ACTIVITIES OF SnS IN TIN - IRON MATTES
By
All rights r eserved INFORMATION TO ALL USERS
The quality of this r epr oduc t i on is d e p e n d e n t u p o n the quality of the c op y s ubmi t t ed. In the unlikely e v e n t that the a u t h o r did not send a c o m p l e t e manuscr i pt and there are missing p a g e s , these will be n o t e d . Also, if material had to be r e mo v e d ,
a n o t e will i ndi cat e the delet ion.
uest
Pr oQues t 10781808
Published by ProQuest LLO (2018). Copyri ght of the Dissertation is held by the Author. All rights reserved.
This work is pr ot ect ed a g a i n s t una ut hor i zed copying under Title 17, United States C o d e Microform Edition © ProQuest LLO.
ProQuest LLO.
789 East Eisenhower Parkway P.Q. Box 1346
A t h e s i s r e s p e c t f u l l y s u b m i t t e d t o t h e F a c u l t y a n d t h e B o a r d o f T r u s t e e s o f t h e C o l o r a d o S c h o o l o f M i n e s i n p a r t i a l f u l f i l l m e n t o f t h e r e q u i r e m e n t s f o r t h e d e g r e e o f M a s t e r o f S c i e n c e i n M e t a l l u r g i c a l E n g i n e e r i n g . G o l d e n , C o l o r a d o D a t e : , 1972 S i g n e d : J o f f r e J u a n G o l d e n , C o l o r a d o D a t e : ^ y I f . 19 72 A p p r o v e d : D r . A.W. S c h l e c h t e n T h e s i s A d v i s o r . A p p r o v e d : fie a d , D e p a r t m e n t / o f M e t a l l u r g i c a l E n g i n e e r i n g .
ACTIVITIES OF SnS Iil TIN - IRON MATTES.
By
Juan E. J o f f r e ,
A B S T R A C T
The vapor p r e s s u r e of SnS a()Ove pure S n S ( c , l ) , and above t i n i r o n ma t t e s was det er mi ne d by means of t he t r a n s n o r t a t i o n method.
I t was confi rmed t h a t - a n a r t from t he o t h e r well known f a c t o r s , such as a uni for m t e mp e r a t u r e in t he r e a c t i o n z one, and a uni form f l o w r a t e - t he i n f l u e n c e of t he geomet ry of t h e r e a c t i o n chamber i s ver y i mp o r t a n t f o r a c c u r a t e d e t e r m i n a t i o n s us i nn t h i s method.
The c a r r i e r nas u t i l i z e d f o r t h e s e d e t e r m i n a t i o n s on t i n and i r o n s u l f i d e s , s houl d be a bl e t o c o n t r o l t he s u l f u r p o t e n t i a l t o n r e v e n t e r r o r s due t o d ec o mpo s i t i o n a n d / o r s e n r e n a t i o n .
The r e s u l t s o b t a i n e d f o r SnS vapor above nure S n S ( c , l ) anree ver y wel l wi t h t he most r e l i a b l e d a t a in t he l i t e r a t u r e . There a r e no a v a i l a b l e da t a on t h e t i n - i r o n m a t t e s .
I t was found t h a t t he SnS-FeS l i q u i d s o l u t i o n behaves a l mos t r e g u l a r l y , and can be r e p r e s e n t e d by:
and,
Thermodynamic r e l a t i o n s were d e r i v e d from t h i s r e n u l a r s o l u t i o n model and a p r a c t i c a l a p p l i c a t i o n t o an SnS - f u m i m o p e r a t i o n i s p r e s e n t e d .
C O N T E N T S
Chapt er no. Page no
A b s t r a c t i i
L i s t o f f i g u r e s ... v i i
L i s t o f t a b l e s ... ix
I I n t r o d u c t i o n ... 1
Tin s me l t i n g p r a c t i c e ... 1
D i s t r i b u t i o n of t i n and i r o n between metal and s l a g ... 2 Recovery o f t i n from s l a g s ... 5. Purpose o f t h i s i n v e s t i g a t i o n ... 6 II L i t e r a t u r e s ur vey ... 7 T i n - s u l f u r system ... 7 I r o n - s u l f u r system ... 13 I r o n - t i n - s u l f u r syst em... ... 15 Ir on s u l f i d e - t i n s u l f i d e syst em ... 16 Hy d r o g e n - s u l f u r system ... 17
I I I The fuming o f t i n from s l a g s and ma t t e s ... 19
D e s c r i p t i o n of t he p r o c e s s ... 19
Matt e f o r ma t i o n ... 23
Thermodynamic e v a l u a t i o n s ... 24
IV Experi ment al a p p a r a t u s and pr o c e du r e ... 34
Choice o f method ... 34 Appar at us ... 35 Experi ment al ... 41 1. M a t e r i a l s , s a mp l e s , and ma t t e p r e p a r a t i o n . 41 2. S u l f u r p o t e n t i a l c o n t r o l ... 43 3. Flowmeter c a l i b r a t i o n ... 47 4. Temperat ure c o n t r o l ... 50 5. Op e r a t i n g p r oc e d u r e ... 52 V Experi ment al r e s u l t s ... 55
Cont ent s - Continued
Chapt er no. Page no,
VI Di s c u s s i on ... 69 1, The t r a n s n o r t a t i o n method ... 69 2, I n t e r p r e t a t i o n of r e s u l t s ... 74 2. 1 Vaoor n r e s s u r e of SnS ov e r nure S n S ( c , l ) ... 74 2 . 2 Vanor p r e s s u r e o f SnS over t i n - i r o n ma t t e s ... 84 3, Thermodynamic c o n s i d e r a t i o n s ... 90 3. 1 " ur e S n S ( c , l ) ... 90 3 . 2 Li qui d t i n - i r o n ma t t e s ... 92 3. 3 Thermodynamic r e l a t i o n s d e r i v e d from t h e r e g u l a r s o l u t i o n model ... 95
3 . 4 C a l c u l a t i o n of t he FeS-SnS phase diagram from vanor n r e s s u r e measurements ... 102
3. 5 The s u h - r e g u l a r s o l u t i o n model a p p l i e d t o t he SnS-FeS system ...•... 106
3. 6 P o s s i b i l i t y of Comnlex f o r ma t i o n ... 110
VII P r a c t i c a l a p p l i c a t i o n ... I l l VIII Concl usi ons ... 119
IX Su g n e s t i o n s f o r f u r t h e r work ... 122 Acknowledgements ... 123 Ref er enc es ... 124 Apnendix I C a l c u l a t i o n s o f vanor n r e s s u r e s in t h e svst em Sn-S . . . . 128 Appendix II C a l c u l a t i o n of tlie SnS-FeS Phase diagram ... 136
Appendix I I I f l at t e a n a l y s i s r e n o r t ... 140
Appendix IV S u l f u r p o t e n t i a l c o n t r o l ... 142
Cont e nt s - Continued
Chapt er no. Page no,
Appendix V
Experi ment al d a t a f o r t h e vapor p r e s s u r e d e t e r m i n a
t i o n s on pure S n S ( c , l ) , and on Sn-Fe ma t t e s ... 146 Appendix VI
T i n - i r o n m a t t e s . Co r r e c t e d e q u a t i o n s f o r t h e SnS
vapor p r e s s u r e s ... 166 Appendix VII
Program SUBREG. The Gauss i an a l g o r i t h m t o c a l c u l a t e
n s i mu l t a n e o u s l i n e a r e q u a t i o n s ... 172 Appendix VIII
Numerical r e s u l t s o f Log Pg^g as a f u n c t i o n o f Sn
c o n t e n t in Sn-Fe m a t t e s , and t e m p e r a t u r e ... 175 Development of a s i mpl e computer program t o
c a l c u l a t e t h e t h e o r e t i c a l volume o f c a r r i e r gas and o i l r e q u i r e d t o fume SnS from t i n - i r o n m a t t e s ... 181
L I S T O F F I G U R E S Fi g. no. Page no 1 2 3 4 5 6 7 8 9 10 10a 11 12 13 14 15 16 17 18 19 20 21 22 23 Tin s me l t i n g f l o w s h e e t ... S h a f t f u r n a c e f o r s l a g fuming ... Vapor p r e s s u r e s in system Sn-S (1000 - 1100°K).... ... Vapor p r e s s u r e s in system Sn-S (1400 - 1500°K).... ... E f f e c t i v e t o t a l vapor p r e s s u r e of t i n in t he system Sn-S as a f u n c t i o n o f s u l f u r p r e s s u r e ... Tot al and p a r t i a l p r e s s u r e s above S n S ( c , l ) ... SnS-FeS phase diagram ... Vapor p r e s s u r e of SnS(g) above i r o n - t i n ma t t e s
( E s t i ma t e d ) ... Experi ment al s e t up ... Phot ogr aph of t he e x pe r i me n t a l a p p a r a t u s ... Furnace used in t he SnS vapor p r e s s u r e d e t e r m i n a t i o n s S a t u r a t i o n ( Re a c t i o n ) system ... SnS-FeS syst em, pj^ g/ p^ r a t i o ve r s us t e mp e r a t u r e f o r t h e SnS and FeS d i s s o c i a t i o n r e a c t i o n s ... C o r r e l a t i o n between r e a c t i o n chamber and f u r n a c e
t e mp e r a t u r e s ... Weight of sample l o s t pe r l i t e r of c a r r i e r gas as a f u n c t i o n of f l o w r a t e (Pure SnS and m a t t e s ) . The a p p a r e n t vapor p r e s s u r e p l o t t e d as a f u n c t i o n of t h e i n e r t gas f l o w r a t e . Schemat i c ... Log Pg^g ve r s u s 1/T p l o t . Sample: Pure SnS( c) .
