Proliferation and expression
of p53 in odontogenic
tumours
- An immunohistochemical analysis
Johanna Wassberger
Mahtab Yarahmadi
Supervisor: Gunnar Warfvinge
Master Thesis in Odontology (30 ECTS)
Malmö University
Programme in Dentistry
Faculty of Odontology
Abstract
Introduction: Ameloblastoma (AB), adenomatoid odontogenic tumour (AOT), ameloblastic fibroma (AF) and odontogenic fibroma (OF) are all odontogenic tumours with an epithelial component. The recurrence rate for these odontogenic tumours varies from low frequencies to quite high frequencies. The aim of this study is to evaluate the expression of Ki-67, p53 and BRAF and the possibility of these antibodies acting as prognostic markers in the recurrence pattern of odontogenic tumours.
Material and method: An immunohistochemical study using Ki67, p53 and BRAF monoclonal antibodies was performed on 29 paraffin blocks from the respective tumours obtained at the department of Oral Pathology in the Faculty of Odontology at Malmö University. Statistical analysis was performed with Kruskal-Wallis one-way ANOVA. Results: In the series of ten AB cases high proliferation activity and a high prevalence of p53 mutations was observated. In the seven AOT cases a moderately high proliferative activity as well as a generally high prevalence of p53 mutation, comparable to AB, was observed. The seven cases of AF and the five cases of OF demonstrated a low proliferative activity and a low prevalence of p53 mutation. The difference between AB and AOT versus AF and OF as two separate groups, showed a significantly higher staining intensity for both Ki-67 (p < 0.001) and p53 (p = 0.001) in AB and AOT as a group.
Conclusion: Ki-67 proliferation index and p53-mutation status can be considered to be a prognostic marker for AB and AOT recurrence. This is, however, not applicable to AF and OF.
Proliferation och uttryck av
p53 i odontogena tumörer
- En immunohistokemisk analys
Johanna Wassberger
Mahtab Yarahmadi
Handledare: Gunnar Warfvinge
Examensarbete (30 HP)
Malmö högskola
Tandläkarprogrammet
Odontologiska fakulteten
Abstrakt
Introduktion: Ameloblastom (AB), adenomatoid odontogen tumör (AOT), ameloblastiskt fibrom (AF) och odontogent fibrom (OF) är odontogena tumörer som innehåller epiteliala komponenter. Frekvensen av recidiv hos dessa varierar från låg förekomst till relativt hög förekomst. Syftet med denna studie är att undersöka om Ki-67, p53 och BRAF kan användas som prognostiska markörer i recidivmönstret hos dessa tumörer.
Material och metod: Studien genomfördes genom immunohistokemi med monoklonala antikroppar av Ki-67, p53 och BRAF på respektive tumör. Tumörerna hämtades från
avdelningen för Oral patologi på Malmö högskola. En statistisk analys utfördes med hjälp av Kruskal-Wallis envägs-ANOVA.
Resultat: I de tio AB-fallen kunde en hög proliferation och en hög prevalens av muterade p53 ses. I de sju fallen av AOT kunde en måttligt hög proliferation och en generellt hög prevalens av muterade p53, jämförbara med värden för AB, ses. De sju fallen med AF och de fem fallen med OF visade båda en låg proliferation och en låg förekomst av muterade p53. Skillnaden mellan gruppen AB och AOT och gruppen AF och OF visade en signifikant högre
infärgningsintensitet för både Ki-67 (p<0.001) och p53(p=0.001) för gruppen med AB och AOT.
Konklusion: Proliferations index med Ki-67 och förekomst av p53-mutationer kan användas som en prognostisk markör för recidiv hos AB och AOT. Det är å andra sidan inte tillämpbart för AF och OF.
Index
Introduction 1
Ameloblastoma 1
Adenomatoid Odontogenic Tumour 1
Ameloblastic Fibroma 2 Odontogenic Fibroma 2 Immunohistochemistry 2 Ki-67 3 p53 3 BRAF V600E 3 Summary of introduction 3 Aim 4 Research questions 4
Materials and Methods 5
Subjects 5
Analysis of tissue samples 5
Immunohistochemical method 5
Immunohistochemical analysis 6
Statistical analysis 6
Results 7
Discussion 11
Materials and Methods 11
Results 12
Ameloblastoma 12
Adenomatoid Odontogenic Tumour 14
Ameloblastic Fibroma 14
Odontogenic Fibroma 14
Benefits and consequences in dental care 15
Ethical considerations 15
Conclusions 17
Introduction
Odontogenic tumours are neoplasms derived from tooth forming cells and tissues in the jaw. Most of these tumours are benign and rarely exhibit malign behaviour (1,2). Odontogenic tumours are classified into three groups according to the composing odontogenic tissues:
- Odontogenic epithelial tumours,
- Odontogenic epithelial/ectomesenchymal (mixed) tumours, and - Odontogenic ectomesenchymal tumours.
Odontogenic epithelial tumours are tumours that do not contain odontogenic
ectomesenchyme, e.g. ameloblastoma and adenomatoid odontogenic tumour. Odontogenic mixed tumours are epithelial odontogenic tumours that contain odontogenic ectomesenchyme, for instance ameloblastic fibroma. Odontogenic ectomesenchymal tumours originate from odontogenic ectomesenchyme that may or may not contain epithelial elements (2,3). Odontogenic tumours are rare, with an assessed frequency of less than 0,5 cases/100,000 individuals every year (1).
Ameloblastoma
Ameloblastoma (AB) is a benign, slow-growing and locally invasive neoplasm of
odontogenic epithelial origin (4). It is by far the most common odontogenic neoplasm in all ethnic groups, representing 11-13% of all odontogenic tumours (2,5). AB is typically a multilocular radiolucency that only occurs in the jaws. Approximately 80% of AB occurs in the mandible with a predilection for the posterior regions (2-4). AB exhibits no gender
predilection and occurs in a wide age range, extending from childhood to late adulthood. Most cases are however diagnosed between 40 and 50 years of age (3,6). The cause of AB is not yet known, but theories state that the tumours can arise from any source of odontogenic
epithelium, e.g. rest of the dental lamina, the enamel organ, from the epithelial lining or an odontogenic cyst, or from the basal cells of the oral mucosa (3,7). There are several types of AB described, based on clinicoradiographic situations, but the two main categories are the conventional solid/multicystic variant (86% of all cases) and the unicystic variant (10% of all AB cases) (2,7). Treatment can be performed by a variety of means, from simple enucleation and curettage, to surgical resection (2-4,6,7). Curettage and enucleation of AB often results in high recurrence rates varying between 50-90% (4,6), whilst bloc resection of AB results in a 15% recurrence rate (2).
