 0.82 to 2.41) were significantly lower than all other stimuli [feed (mean: 7.28, 95% CI: 5.64 to 9.13)(p<0.001); stroke (mean: 5.61, 95% CI: 4.18 to 7.24)(p<0.001); spray (mean: 5.76, 95% CI: 4.31 to 7.42)(p<0.001); control (mean: 4.43, 95% CI: 3.18 to 5.90 (p=0.006)]. When considering comparisons between the different phases, the total mean occurrences of the asymmetric right ear posture were significantly lower in the post-treatment phase of spray (mean: 2.61, 95% CI: 1.67 to 3.76) when compared to the stimulus-treatment phase of spray (p=0.026). 0 5 10 15 20 25 30 35 40 45 50
Pre-treatment Stimulus-treatment Post-treatment
T otal me an d u rati on (s ) of as ymme tr ic ri gh t e ar p er mi n u te Treatment phase
Asymmetric Right Ear
Boredom Control Feed Spray Stroke A B A (a) 0 5 10 15 20
Pre-treatment Stimulus-treatment Post-treatment
T otal me an oc cu rr en ce s of as ymme tr ic r igh t e ar p er mi n u te Treatment phase
Asymmetric Right Ear
Boredom Control Feed Spray Stroke C AB A A A BC (b)
Fig. 9. (a) Mean and upper 95% confidence interval of total duration (s) of time spent in the
asymmetric right ear posture per minute and (b) mean and upper 95% confidence interval of total mean occurrences of asymmetric right ear per minute, by calves in the pre-treatment, stimulus-treatment, and post-treatment phases of boredom, control, feed, spray, and stroke. Significantly different means do not share a letter (A, B, C) and are considered to be significant when p≤0.05. Pairwise comparisons were only made between treatments within
3.1.4. Asymmetric left ear
No significant differences between stimuli treatments within the pre-treatment phase, stimulus-treatment phase or post-treatment phase were observed for the total mean duration of the asymmetric left ear posture.
Significant differences were found between stimuli treatments within the stimulus-treatment phase for median total occurrences of the asymmetric left ear posture after being adjusted for ties (chi-square=10.29, p=0.036)(Fig. 10). The asymmetric left ear posture had the highest median total occurrences during the feed stimulus (median=2.38), followed in decreasing order by the stroke (median=2.28), spray (median=1.75), boredom (median=1.55) and control (median=1.28) stimuli. The median occurrence of the asymmetric left ear was significantly lower during the control stimulus when compared to the feed (p=0.041) and stroke (p=0.004) stimuli. No significant differences were found when considering the pre-treatment phase and the post-treatment phase.
3.1.5. Axial ears
No significant differences between stimuli treatments within the pre-treatment phase, stimulus-treatment phase, or post-treatment phase were observed for the total mean duration or the total mean occurrences of the axial ear posture.
3.2. Stepping behaviour
The analysis of stepping behaviour showed no significant differences between steps taken by each individual leg. The same significant results between treatments were seen when considering front legs versus back legs, left legs versus right legs, and total
Fig. 10. Median of the total occurrences of the asymmetric left ear posture per
minute by calves during the stimulus-treatment phase. Significantly different medians do not share a letter and are considered to be significant when p≤0.05.
0 0.5 1 1.5 2 2.5 3
Boredom Control Feed Spray Stroke
M ed ian of total oc cu rr en ce s of as ymme tr ic le ft e ar p er mi n u te
Stimuli in stimulus-treatment phase
Asymmetric left ear
B
A
steps taken by all legs. As a result, analysis was completed considering total mean steps taken by all legs combined.
