D O C TO R A L D IS S E R TA TI O N I N O D O N TO LO G Y
LORY MELIN SVANBORG
ON THE IMPORTANCE OF
NANOMETER STRUCTURES
FOR IMPLANT
INCORPORATION
IN BONE TISSUE
O n t h e i m p O r t a n c e O f n a n O m e t e r s t r u c t u r e s f O r i m p l a n t i n c O r p O r a t i O n i n b O n e t i s s u e
Malmö University
Faculty of Odontology Doctoral Dissertations 2011
© Lory Melin Svanborg 2011 ISBN 978-91-7104-384-9 Holmbergs, Malmö 2011
lOry melin svanbOrg
On the impOrtance Of
nanOmeter structures
fOr implant incOrpOratiOn
in bOne tissue
Department of Prosthodontics,
Faculty of Odontology at Malmo University
Department of Biomaterials
Institute of Clinical Sciences
The Sahlgrenska Academy at University of Gothenburg
2011
Publikationen finns även elektroniskt, se www.mah.se/muep
To my family and friends, with love. Life is not the days that have passed,
but the days you remember – Carpe Diem...
This thesis represents number 41 in a series of investigations on implants, hard tissue and the locomotor apparatus originating from the department of Biomaterials/Handicap Research, University of Gothenburg and the department of Prosthodontics, Malmö University, Sweden.
1. Anders R Eriksson DDS, 1984. Heat-induced Bone Tissue Injury. An in vivo investigation of heat tolerance of bone tissue and temperature rise in the drilling of cortical bone. Thesis defended 21.2.1984. External examiner: Docent K-G. Thorngren. 2. Magnus Jacobsson MD, 1985. On Bone Behaviour after Irradiation. Thesis defended 29.4.1985. External examiner: Docent A. Nathanson.
3. Fredrik Buch MD, 1985. On Electrical Stimulation of Bone Tissue. Thesis defended 28.5.1985. External examiner: Docent T. Ejsing-Jörgensen.
4. Peter Kälebo MD, 1987. On Experimental Bone Regeneration in Titanium Implants. A quantitative microradiographic and histologic investigation using the Bone Harvest Chamber. Thesis defended 1.10.1987. External examiner: Docent N. Egund.
5. Lars Carlsson MD, 1989. On the Development of a new Concept for Orthopaedic Implant Fixation. Thesis defended 2.12.1989. External examiner: Docent L-Å Broström. 6. Tord Röstlund MD, 1990. On the Development of a New Arthroplasty. Thesis defended 19.1.1990. External examiner: Docent Å. Carlsson.
7. Carina Johansson Techn Res, 1991. On Tissue Reactions to Metal Implants. Thesis defended 12.4.1991. External examiner: Professor K. Nilner.
8. Lars Sennerby DDS, 1991. On the Bone Tissue Response to Titanium Implants. Thesis defended 24.9.1991. External examiner: Dr J.E. Davis.
9. Per Morberg MD, 1991. On Bone Tissue Reactions to Acrylic Cement. Thesis defended 19.12.1991. External examiner: Docent K. Obrant.
10. Ulla Myhr PT, 1994. On Factors of Importance for Sitting in Children with Cerebral Palsy. Thesis defended 15.4.1994. External examiner: Docent K. Harms-Ringdahl. 11. Magnus Gottlander MD, 1994. On Hard Tissue Reactions to Hydroxyapatite-Coated Titanium Implants. Thesis defended 25.11.1994. External examiner: Docent P. Aspenberg.
12. Edward Ebramzadeh MScEng, 1995. On Factors Affecting Long-Term Outcome of Total Hip Replacements. Thesis defended 6.2.1995. External examiner: Docent L. Linder.
13. Patricia Campbell BA, 1995. On Aseptic Loosening in Total Hip Replacement: the Role of UHMWPE Wear Particles. Thesis defended 7.2.1995. External examiner: Professor D. Howie.
15. Neil Meredith BDS MSc FDS RCS, 1997. On the Clinical Measurement of Implant Stability and Osseointegration. Thesis defended 3.6.1997. External examiner: Professor J. Brunski.
16. Lars Rasmusson DDS, 1998. On Implant Integration in Membrane-Induced and Grafter Bone. Thesis defended 4.12.1998. External examiner: Professor R. Haanaes. 17. Thay Q Lee MSc, 1999. On the Biomechanics of the Patellofemoral Joint and Patellar Resurfacing in Total Knee Arthroplasty. Thesis defended 19.4.1999. External examiner: Docent G. Nemeth.
18. Anna Karin Lundgren DDS, 1999. On Factors Influencing Guided Regeneration and Augmentation of Intramembraneous Bone. Thesis defended 7.5.1999. External examiner: Professor B. Klinge.
19. Carl-Johan Ivanoff DDS, 1999. On Surgical and Implant Related Factors Influencing Integration and Function of Titanium Implants. Experimental and Clinical Aspects. Thesis defended 12.5.1999. External examiner: Professor B. Rosenquist. 20. Bertil Friberg DDS MDS, 1999. On Bone Quality and Implant Stability Measurements. Thesis defended 12.11.1999. External examiner:
Docent P. Åstrand.
21. Åse Allansdotter Johnsson MD, 1999. On Implant Integration in Irradiated Bone. An Experimental Study of the Effects of Hyberbaric Oxygenation and Delayed Implant Placement. Thesis defended 8.12.1999. External examiner: Docent K. Arvidsson-Fyrberg.
22. Börje Svensson DDS, 2000. On Costochondral Grafts Replacing Mandibular Condyles in Juvenile Chronic Arthritis. A Clinical, Histologic and Experimental Study. Thesis defended 22.5.2000. External examiner: Professor Ch. Lindqvist.
23. Warren Macdonald BEng, MPhil, 2000. On Component Integration in Total Hip Arthroplasty: Pre-Clinical Evaluations. Thesis defended 1.9.2000. External examiner: Dr A.J.C. Lee.
24. Magne Røkkum MD, 2001. On Late Complications with HA Coated Hip Asthroplasties. Thesis defended 12.10.2001. External examiner: Professor P. Benum. 25. Carin Hallgren Höstner DDS, 2001. On the Bone Response to Different Implant Textures. A 3D analysis of roughness, wavelength and surface pattern of experimental implants. Thesis defended 9.11.2001. External examiner: Professor S. Lundgren. 26. Young-Taeg Sul DDS, 2002. On the Bone Response to Oxidised Titanium Implants: The role of microporous structure and chemical composition of the surface oxide in enhanced osseointegration. Thesis defended 7.6.2002. External examiner: Professor J.-E. Ellingsen.
27. Victoria Franke Stenport DDS, 2002. On Growth Factors and Titanium Implant Integration in Bone. Thesis defended 11.6.2002. External examiner: Associate Professor E. Solheim.
28. Mikael Sundfeldt MD, 2002. On the Aetiology of Aseptic Loosening in Joint Arthroplasties and Routes to Improved cemented Fixation. Thesis defended 14.6.2002. External examiner: Professor N Dahlén.