Adenomatoid Odontogenic Tumour
Adenomatoid odontogenic tumour (AOT) is an uncommon, benign and slow-growing epithelial odontogenic tumour, accounting for 2-7% of all odontogenic tumours (3,8). These tumours occur twice as often in the maxilla compared to the mandible and are found in association with unerupted permanent teeth, usually the canines, or between the roots of anterior teeth (2). The occurrence of AOT is twice as high for women compared to men, where the age range varies from childhood to late adulthood. More than 90% of all AOT cases are found before the age of 30 (3). AOTs rarely show continued growth and never infiltrate the bone like the ameloblastomas do (2). The treatment modality of choice is conservative surgical enucleation with long-term observation (8,9). Recurrences are extremely rare and therefore are the prognosis excellent (3,9).
Ameloblastic Fibroma
Ameloblastic fibroma (AF) is an uncommon and slow growing mixed odontogenic tumour, i.e. an epithelial odontogenic tumour that contains odontogenic ectomesenchyme. The tumour develops from the proliferationof immature mesenchymal and epithelial cells with no hard tissue formation (2,10). AF comprises 1,5-4,5% of all odontogenic tumours (10,11). These tumours appear as a pericoronal radiolucency surrounding crowns of unerupted molars. AB occurs mainly in the posterior mandible (70% of all cases) and has no gender predilection (3,6,10,12). These neoplasms occur mainly in children and adolescents (6). Treatment includes enucleation and curettage of surrounding bone, and removal of the affected teeth (11). The recurrence rate for AF is 18-43,5% after conservative enucleation (13,14).
Approximately 45% of ameloblastic fibrosarcomas develop in the setting of a recurrent AF (7,12,14,15).
Odontogenic Fibroma
Odontogenic fibroma (OF) is an extremely rare benign ectomesenchymal tumour that is characterized by varying amounts of odontogenic epithelium embedded in odontogenic ectomesenchyme. The lesion may evolve from the dental papilla, the dental follicle or from the periodontal membrane, and is therefore associated with the coronal or radicular portion of the tooth (16). OF accounts for 0,1% of all odontogenic tumours (3,16). The lesion has been seen in all age groups, with a mean age of 40 years. OF can occur in both jaws, however more commonly in the mandibular premolar area. The tumour has a female predominance that is twice as high compared to men (3,6). The lesion occurs as a small, well-circumscribed radiolucency that may cause resorption and/or displacement of adjacent vital teeth (2). Treatment of OF is local enucleation or excision, where the lesion readily separates from its bony crypt and shows no evidence of bone infiltration (9). Recurrence is generally very uncommon but may occur (3,9).
Immunohistochemistry
Immunohistochemistry (IHC) is the utilization of monoclonal and polyclonal antibodies for the detection of specific antigen distribution and localisation in cells of the biopsy. The cell membrane has numerous proteins and carbohydrates, i.e. epitopes, recognized by specific associated antibodies. During IHC these antibodies bind to their specific antigens and are then visualized by chromogens as brown colour under microscopy (17,18). IHC is an important and widely used technique in medical research and in clinical diagnostics. The method can be used for a variety of purposes, including predicting the prognosis. This can be achieved by utilizing tumour markers, which are antibodies specific for tumour antigens that are expressed or up regulated in certain cancers (18,19).
There are two different methods of IHC; i) the direct method using only primary antibodies, and ii) the indirect method using primary and secondary antibodies. In the direct method a primary antibody binds directly to the antigen in the cell membrane. In the indirect method a secondary antibody binds to the primary antibody, which in turn is bound to the antigen of interest. The antibodies used are marked with substances, generating some form of stain. When the antigen-bound primary antibody reacts with the secondary antibody, a complex will form and its position will be indicated by a marker on the secondary antibody (18,20). The indirect IHC method, using secondary antibodies, has proven to be more sensitive and more accurate compared to the direct IHC method. The greater accuracy in the indirect IHC method is due to multiple secondary antibodies binding to one primary antibody, thus creating a
stronger signal that makes the antigens easily detectable.The direct method is however a comparably inexpensive alternative because it requires fewer antibodies (21).
Antibodies used for IHC are either monoclonal or polyclonal. Monoclonal antibodies
recognize one single epitope within an antigen and are typically produced from a single B cell of an immunized mouse. Polyclonal antibodies recognize multiple epitopes on one antigen, and are collected from multiple B cell clones that have been activated by the immune response of an immunized animal, e.g. rabbit, that has been injected with a specific antigen that causes an immune response (22,23). The polyclonal antibodies are relatively inexpensive, but have a higher potential for cross reactivity because they recognizes multiple epitopes. Monoclonal antibodies are significantly more expensive than the polyclonal antibodies. However, they have a high specificity to one single epitope that reduces the probability of cross reactivity (24).
Ki-67
Anti-Ki-67 is a monoclonal mouse antibody. This antibody is one of the most useful markers to evaluate cell proliferation activityand is often used in tumour treatment as well as cancer research (25,26). Ki-67 is a protein encoded in the MKI67 gene and necessary for cellular proliferation. The Ki-67 is present during all phases of the cell cycle, except in resting cells where this protein is absent (27). The fraction of Ki-67-positive tumour cells is often
correlated with the clinical course of cancer. Studies have shown the prognostic value of Ki-67 in different types of cancers, e.g. lymphoma and breast cancer (28).
p53
Anti-p53 is a monoclonal mouse antibody and a useful aid for classification of tumours (29). p53 is a protein that is the product of the tumour suppressor gene TP53, and high levels of accumulated p53 is a potential novel marker for malignancy (30). This protein plays an important role in several cellular activities such as the cell cycle, DNA-repair and apoptosis (31). In its mutated form p53 ceases to acts as a cell protector or growth suppressor, and instead acts as a growth promoter and oncogene. TP53 mutations enable genetically damaged and unprepared cells to survive and proliferate, thus resulting in malignant transformation. This mutation has been reported in e.g. breast, lung and colon cancer, and is one of the most common molecular changes identified in human cancer (32).
BRAF V600E
Anti-BRAF V600E is a monoclonal mouse antibody. This antibody is used for identification of mutant BRAF V600E in different kinds of cancers, such as colorectal carcinoma. BRAF mutations are frequent in benign and malignant human tumours and BRAF V600E mutations accounts for the vast majority of BRAF alterations. BRAF is a gene in the DNA that encodes a protein called B-Raf that is active in signal transmission processes that control cell growth, cell proliferation, cell differentiation and release of cellular products (33).
Summary of introduction
AB, AOT, AF and OF have different biological behaviour and recurrence patterns. However, the common denominator is that these odontogenic tumors contain epithelial elements. Therefore, one might speculate if the epithelium might constitute an etiological factor in the tumours pathogenesis and if mutations in the epithelium can be linked to the patterns of
recurrence for the different tumours.
Aim
The aim of this study is to immunohistochemically evaluate the expression of Ki-67, p53 and BRAF in odontogenic tumours. These antibodies are used to detect deviant growth patterns in the epithelial tissues within the odontogenic tumors.