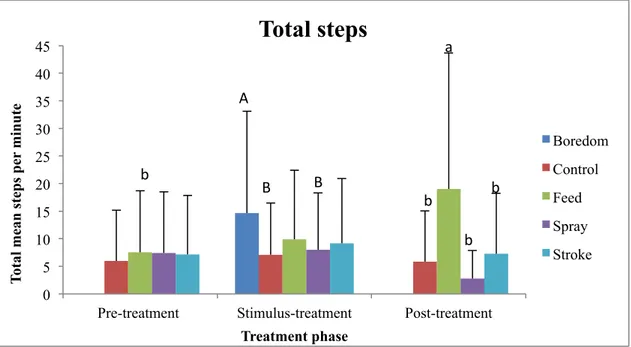
During the stimulus-treatment phase, significant differences in total mean steps of calves were found between stimuli treatments (F=4.09, p=0.006) (Fig 11). Total mean steps of calves during the stimulus-treatment phase of boredom (mean: 14.65, 95% CI: 11.30 to 18.45) were significantly higher than the control (mean: 7.11, 95% CI: 5.13 to 9.40)(p=0.004) and spray (mean: 7.98, 95% CI: 5.94 to 10.32)(p=0.014) stimuli.
When considering comparisons between the different phases, significant differences in total mean steps of calves were found between stimuli treatments and phase (F=5.2, p<0.001) (Fig. 11). Total mean steps of calves during the post-treatment phase of feed (mean: 19.04, 95% CI: 14.18 to 24.61) were significantly higher than total mean steps of calves during the pre-treatment phase of feed (mean: 7.54, 95% CI: 4.61 to 11.18)(p=0.004), as well as all other stimuli during the post-treatment phase [stroke (mean: 7.26. 95% CI: 4.31 to 10.98)(p=0.004); spray (mean: 2.76, 95% CI: 1.13 to 5.11)(p<0.001); control (mean: 5.85, 95% CI: 3.24 to 9.22)(p<0.001)].
3.3. Tail postures
No significant differences were found in tail postures when considering left tail movements versus right tail moments. As a result, each tail posture analysed includes the sum of both the left and right tail movements within that posture.
Fig. 11. Mean and upper 95% confidence interval of the number of total steps taken by
calves during pre-treatment, stimulus-treatment, and post-treatment phases of boredom, control, feed, spray, and stroke. Significant differences when treatment is considered as the main effect are represented by upper case letters (A, B), while significant differences when considering interactions between treatment and phase are represented by lower case letters (a, b). Significantly different means do not share a letter and are considered to be significant when p≤0.05. 0 5 10 15 20 25 30 35 40 45
Pre-treatment Stimulus-treatment Post-treatment
T otal me an s te p s p er mi n u te Treatment phase
Total steps
Boredom Control Feed Spray Stroke B A B b b b b a3.3.1. Tucked tail posture
Significant differences were found between stimuli treatments within the stimulus-treatment phase for median total duration spent in the tucked tail posture after being adjusted for ties (chi-square= 9.54, p=0.049)(Fig. 12a). The highest median total duration spent in the tucked tail postured occurred during the spray stimulus (median=5.94) followed in decreasing order by the boredom (median=3.89), control (median=3.11), stroke (median=2.21) and feed (median=1.02) stimuli. The median duration of the tucked tail posture was significantly higher during the spray stimulus when compared to the feed stimulus (p=0.012). When considering the pre-treatment phase and the post-treatment phase, no significant differences were observed.
Significant differences were found between stimuli treatments within the stimulus-treatment phase for median total occurrences of the tucked tail posture after being adjusted for ties (chi-squared=21.04, p<0.001)(Fig. 12b). The highest median occurrence of the tucked tail position was found during the spray stimulus (median=1.00), followed in decreasing order by the boredom (median=0.55), stroke (median=0.52), feed (median=0.20) and control (median=0.15) stimuli. The median occurrences of the tucked tail posture were significantly higher during the spray stimulus when compared to the control (p<0.001) and feed (p=0.001) stimuli. When considering the pre-treatment phase and the post-treatment phase, no significant differences were observed.
3.3.2. Other tail postures
No significant differences were found during the analysis of all other tail postures. 0 0.2 0.4 0.6 0.8 1 1.2 M ed ian total oc cu rr en ce s of tu ck ed tai l p er mi n u te
Stimuli in stimulus-treatment phase
Tucked tail
(b) 0 1 2 3 4 5 6 7 M ed ian total d u rati on (s ) of tu ck ed tai l p er mi n u teStimuli in stimulus-treatment phase
Tucked tail
(a)
Fig. 12. (a) Median of the total duration (s) of time spent in the tucked tail posture per minute
and (b) median of the total occurrences of the tucked tail posture per minute by calves during the stimulus-treatment phase. Significantly different medians do not share a letter and are considered to be significant when p≤0.05.