C a r r i e r gas: Ng . Rea c t i on chamber: no. 1 ... Col i n and Dr o wa r t ' s p l o t of Log Pg g v e r s u s 1/T
4 22 25 26 27 28 31 32 36 38 38 40 46 51 58 59 60 61 62 63 64 70 75 76
L i s t o f f i g u r e s - Cont i nued
Fi g. no. Page no.
24 Apparat us used by St . C l a i r and c o l l a b o r a t o r s
f o r SnS vapor p r e s s u r e d e t e r m i n a t i o n s ... 79 25 I n f l u e n c e of t he geomet ry of t he r e a c t i o n chamber
and of t h e n a t u r e of c a r r i e r gas on t h e vapor p r e s s u r e measurements of pure S n S ( c , l ) by means of
t h e t r a n s p o r t a t i o n method ... 82 26 Vapor p r e s s u r e of SnS(g) above i r o n - t i n m a t t e s . Simple l e a s t - s q u a r e s f i t l i n e s ... 85 27 Alpha as a f u n c t i o n o f t e mp e r a t u r e ... 87 28 Vapor p r e s s u r e of SnS(g) above i r o n - t i n m a t t e s . Co r r e c t e d l i n e s ... 89 29 T(Log#^^g) ve r s u s p l o t ... 93 30 dC-Funct i on f o r SnS-FeS s o l u t i o n s , and s ymmet ri cal
l i n e o f Logi*^. a t lOOO^C as a f u n c t i o n of Ng^g ... 94 31 Excess and mixing f u n c t i o n s f o r t he f o r ma t i o n of
one mole o f l i q u i d SnS-FeS ma t t e a t v a r i o u s t e mp e r a t u r e s 98 32 A c t i v i t i e s in t he SnS-FeS system a t 80QO and IGOQOC . . . 99 33 The SnS-FeS phase diagram from vapor p r e s s u r e ... 105
measurements
34 Vapor p r e s s u r e o f SnS gas in t i n - i r o n ma t t e s as a --- 112 35 f u n c t i o n of c ompos i t i on and t e mp e r a t u r e ... 113 36 T h e o r e t i c a l volume o f c a r r i e r gas r e q u i r e d t o fume SnS
from one t on o f Sn-Fe ma t t e ... 116 37 T h e o r e t i c a l volume o f o i l r e q u i r e d t o be b u r n t t o
L I S T O F T A B L E S
Tabl e no. Page no,
I Phase r e l a t i o n s in t he Sn-S system ... 7
I I Heats of f o r m a t i o n , f u s i o n , and s t a n d a r d e n t r o p i e s o f Sn-S s p e c i e s ... 8
I I I Sn-S syst em. St a nda r d f r e e e n e r g i e s f o r v a p o r i z a t i o n and r e l a t e d e q u i l i b r i a ... 13
IV Free e n e r g i e s of f o r ma t i o n of i r o n s u l f i d e s p e c i e s . . . . 14
V Thermodynamic p r o p e r t i e s of hydrogen s u l f i d e and s u l f u r gases ... 18
VI Chemical c ompos i t i on of s l a g s t r e a t e d a t t h e P o d o l s ' k s m e l t e r in Russi a ... 23
VII Pt-Pt/10%Rh T.C. vs. NBS Pt27-Pt/10%Rh T. C... 50
VIII Vapor p r e s s u r e of SnS(g) o ve r pure s o l i d SnS as a f u n c t i o n o f t e m p e r a t u r e . C a r r i e r gas : . R. ch. n o . l . 65 IX Vapor p r e s s u r e of SnS(g) ove r pure S n S ( c , l ) and over Sn-Fe l i q u i d ma t t e s as a f u n c t i o n , o f t e m p e r a t u r e . C a r r i e r gas: H2S/H2 m i x t u r e s . Reac. chamber no. 2 . . . . 66
X A l p h a - f u n c t i o n from e x p e r i me n t a l measurements o f SnS vapor p r e s s u r e ... 86
XI Exp r e s s i o ns f o r t h e vapor p r e s s u r e of SnS over pure l i q u i d SnS, and ove r Sn-Fe ma t t e s as a f u n c t i o n of t e mp e r a t u r e ... 88
XII Thermodynamic f u n c t i o n s f o r t h e SnS-FeS l i q u i d syst em . . 100
XI I I The SnS-FeS phase di agram c a l c u l a t e d from vapor p r e s s u r e measurements ... 103
XIV Mal t i ng p o i n t s of ma t t e s ... 104
XV SnS-FeS syst em. S u b - r e g u l a r s o l u t i o n model ... 107
XVI SnS-FeS syst em. S u b - r e g u l a r s o l u t i o n model ... 109
L I S T O F T A B L E S I N A P P E N D I C E S
Tabl e no. Page, no
1.1 Vapor p r e s s u r e s in t h e syst em Sn-S ... 131 1. 2 E f f e c t i v e t o t a l p r e s s u r e in t he system Sn-S
as a f u n c t i o n of Pç ... 134
2
1. 3 Tot al and p a r t i a l p r e s s u r e s of vapor s p e c i e s
over pure S n S ( c ,1 ) 135
I I . 1 C a l c u l a t e d and e x p e r i me n t a l (Haan) v a l u e s f o r
t h e SnS-FeS phase diagram ... 138 I V . 1 SnS-HgS/Hg , and FeS-H2S/H2 i n t e r a c t i o n s .
S u l f u r p o t e n t i a l c o n t r o l ... 145 V . 1 Experi ment al d a t a f o r t h e vapor p r e s s u r e d e t e r
mi n a t i o n s over pure SnS( c ) .
C a r r i e r gas : N2 . Rea c t i on chamber: no. 1 146 V. 2 Experi ment al d a t a f o r t he vapor p r e s s u r e d e t e r
mi n a t i o n s on pure S n S ( c , l ) .
C a r r i e r gas : H2S/H2 m i x t u r e s . Reac t i on chamber: no. 2 148 V. 3 Experi ment al d a t a f o r t h e vapor p r e s s u r e d e t e r
mi n a t i o n s o f SnS over Sn-Fe m a t t e s .
C a r r i e r ga s : H2S/H2 m i x t u r e s . Rea c t i on chamber: no. 2 151 VI. 1 Co r r e c t e d v a l u e s of Log P g ^ g ... 167
I . I N T R O D U C T I O N .
Tin or es a r e i n v a r i a b l y a s s o c i a t e d wi t h i r o n m i n e r a l s . Con c e n t r a t i o n p r o c e s s e s i n ge n e r a l ne v e r l ead t o a c l e a n s e p a r a t i o n o f SnO^ ( C a s s i t e r i t e , t he most commercial t i n or e) from t h e i r o n mi n e r a l s (FegOg Fe^O^, FeSg, CuFe Sgpet c. ) . Removal of i r o n from c o n c e n t r a t e s p r i o r t o s mel t i n g by magnet i c s e p a r a t i o n i s n o t p o s s i b l e i n a l l cas e s becaus e c a s s i t e r i t e i s o f t e n f i r m l y a t t a c h e d t o , or c o a t e d wi t h i r o n o x i d e s .
Leachi ng t h e c o n c e n t r a t e s wi t h HCl d i s s o l v e s i r o n oxi des and amounts of As, Sb, B i , Rb, Cu a r e a l s o removed by t h i s pr ocess. But when t he c o n c e n t r a t e s c o n t a i n much s u l f u r and a r s e n i c as s u l f i d e s and a r s e n i d e s , t h e e f f e c t i v e n e s s o f l e a c h i n g i s r e d uc e d.
During s m e l t i n g s u l f u r may form FeS - CUgS - SnS mat t es' which r e n d e r d i f f i c u l t t he r e c o v e r y of t i n . Fu r t h e r mo r e , s u l f u r i n c r e a s e s t he l o s s of t i n by v o l a t i l i z a t i o n as SnS. Every 1%S may cause v o l a t i l i z a t i o n of 3.72%Sn.
T h e r e f o r e , t o p r e v e n t t he n e g a t i v e a c t i o n of s u l f u r and a r s e n i c d u r i n g s me l t i n g , t h e c o n c e n t r a t e s s houl d be r o a s t e d .
Tin Sme l t i ng P r a c t i c e
Fi gur e 1 g i v e s a ge n e r a l f l o w s h e e t o f t he t i n s m e l t i n g p r o c e s s , Tin c o n c e n t r a t e s - which may have been r o a s t e d a n d / o r l e a c he d - a r e mixed wi t h coke ( o r c o a l ) and l i me s t o n e and f e d t o a r e d u c t i o n f u r n a c e ( b l a s t ,
r e v e r b e r a t o r y , or r o t a r y ) .
B a s i c a l l y , t i n s m e l t i n g i s p r a c t i c e d in two s t a g e s :
a) Sme l t i ng of c o n c e n t r a t e s t o produce crude t i n ( hi gh grade t i n ) and a t i n -r i c h s l a g \/hich may c o n t a i n f-rom 10 t o 25 % Sn.
b) Sme l t i ng of t he t i n - r i c h s l a g under s t r o n g e r r e du c i n g c o n d i t i o n s t o p r o duce a t i n - i r o n a l l o y o r " har dhead" which c o n t a i n s about BOMSn and 20%Fe
( m i s c i b i l i t y gap compos i t i on) and a f i n a l si an t o d i s c a r d .
Thi s second si an i s f r e q u e n t l y n o t low enounh in t i n t o d i s c a r d and may be t r e a t e d agai n b e f o r e bei ng s e n t t o wa s t e .
The crude t i n from t he f i r s t s t a n e i s d r e s s e d t o produce pure metal and an i r o n - b e a r i n g d r o s s . Iron d r o s s e s and hardheads from t he second s t a n e a r e r e c y c l e d t o t he c o n c e n t r a t e s me l t i n g s t a n e where t h e i r i r o n c o n t e n t a c t s t o reduce t i n . All fumes ( r e c o v e r e d as SnOg) a r e a l s o r e c y c l e d t o t he f i r s t s t a ge.