We hope that the findings from our study will provide information on whether or not these antibodies can be used as clinical prognostic markers for the recurrence pattern of AB, AF, AOT and OF. In turn this might help evaluating the degree of invasiveness necessary when treating these tumours as well as deciding the appropriate recall frequency after treatment.
Research questions
● Can different mutation patterns be found in various odontogenic tumours? ● Can mutation patterns found in odontogenic tumours act as prognostic biological
Materials and Methods
SubjectsSubjects were randomly selected from the database atthe department of Oral Pathology in the Faculty of Odontology at Malmö University. Based on letters of referral, ten of the most recent subjects of AB, AT, AOT and OF were selected from the year 2000 and onwards. Every selected subject was anonymized and instead identified by a number. We did not consider parameters such as gender, age, tumour localisation or ethnicity. Subjects with an uncertain diagnosis, or decalcified biopsies were excluded. Only AB subjects with the
diagnosis “solid and multicystic ameloblastoma” were selected. AF, AOT and OF had no sub-diagnostic considerations. Initially ten subjects were selected for each tumour, making a total of 40 subjects in this study, ten of each tumour. In the later stage of the procedure some subjects were excluded and replaced with new ones, although all excluded subjects could not be replaced. This is further addressed in the sub-heading ‘Analysis of tissue samples’.
Analysis of tissue samples
A number of subjects had several biopsies available with varying amounts of tissue. The biopsy containing most tissue was selected to represent the subject. By examining the already existing haematoxylin and eosin stained slides (H&E-slides) for all subjects, a total of 22 biopsies were excluded due to the following reasons:
- AB: 1 biopsy was decalcified and 6 slides were lent to other projects and thus
unavailable to us . These excluded biopsies were replaced with 7 new ones, resulting in a total of 10 AB biopsies.
- AF: 2 slides were unavailable and 1 had an uncertain diagnosis. These slides could not be replaced due to insufficient number of biopsies that met the inclusion criterias, resulting in a total of 8 AF biopsies.
- AOT: 4 biopsies were defective in appearance and 3 slides were unavailable. All but one slide could be replaced with new ones, resulting in a total of 9 AOT biopsies. - OF: 3 slides were unavailable and 2 slides had an uncertain diagnosis, resulting in a
total of 5 OF biopsies.
The total number of subjects came to 32 instead of the initial 40. Immunohistochemical method
New microscopic slides (3μm thick) from each biopsy were prepared for the IHC procedure. The slides were then dried in 60 degrees for one hour.
The slides were deparaffinized and rehydrated in an ethanol series. Antigen retrieval was performed using a pressure cooker (110 degrees in 7 minutes for BRAF and 95 degrees in 20 minutes for Ki-67, p53 and cytokeratin staining using AE1/AE3 to further reveal epithelial elements in the tissue section). Afterwards the slides were placed in TBS (pH 7,6) for five minutes, incubated in Peroxidase-blocking solution (HPBK) for another five minutes and then washed. ‘DAKO BLOCKING SYSTEM’ was used in the IHC procedure since it was readily available at the university and easy to use. First the slides were incubated in a Background sniper for 10 minutes, washed, incubated with the primary antibody, diluted with ‘DAKO
diluent’ for 24 hours in 4oC ([BRAF]= 1:200) or 30 minutes in 20oC ([Ki-67]=1:200 and
[p53]=1:50). The slides were washed, incubated in Mach1 secondary Mouse probe for 15 minutes and rinsed off, to enable binding of the colouring agent. Mach1 universal horseradish peroxidase polymer (HRP) was added and the slides were incubated for 30 minutes, washed and incubated again with diaminobensidine (DAB), containing DAB chromogen and Betazoid DAB buffer, for 10 minutes. After washing the slides, they were counter stained with
hematoxylin (HTX), washed thoroughly, dehydrated and mounted. Immunohistochemical analysis
All slides were examined in a microscope simultaneously by the authors. An oral pathologist at the department of Oral Pathology, Malmö University, subsequently examined the slides. The staining was classified according to a semi-quantitative grading method as follows: 1: Negative staining
2: Sporadically positive staining 3: Semi-fully positive staining 4: Fully positive staining. Statistical analysis
The statistical analyses was performed in the statistic software programme SPSS with
Kruskal-Wallis one way ANOVA. AB and AOT as a group was compared with AF and OF as a group with regards to Ki-67 and p53 staining.
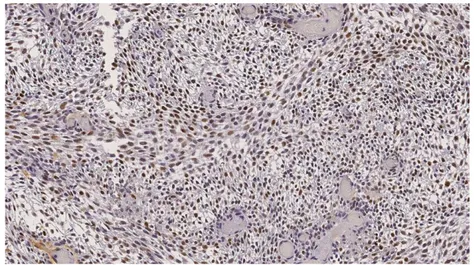
Results
AB and AOT showed positive Ki-67 and p53 immunostaining to a moderate to high degree corresponding to staining values 3 and 4 (figure 6 and 7), which is illustrated in figure 1 and 2. AF showed positive Ki-67 and p53 immunostaining, although to a lower degree
corresponding to staining values 2 (figure 5) compared to AB and AOT, which is illustrated in figure 1 and 2. OF presented ambiguous results leaning towards negative staining
corresponding to staining value 1 (figure 4) for p53 and Ki-67, which is also illustrated in figure 1 and 2.
Figure 1 The four different tumours and the degree of staining with Ki-67. The degree of staining was evaluated
as following: 1: Negative, 2: Sporadically positively stained, 3: Semi-fully positively stained, 4: Fully positively stained.
Figure 2 The four different tumours and the degree of staining with p53. The degree of staining was evaluated as
following: 1: Negative, 2: Sporadically positively stained, 3: Semi-fully positively stained, 4: Fully positively stained.
Figure 3. Odontogenic fibroma: Brown AE1/AE3 staining identifying odontogenic epithelium in the tissue
Figure 4. Odontogenic fibroma: p53 staining illustrating value 1, Negative staining.
Corresponding staining values were used for Ki-67.
Figure 5. Ameloblastma: p53 staining illustrating value 2, Sporadically positively stained cells (arrows).
Corresponding staining values were used for Ki-67.
Figure 6. Ameloblastoma: p53 staining illustrating value 3, Semi-fully positively stained tissue (arrows).
Figure 7. Ameloblastoma: p53 staining illustrating value 4, Fully positively stained.