29. Christer Slotte DDS, 2003. On Surgical Techniques to Increase Bone Density and Volume. Studies in the Rat and the Rabbit. Thesis defended 13.6.2003. External examiner: Professor C.H.F. Hämmerle.
30. Anna Arvidsson MSc, 2003. On Surface Mediated Interactions Related to Chemo-mechanical Caries Removal. Effects on surrounding tissues and materials. Thesis defended 28.11.2003. External examiner: Professor P. Tengvall.
31. Pia Bolind DDS, 2004. On 606 retrieved oral and cranio-facial implants. An analysis of consecutively received human specimens. Thesis defended 17.12. 2004. External examiner: Professor A. Piattelli.
32. Patricia Miranda Burgos DDS, 2006. On the influence of micro-and macroscopic surface modifications on bone integration of titanium implants. Thesis defended 1.9. 2006. External examiner: Professor A. Piattelli.
33. Jonas P Becktor DDS, 2006. On factors influencing the outcome of various techniques using endosseous implants for reconstruction of the atrophic edentulous and partially dentate maxilla. Thesis defended 17.11.2006. External examiner: Professor K. F. Moos
34. Anna Göransson DDS, 2006. On Possibly Bioactive CP Titanium Surfaces. Thesis defended 8.12.2006 External examiner: B. Melsen
35. Andreas Thor DDS, 2006. On platelet-rich plasma in reconstructive dental implant surgery. Thesis defended 8.12.2006. External examiner: Prof E.M. Pinholt.
36. Luiz Meirelles DDS MSc, 2007. On Nano Size Structures For Enhanced Early Bone Formation. Thesis defended 13.6.2007. External examiner: Professor Lyndon F. Cooper. 37. Pär-Olov Östman DDS, 2007. On various protocols for direct loading of implant-supported fixed prostheses. Thesis defended 21.12.2007. External examiner: Prof B Klinge
38. Kerstin Fischer DDS, 2008. On immediate/early loading of implant supported prostheses in the maxilla. Thesis defended 8.2.2008. External examiner: Professor Kristina Arvidson Fyrberg
39. Alf Eliasson 2008. On the role of number of fixtures, surgical technique and timing of loading. Thesis defended 23.5.2008. External examiner: Kristina Arvidson-Fyrberg. 40. Victoria Fröjd DDS, 2010. On Ca2+ incorporation and nanoporosity of titanium surfaces and the effect on implant performance. Thesis defended 26.11.2010. External examiner: Professor J. E. Ellingsen
41. Lory Melin Svanborg DDS, 2011. On the importance of nanometer structures for implant incorporation in bone tissue. Thesis to be defended 01.06.2011. External examiner: Associate professor Christer Dahlin
O n the im portance of nanom eter
structures for
im plant incorporation in bone tissue
Lory Melin Svanborg
Department of Prosthodontics, Faculty of Odontology at Malmo University
Department of Biomaterials Institute of Clinical Sciences
The Sahlgrenska Academy at University of Gothenburg
2011
On the importance of nanometer
structures for
abstract
introduction
Replacing lost teeth with dental implants is today a reliable treatment method associated with good long-term clinical results. Different surface modifications alter the surface topography at micro- and nanometer level of resolution as well as chemical properties, which have shown to be of importance for osseointegration. Research within the field of implantology is still intense and aims at further improving the implant properties to achieve successful treatments for patients with compromised bone as well as developing a surface that provides faster integration to shorten the treatment period. Furthermore, more basic science data is needed to increase our understanding of the mechanisms involved in osseointegration.
The significance of the surface topography on the micrometer level for implant integration is well known. However, the knowledge of how and to what extent nanostructures may be of importance in early bonehealing and osseointegration remains to be investigated.
aim
The overall aim of this thesis was to describe a technique to characterize commercial oral implants on the nanometer level when nanostructures are applied on a microroughness and to investigate whether or not the nanometer surface roughness was correlated to the more well-known micrometer roughnesss; to study the real-time initial cellular interactions of human osteoblasts and fibroblasts to different implant surfaces with and without a coat of nanocrystalline hydroxyapatite; and to evaluate the early bone response to a nanocrystalline hydroxyapatite coating (nano-HA) applied on smooth cylindrical and moderately rough screw-shaped implants.
materials and methods
Twelve different commercial screw-shaped dental implants with different surface modifications were examined using optical interferometry together with Gaussian digital filters and scanning electron microscopy (SEM). Human osteoblasts and fibroblasts were used when investigating the initial cell-surface interaction to different surfaces modifications with optical tweezers (OT) and quartz crystal balance with dissipation monitoring (QCM-D). To evaluate the effect of nanocrystalline hydroxyapatite (nano-HA) compared to nanosized particles of titanium in early bone response, smooth cylindrical titanium implants with no microroughness were inserted in rabbit tibia. The implant surfaces were examined using atomic force microscopy (AFM) and interferometry. To evaluate the biological response, histological analyses including bone contact (BIC) and bone area (BA), as well as qualitative analysis were performed. Furthermore, screw-shaped sandblasted and acid etched titanium implants coated with nano-HA of different thicknesses and un-coated controls were evaluated in rabbit tibia as well as femur. Interferometry, SEM and X-ray Photoelectron Spectroscopy (XPS) were used to characterize the implant surface topography and chemical composition. Biomechanical and histological evaluations including BA, newly formed bone and qualitative evaluations were performed.
results
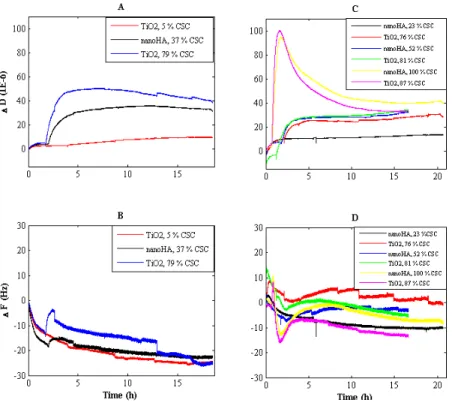
The studies showed that it is possible to characterize the surface nanoroughness of commercially dental implants using interferometry. A 1x1µm Gaussian filter was found useful to identify nanoroughness in terms of height deviation. It was demonstrated that the implants do have distinct roughness on the nanometer level of resolution and that the nanoroughness is not correlated to the microroughness when comparing mean surface roughness (Sa). Significant differences in Sa on the nanometer scale were found among some of the implants investigated. However, to detect specific nanostructures an additional SEM examination is necessary. The results from optical trap experiments showed that both osteoblasts and fibroblasts responded in a similar way towards most of the surfaces.
No difference in initial cell attachment could be detected between the surfaces when using the QCM-D technique.
A nano-HA coating applied on smooth cylindrical implants did not enhance bone responses in terms of bone contact (BIC) and bone area (BA) values as compared to nano-titania.