D i s t r i b u t i o n of Tin and I r on Between Metal and Sl ag
The f r e e e n e r g i e s o f f o r ma t i o n of t he l ower oxi des of i r o n and t i n a r e ver y s i m i l a r :
FG(1) + ‘'^°2 (n) " FeO(T)
AGp = - 55, 620 + 10.83T , f o r T = 1808 t o 2000°K^^^
^ " ( 1) + %0 2(o) = SnO(i)
AGp = - 69, 336 + 2 5 . 7T , f o r T = 1143 t o 18730K^^'
T h e r e f o r e , a compl et e r e d u c t i o n of t i n as f a i r l y pure metal from t he si an i s i mp o s s i b l e because r e d u c t i o n o f i r o n occur s a t t he same t i m e , so t h a t an iron- t i n a l l o y r e s u l t s .
The e q u i l i b r i u m d i s t r i b u t i o n of t i n and i r o n between met al and s i an phases may be e x p r e s s e d by t he f o l l o w i n g r e a c t i o n :
^^( me t a l ) ^*^^(slag) ~ ^ ^ ( me t a l ) ^ ^ ^ ^ ( s l a n ) * f o r which t h e e q u i l i b r i u m c o n s t a n t K i s given by:
and,
K = *Sn ®FeO
*Fe
IlETAL ®SnO SLAG
k = wt%Sn wt%Fe wt%Fe METAL wt%Sn SLAG ( r e f , 3) where k i s t he d i s t r i b u t i o n c o e f f i c i e n t . P r a c t i c a l e x p e r i e n c e ( Re f s , 2 , 3 , 4 ) i n d i c a t e s t h a t k v a r i e s from about 50 f o r a ha r dhead o f about m i s c i b i l i t y nap compos i t i on t o about 300 f o r crude t i n .
To summari ze; compl et e removal of t i n metal from t he wa st e s l a g , a n d t he p r o d u c t i o n of a met al wi t h a low c o n t e n t of i r o n c o n s t i t u t e a most d i f f i c u l t problem i n t i n m e t a l l u r g y .
Tin i s l o s t in s l a n n o t onl y by i n c o mp l e t e r e d u c t i o n from i t , bu t t h e r e are a l s o mechani cal and p h y s i c a l l o s s e s .
Mechani cal l o s s e s a r e caused by i n c o mp l e t e s e t t l i n n o f t i n d r o p l e t s from s l a n t o mol t en m e t a l .
Ph y s i c a l l o s s e s can be caused by d i s p e r s i o n and f or ma t i o n o f ver y f i n e s u s p e n s i o n s of t i n i n t h e s l a g , Accordi nn t o Murach^^), t i n s o l u b i l i t y in s l a n s is n o t a d mi t t e d as a t r u e f a c t as y e t .
The l o s s e s of t i n in s l a n a r e i n f l u e n c e d by t h e chemi cal compos i t i on of s l a n s i n c e i t d e t e r mi n e s t he degr ee of r e d u c t i o n of SnO t o m e t a l , t he s p e c i f i c g r a v i t y , v i s c o s i t y , me l t i n g p o i n t , and s u r f a c e p r o p e r t i e s of s l a n s .
CONCENTRATE ' CARBON
HARDHEADS FLUX
DROSS FUMES
CRUDE TIN SLAG I FUÎ1E I
DROSS PURE TIN
HARDHEADS
DISCARD
Recovery of Tin from Sl ans
Tin c o n t e n t s i n s l a n s from f i r s t - s t a n e s me l t i n n vary from 3 . 5 t o 25%Sn, Thi s wide ranne depends on t he methods and t e mp e r a t u r e s of smel- t i n q , c omposi t i on of c o n c e n t r a t e s and s l a n s , and on many o t h e r f a c t o r s . This pr i mar y s l a n i s r e - s m e l t e d t o produce a hardhead and a f i n a l s l a n , as was men t i o n e d above,
Co n s i d e r i n n t he hiqh p r i c e of t i n ( ar ound $ 1 , 7 0 / f i n e pound in t h e l a s t f i v e y e a r s ) , a l o s s of 3%Sn in s l a n i s a c o n s i d e r a b l e l o s s and t h e f i n a l t i n con c e n t r a t i o n in wa st e s l a n s i s i n many cases n r e a t e r t han in t he o r e from which i t was e x t r a c t e d . T h e r e f o r e , any e f f o r t towards t h e r e d u c t i o n o f t i n l o s s e s i n s l a g s i s e x t r e me l y i mp o r t a n t f o r t i n - p r o d u c i n n c o u n t r i e s .
Tin can be r e c ov e r e d from s l a n s by:
a) r e s m e l t i n n t he s l a n t o produce hardhead and a s l a n l ower in t i n , b) mi ne r a l d r e s s i nn met hods ,
c) h y d r o m e t a l l u r g i c a l met hods , and d) v o l a t i l i z a t i o n p r o c e s s e s .
The f i r s t t h r e e methods a r e e x p l a i n e d i n d e t a i l by M u r a c h ^ ^ \ l i r i g h t ^ ^ \ Bel - y a y e v ^ ^ ) , and o t h e r s . They a l l have some drawbacks or l i m i t a t i o n s as f a r as e f f i c i e n c y and c o s t a r e c oncer ned. The f o u r t h , r e c o v e r y of t i n from s l a n s by v o l a t i l i z a t i o n , i s assuming i n c r e a s i n n i mpor t ance and i s based on t he v o l a t i l i t y of SnO and SnS,
Purpose of t h i s I n v e s t i n a t i o n
Format ion of SnS from s t annous oxi de c o n t a i n e d in s l a p s can be e f f e c t e d by exchange r e a c t i o n s v/ith s u l f i d e s of t he o t h e r m e t a l s , u s u a l l y p y r i t e s . The r e a c t i o n p r e s e n t i m t he f i n a l r e s u l t i s ni ven belo\/:
^"' ’ ( s l a q ) ^ v o l a t i l e ) ' ' ®° ( sl an)
Along v/ith t he t i n s u l f i d e f o r ma t i o n from SnO in t he s l a n , an i r o n - t i n mat t e i s for med, p r i n c i p a l l y when t h e FeS i s i n e xces s (as i s u s u a l l y t he c a s e ) . SnS s o l u t i o n i n t h i s ma t t e i s accompanied by a d e c r e a s e in i t s a c t i v i t y , and t h e r e i s a drop in vapor p r e s s u r e of s t a nn ou s s u l f i d e which would reduce i t s v o l a t i l i t y .
To what e x t e n t i s t h i s v o l a t i l i t y reduced?
What would be t he a c t i v i t y of t i n s u l f i d e in t h i s l i g u i d ma t t e ?
There i s n o t a s p e c i f i c answer t o t h e s e q u e s t i o n s so f a r and onl y e s t i m a t e s can be made based on c e r t a i n p r o p e r t i e s of t h e s yst em.
The phase dianram of t he SnS-FeS s yst em i s k n o w n ^ ^ \ b u t no i n f o r m a t i o n e x i s t s about t h e thermodynamic p r o p e r t i e s of SnS in t i n - i r o n m a t t e s .
T h e r e f o r e , t h e purpose of t h i s i n v e s t i n a t i o n \/as t o o b t a i n t h e answers t o t he above q u e s t i o n s under t he s i m p l e s t a s s umpt i ons and c o n d i t i o n s p o s s i b l e , as a p r e l i m i n a r y s t e p toward more s o p h i s t i c a t e d s t u d i e s f o r a b e t t e r u n d e r s t a n d i n n o f t h e thermodynamics of t i n fuminn from s l a n s and m a t t e s .
I I . L I T E R A T U R E S U R V E Y
Tin - S u l f u r Svstem
The S n - S system was s t u d i e d by Al ber s and c o l l a b o r a t o r s (9) and by G,
T h e i r r e s u l t s a r e summarized in t a b l e I ,
Tabl e I
Phase R e l a t i o n s in t he S n - S System
PHASE SYMMETRY CHARACTERISTICS
SnS / g S n S g <%SnS_ Orthorhombi c Hexaqonal Cubi c ? Occurs in n a t u r e as f l e r z e n b e r q u i t e ( P o t o s i , B o l i v i a ) . Near l y s t o i c h i o m e t r i c p ha s e , ‘l e l t s a t 8700C. f l e l t s a t 7580C t o l i q u i d SngS. and ocSnSg. P o s s i b l e e x i s t e n c e of a hinh t e m p e r a t u r e polymorph. On h e a t i n q i n v e r t s t o AiSnSp a t 602OC. f l e l t s a t about SGO^C. Repor t ed by Al bers (?)