Discussion
Materials and Methods
In the assessment of the materials and methods used in this study we found a number of strengths and limitations.During the entire study we have had professional supervisors working alongside us, providing histopathological knowledge.In the IHC procedure we used well documented antibodies, suitable to our purpose (25-32). Ki-67, p53 and BRAF V600E monoclonal antibodies were used because they all have high specificity to their respective epitope. This reduces the probability of cross reactivity when performing IHC-staining(24). We used the indirect IHC-method with secondary antibodies. This provided us with a greater accuracy since two or more secondary antibodies bind to one primary antibody, therefore amplifying the signal, which makes the antigens more easily detectable (21). However, the indirect IHC method requires additional steps and control procedures (34) , which can imply a higher probability for error. There are a number of factors that can lead to incorrect results when using the IHC method, i) choosing an inappropriate antibody for the given clinical application, ii) wrong concentration of antibodies, which can lead to false-positive signals from high concentration, and iii) false-negative result when using a non-optimized antigen retrieval method. In our study a biomedical scientist at the department of Oral Pathology, Malmö University, prepared antibody concentrations.
Initially we chose four odontogenic tumours for this study: ameloblastoma, adenomatoid odontogenic tumour, calcifying epithelial odontogenic tumour and odontogenic cancer. When gathering the subjects for the study we realized that there were not enough tissue specimens available for odontogenic cancer and calcifying epithelial odontogenic tumour. Therefore, we switched out these odontogenic tumours to ameloblastic fibroma and odontogenic fibroma. A number of tissue subjects could not be included due to reasons discussed in ‘Materials and Methods’ and the final number of specimens came to 32 biopsies instead of the 40 biopsies we initially intended to include in this study.
One of the exclusion criterias we used for the subjects were decalcified biopsies, for the reason that the decalcifying process uses formic acid, which changes the structure of the proteins in the tissue subjects. This makes it difficult for the antibodies to bind correctly to the epitopes and therefore lowering their detectability under microscope. In general, the letters of referral contained information about the tissue specimens decalcification status. However, this was not always guaranteed because the decalcification process (that sometimes is performed during the embedding process of the tissue subject) is not always mentioned in the letters of referral. This can be viewed as a source of error in our study.
There are two negative aspects where BRAF V600E were used in this study, i) the miscalculation of the amount of BRAF V600E antibodies that was required for the 32 subjects, and ii) that ‘DAKO Blocking System’ was used instead of the BRAF V600E detection kit recommended by the manufacturer. The amount of BRAF V600E antibodies only sufficed for 18 subjects, that had faulted the IHC-staining, thus we did not achieve the desired result. An additional purchase of BRAF V600E antibodies and the required detection kit exclusive for the BRAF V600E antibodies, was not held within our budget. We believe that using the ‘DAKO Blocking System’ instead of the BRAF V600E detection system might have negatively influenced the IHC outcome of the 18 faulting slides.We used the
recommended detection system for p53 and Ki-67 and obtained adequate IHC staining results. The IHC procedure was performed manually, which likely represents a source of error in the study. The IHC staining of the slides might have come out better if it was performed automatically by an IHC staining system.
Our initial analyses of the IHC staining of the slides were uncertain, since we are not trained professionals in oral pathology. We went over our analysis a second time and then
re-evaluated the IHC staining for each slide with a professional oral pathologist.
The optimal scenario for our study would be if we had specimens of both the primary tumours and their recurrent tumours.This would enable us to draw more confident
conclusions about the antibodies used as prognostic markers for the recurrence of the studied tumours. But since the letters of referral for the selected subjects did not contain such
information, this study design was not an option. Results
The difference between AB and AOT versus AF and OF as two separate groups, showed a significantly higher staining intensity for both Ki-67 (p < 0.001) and p53 (p = 0.001) in AB and AOT as a group.
The co-expression of Ki-67 and p53 indicate high levels of cell proliferation and mutation in the TP53 gene, which in turn stipulates the aggressive nature of the tumour (35). Tumours develop through a series of multiple steps of genetic alterations (36). The TP53 gene is a tumour suppressor gene situated on chromosome 17p13. This gene is thought to play an important role in the regulation of cell proliferation and is a common cellular target in cancers (36,37). Its gene product, the p53 protein, is a transcriptional factor that plays an important role in response to cellular damage by inducing the cell cycle to arrest to allow DNA repair or apoptosis if the DNA is irreversibly damaged (36). The accumulation of p53 protein (due to the rapid cell turnover during a short period of time) is associated with increased cell
proliferation and malignant transformation in cancers (35-37). The p53 protein has a tendency to be over-expressed as the biologic behavior of odontogenic tumours become more
aggressive (35).
The same can be said for Ki-67, which also tends to be overexpressed in odontogenic
tumours of more aggressive nature (35,38). Ki-67 is a protein required for maintaining the cell cycle. This protein is expressed in all phases of the cell cycle (G1, S, G2 and M phase), but absent in the resting (G0) cells (38). Ki-67 is the most reliable and the most used marker of cellular proliferative activity and for determining the proliferation rate in tumours (35,38-40) . Ki-67 may possibly constitute a marker of the tumours biologic behavior and local
invasiveness and thus help determining the risk of tumour recurrence (38,41-43).
Ameloblastoma
An important aspect of AB is its locally invasive biological behavior, causing bone destruction and soft tissue invasion as well as its high recurrence rate (35,38,39,44) . The recurrence is strongly affected by the choice of surgical treatment, where the risk of recurrence is significantly reduced by radical surgical treatment compared to conservative surgical treatment (35,38). In our study, the Ki-67 staining was positive in all subjects and the semi-quantitative analysis of the immunoreaction showed a moderate staining reaction in the majority of the subjects. A study by Abdel-Aziz et al (39) found that there were significant differences between the recurrent and nonrecurrent forms of AB; with the highest epithelial
proliferative activity (Ki-67 expression) detected in the recurrent AB. Additionally, a study by O’Leary et al (41) found that Ki-67 was significantly higher in the recurrent tumours, thereby suggesting that Ki-67 can be considered a predictive marker of AB invasiveness and
recurrence. Other studies (45,46) on AB have shown that the lowest proliferative activity is found in the unicystic ameloblastoma, which is clinically less aggressive and can successfully be treated by enucleation and curettage. However, studies by Meer et al and Rosenstein (47,48) detected high values of proliferative activity in the unicystic ameloblastoma comparing with the conventional solid multicystic type, the former being clinically less recurrent than the latter. This suggests that the unicystic ameloblastomas low recurrence rate (despite of its high proliferative activity) can be explained by its greater accessibility and ease of surgical removal.
In our study, the semi-quantitative analysis of the p53 immunoreaction in the AB subjects showed positive staining in a moderate to high degree in the majority of the subjects. In normal cells the p53 protein is a cell regulator and inactivation of this protein is one of the most common genetic changes in human cancers (44,49) . In a study by Gadbail et al (49) they found that the TP53 gene mutation has a tendency of being increasingly expressed as the biologic behavior of the odontogenic cysts and tumours becomes more aggressive.