Screw-shaped sandblasted and acid etched titanium implants with applied nanothick (~20nm) coating of nano-HA with similar Sa values on both micro- and nanometer scale of resolution presented similar removal torque values, BA values and showed similar amounts of newly formed bone as compared to un-coated controls when placed in cortical bone. The same result was demonstrated in trabecular bone with a submicron thick coating of nano-HA onto sandblasted and acid etched screw-shaped implants.
conclusions
Within the limits of the studies in this thesis, it was demonstrated that commercially available oral implants do have nanoroughness of various amounts and that the nanoroughness is not correlated to the microroughness.
It was demonstrated possible to observe cell attachment using optical trapping and QCM-D, however no obvious differences between the surfaces could be detected.
A nano-HA coating applied on cylindrical titanium implants did not enhance early bone response compared to a nano-titania coating when evaluated in cortical bone. Furthermore, sandblasted and acid etched screw-shaped implants with applied coatings of different thicknesses of nano-HA perform similar as un-coated controls when evaluated in cortical and trabecular bone.
Correspondence:
Lory Melin Svanborg, Department of Prosthodontics, Faculty of Odontology, Malmö University. Sweden. E-mail: lory.svanborg@mah.se
this thesis is based on the following five papers:
Surface characterization;
I. “Surface characterization of commercial oral implants on the nanometer level.”
Svanborg LM, Andersson M, Wennerberg A.
J Biomed Mater Res B Appl Biomater. 2010 Feb;92(2):462-9.
In vitro study;
II. “Using optical tweezers and QCM-D to investigate initial human osteoblast and fibroblast attraction/adhesion towards different implant surfaces”
Svanborg LM, Westas E, Wang L, Duran R, Mustafa K, Wennerberg
A, Andersson M. In manuscript.
In vivo studies;
III. “Effect of hydroxyapatite and titania
nanostructures on early in vivo bone response.”
Meirelles L, Melin L, Peltola P, Kjellin P, Kangasniemi I, Currie F, Andersson M, Albrektsson T, Wennerberg A.
Clin Implant Dent Relat Res. 2008 Dec;10(4):245-54.*
IV. “The effect of hydroxyapatite nanocrystals on early boneformation surrounding dental implants.”
Svanborg LM, Hoffman M, Andersson M, Currie F, Kjellin P,
Wennerberg A. Int J Oral Maxillofac Surg. 2011 Mar;40(3):308-15. Epub 2010 Dec 15.
V. “Evaluation of early bone healing on sandblasted and acid etched implants coated with nanocrystalline hydroxyapatite - An in vivo study in rabbit femur”
Svanborg LM, Meirelles L, Franke-Stenport V, Kjellin P, Currie F,
Andersson M, Wennerberg A. Submitted.
* This paper has previously appeared in a Ph D thesis by Luiz Meirelles (2007).
L melin participated in surgery of animals, as well as in surface and histological analyses.
table Of cOntents
ABSTRACT ... 11
INTRODUCTION ... 17
Background and need for further studies on implant surfaces ...17
Implant material, design and surface properties ...18
Microroughness ...21
Methods to alter microtopography ...21
Nanoroughness and nanostructures ...25
Some methods to alter nanotopography ...26
Nanostructures and biological response ...29
New possibilities to evaluate cell response to nanostructures .31 Summary and relevance of the present thesis ...32
AIMS ... 34
MATERIALS AND METHODS ... 35
Implants and components ...35
Implant design and surface preparation ...35
Methods for surface characterization ...36
Atomic Force Microscope ...36
Optical interferometry ...37
Gaussian filter and surface parameters ...37
Scanning Electron Microscopy ...39
X-ray Photoelectron Spectroscopy ...39
Powder X-ray Diffraction (PXRD) ...39
Methods for evaluation in vitro ...40
Cells and culture ...40
Optical trapping ...40
Quartz Crystal Microbalance with dissipation monitoring (QCM-D) ...42
Methods for evaluation in vivo ...42
Animals and surgical technique ...42
Removal torque analysis ...43
Histological specimen preparation and evaluation ...43
Data analysis/statistics ...44 RESULTS ... 45 Surface characterisation ...45 SEM... ...45 Interferometry characterization ...46 AFM... ...48 XPS and PXRD ...48 Evaluation in vitro ...49 Optical trap ...49 QCM-D ...49 Evaluation in vivo ...52
Removal torque analysis ...52
Histological evaluation ...52
DISCUSSION ... 53
Implant material, design and surface characteristics ...53
Evaluation of surface characteristics ...53
AFM... ...53
SEM and XPS ...54
Optical interferometry ...54
Gaussian filter and surface parameters ...54
Methods and results in vitro ...55
Optical trap ...56
QCM-D ...56
Methods and results in vivo ...58
Animals and surgical technique ...58
Removal torque analysis ...58
Histological specimen preparation and evaluation ...59
Summary and topics for future research ...61
CONCLUSIONS ... 63
POPULäRVETENSKAPLIG SAMMANFATTNING ... 65
ACKNOWLEDGEMENTS ... 68
REFERENCES ... 71
intrODuctiOn
background and need for further studies on
implant surfaces
“Osseointegration is a process whereby clinically asymptomatic rigid fixation of an alloplastic material is achieved and maintained in bone during loading”. This definition of osseointegration based on stability instead of histology was suggested by Zarb & Albrektsson 19911. Implant treatment should aim to achieve predictable clinical
results with controlled clinical implants to ensure an adequate treatment for the patients with the goal to restore function and to the greatest possible extent, aesthetics.
Titanium has been used as an implant material since the discovery of osseointegration in the 1960´s by Professor Per-Ingvar Brånemark and others. Titanium implants have since then been used in different applications, for example; amputation prostheses, hip replacements, pacemakers and oral implants. Dental implants have become a common, often uncomplicated treatment method to replace lost teeth. Today, many millions of oral implants are placed in patients over the world annually. High clinical success rates, commonly over 90% have been reported for different implant surfaces after more than 10 years in function2-5. The mandible is often associated with
higher success rates than the maxilla due to greater variations of bone quality in the latter. Albrektsson et al.6 proposed criteria for
implant success to be absence of pain, infection, neuropathy, implant mobility and radiographic peri-implant radiolucency. Furthermore, no more than 1mm bone loss during the first year after insertion was accepted and thereafter less than 0.2mm bone loss annually. During the years, techniques have been developed to improve the
implants, with respect to design and surface properties, and thereby the success rate has increased. However, many patients with poor bone quality and or quantity would benefit from improved implant designs and/or specific surface modifications. Six factors have previously been suggested to be of importance for osseointegration; the biocompatibility of the implant material, the implant macro design, the implant surface properties, the status of the bone (quality, quantity and health), surgical technique and implant loading conditions7. It has been demonstrated possible to promote
implant incorporation in bone by altering surface properties such as; physical (e.g. energy, wettability), chemical (e.g. ion incorporation), mechanical (e.g. hardness, yield strength) and structure (topography, geometry, roughness) of the implants 8-13. The research within the
field of biomaterials and implantology is still intense and aims at further improving oral implants. Lately, there has been a growing interest in how the presence of nanometer structures on an implant surface influences the early bone healing process and the subsequent osseointegration.