o fO tn <U o 0» CL 1/3 c C3 4- O in CJ CL O 5- 4-> C LU -a i-fC "D C ft! 4-> CO *u c ft! c o 4-) fC E L-O Ll. 4 -o to 4-> ft! O CD z : CL o CO cr o c\j o fC Ü o <+ -O <4-DC
<3
CD O E ft! U ft: u CD O E c CO cu cr. -a o c\i \ CO r— ft) u CO CO O C\J <1 CO LU c to I 1 I 1 1 1 1 1 1 1 1 1 1 1 1 o 1 1 O 1 1 o LO 1 O 1 LO 1 LO O 1 LO 1 1 CM LO 1 1 1 1 CO 1 T—1 1 1 O 1 1 1 1 CO 1 O 1 CO 1 CO cr> 1 1 O 1 LO CO 1 r—f 1 CM 1 t-H 1 I 11 t-H 1 00 1 11 CD 1 LO 1 r~s 1 CO 1 t-H 1 1 1 CM 1 1 1 1 C\J 1 + 1 CM 1 CM 1— 1 CM 1 1— 1 1— CO 1 h- 1 CO 1 CO 1 1 CO 1 1 I 1 C 1 1 1 O 1 o r-H t O LO 1 t-H 1 t-H t-H LO 1 • 1 to 1 1 • CO 1 1 ^ 1 r-H. «—1 1 f—1 CO 1 LO I CO CO 1 1 • CM 1 CO 1 •T—* 1 CO 1 1 t—H + CGI 1 1 1 + t-H , + 1— OI 1— h-l H— 1 1 k— CJ •! 1-HLO 1 CO h- 1 CO <0- OI CO 1 1 LOLO 1 • O 1 • O I ^ +11 1 CO t-:: 1 CO r-H 1 c I 1 11 k— 1 k— k— 1 1— 1 CO 1 CO CO 1 CO 1 1 1 1 1 1 1 I O 1 o O 1 1 CM 1 t-H 1 t-H *—t 1 t-H 1 1 " k— 1 COCM 1 CO 1 1 CMLO 1 1 1 LO O I 1 • k— 1 LO 1 • t-H 1 r-^LO 1 CO + 1 1 • 1 + O I + CvJ 1 f—1 1 CO t-H| CO 1 CO »l LO • 1 • CM.I • cr.i «e 1 1 CO 1 1 1 CO +*l o. I 1 I (D 1— 1 1 1 LO 1 1 1 1 1 1 1 1 LO OI 1 1 CO r^i 1 11 1 1 11 in 1 O 1 1 cr 1 o CJ 1 1 1 1 1 fO O I LO r-. 1 1 S- LÛ 1 LO r—1 1 1 I 1 1 1 4-> i-f| I 1 CM 1 1 1 1 CO 1 1 1 1 o o • LO 1 CJ 1 1 CO JO CO 1 1 1 1 CO CO to CO 1 1 1 11 1 O LO 1 LO LO LO t-4|1 CJ O I LO O CM OI <+ CM 1 1 1 CM OI o O I CO I—f +11 1 t—f +1 <+ +11 1 r-H +1 1 1 11 o ^ 1 1 o o 1 o 1 1—1 CD O 1 o 1 1 LO CJ 1 1 1 1 LO 1 CM t-H 1 1 1 1 +1 1 1 "— 1 1 ___ '—. 1 ___ <U 1 (D u , 1 (j D +-> 1 u >, r 1 to to ^ 1 to 0 JC 1 C L- C 1 c C 1 c 0 1 co c to 1 to to 1 to + -> c o u•o Q) 4-> C o o CJ JD CL rr o CO CTi O CM O I -GJ O E (_) O 4 - < <0^4-< U J 00 CD O CM to CO cr, O CNJ
<3
to LxJ CL to O E fC o o o + -> c E cr o TO fO u o E fO o o o o LO o co CM o co CM 1 1 1 CO CO CO LO o c r f—i CM CM 1—1 1 + CM CM 1—1 1— »— CM CO CO CO 1 1 CJ o CD O 1 1—1 t—1 r—1 LO • 1 r-H t—1 <+ k— O LO CM I ■ 1 + + o k— k - k— '—1 r-H c r CO . LO T—1 CO LO r-H r-H • LO LO CO f-H CO k— 1— CO CO 1 1 c C CM r-H t—1 1 • k— CM CO LO o o c r + + l-H l-H. CO LO LO CO LO LO CO 1 1—1 1 1 1 1 1 1 1 1 1 t-H • li eu L - — 1 c r , CM OD i -CM cu pH. o tn LO -H CM -H CO t-H CU -K -K » CD o o --H C 4 -o o o o c <U X3 CO 1 o O CD o S- C LO o O C o fO CM Ch. 1 1 44 1 41 c +-> fO +L> E o <U C cu CM S-to to to Lj_ LU c c c u ■K to to to ■K -KSi nce s t a n no us s u l f i d e (SnS) i s t he compound of i n t e r e s t t o t h i s i n v e s t i g a t i o n , a l i t e r a t u r e s ur vey \/as conduct ed on t he thermodynamic n r o p c r t i e s of t h i s s p e c i e s . Tabl e II ni ves t h e h e a t and s t a n d a r d e n t r o p i e s f o r t he Sn-S s p e c i e s as qi ven by Ke11ey ^ ^ ^ ^.
f 15)
Hsi ao and Sc hl echt en^ s t u d i e d t h e v o l a t i l i t y of SnS in a vacuum f u r n a c e . T h e i r r e s u l t i s qiven in t he f ol l ov/ i nq e q u a t i o n :
Log , (mm Hg) = -8380/ T +6. 728
Temper at ur e ranqe: 503° - 704OC ( c oul d he e xt e nd e d up t o 870°C) P r e s s u r e r a n q e , log p^ ^ , (mm Ho): - 4 , 0 0 t o 1,91 .
A. W. R i c h a r d s s t u d i e d t he h e a t and f r e e e n e m y of f o r ma t i o n and v a p o r i z a t i o n o f s t annous s u l f i d e u s i nq t he e n t r a i n me n t method. Based on t h i s s t u d y , he made a new e s t i m a t e of t he e n t r o p y of SnS. His d a t a a r e summarized as f o- 11ows: SnS(c) = SnS(^, Log Pg^g , atm = - 1 0 , 4 7 0 / T + 7. 088 (± 0 . 02) gO ^ ^ 9 8 ( Sn S, ^ t ) = 19. 4 ± 1 e . u . compared t o 18. 2 +1. 5 e . u . ( Ki r e e v ^ ^ ^ ^ ) .
AHf298(SnS^^j ) ^ " 24. 34 ± 1.1 Kcal / mol e. compared t o - 24. 7 Kcal/mole ( S u d o / ^ ^ ^ ) .
S t , C l a i r , S h i b l e r , a n d Sol e t ^ ^ ^ ^ o b t a i n e d t he vapor p r e s s u r e of SnS by means o f t h e t r a n s p i r a t i o n method, and c a l c u l a t e d t he h e a t and f r e e e ner gy o f vapo r i z a t i o n above and below t he me l t i n o p o i n t :
For SnS, \ = SnS, \
( c ) (n)
AC = - 1 , 0 6 - 3 . 6 . 1 D ‘ ^T ,
Ah”u|,, = 5 1 , 3 5 5 - 1.06T - 1 . 8 ‘ IO‘ V ( c a l / m o l e ) .
AG°ubi = 5 1 , 3 5 5 + 2.44T Loq 7 + I . S - I O ' V - 4 6 . 0 2 7 ( c a l / m o l o ) ,
Loq , atm = - — - ,533Loq 7 - 3 . 9 3 . 1 0 ' ' ’7 + 1 0 . 0 59 .
For T S n S ( , ) = SnS(^) AC = - 9. 02 , AH°g = 50, 590 - 9. 027 , ( c a l / m o l e ) , AG°g = 50,590 + 20. 777 Loq 7 - 99. 41 ( c a l / mo l e ) Loq , atm = - - 1 1 :2^ - 4. 54 Loq 7 + 21. 729
The v a por p r e s s u r e of SnS in e q u i l i b r i u m with mol t en SnS i s , a c c o r d i n g t o Kl ushi n and Chernykh^^^) ( q yo t e d by G, J . danz^^l^i, e x p r e s s e d as f o l l o w s :
Log Pg^g , (mm l!n) = -
2222
. + 9, 551The e x i s t e n c e of o t h e r gaseous s p e c i e s t han SnS was u n c e r t a i n u n t i l P., Colin ( 2 2)
and J . Drowart^ ' made a thermodynamic s t u d y of SnS in 1964 us i n q a mass s p e c t r o m e t e r .
They found t h a t t h e maj or components of t h e t i n - s u l f u r vapor a r e gaseous SnS and SngSg , t he l a t t e r in a v e r y low p r o p o r t i o n . P o s s i b l e t r i m e r Sn^S. and t e t r a m e r Sn^S^ m o l e c u l e s , whose i n t e n s i t y r e l a t i v e t o SnS was equal t o , or s m a l l e r t han 5*10 ^ ^ coul d not be d e t e c t e d . T h e i r summarized d a t a a r e qi ven
Molecule Temp. Ranqe H D? = Di s s oc . o n e r q i e s S Ui) I « 2qg M °K Kcal/mole SnS 815 - 1005 52. 6 ± 1. 6 110.1 ± 3 Kc al / mole SngSg 815 - 1005 56. 5 ± 5
Thus, t h e thermodynamic d a t a ni ven hv Ri c h a r d s ^ ^ ^ ^ , S t , C l a i r e t _ a n d Kl ushi n and C h e r n i k l / ^ ^ ) not r e f e r r e d t o t he p a r t i a l p r e s s u r e s of SnS,^^ b ut t o t h e t o t a l vanor p r e s s u r e of t i n as a s u l f i d e ; i . e .
^T( sns ) ” ^9n(n) ^SnS(n) ^ PgPgSg (°)
I t can be assumed, w i t h o u t i n t r o d u c i n g a s i n n i f i c a n t e r r o r , t ’n a t :
^ ”y s n s ) • as w i 11 be shown l a t e r .
T h e o r e t i c a l thermodynamic c o n s i d e r a t i o n s , based mos t l y on Col i n and Dr owa r t ' s
i n v e s t i g a t i o n s ^ 2 2 ) ^ qi ven by H. H. Kellong^^^) 1966,
The s t a n d a r d f r e e e n e r g i e s f o r v a p o r i z a t i o n and r e l a t e d e q u i l i b r i a f o r t he S n - S s y s t e m, a r e qi ven in t a b l e I I I . These e q u a t i o n s were c a l c u l a t e d by a l e a s t - s q u a r e s f i t t o t he dat a t a b u l a t e d in K e l l o g g ' s p a p e r .