Overexpression of p53 can promote cell proliferation in odontogenic tumours and moderate or weak p53 expression could be due to slow and expansive growth of tumours. A study by Kumamoto et al (36) concluded that elevated expression of p53, MDM2 and p14ARF in benign and malignant AB suggests that alteration of the p53-MDM2-p14ARF cascade is involved in the malignant transformation of odontogenic epithelium and that p53 might be of some significance in the neoplastic changes of epithelium in AB.
Several studies use Ki-67 and p53 as markers for odontogenic tumours biologic behaviour, local invasiveness and rate of recurrence. It can therefore be stipulated that Ki-67 and p53 expressions can possibly be used as prognostic markers in odontogenic lesions (35). If applying this statement to our study subjects, one might interpret that the moderately to high staining reactions of Ki-67 och p53 implies that these tumours were indeed aggressive in nature and in need of frequent supervisory controls.
There are several recent studies showing the presence of BRAF mutations in AB. Fregnani (50) investigated the immunoexpression of BRAF-V600E in 73 AB cases and found that 46% of the cases demonstrated BRAF mutations in the epithelium. Additionally they found a significant association between BRAF V600E expression and recurrence. In another study by Sweeny et al (51) on the identification of recurrent SMO and BRAF mutations in AB, they reported similar figures with 46% of the 28 subjects testing positive for BRAF V600E mutations. However a study by Brown et al (52) demonstrated BRAF V600E mutations in 62% of 50 analyzed AB cases. Similar figures were found in a study by Kurppa et al (53), which reported BRAF V600E mutations in 63% of the 24 analysed AB cases.
Studies by Brown et al (52), Sweeny et al (51), Kruppa et al (53), and Heikinheimo (54) conclude that BRAF V600E mutations is the most frequent genetic alteration in AB and also suggest that this mutation is associated with more aggressive behavior of AB. They also support that BRAF V600E mutation may have implications for diagnosis and prognosis of AB, and suggest the future use of BRAF inhibitors for targeted therapy of this neoplasm, because BRAF V600E mutations seems to play a role in AB pathogenesis.
In the light of these findings we wanted investigate if BRAF V600E mutations occurred in AB as well as in other odontogenic tumours with epithelial components, i.e. AF, AOT and OF. However, we were not able to perform this analysis because of the faulted BRAF procedure discussed earlier under ‘Materials and Methods’. Nonetheless, we believe that
BRAF mutations can become a great aid in determining the prognosis and therapy of AB as well as other odontogenic tumours of epithelial origin. Even though our BRAF V600E stainings could not be implemented as we had hoped for, it is not ethically viable to not convey our results. The planning of the BRAF V600E IHC procedure was not optimal in terms of costs, equipment and the appropriate amount of antibodies needed for all subjects. When searching for studies on odontogenic tumours and IHC with Ki-67, p53 and BRAF V600E we found that there were a lack of studies made on AF, AOT and OF compared to AB. This is probably due to AB being the most common odontogenic tumour. More studies on Ki-67, p53 and BRAF V600E mutations are needed for other odontogenic tumours of epithelial origin.
Adenomatoid Odontogenic Tumour
The staining of p53 and Ki-67 were positive in most of the subjects. There was moderate to high staining reaction of p53 and weak to moderate staining reaction of Ki-67. These results are comparable to those of AB, which is similar to the findings in a study by Zulfin Shaikh et al (55). However, other studies have found that the amount of p53 and Ki-67 is significantly higher in AB than in AOT, which is inconclusive with our results (56-59). The study by Reichart et al (58) concludes that lower levels of p35 and Ki-67 in AOT would suggest that AOT has a hamartomous behaviour rather than neoplastic behavior similar to AB (59,60). Nevertheless, the results in our study propose that the AOT has neoplastic behaviour much like the AB. Our findings oppose the results of several other studies, therefore the validity of our results regarding AOT might be considered questionable. Although it is conceivable that AOT has hamartomous behavior (based on the low staining reactions to p53 and AOT and low rate of recurrence), continuous follow-up may be necessary (56,59,61-63).
Ameloblastic Fibroma
AF has a neoplastic behavior and as such one might expect high proliferative activity in the tumour; but staining indicates otherwise (64-66).A higher positive staining reaction of p53 and Ki-67 can be a predictor of the tumour's aggressiveness, local invasiveness and its potential to transform to malignant ameloblastic fibrosarcoma (AFS). Therefore it is assuring that the p53 and Ki-67 showed low positive staining values in this study.Studies have shown that low staining values are due to the low mitotic activity of the tumour. These studies show positive staining in the mesenchymal parts of AFS, but no staining of the epithelium.
Meanwhile there was almost no staining at all in the AF specimens (64,65,67-71).Studies evaluating the risk of recurrence of AF when displaying positive staining reactions for p53 and Ki-67 have shown that Ki-67 expression in stromal cells appear to be a significant marker for the invasiveness and recurrence of AF, thus constituting a prognostic factor (44,67). Although very rare, there is a risk of recurrence and malignant transformation of AF.
Therefore, invasive surgical removal of the tumour as well as continuous follow-up might be necessary to prevent future suffering of the patient (65,66,72).
Odontogenic Fibroma
In this study OF displayed a low and vague expression of p53 and Ki-67. Staining with cytokeratin AE1/AE3 (figure 3) was performed in order to reveal the epithelial islands and therefore creating a clarifying epithelial template used when tracing epithelium in subjects stained with p53 and Ki67 respectively. Low expression of p53 and Ki-67 was also found in other studies. However, there are only a few studies made on p53 and Ki-67 in OF (73,74).
OF only contains small quantities of epithelial cells, thus its scarce staining. The antibodies used in this study are molecular markers for assessment of cell proliferation and indicative of the aggressiveness of the tumour, as well as treatment results, in epithelial cells (16,73). Usually the epithelial cells in OF seem to be inactive, and will therefore not show a positive staining reaction to Ki-67 and p53 (16,75,76).
Recurrence of OF is rare and mostly due to inadequate removal of the primary tumour. Malignant transformation of the tumour is also rare. A conservative treatment with curettage and enucleation is therefore usedwhen removing the tumour. Even though OF is benign, follow-up is recommended (16,75-79).
Benefits and consequences in dental care
The aim of this study was to do an IHC evaluation of Ki-67, p53 and BRAF in different odontogenic tumours. In this study we found that Ki-67 and p53 was detected in AB, AF and AOT, but not in OF. BRAF, could however not be fully evaluated.
As discussed above, we found that our results are consensual with several other similar studies. These studies also show, as we anticipated and hoped, that p53, Ki-67 and BRAF V600E markers in odontogenic tumours can possibly be of prognostic value in determining the tumour's aggressiveness, local invasiveness and the risk of recurrence. We believe that identification of specific mutations and cell activity registration may be a more integrated part of the diagnosis and treatment of odontogenic tumours in the future, and help determine the need for follow-up after tumour removal.