A
B
A
B B
3.4. Other behavioural observations
Analysis of lying behaviour determined that there was a significant difference between stimuli during the stimulus-treatment phase (p=0.01). Overall, calves laid down a total of five times during the boredom stimulus treatment, compared to once during the stroke stimulus and once during the control stimulus. No lying was observed during the spray and feed stimuli.
During all experimental sessions occurrences of urination and defecation were rare and as a result, these observations were excluded from the final analysis.
4. Discussion
The results from this study demonstrate how the body language of calves, namely ear posture, tail posture and stepping behaviour, change depending on the stimulus that the animal is experiencing. More importantly, as previous research has linked stimuli to emotions (Westerath et al., 2014), this study establishes a link between the body postures of dairy calves and their emotions, and addresses how through observing body language as a whole, an animal’s emotional state can be better understood.
4.1. Ear posture
The duration and occurrences for all ear postures, except for the backward ear posture, followed similar trends throughout treatments. As such, results of ear postures will be discussed generally, without the distinction between duration and occurrences, unless otherwise specified.
Forward ears: During the high arousal stimulus of feed, the forward ear posture was significantly higher than during the low arousal stimuli, boredom and stroke, and the control. Though not statistically significant from the other stimuli, the forward ear posture was also high during the spray stimulus, similar to what was observed during feed. As the stimuli spray and feed are both assumed to elicit high arousal in the calves, these results suggest that while the forward ear posture may be influenced by emotional valence, it appears to be more heavily influenced by the overall arousal level of the animal’s emotional state and is more prevalent in situations of high arousal.
These results are somewhat contradictory when compared to other studies. Reefmann et al. (2009) found a higher proportion of the forward ear posture in sheep that were separated from group members when compared to sheep that were fed hay. However, arousal levels of the sheep were not taken into consideration and species-specific behavioural responses may explain the differences in results observed in the current study. In the predictions made by de Oliveira and Keeling (2018) the forward ear posture was plotted in Q3 (negative, low arousal) of the dimensional model of core affect, whereas the spray and feed stimuli of the current study corresponded to Q4 and Q1 respectively (both high arousal). While de Oliveira and Keeling (2018) found the forward ear posture to be expressed the least during feeding, cows in this study were observed while eating their standard feed of roughage, and it is possible
that this was not as highly desirable as the concentrate feed used in the current study. However, as Lambert and Carder (2019) also found a lower expression of the forward ear posture when cows were eating a desirable feed of concentrates, it is possible that the higher expression of forward ears during the feed stimulus of the current study is the result of another factor. One such factor could be the experimental design of the current study. As the stimulus of feed was presented to the calf every other 15 seconds during the six minute stimulus-treatment phase, it is possible that the forward ear posture occurred during the time that the calf could not access the concentrate. This may have elicited frustration in the calves and would then be more in line with the results from Lambert and Carder (2019), which suggested that forward ears are an indication of negative, high arousal. While this is still in contrast to the prediction of de Oliveira and Keeling (2018), where the forward ear posture corresponded to Q3 of the dimensional model of core affect, it was also correlated to the activity of queuing in their study, which loaded in both Q3 and Q4, the negatively valenced quadrants. Therefore, as the occurrences of the forward ear posture during the spray stimulus in the current study were significantly different from the boredom stimulus, it is possible that forward ears in calves accurately depicts an emotional state that lies in Q4 of the core affect model.