This thesis will concentrate on surface topography with particular direction towards the potential importance of nanometer topography for enhanced implant incorporation in bone tissue.
implant material, design and surface properties
Titanium is the most commonly used material for bone integrated implants due to its excellent biocompatibility. According to the Oxford Dictionary of National Biography, titanium was first discovered by William Gregor in 1791. This metallic element is the 9th most common element in the earth crust and titanium may be
extracted from the minerals rutile (TiO2) and ilmenite (FeTiO3). Rutile is the most natural and common form of TiO2 and contains iron, niobium and tantalum. Anatase and brookite are two more rare forms of TiO2. There are four grades of commercially pure titanium (c.p. Titanium) which consists of approximately 99 weight percent of pure titanium but differ in the amount of oxygen, iron, carbon nitrogen and hydrogen. The difference in concentration has a significant impact on the physical and mechanical properties; for example titanium have high strength-to-weight ratio, high melting
point and low electrical and thermal conductivity. Titanium forms a very stable surface oxide within nanoseconds when exposed to air, which makes the metal highly chemical and corrosion resistant. This is often claimed to be the reason for the good biocompatible properties of titanium. The native oxide is 2-6 nm thick and consists mainly of TiO2. C.p. Titanium exists in two different crystal structures; hexagonal α-structure and a cubic β-structure. The former is prevalent in room temperature while the latter is formed by heating to 883˚C or more. To maintain the β-structure at room temperature, alloy elements are needed. Titanium alloys may contain both α- and β-structure, such as Ti-6Al-4V (titanium grade 5), which has good mechanical properties as well as adequate toughness and good corrosion resistance14,15. It has been reported that titanium
alloys present similar biological response as to c.p. titanium16. In
contrast, previous in vivo studies have demonstrated c.p. titanium to have significantly increased bone response as compared to titanium alloy samples17-20. However, human studies on titanium grade 5
implants (Ti-6Al-4V) have shown good clinical results, indicating good biocompatibility21,22. Implants manufactured from zirconia
ceramics have demonstrated to be biocompatible, showing similar biological response as titanium and to have mechanical properties that make them suitable as materials for dental implants23,24. Most
commercial dental implants available today are still manufactured from c.p. titanium grade 4.
It was suggested that the inferior bone response to the titanium alloy was due to leakage of aluminum ions. Ion release from different implant surfaces have previously been reported in the literature, however, not in toxic concentrations25,26. For example, Wennerberg
et al.27 investigated if there was a correlation between surface
roughness and ion release in vitro but found no such differences between the smoothest and roughest surface. However, in vivo, increased amounts of titanium were observed in the peri-implant bone after 1 year of implantation compared to after 12 weeks. Further, ion leakage from nanosized hydroxyapatite crystal (nano-HA) coated surface have been reported when inserted in rat. The released ions were shown to be eliminated from the body via blood and liver and the authors concluded that the nano-HA coating had a low probability to be a biological risk factor28.
Surface properties have been shown to be of importance to alter bone responses and establish adequate osseointegration. Since the tissue mainly interacts with the outermost anatomic layers of the implant surface29, the surface may be of greater importance than
the bulk material characteristics. There are many different ways of modifying the implant surface and thereby changing the surface topography and/or chemistry. Commonly, it is difficult to change one surface characteristic without affecting the others.
Some techniques, for example; plasma spraying, coatings and ion deposition, will add material to the implant surface. In contrast, there are techniques that will remove material from the surface e.g.; blasting, etching and oxidation. Surface modification is further created by a combination of different techniques. To be able to properly evaluate the effect of the surface properties in bone healing, the surfaces must be characterized in a standardized manner. Topography as well as chemistry must be described.
Surface topography is scale dependent; what may be considered a rough surface on a container ship is certainly not equal to what is considered rough on a dental implant. Overall, surface roughness is measurable on the mm, µm and nm length scales. Even picometer roughness may exist, however, its possible importance for implant take being beyond our present knowledge. For dental implants, mm roughness represents the design of the implant (i.e. overall shape and thread design); µm surface roughness is known to influence osseointegration whereas the precise importance of the nanometer roughness remains to be investigated.
Different implant designs have been tested in the treatment of edentulous patients. Threaded implants have proven superior to cylindrical designs in long-term studies30,31, due to improved load
distribution32. Therefore, threaded screw shaped implants dominate
the commercial market today. Different thread designs and implants with micro-threads with the aim to preserve the marginal bone level are commercially available33.
As mentioned, micrometer roughness is well-known and several in vitro, in vivo as well as human studies have shown that surface roughness have an impact on osseointegration and biomechanical fixation9,11,34-36. However, changes made on surface roughness at
the micrometer level may simultaneously, if accidentally, result in changes at the nanometer level; hence it may be difficult to separate the importance of micro- and nanoroughness for implant integration.
microroughness
On the micrometer level of resolution, dental implants may be divided into groups by the average surface roughness (S)a; smooth (Sa <0.5µm), minimally rough (Sa 0.5<1µm), moderately rough (Sa 1-2µm) and rough (Sa >2µm), as suggested by Wennerberg and Albrekstsson 200437. Implants with an isotropic, i.e. one without
dominating direction of the surface irregularities, an Sa value of approximately 1.5µm and an increased surface area enlargement (Sdr) of approximately 50% have shown stronger bone integration than smoother or rougher implants38.
Methods to alter microtopography
Turning. The original Brånemark (Nobel Biocare) implant was a turned titanium screw with no further surface treatment37. It had
a minimally rough surface and was for a long time the most used implant with good long-term clinical results39,40. Today most of the
commercial implants available are minimally to moderately rough41.
Grit-blasting with various hard ceramic particles such as alumina (AlO3), titanium oxide (TiO2), silica or calcium phosphate is one way of roughening the implant surface. The size of the blasting particles determines the roughness created and the blasting particles should be chemically stable and biocompatible. Several in vivo studies have shown significantly improved bone-to-implant contact (BIC) for TiO2 blasted implants as compared to machined ones34,35,42
and clinical studies have demonstrated good results with implants in function 5 and 10 years after implantation43-47. Figure 1a shows
a SEM image of a TiO2 blasted surface (TiOblast™, Astra Tech AB, Sweden). Blasting with calcium phosphates, such as hydroxyapatite and beta-tricalciumphosphates have demonstrated higher BIC values when compared to machined surfaces in some in vivo studies48,49.
a) TiOblast™ b) Osseotite® c) TiUnite™
Figure 1. SEM images in x3000 magnification of common surface modifications.
Acid-etching of a surface with strong acids such as HCl, H2SO4, HNO3 and HF creates an isotropic surface that may enhance osseointegration in vivo50,51. The Osseotite surface (3i Biomet, USA) is produced with
a mixture of HCl and H2SO4 which creates a dual-acid etched surface when heated (Figure 1b). High clinical survival rates >97%, 3 years after implantation have been reported52 for this surface. Etching with
hydrofluoric (HF) acid roughens the surface and in addition changes the surface chemical composition by fluoride incorporation, which, in vivo, has been demonstrated favourable for the osseointegration8,53.