The findings in this study answer our research questions, i.e. “Can different mutation patterns be found in various odontogenic tumours?” and “Can mutation patterns found in odontogenic tumours act as a prognostic biological markers and thereby influence the choice of therapy?”.
Often when removing tumour tissue for histopathological analysis, the whole tumour with margins (invasive procedure) is removed. Thus it might not be of importance to know the need for invasiveness in surgery. However, if the clinician has knowledge about the biological nature of the tumour, he or she can preserve jaw tissue by not removing a margin of bone around the tumour. More importantly, a reliable prognostic marker can be used when planning the recall frequency for controls, based on the tumours risk of recurrence and potential for malignant transformation.
Being able to predict the tumour's biological pattern can be financially and timely beneficial for the patient, as well as the dentist and the general society. For the individual patient and general society this might mean appropriate follow-up frequence and less “sick days” from school or work. For the dentist this might imply saving time and conducting efficient dental care.
The previously mentioned studies have all speculated about the possibility of using BRAF V600E mutation inhibition substances for the treatment of AB (50-54). Hypothetically, this suggests that a substance created to inhibit p53 mutations might be an efficient treatment for tumours of aggressive nature. Thus reducing the risk of recurrence. However, more research on this subject is required.
Ethical considerations
All patients in this study were anonymized and instead given an identification number. The personal information about the subjects was held in confidentiality at at the department of Oral Pathology in the Faculty of Odontology at Malmö University. This study had no
influence on the patient's privacy, treatment or impairment; hence no ethical approval was required. All patients registered in the database had given their permission for the biopsies to be saved in the biological bank at the department of Oral Pathology in accordance with Swedish Law (80).
Identification of gene mutations associated with disease implicates a multitude of
possibilities in the medical technical arena, however not necessarily for the individual patient. An interesting hypothetical concept is that with the use of future research one could perform DNA sequencing to map out mutations that can be linked to, or confirm, the risk of recurrence of odontogenic tumours. In this quest for mutations linked to odontogenic tumours one might come across other mutations linked to incurable or latent diseases in your genome. Are you, as a clinical practitioner, obligated to inform the patient about these findings - even if the mutations or plausible diseases are not affecting the patient at the time? The medical technical area is not always in line with the ethics that constitutes an important fundament in health care. The debate that interplays between medical technique and human ethics is a never-ending story and at times highly philosophical without any rights and wrongs. Regardless of which side of the debate one sympathizes with, it is indeed interesting to follow what is to come in the world of medical genetics.
Conclusions
The main conclusion of this thesis is that Ki-67 proliferation index and p53-mutation might be considered prognostic markers for recurrence of AB and AOT, but not for AF or OF.
However it may be difficult to apply these findings clinically, since the removal is often done with broad tumour margins to avoid recurrence.
The degree of invasivness when treating odontogenic tumours needs further investigation in order to perform adequate tumour removal while still minising the risk of tumour recurrence.
References
(1) Garg K, Chandra S, Raj V, Fareed W, Zafar M. Molecular and genetic aspects of odontogenic tumors: a review. Iran J Basic Med Sci. Iran J Basic Med Sci 2015 Jun;18(16):529-36.
(2) Morgan PR. Odontogenic tumors: a review. Periodontology 2000 2011;57:160-176.
(3) CHAPTER 6 - Odontogenic Tumours. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Pathology and Genetics of Head and Neck Tumours - WHO/IARC Classification if Tumours. 3rd edition ed.: World Health Organization; 2005. p. 283-327.
(4) McClary A, West R, et al. Ameloblastoma: a clinical review and trends in management. Eur Arch Otorhinolaryngol 2016 Jul;273(7):1649-61.
(5) Wood N, Goaz P. Bony Lesions. Oral and Maxillofacial Lesions. 5th edition ed. St. Louis, Missouri: Mosby-Year Book; 1997. p. 289-290,337-338.
(6) Regezi JA, Sciubba JJ, Jordan R. Oral pathology: clinical pathologic correlations. 6th edition ed. St. Louism Mo.: Elsevier/Saunders; 2012.
(7) Neville B, et al. Tumors of Odontogenic Epithelium. Oral and Maxillofacial Pathology. 3rd edition ed. Philadelphia: W.B. Saunders; 2008. p. 702-728.
(8) Kalia V, Kalra G, Kaushal N, Sharma V, Vermani M. Maxillary adenomatoid odontogenic tumor associated with a premolar. Annals of Maxillofacial Surgery 2015;5(1):119-122.
(9) Handschel J, Depprich R, Zimmermann A, Braunstein S, Kübler N. Adenomatoid odontogenic tumor of the mandible: review of the literature and report of a rare case. Head & Face Medicine 2005;1(3).
(10) Munde A, Karle R, Kale U. Ameloblastic fibroma in one-year-old girl. J Oral Maxillofac Pathol 2013 Jan-Apr;17(1):149.
(11) Vij R, Vij H. Ameloblastic fibroma: an uncommon entity. BMJ Case Rep 2013 Jul;9;2013. (12) Chapter 29: Odontogenic Cysts and Tumors. In: Miloro M, Ghali G, Larsen P, Waite P, editors. Peterson's Principles of Oral and Maxillofacial Surgery. 3rd edition ed. Shelton, CT: PMPH-USA Ltd.; 2011. p. 635-649.
(13) Manzon S, Philbert R, Bush B, Zola M, Solomon M. Treatment of a recurrent ameloblastic fibroma. N Y State Dent J 2015 Jan;81(1):30-32.
(14) Kulkarni R, Sarkar A, Goyal S. Recurrent Ameloblastic Fibroma: Report of a Rare Case . Case Reports in Dentistry 2013;2013.
(15) Ealla K, Basavanapalli V, Velidandla S, Manikya S, Ragulakollu R, Danappanavar P, et al. Ameloblastic Fibroma of the Maxilla with Bilateral Presentation: Report of a Rare Case with Review of the Literature. Case Reports in Pediatrics 2015;2015.
(16) Chhabra V, Chhabra A. Central odontogenic fibroma of the mandible.<br />. Contemporary Clinical Dentistry 2012;3(2):230-233.
(17) Rajendran R. Shafer's textbook of oral pathology. 6th edition ed. India: Elsevier; 2009. (18) Duraiyan J, Govindarajan R, Kaliyappan K, Palanisamy M. Applications of
immunohistochemistry. Journal of Pharmacy & Bioallied Sciences 2012;4(Suppl 2):S307-S309. (19) Mohan H. Essential pathology for dental students. 3rd edition ed. New Delhi: Jaypee brother's medical publishers; 2005. p. 14-15.
http://www.ne.se.proxy.mah.se/uppslagsverk/encyklopedi/l%C3%A5ng/immunhistokemi. Accessed 21/10, 2016.