The pr e s e nc e of SnS. in SnS samples i s n o t harmful t o SnS becaus e SnS^ de composes a t low t e mp e r a t u r e s t o SnS and a comnlex mi x t u r e of pol ya t omi c s u l f u r mo l e c u l e s . I f , i n t u r n , samples c o n t a i n a l s o SnCL, t h e s e a r e s i m u l t a n e o u s l y r educed t o SnS as shov/n by e f f u s i o n o f S0« mo l e c u l e s . These p r o c e s s e s were
(2 2)
Tabl e I I I
S n - S System. St a nd a r d Free Energy f o r V a p o r i z a t i o n and Re l a t e d Eq u i 1i b r i a ^ ^ ^ ) R E A C T I 0 Î1 AG^ = A . T + B ( c a l / mo l e ) A B Temp. Ranne, °K ± x ( c a l ) S n ( l ) = Sn(g) - 2 3 . 8 3 +70,190 505 - 1500 6. 05 S n ( l ) + = SnS(c) +24.7927 - 4 3, 704 493 - 1143 32. 41 S n ( l ) + = SnS( l ) +14.6092 - 32, 042 1143 - 1500 22. 43 SnS(c) = SnS(g) - 3 5. 35 7 +50,671 493 - 1143 10.396 SnS( l ) = SnS(g) - 2 6. 1126 +40,102 1143 - 1500 6. 117 2SnS(g) = Sn^S^j ^j +37.96 - 4 6 , 8 8 8 1100 - 1500 34. 33 I r o n - S u l f u r Svstem Ro s e n q v i s t ( 2 4 ) s t u d i e d t he i r o n - s u l f u r syst em based on d a t a s u r v e ye d by Hansen^^^) and t he work on t hermal a n a l y s i s by densen^^^) The f r e e e n e r g y o f f o r ma t i o n of FeS i s given below:
^ FS(c) ^ 2 (q) " ^
AGp = - 71, 500 + 25. 25 T ( c a l ) (500 - 988°C). (27)
Which a g r e e s ver y \ / el l wi t h d a t a o b t a i n e d by Sudo^ .*
and by Alcock and R i c h a r d s o n ^ ^ ^ ^ :
AGp = - 71,820 + 25. 12 T ( c a l ) .
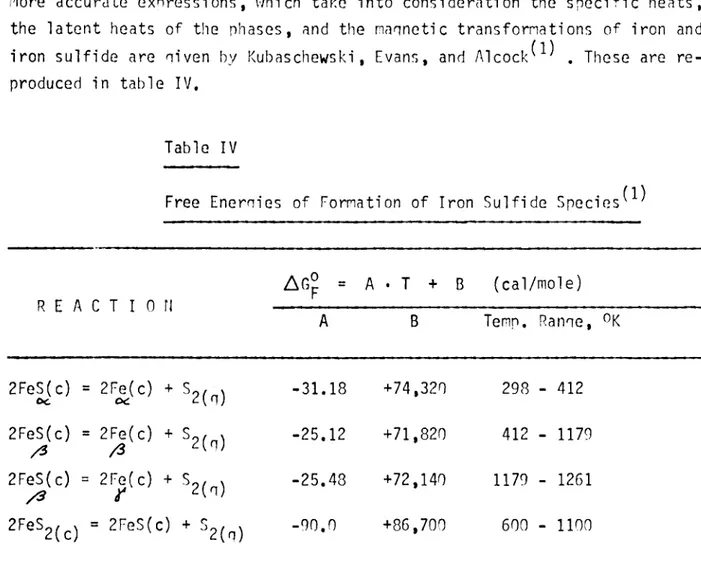
More a c c u r a t e e x n r e s s i o n s , which t a k e i n t o c o n s i d e r a t i o n t he s p e c i f i c h e a t s , t he l a t e n t h e a t s of t he p h a s e s , and t h e maqnet i c t r a n s f o r m a t i o n s of i r o n and i r o n s u l f i d e a r e niven by Kubaschewski , Evans, and Alcock^^^ , These ar e r e produced in t a b l e IV.
Tabl e IV
Free E n e m i e s of Format ion of I r on S u l f i d e Speci es ^^^
R E A C T I O N AGp = A • T + B ( c a l / m o l e ) A B Temp. Ranqe, 2FeS(c) oc = 2Fe( c) + - 3 1 . 1 8 +74,320 298 - 412 2FeS(c) = + ^2 (q) - 2 5. 12 +71,820 412 - 1179 2FeS{c) / 3 = 2Fg(c) + - 2 5 . 4 8 +72,140 1179 - 1261 = 2FeS(c) + - 9 0 . 0 +86,700 600 - 1100
fis)
Hsi ao and Sc h l e cht en^ ' gi ve t he f o l l o w i m appr oxi mat e e x p r e s s i o n f o r t he FeS va por p r e s s u r e :
Loq , (nm Hn) = - 10, 850/ T + 4. 162
Te mper at ur e ranne: 804 - lOOG^C.
P r e s s u r e r a n n e , Loq Pp^g , (mm Ho) = - 5 . 8 4 t o - 4 . 2 6
Ppes » (mm Hq) = 1 . 4 4 » 10"^ t o 5 . 4 9 ' 1 0 " ^
which shows t he e x t r e me l y low n r e s s u r e of t h i s s u l f i d e . To c o r r o b o r a t e t i n ' s , Ro s e n qv i s t ^^^ ) s t a t e s t h a t t he vapor p r e s s u r e of FeS i s t oo low t o be measu red d i r e c t l y . Advantaqe of t h i s f a c t i s t a ken t o s t u dy t he v a p o r i z a t i o n of SnS in Fe-Sn m a t t e s .
I r on - Tin - S u l f u r Svstem
The t e r n a r y syst em was i n v e s t i n a t e d by Ourach and Li k h n i z -f29)
kaya' ' i n 1938, Accordi nn t o t h e s e a u t h o r s , t he i r o n - t i n ma t t e c o n t a i n s l e s s s u l f u r t han coul d be e x n e c t e d in s o l u t i o n s of FeS and SnS, T h e r e f o r e , t he y be l i e v e d t h a t t i n in ma t t e s i s n r e s e n t a l s o as d i s s o l v e d m e t a l . Thev showed a m i s c i b i l i t y nap between 0%Fe and 50%Fe whi ch, a c c o r d i n n t o t h e now wel l e s t a b l i s h e d l i m i t s of t he m i s c i b i l i t y nap r e qi on in t he i r o n - t i n s ys t e m, i s wronq. The c o r r e c t l i m i t s o f t h a t m i s c i b i l i t y qap a r e 29%Fe and 50%Sn^‘^ .
f1oh^^^^ i n v e s t i q a t e d t h e phase r e l a t i o n s i n t h i s syst em a t GOOOC by t he r i n i d s i l i c a t ube method and found t h a t t h e s t a b l e phases a t t h i s t e mp e r a t u r e a r e : p y r r h o t i t e (Fe^_^S) and n y r i t e (FeS^) on t h e Fe-S j o i n ; h e r z e n b e r q u i t e ( S n S ) , Sn^Sg, and SnSg on t he Sn-S j o i n ; and -Fe s o l i d s o l u t i o n wi t h up t o 6at%SnS, hexaqonal FeSn and l i q u i d on t he Fe-Sn j o i n , flo t e r n a r y compounds e x i s t a t GOQOC.
S o l i d s o l u t i o n s between phases such as FeS. and SnS, Sn S . , S n . S . , and FeS ar e r e s t r i c t e d t o l e s s t han 1%, whereas t h e mutual s o l u b i l i t i e s between SnS and FeS exceed 1%. Tlie s o l u b i l i t y of s u l f u r in e i t h e r Fe or FeSn as well as
t h a t o f t he me t a l s in l i q u i d s u l f u r was r e p o r t e d t oo small t o be d e t e c t e d ^ . There i s no o t h e r a c c o un t on t h i s svst em in t he l i t e r a t u r e .
I r o n S u l f i d e - Tin S u l f i d e Svstem
The l i t e r a t u r e ni ves onl y one r e f e r e n c e on t h i s s yst em. The phase di anram was s t u d i e d bv llaan^^^ i n Germany ( 1913) . The nraph o b t a i n e d
{31 )
by Haan i s niven in Phase Dianrams f o r Cer ami st s^ (1964) and t h e r e a r e no o t h e r d a t a a v a i l a b l e .
Tha f o l l o w i n q d a t a can be o b t a i n e d from t he phase dianram;
SnS m e l t i n q p o i n t a t 87Q0C, FeS m e l t i n q p o i n t a t I I 88OC . E u t e c t i c t e mp e r a t u r e a t 785®C a t 15%FeS (85 wt%SnS),
No s o l i d s o l u b i l i t y has been d e t e c t e d .
L a t e r , J . P . Counhlin^^^^ de t er mi ne d t he m e l t i n q p o i n t of FeS as 11950C. This v a l u e i s qi ven by Kubaschewski et , ^^ * and w i l l be t aken f o r c a l c u l a t i o n s i n t h i s work s i n c e i t i s b e l i e v e d t o be t he most r e l i a b l e .
( 33)
Davey and Fl ossbach^ ' made an e s t i m a t i o n of t he vapor p r e s s u r e o f SnS over (23) t i n - i r o n ma t t e s based on t h i s phase di anram and on d a t a ni ven by Kelloqn^ . They ni v e t he f o l l o wi n n e x p r e s s i o n f o r t h e vapor p r e s s u r e s of SnS o ve r Sn-Fe ma t t e s ;
Loq Pj 5 = - 8650/ T + 5. 62 + Loq Ng ^ + 230. 0 . Up ^ /T
f o r a t e m p e r a t u r e ranqe of 1000 - 1500OK.
The v a l u e 230. 0 c or r e s po n ds t o a c o n s t a n t (B) when assuminq r e q u l a r s o l u t i o n b e h a v i o r o f t h e l i q u i d phase:
and t o an e u t e c t i c c omposi t i on of 18wt%FeS,
The f o l l o w i n q c h a p t e r w i l l p r e s e n t some c a l c u l a t i o n s based on Davey' s e s t i m a t e s .