The effect of surface modification with fluoride ions is suggested to be increased proliferation and differentiation of mesenchymal cells54.
Osseospeed™ (Astra Tech AB, Sweden) and SLA (Straumann,
Swizerland) are examples of implants manufactured by a combination of blasting and etching procedures. (Figure 2a and b shows SEM images of the two surfaces). Both implants with good clinical results reported in the literature55-57. Hypothetically the blasting procedure creates a
surface roughness for good mechanical fixation and the additional etching smoothens out sharp peaks. Commonly, this combination of techniques creates a moderately rough surface.
a) b)
Figure 2. SEM images in x3000 magnification of the Osseospeed™ surface (a),
which is created by blasting followed by HF etching. Figure b) shows the SLA® surface modified by sand-blasting and etching with H2SO4/HCL.
Anodization produces a micro- and or nano-porous oxide layer on the titanium surface. The appearance of the layer from the anodization process depends on current density, concentration and composition of the acids used in the electrolyte solution and the temperature. In vivo studies have demonstrated increased bone response with higher biomechanical and histological values for anodized surfaces compared to machined ones58,59. In a study by
Jungner et al (2005)60, higher clinical success rates were reported
for the oxidized TiUnite™ surface (Nobel Biocare, Sweden) when compared to a turned titanium implant (Mark III, Nobel Biocare, Sweden). A 5 year follow up study demonstrated good clinical results (success rate >97%) in soft bone quality despite immediate loading61. Mechanical interlocking due to bone ingrowths in the
surface pores and biomechanical bonding have been suggested to be the reason for the increased performance of the anodized surface62,63.
Figure 1c shows a SEM image of the moderately rough TiUnite™ surface in a x3000 magnification.
To modify the surface chemical composition, ion incorporation with magnesium, calcium or phosphorus into the oxide layer has been reported as well64. Calcium incorporation to an anodized
surface increased biomechanical fixation in vivo65. A minimally
rough anodized surface with calcium incorporation is manufactured by Ospol AB (Malmö, Sweden).
Plasma spraying is a method where particles, hydroxyapatite (HA) or titanium are projected on the surface through a plasma torch at very high temperature. The particles condense and fuse together on the surface thereby creating a coat. Titanium plasma spraying has displayed better bone integration in vivo as compared to smoother implants66. However, clinical studies have been unable to confirm
these results and have shown disadvantages with increased marginal bone resorption67,68.
Previous in vitro and in vivo studies on HA plasma sprayed titanium implants have demonstrated stronger initial bone response compared with conventional titanium implants. Further, studies comparing implants with a nanothick calcium phosphate coating and plasma sprayed calcium phosphate coated (PSCaP) implants in vivo, demonstrated significant increased early bone response and torque values to the PSCaP surface69,70.
32
contact (BIC) for TiO2 blasted implants as compared to machined
ones34,35,42
and clinical studies have demonstrated good results with implants in function 5 and 10 years after implantation43-47
. Figure 1a shows a SEM image of a TiO2 blasted surface (TiOblast™,
Astra Tech AB, Sweden). Blasting with calcium phosphates, such as hydroxyapatite and beta-tricalciumphosphates have demonstrated higher BIC values when compared to machined surfaces in some in vivo studies48,49
.
a) TiOblast™ b) Osseotite® c) TiUnite™
Figure 1. SEM images in x3000 magnification of common surface modifications.
Acid-etching of a surface with strong acids such as HCl, H2SO4, HNO3 and HF creates an isotropic surface that may
enhance osseointegration in vivo50,51
. The Osseotite®
surface (3i Biomet, USA) is produced with a mixture of HCl and H2SO4 which
creates a dual-acid etched surface when heated (Figure 1b). High clinical survival rates >97%, 3 years after implantation have been reported52
for this surface. Etching with hydrofluoric (HF) acid roughens the surface and in addition changes the surface chemical composition by fluoride incorporation, which has, in vivo, been demonstrated favourable for the osseointegration8,53
. The effect of surface modification with fluoride ions is suggested to be increased proliferation and differentiation of mesenchymal cells54
. Osseospeed™ (Astra Tech AB, Sweden) and SLA®
(Straumann, Swizerland) are examples of implants manufactured by a combination of blasting and etching procedures. (Figure 2a and b shows SEM images of the two surfaces). Both implants with good clinical results reported in the literature55-57
. Hypothetically the blasting procedure creates a surface roughness for good mechanical fixation and the additional etching smoothens out sharp peaks.
However, long-term results of plasma sprayed implants have been unfavourable and associated with failure. Plasma spraying with HA particles creates a 50-200µm thick coat but with poor adhesion to the bulk material and this is believed to be the reason for the long-term negative clinical results of such implants71. However, it was
never clarified whether the positive initial bone response was due to the proposed bioactivity of HA, to possible differences in surface topography when comparing coated with non-coated implants; the Ra value for HA coated implants were at the time reported to be 1.6µm or more while a machined titanium implant is in the range of 0.5-1µm72. Furthermore, that the thicker HA-coated implants
have a greater press fit when inserted in the same size defects as the controls, which may give the former a better initial stability.
Calcium phosphate coatings (CaP). Different methods are available to coat implant surfaces with calcium phosphates; for example, plasma spraying, sol-gel coating, sputter deposition, electrophoretic deposition, biomimetic precipitation etc. The Ca/P ratios may differ in these coatings, phases present may include tricalcium phosphate, tetracalcium phosphate, octacalcium phosphate and hydroxyapatite (HA). The CaP ratio determines the degradation rate of the CaP. Pure crystalline HA is known to be the most stable and strongest among these phases. It has been demonstrated that the biological response in vitro and in vivo may differ towards differently composed CaPs; Osteoblast adhesion was evaluated towards CaP coatings with different CaP ratios (samples with CaP ratios between 0.5 and 2.5) and results demonstrated increased osteoblast adhesion with the greater CaP ratios (up to 2.5). Changes in chemistry of the different CaP ratios may have altered the osteoblast adhesion, since many proteins for osteoblast adhesion (e.g.vitronectin) have calcium binding sites which may have influenced the initial protein adsorption. Furthermore, different topography .e.g. grain and pore size) in the nanometer range between the CaP ratios may have altered the surface wettability, energy and nanometer roughness and thereby influenced the osteoblast response73. Yang et al74. compared
electrochemically deposited nano-hydroxyapatite (EDHA) and biomimetically deposited CaP (BDCaP) in vivo and found signi-ficantly higher bone in contact (BIC) values and bone area for
the EDHA after 6 and 12 weeks of healing. SEM characterisation showed that BDCaP crystals were flake like (~100nm thick and ~7μm in length), whereas the EDHA crystals were rod like with a hexagonal cross section (~70nm in diameter). The results were later confirmed by the same group75. Results as these suggest that the
apatite may be advantageous over other calcium phosphates. As mentioned previously, plasma spraying is one method to coat implants with HA, although with some disadvantages. New techniques have been developed to produce thinner CaP coatings, in the submicron range, to improve the properties and thus minimize potential problems with coat loosening.
nanoroughness and nanostructures
Surface micro topography including surface roughness of today’s available dental implants is well known, however, the characteristics of the implants on the nanometer scale of resolution are much less known. All surfaces possess nanoroughness, however not all of them have defined nanostructures. Nanostructured materials are defined in the literature as materials containing structural elements with dimensions in the range of 1 to 100nm. Furthermore, nanocoatings are defined as single or multilayer coatings with a thickness in the range of 1 to 100nm76.