Within the feed stimulus, the duration of the forward ear posture remained high throughout the post-treatment phase and showed a trend of being significantly higher than the duration of forward ears during the pre-treatment phase, suggesting a carry-over effect. During the post-treatment phase of feed the calves were likely in the same positive, high arousal emotional state as they were while experiencing the stimulus-treatment of feed, and may have anticipated receiving more concentrates. Furthermore, in terms of the duration of ear postures, feed was the only stimulus throughout the study that was found to be significantly different from the control. This could indicate that feed was the most successful at changing the emotional state of the dairy calves. While it is well known that concentrates are desirable to calves (Westerath et al., 2014), these results highlight precisely how stimulating a feed reward can be to calves and how a state of high arousal produced through receiving desirable feed can persist even after the feed is removed. In contrast to these results, calves expressed a significantly lower occurrence of both the forward and backward ear postures in the post-treatment phase of spray when compared with the stimulus-treatment phase of spray. This indicates that while the spray stimulus may have elicited a negative, high arousal state in the calves, it did not seem to have any prolonged effects after it was removed. Contrary to the current study, Lambert and Carder (2019) found that the ear postures of cows did not show any carry-over effects when access to concentrates or woodchips were removed. It is possible that the calves in the current study found the feed of concentrates to be more arousing due to their novelty, as opposed to an older cow that has more experience with this type of feed. This may be important from an animal welfare perspective, as depending on the situation, calves exposed to either positive or negative stimuli may be more prone to long-term effects, where welfare could either be significantly improved, or significantly diminished.
Backward ears: When compared to the forward ear posture, the opposite results were seen for the backward ear posture. The duration of backward ears was highest during the low arousal stimuli, boredom and stroke, and the control, while lowest during feed. These results seem logical, as the backward ear posture was expressed more
during occasions where the forward ear posture was expressed less. As de Oliveira and Keeling (2018) predicted that backward ears would be expressed in Q2 of the core affect model, this hypothesis is supported. However, the prediction that backward ears would also be observed in the Q1 quadrant (feed) was not found in the current study. One potential reason for this might be the differences in classification of backward ears that were used. While de Oliveira and Keeling (2018) identified both backwards ears “up” and backwards ears “down”, the current study did not specify between these two positions. Though this was necessary due to time constraints, it is possible that considering backward ears as one posture instead of two separate ones may have influenced the final results observed. It could be interesting to analyze the backward ear posture data of the current study as two separate postures, and determine whether or not this truly has an effect on the results.
While not statistically significant from other stimuli, the backward ear posture had a similar low duration during the negative high arousal spray stimulus as during the positive high arousal feed stimulus. These results, combined with the high duration of the backward ear posture for the low arousal stimuli, could indicate that, like the forward ear posture, backward ears may be a more general indication of an animal’s low arousal level, as opposed to its emotional valence.
The overall dominance of the backward ear posture in the majority of stimuli treatments is in keeping with the results from de Oliveira & Keeling (2018), where the backward ear posture was the only body posture of those studied that loaded highly on two components of the dimensional model of core affect. The authors suggested that this ear posture may have a low specificity and that cows might use it in a wide variety of situations (de Oliveira and Keeling, 2018). A similar study also came to the conclusion that backward ears tend to be the predominant posture expressed, as the ear posture EP1, which resembles the backward ear posture in this study, was performed the most by cows in the absence of any stimuli (Lambert and Carder, 2019). The only stimulus treatment the backward ear posture was not predominate in was the feed stimulus, which might suggest that the reward of feed created a motivation or desire strong enough to override the biological preference (within the experimental environment) of that ear posture.
Asymmetric ears: An interesting result of this study was the higher duration of time spent in the asymmetric right ear posture during the positive feed stimulus then in all other stimuli treatments. The expression of this posture also increased during the positive stroke stimulus and occurred significantly less than all other stimuli, including the control, during the negative boredom stimulus. This suggests that the asymmetric right ear posture is more likely to be observed when a calf is experiencing a positive emotion versus when a calf is experiencing a negative emotion, and may have a tendency to be more prevalent if that positive emotion is accompanied by a high arousal emotional state. These results support the hypothesis that an asymmetric right ear posture could be expected during a positive low arousal stimulus, such as stroke, though as stroke was only significantly different from the boredom stimulus, caution must be taken when interpreting these results. While it was not predicted to observe the asymmetric right ear posture during the positive high arousal stimulus of feed, this posture coincided with the activity of feed in the study by de Oliveira and Keeling (2018), and suggests that an asymmetric right ear posture and the activity of feeding may be biologically connected. As previously discussed in regard to forward ears, though de Oliveira and Keeling (2018) did not observe the asymmetric right ear posture during feed in their study, this may be a result of the cows consuming a