Hvdronon - S u l f u r Svstem
Thermodynamic d a t a on t h i s system were r e q u i r e d t o c a l c u l a t e t!ie HgS/H. r a t i o s f o r t he mi x t u r e s of t h e s e two e a s e s a c t i n n as a c a r r i e r nas in t he t r a n s p i r a t i o n method, and in t h i s manner ma i n t a i n a c o n t r o l l e d s u l f u r p o t e n t i a l so as to p r e v e n t any decompos i t i on of t he s u l f i d e mi xt u r e s d u r i n n t h e p r o c e s s .
Kubaschewski e t a l [ ^ ^ l i s t t he f o l l o wi n n dat a f o r t h e f o r ma t i o n of H.S nas;
^"2{q) '^2(n)---AGp = - 4 0, 2 10 + 7.27T Loq T - 1.21T (+ 700 c a l ) f o r T = 298 to 175Q0K o r : AGp = - 43, 160 + 23.61T (± 1000 c a l ) f o r T = 293 to ISOO^K .
E l l i o t t and Ol e i s e r ^ ^ ^ ^ ni ve f o r t h e same e q u a t i o n a t a b l e of v a l u e s , from which a l e a s t - s q u a r e s f i t c a l c u l a t i o n n i v e s :
AGp = -43, 006 +23.407T (± 21. 2 c a l ) f o r T = 900 t o 1600OK.
Haner^^^) made a c a r e f u l s u r ve y o f t he l i t e r a t u r e on t h e h y d r o n e n - s u l f u r s y s tem and c a l c u l a t e d e q u a t i o n s o f AG^ of ILS, \ , MS, \ , and S, \ s p e c i e s a t
F 2 (n) (n) (n) / . . t
hiqh t e mp e r a t u r e s based on v a l u e s of AGp niven in t he dAilAF t a b l e s ' ' ' and g i ve s t he e q u a t i o n s f o r AG^ of S^, \ and S . , \ from Ri char ds on and J e f f e s ^ ^ ^ ^
F 6 (n) 8 (n)
These e q u a t i o n s , al onq \ / i t h above ni ven d a t a a r e l i s t e d i n Tabl e V.
The r e f e r e n c e s t a t e s f o r s u l f u r and hvdronen a r e t he i d e a l S . , \ and i d e a l 2 (q)
^^2(q) r e s p e c t i v e l y in a l l t he e q u a t i o n s qiven in Tabl e V.
I t can be seen t h a t f o r t he f o r ma t i o n of ILS, x , t h e anreement in a l l cas es f 1 )
i s qood. The va l ue s qiven by Kubaschewski fJL' were t aken f o r c a l c u l a t i o n s i n t h i s work s i n c e t hev a r e based on most r e c e n t d a t a .
Tabl e V
Thermodynamic e r n n e r t i e s of llydronen S u l f i d e and S u l f u r Oases
SPECIES AGp , c a l o r i e s Temp. Ranqe, °K SOURCE
REFERENCE. " 2^ ( 0 ) - 2 0, 105 (; + 3.635T LoqT - .605T t 350 c a l ) 298 - 1750 1 , 12 - 2 1, 580 + 11.805T (+ 500 c a l ) 298 - 1800 1 , 36 - 21, 580 + 11.80 T (+ 500 c a l ) 298 - 1723 14. 37 - 21, 570 + 11.79 T (± 200 c a l ) 900 - 1700 34, 35 "S( 9) +20,070 - 3.70T (+ 300 c a l ) 900 - 1700 34, 35 S(n) +51,170 - 14.44T (± 1000 c a l ) 900 - 1700 34, 35 ^6 (q) - 6 6, 450 + 73.74T (+ 3000 c a l ) 298 - 1300 34, 35 - 99, 200 + H 3 . 1 6 T ( + 4000 c a l ) 298 - 1300 34, 35
I I I . T H E F U f1 I M 0 O F T I M F R O M
S L A G S A N D M A T T E S
Apart from t i n , s l a n s from t i n s mo l t i n q c o n t a i n c o n s i d e r a b l e q u a n t i t i e s of , Zn, Cu; as well as r a r e me t a l s which a r e v a l u a b l e t o t he economy of t he t i n - p r o d u c i n q c o u n t r i e s .
As s t a t e d above (Ch. I ) , and as p r a c t i c a l e x p e r i e n c e has shown, amonn a l l t he e x i s t i n q p r o c e s s e s t o r e c o v e r t i n from s e c o n d - s t a o e s l a n s , t h e most e f f e c t i v e and e c o no mi c a l l y advant aneous i s t he s l a q fuminn p r o c e s s .
D e s c r i p t i o n o-^ t he Pr ocess
The e s s e n c e of t h e p r o c e s s c o n s i s t s i n t he c on v e r s i o n of SnO i n s l a q s i n t o t he more v o l a t i l e compound SnS, which i s t hen r e c o v e r e d as a d u s t in t he form of SnCU.
S t u d i e s on t he v o l a t i l i t y of SnS a r e no t s c a r c e ; some of them ( t h e i mp o r t a n t ones) were a l r e a d y c i t e d in Chapt er I I .
Some of t he works a t a p i l o t p l a n t o r a t i n d u s t r i a l l e v e l ni ven in t he l i t e r a t u r e a r e ment i oned below;
A devel opment of a pr oc e s s of t i n fuminn a p p l i c a b l e t o t h e e nr i c hme n t of low- qr ade B o l i v i a n and German t i n c o n c e n t r a t e s by Lanne and B a r t h e l ^ ^ ^ ) .
Be l y a y e v ^ ^ ) , Murach^^^, Kolodin^^^^ d e s c r i b e t he fuminn pr oc e s s i n d e t a i l . Wri qht ^^) a l s o q uot e s t h e s e a u t h o r s in hi s book.
l i r i q h t h i ms e l ^ conduct ed a o i l o t - n l a n t i n v e s t i n a t i o n , us i nn a m o d i f i c a t i o n of t h e Ko l od i n- t vn e f u r n a c e , b u i l t bv t he I n s t i t u t e of Mininn and Metal 1u r n i c a l I n v e s t i n a t i o n s a t Or ur o, B o l i v i a . There i s no p u b l i s h e d a c c o u n t of t h i s .
Format ion of SnS ^rom s t annous oxi de in si an can be e f f e c t e d by exchan- qe r e a c t i o n s v/ith s u l f i d e s of o t h e r m e t a l s :
SnO + MS SnS + MO
where MS could he Al^S^, CaS, ZnS, or f e S , t he most e f f e c t i v e and most e x p e n s i v e bei nq Al^Sg.
Si nce n y r i t e , F e S. , t r a n s f o r ms e a s i l y t o FeS a t t e mp e r a t u r e s above GOO^C, and as i t i s t he c h e a p e s t , i t i s most commonly used f o r t i n s u l f i d i z a t i o n .
Si a n from s e c o n d - s t a n e t i n s me l t i n n i s m e l t e d , or k e ot in t he molten s t a t e , in a r e v e r b e r a t o r ' / or r o t a r v f u r n a c e , and wlien in t h e mol t en s t a t e i t i s c a r r i e d in s t e e l l a d l e s ( b a t c h e s of G - 8 t o n s ) and poured i n t o t h e fuminn f u r n a c e where i t i s blown by a mi x t u r e of coal d u s t , or o i l , a i r , and p y r i t e . The p y r i t e mixes wi t h t he s l a q and decomposes wi t h f o r ma t i o n o f f e r r o u s s u l f i d e which e x t r a c t s t h e t i n from i t s s i l i c a t e s in t he s i an:
ZFeSg = = 2FeS + S.
Zi nc oxi de in s l a q r e a c t s 'with CO a c c o r d i n q t o:
' -(coal o r o i l ) ^ ^2 " "
ZnO + CO Zn + CO.
This reduced Zn v a p o r i z e s and i s t r a p p e d as ZnO in t he e l e c t r o s t a t i c p r e c i p i t a t o r s when t h e e x h a u s t f u r n a c e gases are c l e a n e d .
Lead i s removed from s l a n as PbO, PbS, and Pb vapor .
Rare el ement s t h a t form v o l a t i l e s p e c i e s a r e a l s o removed as vapors from t he s l a g and t r a p p e d as d u s t .
The f u r n a c e used in t i n fuminn i s b a s i c a l l y t he same as f o r t h e z i n c fuminq p r o c e s s . I t i s a w a t e r - j a c k e t e d r e c t a n n u l a r s h a f t - f u r n a c e , as shown i n Fi q. 2, 'with 12 c o n v e r t e r - t y p e t u y e r e s (G on each s i i o r t s i d e of t he f u r n a c e ) throunli wliich t he c o a l , or o i l and p y r i t e s a r e blown i n t o t he f u r n a ce by compressed a i r . The h e a r t h of t h e f u r n a c e i s a c a s t i r o n p l a t e wi t h 12 w a t e r - c o o l e d s t e e l pi ne s c a s t i n t o i t . I t is i n s t a l l e d on G c a s t - i r o n s u p p o r t s . Blowinq be qi ns a t t he time t he s l a q i s bei nq poured i n t o t he f u r n a c e and con t i n u e s f o r about 2 t o 3 ho ur s .
The p r o c e s s , a c c o r d i n n t o Bel yaye v^^^, can be d i v i d e d i n t o t h r e e p e r i o d s : a) ! l e a t i nq- up of t he s l a n . b) Reduct i on and s u l f i d i z a t i o n . c) Reheat i nq o-^ t he s l a n b e f o r e t a p p i n q . Sl an t e mp e r a t u r e s a r e from 1150 t o 1300^0, The o p e r a t i o n c o n d i t i o n s , as summarized by l l r i n h t(
6
) a r e : Furnace char ne Du r a t i o n of c y c l e Coal consumption(When o i l i s not used) P y r i t e consumption Ai r s uppl y approx. 8 . 5 t o n. 2 - 3 h o u r s . 18wt% of s l a n t r e a t e d 4,lwt% of s l a q , 67% of t h e o r e t i c a l f o r compl et e combust i on.