Numerous in vitro studies have reported altered cell responses to differently nanostructured surfaces, however, not many studies have focused on a fundamental understanding of these observations. Therefore, the exact biological role of nanostructures is unknown. One simplified explanation may be that nanostructures attempt to mimic the biological tissues and therefore act in the same arena as the natural tissues and its extra cellular matrix.
Bone is a specialized form of connective tissue, composed by approximately 67% inorganic material, mainly hydroxyapatite (Ca10(PO4)6(OH)2); 33% organic material such as collagen (type I,V,XII), non-collagenous proteins (e.g. osteopontin, osteocalcin, phosphoproteins, bone sialoproteins, proteoglycans, glycoproteins) and water. Bone tissue may be divided into three levels of structure; nanostructures including non-collageneous proteins, collagen and hydroxyapatite. Microstructures including cells, lamella and osteons
represent the second level. The third level including cancellous and cortical bone may be termed macrostructure. Cortical and cancellous bone is often distinguished by their degree of porosity or density, where the latter has increased porosity due to the being tissue is differently arranged with trabecula and marrow spaces77,78.
There are many ways of applying nanostructures on surfaces, for example; sol-gel coating, anodization, discrete particle deposition, ion beam deposition, alkali treatment (NaOH), lithography and printing technique. Some techniques will be described further below;
Some methods to alter nanotopography
Sol-gel coatings. Sols are dispersions of colloidal particles (with diameters of 1-100nm) in a liquid. A gel is an interconnected, rigid network with pores of submicrometer dimensions. During the Sol-Gel process, a liquid with a specific composition (i.e. the Sol) is converted into a solid gel phase. The particle size and aggregation is altered by the aging time, hours or days, of the Sol. Thin coatings can be deposited onto a surface by dip- or spin coating techniques. Drying removes the remaining liquid and the sample is finally heat-treated to sinter the coating. The coat thickness can be altered by the number of layers added to the surface sample. The procedure makes it possible to produce coatings of titanium, hydroxyapatite or combination of both79. In vitro studies have shown increased
osteoblast adhesion and differentiation to Sol-gel coated HA titanium surfaces with a coat thickness of ≤ 1μm compared to controls80,81.
Discrete calcium deposition (DCD) makes it possible to deposit nanometer sized calcium phosphate (CaP) crystals onto a surface. This is achieved by applying a suspension of CaP crystals in the size range of 20-100nm onto the implant surface. This can be achieved using dip coating, where the implant is immersed into the suspension followed by withdrawal as a certain speed, using spin coating, where the implant is spun during or after deposition of the dispersion or by the use of spraying techniques. After the deposition step the implant is subjected to a drying procedure and optionally heat treatments. The result is a non-confluent layer of CaP crystals, with an approximately 50% coverage of the implant surface and the remaining surface being the bulk material82. The NanoTite™ (3i,
Implant innovations, USA) is a commercially available implant with a surface produced by DAE and DCD83.
Nanocrystalline hydroxyapatite coatings may be produced by molecular self-assembly synthesis. Nanoparticles of hydroxyapatite is prepared by mixing H3PO3 and Ca(NO3) to a Ca/P ratio of 1.67 in the presence of a liquid crystalline phase. The crystalline phase limits particle growth to ~5nm. When HA particles have formed, the liquid crystalline phase is dissolved and the particles can be deposited onto a surface using DCD. The sample is dried in room temperature followed by heating in nitrogen atmosphere at 550˚C for 5 minutes to eliminate surfactants on the surface and the procedure results in a nanocrystalline HA coat (nano-HA). The thickness of the coat may be altered by adding more layers through multiple dipping of the sample84. Nanocrystalline HA may be advantageous since it resembles
the nanostructure of the inorganic material in bone. Results reported by Göransson et al.85 demonstrated that surfaces coated with
nano-HA, induced earlier CaP formation and in addition increased bone cell response compared to a blasted control surface in vitro. An in vivo study in rabbit tibia have demonstrated significantly increased bone formation to electro-polished cylindrical titanium implants coated with the technique described when compared to uncoated controls86.
TiO2-nanotubes are manufactured by electrochemical anodization. Oh et al.87 fabricated TiO
2 nanotubes by anodization in 0.5%
hydrofluoric acid solution at 20V for 30 minutes in room temperature followed by annealing at ~550˚C. This method produces anatase phase nanotubes with an outer diameter of ~100nm, inner diameter of ~80nm and a height of ~250nm. Increased osteoblast adhesion on the TiO2 nanotube surface compared to non-modified TiO2 was reported in that study87. Increased human osteoblast adhesion,
proliferation and differentiation were found on a surface with TiO2 nanotube morphology compared to a polished titanium control. The nanotube surface was more hydrophilic and had increased surface roughness compared to control surface88. Significantly increased
bone bonding strength to TiO2 nanotubes have beee demonstrated after 4 weeks of implantation in rabbit tibia and histological results
28
whether the increased bone response is due to the surface attached fluoride ions, the different surface topography or maybe a combination of both. Figure 3a and b shows SEM images of the two surfaces, with nanostructures present on the Osseospeed™ surface.
a) Osseospeed™ c) SLActive®
b) TioBlast™ d) SLA®
Figure 3a-d. Shows SEM images of the Osseospeed™(a) and SLActive®
surface (c) and their predecessors TioBlast™ and SLA® in figure b) and d) respectively.
The SLActive® implant has displayed strong bone response in vivo
compared to its predecessor SLA® and this was explained by
SLActive® having a more hydrophilic surface. However, surface
roughness differs between the two implants both on micro as well as nanometer levels of resolution41
Certainly, some techniques, such as etching, performed in an attempt to alter micro roughness may simultaneously affect roughness on the nanometer level of resolution.
whether the increased bone response is due to the surface attached fluoride ions, the different surface topography or maybe a combination of both. Figure 3a and b shows SEM images of the two surfaces, with nanostructures present on the Osseospeed™ surface.
a) Osseospeed™ c) SLActive®
b) TioBlast™ d) SLA®
Figure 3a-d. Shows SEM images of the Osseospeed™(a) and SLActive®
surface (c) and their predecessors TioBlast™ and SLA® in figure b) and d) respectively.