(21) Ross M. Histology- A text and atlas with correlated cell and molecular biology. 5th edition ed. Baltimore: Lippincott Williams & Wilkins; 2006.
(22) abcam. A comparison between polyclonal and monoclonal. 2016; Available at:
http://www.abcam.com/protocols/a-comparison-between-polyclonal-and-monoclonal. Accessed
10/21, 2016.
(23) Sigma-Aldrich. Alphabetical Index Monoclonal & Polyclonal Antibodies. 2016; Available
at:
http://www.sigmaaldrich.com/life-science/cell-biology/antibodies/antibody-products.html?TablePage=14574648. Accessed 10/21, 2016.
(24) Pacific Immunology. Polyclonal vs monoclonal antibodies. 2016; Available at:
https://www.pacificimmunology.com/resources/antibody-introduction/polyclonal-vs-monoclonal-antibodies/. Accessed 10/21, 2016.
(25) DAKO Agilent Pathology Solutions. Ki-67 Antigen: Clone MIB-1. 2016; Available at:
http://www.dako.com/se/ar38/p104960/prod_products.htm. Accessed 10/10, 2016.
(26) Ai Y, Shao Y, Li H, Xue W, Quan F, Wu S. Ki-67 is overexpressed in human laryngeal carcinoma and contributes to the proliferation of HEp2 cells. Oncol Lett 2016;12(4):2641-2647. (27) Bullwinkel J, Baron-Lühr B, Lüdemann A, Wohlenberg C, Gerdes J, Scholzen T. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J Cell Physiol 2006 Mar;206(3):624-635.
(28) Ahlin C, Fernö M, Amini R, Tolokiene E, Blomqvist C, Bergh J, et al. Ki-67 och cyklin A – prognostiska faktorer vid bröstcancer. Läkartidningen 2016;107(10):672-675.
(29) DAKO Agilent Pathology Solutions. p53 Protein: Clone DO-7. 2016; Available at:
http://www.dako.com/se/ar38/p105510/prod_products.htm. Accessed 10/10, 2016.
(30) Sigma-Aldrich. Monoclonal Anti-p53 antibody produced in mouse. 2016; Available at:
http://www.sigmaaldrich.com/catalog/product/sigma/p5813?lang=en®ion=SE. Accessed 10/10,
2016.
(31) NE N. p53. 2016; Available at:
http://www.ne.se.proxy.mah.se/uppslagsverk/encyklopedi/l%C3%A5ng/p53. Accessed 10/10, 2016.
(32) Yemelyanova A, Vang R, Kshirsagar M, Lu D, Marks M, Shih I, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Modern Pathology 2011;24:1248-1253. (33) Barras D. BRAF Mutation in Colorectal Cancer: An Update. Biomarkers in Cancer 2015;7(S1):9-12.
(34) abcam. Direct vs indirect detection in IHC. 2016; Available at:
http://www.abcam.com/kits/direct-and-indirect-detection-in-ihc. Accessed 10/10, 2016.
(35) Gadbail A, Patil R, Chaudhary M. Co-expression of
Ki-67 and p53 protein in ameloblastoma and keratocystic odontogenic tumor. Acta Odontol Scand 2012;70(6):529-535.
(36) Kumamoto H, Izutsu T, Ohki K, Takahashi N, Ooya K. p53 gene status and expression of p53, MDM2, and p14 proteins in ameloblastomas. J Oral Pathol Med 2004;33(5):292-299.
(37) Carvalhais J, Aguiar M, Araújo V, Araújo N, Gomez R. p53 and MDM2 expression in odontogenic cysts and tumours. Oral Dis 1999;5(3):218-222.
(38) Olimid D, Florescu A, Cernea D, Georgescu C, Mărgăritescu C, Simionescu C, et al. The evaluation of p16 and Ki67 immunoexpression in ameloblastomas. Rom J Morphol Embryol
2014;55(2):363-367.
(39) Abdel-Aziz A, Amin M.
EGFR, CD10 and proliferation marker Ki67 expression in ameloblastoma: possible role in localrecurr ence. Diagn Pathol 2012;7(14).
(40) Migaldi M, Sartori G, Rossi G, Cittadini A, Sgambato A.
Tumor cell proliferation and microsatellite alterations in human ameloblastoma. Oral Oncol 2008;44(1):50-60.
(41) O’Leary T, Frisman D. In: O’Leary T, editor. Antigenes. In Advanced diagnostic methods in pathology: Principles, practice and protocols. 1st edition ed. Philadelphia: Saunders; 2003. p. 35-91. (42) Ahlem B, Wided A, Amani L, Nadia Z, Amira A, Faten F.
Study of Ki67 and CD10 expression as predictive factors of recurrence of ameloblastoma. Eur Ann Otorhinolaryngol Head Neck Dis 2015;132(5):275-279.
(43) Rizzardi C, Leocata P, Ventura L, Zweyer M, Brollo A, Schneider M, et al. Apoptosis-related factors (TRAIL, DR4, DR5, DcR1, DcR2, apoptotic cells) and proliferative activity in ameloblastomas. Anticancer Res 2009;29(4):1137-1142.
(44) Slootweg P. p53 protein and Ki-67 reactivity in epithelial odontogenic lesions. An immunohistochemical study. J Oral Pathol Med 1995;24(9):393-397.
(45) Sandra F, Mitsuyasu T, Nakamura N, Shiratsuchi Y, Ohishi M. Immunohistochemical evaluation of PCNA and Ki-67 in ameloblastoma. Oral Oncol 2001;37(2):193-198.
(46) Funaoka K, Arisue M, Kobayashi I, Iizuka T, Kohgo T, Amemiya A, et al. Immunohistochemical detection of proliferating cell nuclear antigen (PCNA) in 23 cases ofameloblastoma. Eur J Cancer B Oral Oncol 1996;32B(5):328-332.
(47) Meer S, Galpin J, Altini M, Coleman H, Ali H. Proliferating cell nuclear antigen and Ki67 immunoreactivity in ameloblastomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003;95(2):213-221.
(48) Rosenstein T, Pogrel M, Smith R, Regezi J. Cystic ameloblastoma-behavior and treatment of 21 cases. J Oral Maxillofac Surg 2001;59(11):1311-1316.
(49) Florescu A, Simionescu C, Ciurea R, Pitru A. P53,
Bcl-2 and Ki67 immunoexpression in follicular solid ameloblastomas. Rom J Morphol Embryol 2012;53(1):105-109.
(50) Fregnani E, da Cruz Perez D, Paes de Almeida O, Fonseca F, Soares F, de Castro Junior G, et al. Braf- V600E expression correlates with ameloblastoma aggressiveness. Histopathology 2016. (51) Sweeney R, McClary A, Myers B, Biscocho J, Neahring L, Kwei K, et al.
Identification of recurrent SMO and BRAF mutations in ameloblastomas. . Nat Genet 2014;46(7):722-725.