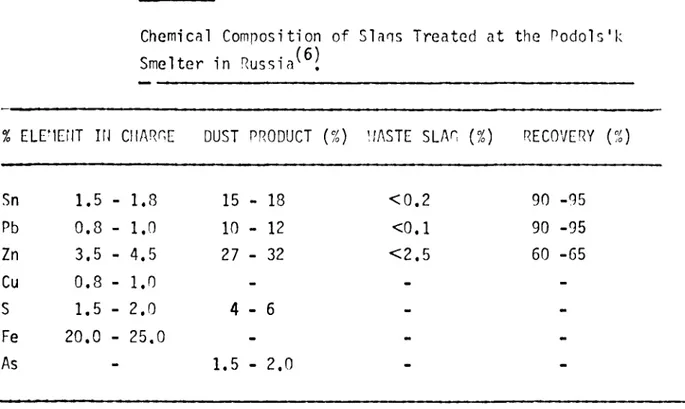
Typi ca l c har ne and p r o d u c t comp o s i t i on s a r e niven in Ta bl e VI.
P r i v a t e c o m m u n i c a t i o n ^ n i v e s t he f o l l o wi n n r e c e n t d a t a from a Russi an and a B o l i v i a n p l a n t r e s n e c t i v e l v : Russi an p l a n t ; B o l i v i a n n i a n t : char ne wa s t e s l a n c har ne wast e s l a n avne. l%Sn 0 . 1 - 0.09%Sn. avne. G%Sn 0,5%Sn.
Ç=3
a
FIG. 2. SHAFT FURNACE FOR SLAG FUMING.
1. COOLING PLATES 3. TUYERES
2. HEARTH 4. FLUE.
Tabl e VI.
Chemical Composi tion of Sl ans T r e a t e d a t t he Po d o l s '
(6)
Sme l t e r in Russi a
% ELEMENT IN CHARGE DUST PRODUCT (%) WASTE SLAG {%) RECOVERY (%)
Sn 1 . 5 - 1. 8 15 - 18 < 0 . 2 90 -95 Pb 0 . 8 - 1.0 10 - 12 < 0 . 1 90 -95 Zn 3. 5 - 4. 5 27 - 32 < 2 . 5 60 -65 Cu 0 . 8 - 1.0 - - -S 1. 5 - 2 . 0 4 - 6 - -Fe 20 . 0 - 25. 0 - - -As - 1. 5 - 2. 0 - -Matte Formation
The use of e xc e s s n y r i t e or s u l f u r blown i n t o t h e molten s l a q r e d uc e s t he r e c o v e r y of t i n as t he v o l a t i l e Sn s u l f i d e , be caus e of t h e f o r ma t i o n o f an i r o n - t i n ma t t e due t o undecomposed f e r r o u s s u l f i d e . Thi s ma t t e c o n s i s t s n o t onl y o f FeS and SnS b u t a l s o o f t h e s u l f i d e s of t h e o t h e r me t a l s p r e s e n t i n s l a n (Pb, Cu, Zn, e t c . ) .
Ma t t e , bei nn h e a v i e r t han s l a n , c o l l e c t s under t h e l a t t e r i n t he h e a r t h of t h e fuminq f u r n a c e and i s n e r i o d i c a l l y t apped as i t a c c u mu l a t e s .
I t i s e v e n t u a l l y t r e a t e d a n a i n t o v o l a t i l i z e t he SnS, e i t h e r s e p a r a t e l y , or mixed wi th new b a t c h e s of s l a n .
I t i s o f q r e a t i n t e r e s t t o t h e t i n m e t a l l u r n i s t t o know t h e e x t e n t t o which SnS v o l a t i l i t y i s hamnered by t h e s o l u t i o n of s t a nn ou s s u l f i d e in t h i s m a t t e , so as t o de t e r mi ne t h e optimum c o n d i t i o n s f o r t h i s p r o c e s s .
I t i s a d v i s a b l e a t t h i s p o i n t t o a n a l y s e in more d e t a i l t he d a t a a v a i l a b l e on t he t i n - s u l f u r svst em.
Thermodvnamic Ev a l u a t i o n s
Fi our es 3 and 4 were o b t a i n e d by c a l c u l a t i o n s from dat a qi ven in Tabl e IV, Chapt er I I . Complété c a l c u l a t i o n s a r e niven in Appendix I . The s t a b i l i t y r e n i o n s , deoendi nn on t he p a r t i a l p r e s s u r e of S^, a r e i n d i c a t e d a t t h e top of t h e two f i n u r e s . I t can be seen t h a t , as t he t e mn e r a t u r e i n c r e a s e s , t he p a r t i a l p r e s s u r e s of Sn^Sp naseous s n e c i e s a l s o i n c r e a s e in i mpor t a n c e as do t h os e of SnS/^\ , b u t t h e p e r c e n t a n e of SnpSp/^x r e l a t i v e t o t h a t of SnS^^j d e c r e a s e s .
The e f f e c t i v e t o t a l n r e s s u r e of Sn - S naseous s p e c i e s i s p l o t t e d a n a i n s t s u l f u r p r e s s u r e in Fi n, 5, and a n a i n s t 1/T in Fi n, 6 , The p a r t i a l p r e s s u r e s of SnS^^j and Sn^Sp^^j ve r s us 1/T a r e a l s o ni ven in Fi n, 6 , t o show t h e im p o r t a n c e of Pg^^g over t h e t o t a l p r e s s u r e and t he very smal l c o n t r i b u t i o n s of SnpSpÇq) and of Sn^^^ t o t he e f f e c t i v e t o t a l , p r e s s u r e , ni ven as:
' T(SnS) ^ ^ '
T h e r e f o r e , Pg^ and pg^ g can be n e n l e c t e d w i t h o u t i n t r o d u c i n n any s i n n i f i - c a n t e r r o r t o t he thermodynamic p r o p e r t i e s of :
The s t a b i l i t y zones of i n t e r e s t t o t h i s i n v e s t i n a t i o n a r e ttie zones t o t he r i q h t of t he 5 n ( l ) - S n S ( c , l ) l i n e , t h a t i s ; t he zones a t which t he vapor p r e s s u r e s of SnS/ ^ and $^2^ 2 ( 0 ) i nd e p e n de nt of s u l f u r nas p r e s s u r e s s i n c e no de c omn os i t i o n of SnS, ,x i s d e s i r e d ,
C\J o o oo o n C-o> O) C\J CO cv CVI CO CO *o LD 00 o (O 00 or> CO in VO I CO E +-> ro CO CO CL CD o CO I c CO CO > CO CO UJ c: Z3 CO CO LU cr c_ c: o c_ < lu E 5 0 *1
o o o o ^ LT> O Cl LU en D> CT) co CM CM ro LD t/) Oî cc I n un o r-4 CM V? CL Cr O cr I (y? > -(y? œ LU cr. =) cc cc LU cr c^ cr c c U t? d b 0 1
o o o o o c o o LD CVJ < T ) in (/) CL 00 Q. CL LD n c r-4 E 4-> fO C\J vo CL cr O a: =) oo l/l LU CL: CL oc I/O u_ o o H -O to <c to I c to LU I— to > to H— Ll_ O LU C:: ro to to LU CL: CL CL o CL-c h -o (_) LU LU LD LU B ( s u s ) i d
E +J (t3 o> o
1
T(SnS) SnS 2 3 - 4 7 6 8 9 10 11 10*/ I OK- 1
The p e r c e n t a g e of ove r t he e f f e c t i v e t o t a l p r e s s u r e rannes between ^"2^2( n)
2 , 4 t o 4% ( i n t he f l a t zones) a t t e mp e r a t u r e s between 1500 t o IOOQOk i n t h a t o r d e r . In a l l cas e s p~ ^ i s l e s s t han 1% of t h e t o t a l p r e s s u r e when i t
^^2^2( n) i s de pendent on t he s u l f u r p r e s s u r e ( F i n s , 3 , 4 , and 5 ) , C a l c u l a t i o n s a r e gi ven in Appendix I , T h e r e f o r e , f o r t he p r e s e n t work, t h e t o t a l vapor p r e s s u r e of SnS s p e c i e s w i l l be c o n s i d e r e d as bei ng t he vapor p r e s s u r e of SnS^^^ o n l y , n e g l e c t i n g t he o t h e r gaseous s p e c i e s .
Crude e s t i m a t i o n s based on t h e thermodynamic p r o p e r t i e s of FeS and SnS, and on t h e FeS-SnS phase diagram a r e p r e s e n t e d below.
The p r e s s u r e of SnS v a por over t h e mat t e i s p r o p o r t i o n a l t o i t s a c t i v i t y t h e r e i n , s ay:
= c
'^"^lATTE
'
^''^flATTE
where c = c o n s t a n t a t each t e mp e r a t u r e = .
(2)
R e f e r r i n g a gai n t o t he s u l f i d i z i n g r e a c t i o n of SnS from s l a g s , and a p p l y i n g i t t o t h e ma t t e f o r ma t i o n :
S"0(SLAG) '"°^(!1ATTE) ~ ^"^{MATTE) * '"°°{SLAG) (1) f o r which:
K =
S u b s t i t u t i n g (2) i n t o ( 3 ) :
®SnS *FeO (3)
®FeS
MATTE *SnO SLAG
'SnS MATTE ‘SnS,
1ATTE FeSMATTE
'SnO TeO
Th e r e f o r e : a. PsnS ~ ^ * ^FeS ^"^MATTE ^ Î1ATTE ‘SnO ®FeO SLAG
Hence, t h e vapor p r e s s u r e of nure SnS i s h i g h e r , t h e h i g h e r t he a c t i v i t y o f SnO i n t he s l a g , and t h e lower t he FeO c o n t e n t t h e r e i n .