The SLActive®
implant has displayed strong bone response in vivo
compared to its predecessor SLA®
and this was explained by SLActive®
having a more hydrophilic surface. However, surface roughness differs between the two implants both on micro as well as nanometer levels of resolution41
Certainly, some techniques, such as etching, performed in an attempt to alter micro roughness may simultaneously affect roughness on the nanometer level of resolution.
showed greater BIC values, new bone formation and calcium and phosphorus levels on the nanotube surface89.
As previously mentioned, it is often difficult to change one surface characteristic, without affecting others. The Osseospeed™ surface has been demonstrated to promote the bone response compared to its predecessor TioBlast™. However, it is not known whether the increased bone response is due to the surface attached fluoride ions, the different surface topography or maybe a combination of both. Figure 3a and b shows SEM images of the two surfaces, with nanostructures present on the Osseospeed™ surface.
b) TioBlast™ d) SLA®
Figure 3a-d. Shows SEM images of the Osseospeed™(a) and SLActive® surface
(c) and their predecessors TioBlast™ and SLA® in figure b) and d) respectively.
The SLActive® implant has displayed strong bone response in
vivo compared to its predecessor SLA® and this was explained by
SLActive® having a more hydrophilic surface. However, surface
roughness differs between the two implants both on micro as well as nanometer levels of resolution41(figure 3b and d).
Certainly, some techniques, such as etching, performed in an attempt to alter micro roughness may simultaneously affect roughness on the nanometer level of resolution.
Nanostructures and biological response
Water, ions and subsequently proteins will adsorb onto the implant surface within milliseconds after insertion, the latter further interacting with cell-membrane receptors. It is suggested that nanomaterials affect protein interactions due to the unique surface properties, such as; increased surface area, surface roughness, altered electron distributions etc. Increased surface area will provide more available sites for protein adsorption and thus affect the amount of cellular interactions90. For example; Scopelliti and
coworkers91 found that an increase in nanometer roughness, from
15 to 30nm, resulted in a significant increase in protein adsorption on nanostructured titanium devices. The authors suggested that proteins get trapped inside nanometer sized pores and thereby promoted protein aggregation. In vitro results reported by Liu et al.92
demonstrated enhanced osteoblast adhesion, alkaline phosphatase activity, and calcium-containing mineral deposition to nanophase titania/PLGA composites that had surface roughness values closer to that of natural bone. Webster et al.93 found enhanced osteoblast
adhesion with increased nanoroughness. In the same study it was observed that greater concentrations of vitronectin was adsorbed onto the nanostructured surfaces while greater concentrations of laminin was found on the control surfaces. Unfold vitronectin may promote the availability of RGD (Arginine-Glycine-Aspartic acid) sequences that could enhance osteoblast adhesion94. Increased
wettability or hydrophilicity has also been associated with increased protein adsorption and subsequently enhanced cell adhesion on biomaterials95. De Oliveira and Nanci96 found that nanotexturing
of titanium-based surfaces up-regulates the early expression of bone sialoprotein (BSP) and osteopontin (OPN). The expression of BSP is generally believed to initiate newly differentiated osteoblasts that correspond with initial bone mineralization97. OPN is involved in cell
adhesion, migration, and in the regulation of mineral deposition98.
Webster et al.93 demonstrated significantly greater osteoblast
compared to conventional formulations of the same ceramics after 4h of culturing. This study further revealed that fibroblast adhesion was significantly lower to the nanostructured surfaces which was explained by significantly increased adsorption of vitronectin on the nanostructured surfaces, while increased concentrations of laminin was found on the controls as mentioned previously. Further studies by Webster and co-workers99 demonstrated significantly greater
osteoblast proliferation, more synthesis of alkaline phosphatase and deposition of calcium-containing minerals by osteoblasts cultured on nanophase ceramics compared to conventional ceramics after 21 and 28 days. Increased osteoblast adhesion and significantly more calcium and phosphorus deposition have been reported to metals with nanotopography (Ti, Ti6A14V, CoCrMo) compared to conventional metals100,101. De Oliveira et al.102 reported enhanced osteogenesis on
titanium discs with chemically produced nanotopography. Increased osteoblast response on nanometer carbon fibres have been reported in the literature103,104. Sato and co-workers105 found significantly
greater osteoblast adhesion on nano-titanium and nano-HA coatings compared to uncoated controls. Puckett et al.106 investigated bacteria
adhesion towards different nanostructured surfaces and found significant differences in bacteria adhesion, indicating that different surfaces may be more prone to microbial colonisation and that it may be possible to influence the risk of microbial colonisation by altering the surface topography and thereby decreasing the risk of complications of peri-implant infections.
Numerous in vitro studies have shown increased cell response to different nanostructured surfaces and it is clear that nanostructures affect the cell response in different ways. Further, several in vivo studies have investigated the importance of different nanometer sized structures on different kinds of implant design in early bone healing and some have confirmed the in vitro result by demonstrating positive biological effects. Bjursten et al.89 investigated different
surfaces, TiO2 gritblasted and TiO2 nanotube surfaces. Discs were implanted in rabbit tibia, and the results showed significantly improved bone bonding strength, increased bone to implant contact (BIC) and new bone formation to TiO2 nanotubes compared with the TiO2 gritblasted surface. Dual acid etched, rectangular titanium
plates were modified with discrete crystalline deposition (DCD) of CaP nanocrystals and implanted in rat femur. Results from this study demonstrated the modified surface to have significantly higher disruption forces than non-DCD samples82. Few human studies
with nanostructured surfaces have been performed. Dual acid etched (DAE) micro-implants with and without nanometer-scale CaP coating were placed in the posterior maxilla. Results from this study revealed significantly higher bone to implant contact (BIC) for the test implant compared with the control after 4 and 8 weeks of healing107. SEM was the only method for surface characterization.
Similar results were reported in a study by Orsini et al.108 for
the same type of implant surface. Telleman et al.83 inserted mini-
implants in grafted bone with one part of the implant extended into native maxillary bone. The authors confirmed previously reported results by concluding that the CaP coated DAE surface increased the healing properties in the native bone while the difference was not seen in the bone graft area after 3 months of healing, when compared to the DAE surface alone. The surface roughness was described on the micrometer level of resolution and further with SEM. A human study investigating soft-tissue adhesion and marginal bone response, found significant increase in soft-tissue adaptation to nanoporous TiO2 after 14 weeks of healing compared to the control implant, possibly due to reduced marginal bone resorption109. These results
indicate that nanostructures may also be advantageous in peri-implant soft tissue healing.
Results like these suggests that nanostructures may be clinically advantageous for shortening healing times, allowing earlier loading of dental implants and presenting increasing treatment success in patients with compromised bone.