(52) Brown N, Rolland D, McHugh J, Weigelin H, Zhao L, Lim M, et al. Activating FGFR2-RAS-BRAF mutations in ameloblastoma. Clin Cancer Res 2014;20(21):5517-5526.
(53) Kurppa K, Catón J, Morgan P, Ristimäki A, Ruhin B, Kellokoski J, et al.
High frequency of BRAF V600E mutations in ameloblastoma. J Pathol 2014;232(5):492-498. (54) Heikinheimo K, Kurppa K, Elenius K. Novel targets for the treatment of ameloblastoma. J Dent Res 2015;94(2):237-240.
(55) Shaikh Z, Niranjan K. Cell cycle aberration in ameloblastoma and adenomatoid odontogenic tumor: As evidenced by expression of p53 and survivin. Indian J Dent Res 2015;26(6):565-570. (56) Galvão Barboza C, Pereira Pinto L, de Almeida Freitas R, de Lisboa Lopes Costa, A., Batista de Souza L. Proliferating cell nuclear antigen (PCNA) and p53 protein expression in ameloblastoma and
adenomatoid adontogenic tumor. Braz Dent J 2005;16(1).
(57) Salehinejad J, Zare-Mahmoodabadi R, Saghafi S, Jafarian A, Ghazi N, Rajaei A, et al.
Immunohistochemical detection of p53 and PCNA in ameloblastoma and adenomatoid odontogenic tumor. Journal of Oral Science 2011;53(2):213-217.
(58) Reichart P, Philipsen H, Khongkhunthian P, Sciubba J. Immunoprofile of the adenomatoid odontogenic tumor. Oral Dis 2016.
(59) Razavi S, Tabatabaie S, Hoseini A, Hoseini E, Khabazian A. A comparative
immunohistochemical study of Ki-67 and Bcl-2 expression in solid ameloblastoma and adenomatoid odontogenic tumor. Dent Res J (Isfahan) 2012;9(2):192-197.
(60) Sempere F, Martinez M, Sirera B, Marco J. Follicular adenomatoid odontogenic tumor: immunohistochemical study. Med Oral Patol Oral Cir Bucal 2006;11:305-308.
(61) Crivelini M, Soubhia A, Felipini R. Study on the origin and nature of the adenomatoid odontogenic tumor by immunohistochemistry. J Appl Oral Sci 2005;13(4).
(62) Angiero F, Crippa R. Adenomatoid Odontogenic Tumor: A Case Report with Immunohistological Profile. Anticancer Res 2013;33(6):2673-2677.
(63) Martínez A, Mosqueda-Taylor A, Marchesani F, Brethauer U, Spencer M.
Adenomatoid odontogenic tumor concomitant with cystic complex odontoma: case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108(4):e25-e29.
(64) Galvão C, Gomes C, Diniz M, Vargas P, de Paula A, Mosqueda-Taylor A, et al. Loss of heterozygosity (LOH) in tumour suppressor genes in benign and malignant mixed odontogenic tumours. J Oral Pathol Med 2012;41(5):389-393.
(65) Williams M, Hanna E, El-Naggar A.
Anaplastic ameloblastic fibrosarcoma arising from recurrent ameloblastic fibroma: restricted molecula rabnormalities of certain genes to the malignant transformation. Williams MD1, Hanna EY, El-Naggar AK. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104(1):72-75.
(66) Sano K, Yoshida S, Ninomiya H, Ikeda H, Ueno K, Sekine J, et al.
Assessment of growth potential by MIB-1 immunohistochemistry in ameloblastic fibroma and related lesions of the jaws compared with ameloblastic fibrosarcoma. J Oral Pathol Med 1998;27(2):59-63. (67) Takeda Y. Ameloblastic fibroma and related lesions: current pathologic concept. Oral Oncol 1999;35(6):535-540.
(68) Huguet P, Castellvi J, Avila M, Alejo M, Autonell F, Basas C, et al.
Ameloblastic fibrosarcoma: report of a case. Immunohistochemical study and review of the literature. Med Oral 2001;6(3):173-179.
(69) Gilani S, Raza A, Al-Khafaji B. Ameloblastic fibrosarcoma: A rare malignant odontogenic tumor. Eur Annals of Otorhinolaryngology, Head and Neck Diseases 2014;131(1):53-56.
(70) Pontes H, Pontes F, Silva B, Cury S, Fonseca F, Salim R, et al.
Immunoexpression of Ki67, proliferative cell nuclear antigen, and Bcl-2 proteins in a case of ameloblasticfibrosarcoma. Ann Diagn Pathol 2010;14(6):447-452.
(71) Ponnam S, Srivastava G, Smitha B. Ameloblastic Fibroma. J Oral Maxillofac Pathol 2012;16(3):444-445.
(72) Hu Y, Deng M, Yuan L, Niu Y. Ameloblastic fibrosarcoma of the mandible: A case report and mini review. Exp Ther Med 2014;8(5):1463-1466.
(73) Mosqueda-Taylor A, Martínez-Mata G, Carlos-Bregni R, Vargas P, Toral-Rizo V, Cano-Valdéz A, et al. Central odontogenic fibroma: new findings and report of a multicentric collaborative study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2011;112(3):349-358.
(74) Tan S, Yeo J, Kheem Pang B, Petersson F. An intraosseous sclerosing odontogenic tumor predominantly composed of epithelial cells: relation to (so-called) sclerosing odontogenic carcinoma and epithelial-rich central odontogenic fibroma. Oral Surg Oral Med Oral Pathol Oral Radiol
2014;118(4):e119-e125.
(75) Monteiro L, Marins M, Pacheco J, Salazar F, Magalhães J, Vescovi P, et al.
Er:YAG Laser Assisted Treatment of Central Odontogenic Fibroma of the Mandible. Case Rep Dent 2015;2015.
(76) Hrichi R, Gargallo-Albiol J, Berini-Aytés L, Gay-Escoda C. Central odontogenic fibroma: Retrospective study of 8 clinical cases. Med Oral Patol Oral Cir Bucal 2012;17(1):e50-e55.
(77) Covani U, Crespi R, Perrini N, Barone A. Central odontogenic fibroma: A case report. Med Oral Patol Oral Cir Bucal 2005;10(Suppl2):e154-e157.
(78) Pippi R, Santoro M, Patini R. The central odontogenic fibroma: How difficult can be making a preliminary diagnosis. J Clin Exp Dent 2016;8(2):e223-e225.
(79) El Harti K, Oujilal A, El Wady W. Central odontogenic fibroma of the maxilla. Indian J Dent 2015;6(4):217-220.
(80) Swedish Parliament. Law (2002:297) about saving biological material in health care etc. (Lag (2002:297) om biobanker i hälso- och sjukvården m.m.). 2002;2002:297.