New possibilities to evaluate cell response
to nanostructures
Whether or not nanostructures, of any kind, on the implant surface are clinically relevant is not yet known, even if a few studies, previously mentioned, may suggest so. Furthermore, the mechanism behind the increased cell response to nanostructured surfaces is not fully understood. Increased understanding of the initial interaction between cells and implant surface material is of interest
to further map the mechanism behind the varying cell and tissue response towards different surface modifications, at the micro- as well as nanometer levels of resolution. Increased understanding of the initial cell response would be of great value to further develop more optimal implant surfaces. However, there has been a lack of suitable methods to investigate and monitor real-time initial cell-surface interactions. The most commonly used in vitro technique to evaluate cell response including attachment, spreading, calcium and phosphorus deposition etc. towards different surfaces is cell culturing.
Two promising methods to in real-time evaluate implant surfaces in vitro, are optical trapping (OT) and quartz crystal micro balance with dissipation monitoring (QCM-D). The optical trap or optical tweezers was discovered in the early 1970s110,111 and has since then
been used for manipulating single cells as well as investigating biophysical/biomechanical systems. The traps can be used in different applications such as transporting cells to specific locations, cell sorting, transporting foreign materials into cells as well as studying cell interactions and cell adhesion112-118. The advantages of
using the optical trap include non-contact force for manipulation of cells and the possibility of measuring interaction forces in the piconewton range119. The QCM-D has become a popular tool to
study bimolecular adsorption onto different surfaces. The technique enables in situ measuring without invasive labelling and is also capable of monitoring the viscoelastic properties of the adhered layer. The method has previously been used to study protein absorption and only a few studies have aimed at studying cell-surface interactions120-125.
summary and relevance of the present thesis
Nanostructures have been suggested to be important in the osseo-integration process since it is on the nanometer level that ions and proteins attach to the surface which may govern the following cell response and thereby the early bone healing around the implant. However, our knowledge of the exact mechanisms behind demonstrated in vitro and in vivo results and to what extent nano-structures influence the early bone healing and subsequently the osseointegration process is still very limited. Two novel promising
methods were evaluated in this thesis to investigate the possibility to detect differences in the initial cell adhesion towards different surfaces.
There are a number of ways to alter implant surface properties, such as chemistry and topography, but as mentioned, it is difficult to change one without affecting the other. Hence, it is hard to evaluate and interpret from what precise characteristics the altered biological response is influenced. To evaluate the effect of nanostructures in absence of micro topography, cylindrical implants with applied nanostructures with different properties were investigated in this thesis.
Processes aiming at modifying the surface at the micrometer level, will most certainly affect the topography at the nanometer level. Therefore, to fully understand the relationship between the properties of an implant surface and its osseointegration behaviour, a characterization at the nanometer level of resolution is relevant. The first study in this thesis aimed at characterizing nanometer topography on screw-shaped dental implants, with different surface modifications.
Calcium phosphate coatings have shown increased biological responses and some studies have suggested that the hydroxyapatite may be more advantageous than other calcium phosphates. Blasted and acid etched screw-shaped implants have previously demonstrated good biocompatibility and clinical results with high success rates. The possibility of nanometer coatings to further promote bone healing when applied on a blasted and etched screw-shaped implant has not been investigated. Therefore, in vivo studies in this thesis aimed at investigating if additional nanostructures on micrometer rough implants may improve the biological response in bone of different qualities.
aims
Surface characterization;
• To describe a technique to characterize commercial oral implants on the nanometer level and to investigate whether or not the nanometer surface roughness was correlated to the more well-known micrometer roughness on the implants. Real-time in vitro;
• To study the real-time initial cellular interactions of human osteoblasts and fibroblasts to different implant surfaces with and without a coat of nanocrystalline hydroxyapatite using optical trapping and QCM-D.
In vivo bone response;
• To evaluate the early bone response to a nanocrystalline hydroxyapatite coating compared to a nano titanium coating when deposited on cylindrical titanium implants without microstructures.
• To investigate whether nanometer thick coatings of hydroxyapatite nanocrystals applied on moderately rough surfaces may enhance early bone healing on screw-shaped implants and to evaluate if the thickness of the coat influences healing.
• To investigate whether a sub-micron coating of hydroxyapatite nanocrystals applied on a moderately rough surface may enhance early bone healing on screw-shaped implants in trabecular bone.
materials anD methODs
implants and components
Implant design and surface preparation
In study I, commercial dental implants of different brands with 12 different surface modifications were used. Rectangular cubes were manufactured from commercially pure (grade 4) titanium (Ti) for study II. Four samples of each surface and four different surface modifications were used. 1) Oxidized Ti, 2) Oxidized Ti coated with nanocrystalline HA (nano-HA), 3) Turned Ti and 4) Turned Ti coated with nano-HA. The nano-HA coating was performed using a technique described by Kjellin et al84. Cylindrical implants
sized 8 mm in length and 3.5 mm in diameter manufactured from commercially pure titanium (grade 3) were used in study III. Each implant had an upper threaded part fixed in a plate to stabilize the implant. The whole implant device was fixed in the bone through two side holes for fixating screws. 20 implants were divided in two groups; one group was coated with nano-HA. The other group was coated with nano-titania (provided by MetAlviveTM, Vivoxid, Turku,
Finland). For study IV and V screw shaped implants with a length of 8.5 mm and a diameter of 3.5 mm were manufactured from commercially pure titanium (grade 4). The surfaces were blasted with hydroxyapatite particles 6.5-9µm in size and then acid etched. The test surfaces in study IV were coated with two different thicknesses of nanocrystalline hydroxyapatite, deposited as described by Kjellin at al84 and in study V a modified technique was used, which resulted
Paper: I II III IV V 12 commercially available dental implants with different
surface modifications.
Turned Ti
Turned Ti + nano-HA
Oxidized Ti
Oxidized Ti + nano-HA
Cylindrical implant + nano-HA
Cylindrical implant + nano-titanium
Sandblasted/Acid etched surface
Sandblasted/Acid etched + nano-HA single coat
Sandblasted/Acid etched + nano-HA double coat
Sandblasted/Acid etched + submicron thick nano-HA coat Table 1. Titanium surface modifications used in the studies in this thesis.
methods for surface characterization
Atomic Force Microscope
With an Atomic force microscope (AFM) it is possible to characterize surfaces near the atomic level and therefore suitable to evaluate nanometer structures. The AFM consists of a cantilever with a sharp tip (probe) at its end that is used to scan the surface. The cantilever is typically silicon or silicon nitride with a tip radius of curvature on the order of nanometers. When the probe is close to the surface, forces between the probe and the surface lead to a deflection of the cantilever according to Hooke›s law. The AFM can be operated in several different modes, contact and non-contact modes, depending on the application. In a tapping mode, AFM images are produced by imaging the force of the intermittent contacts of the probe with the surface. The cantilever is driven to oscillate near its resonance frequency and the amplitude of this oscillation is approximately 100 to 200nm. Due to the interaction of forces acting on the cantilever when the probe comes close to the surface for example, Van der Waals force, dipole-dipole interaction and electrostatic forces, cause the amplitude of this oscillation to decrease as the probe gets closer to the surface. The tapping mode reduces the damage done to the surface and the probe compared to contact mode. The maximal lateral measuring area is 100x100µm and resolution about 100pm. Maximal vertical distance