Now t h e vapor p r e s s u r e o f pure SnS can be c a l c u l a t e d from d a t a gi ven in Tabl e I I I (Ch, I I ) , and t he a c t i v i t y of SnS i n i r o n - t i n ma t t e s can be o b t a i n e d from t h e phase di agram given by Haan^^) and t he e n t h a l p y o f f u s i o n o f FeS^^^, Thus, t h e p a r t i a l p r e s s u r e of SnS vapor ove r Sn-Fe ma t t e s i s e s t i m a t e d t o be r e p r e s e n t e d by t h e f o l l o wi n g e q u a t i o n s :
Log PsnS = - —
+
f'sns +
'
"leS
(4)
AG° 2
PsnS
^ ~ pj^
^^SnS
• ''VeS
*
Where and AG° a r e t h e f r e e ener gy of v a p o r i z a t i o n and s u b l i m a t i o n r e s -
V s '
p e c t i v e l y . T h e i r e x p r e s s i o n s a r e gi ven in Tabl e I I I ( C h . I I ) ,
The compl et e d e r i v a t i o n of t h e s e two e q u a t i o n s i s gi ven i n Appendix I I , al ong wi t h t h e c a l c u l a t i o n o f t he phase diagram knowing t h e t e mn e r a t u r e s of f u s i o n , t h e e u t e c t i c t e mp e r a t u r e and c o mp o s i t i o n , and t h e e n t h a l p y o f f u s i o n of FeS assuming a r e g u l a r s o l u t i o n b e h a v i o r of t he l i q u i d phase and t h a t t h e r e i s no s o l i d s o l u b i l i t y i n t h e s ys t e m. The agreement of t h e s e r e s u l t s wi t h t h e d i a gram in t h e l i t e r a t u r e a ppea r s t o be good. Fi gur e 7 shows t h e phase diagram as o b t a i n e d by Haan^^^, and as c a l c u l a t e d assuming t h e r e g u l a r s o l u t i o n model f o r t h e l i q u i d phase.
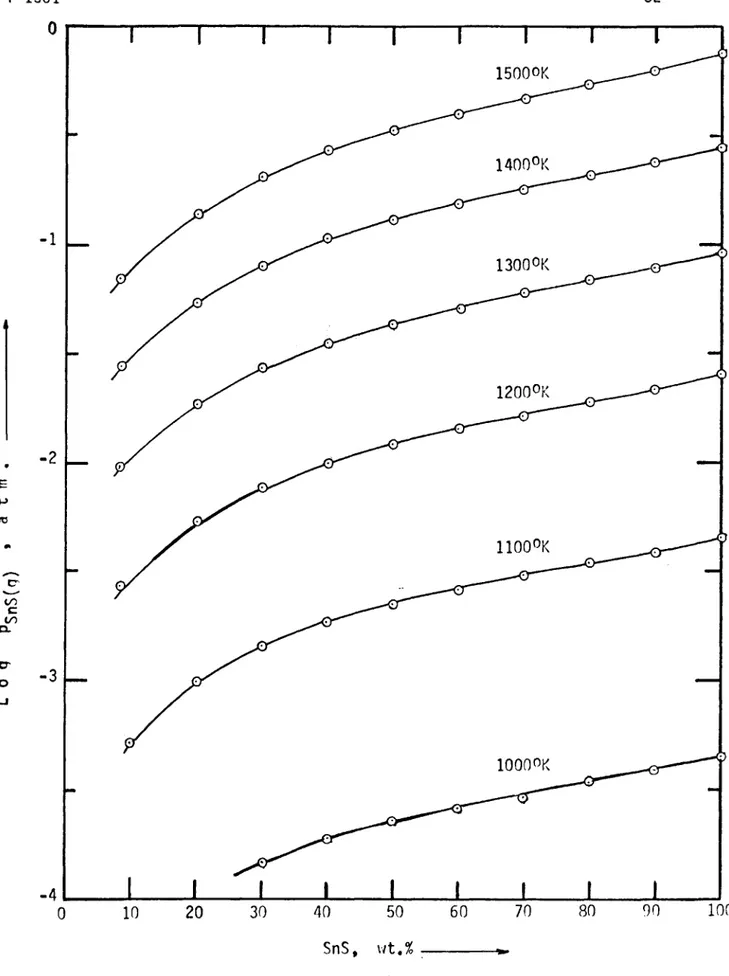
Fi gur e 8 shows t he v a r i a t i o n of t he p a r t i a l p r e s s u r e of SnS as a f u n c t i o n of SnS c o n t e n t i n t he ma t t e s f o r d i f f e r e n t t e mp e r a t u r e s as c a l c u l a t e d from equa t i o n s ( 4 ) , and ( 5 ) ,
in CO cri CO o cr. CO CM o c CD o o ro CM O O O CO O o o o CM cr o oo o o o CO CO CD D: CO <0 LU CO < CO 0) CO c CO CO vr D: c >-LU < cr a LU cr =3 LU O CL _ J X < LU C J
o □
cCJ <3 CO Do '3WniVo3dW31E 4-> TO OO c tr> CL cr o
0
1
2 3 -4 100 90 80 70 60 20 30 40 50 10 0 SnS, wt,%The a c c ur a c y o f t h e s e e s t i m a t e s i s dependent upon t h e a c c ur a c y t o which t he phase dianram was determined i n 1 9 1 3, and t h e v a l i d i t y o f t he r e n u l a r s o l u t i o n as sumption f o r t h e l i q u i d p has e. T h e r e f o r e , t he d e t e r m i n a t i o n o f t he vapor p r e s s u r e s o f SnS o ve r Fe-Sn ma ttes i n t h e l a b o r a t o r y i s not o n l y j u s t i f i e d but n e c e s s a r y f o r a b e t t e r c o n t r o l o f t h e t i n fuminq p r o c e s s .
IV. E X P E R I M E N T A L A P P A R A T U S
A N D P R O C E D U R E
Choice of Method
The t r a n s p o r t a t i o n ( t r a n s p i r a t i o n ) method was s e l e c t e d b e caus e i t r e q u i r e s a r e l a t i v e l y s i mp l e d e s i qn of a p p a r a t u s and be caus e i t has proved t o be s u c c e s s f u l i n meas ur i nq vapor p r e s s u r e s of a number o f e l e m e n t s , t h e i r h a l i d e s , o x i d e s , and s u l f i d e s , ove r a wide ranne of vapor p r e s s u r e s
-4
(between 10" t o lO"" mm Hn). I t has a l s o been e x t e n s i v e l y used t o d e t e r mi n e t he p a r t i a l p r e s s u r e s of v o l a t i l e components of an a l l o y o v e r t h e l i q u i d , or s o l i d a l l o y .
The l i t e r a t u r e q i v e s numerous a cc ount s of i n v e s t i n a t i o n s u s i n q t h i s method. There a r e ve r y many, and onl y t h e c l a s s i c a l and most i mp o r t a n t t o t h i s s t u d y a r e l i s t e d h e r e .
I t i s a dynamic met hod, by which a measured volume of an i n e r t nas ove r t he s o l i d or l i q u i d ma t t e becomes s a t u r a t e d wi t h t i n s u l f i d e v a p o r s . Thi s c a r r i e r gas i s pa s s e d a t a c o n s t a n t v e l o c i t y o v e r t h e s u b s t a n c e in t h e s a t u r a t i o n cham b e r , a t a c o n s t a n t t e m p e r a t u r e , and c a r r i e s away t he v o l a t i l e component s.
The vapor p r e s s u r e i s t hen de t e r mi ne d from t he l o s s in we i n h t of t he sample p e r u n i t volume of c a r r i e r nas.
The i mp o r t a n t c o n d i t i o n s of t h e t r a n s p o r t a t i o n method a r e : ( i ) A uni f or m t e mp e r a t u r e zone in t h e f u r n a c e , and
( i i ) t he geomet ry o f t he r e a c t i o n o r s a t u r a t i o n chamber.
Apparat us
The t r a n s p o r t a t i o n method t o de t e r mi ne t he vapor p r e s s u r e s of t i n s u l f i d e r e q u i r e d an a p p a r a t u s s u i t a b l e t o :
a) d e t e r mi n e t h e l o s s in we i n ht of a sample i n c o n t a c t wi t h a movinn qas pha se a t c o n s t a n t f l o w r a t e , a t a un i f or m, e l e v a t e d t e m p e r a t u r e , and
b) change f l o w r a t e s and t e mp e r a t u r e s f o r d i f f e r e n t s e t s of e x p e r i me n t s .
The a p p a r a t u s u t i l i z e d f o r t h i s purpose i s shown s c h e m a t i c a l l y i n Fi g . 9 , and a phot ogr aph i s gi ven in Fi g. 10, I t c o n s i s t e d o f two nas p u r i f i c a t i o n s y s t e ms , a gas mixing s ys t e m, a s a t u r a t i o n , and a c o n d e n s a t i o n s yst em.
Ni t r o ne n was used a t a high f l o w r a t e t o purge t h e f u r n a c e F3 and t he gas t r a i n s . I t was a l s o used as a c a r r i e r nas f o r measurements of SnS vapor p r e s s u r e s
over pure SnS(c) t o prove t h e r e l i a b i l i t y of t he a p p a r a t u s . Su r f a c e o x i d a t i o n took p l a c e in t he f i r s t e x p e r i me n t s . T h e r e f o r e n i t r o n e n was pass ed ove r pure copper t u r n i n g s a t 500^0 ( f u r n a c e FI) and t hen t hr ough two dr yi ng chambers c o n t a i n i n g s i l i c a gel and anhydrone (Mg(C10^)2) r e s p e c t i v e l y ( DI , D2) t o r e move t he w a t e r va por . Thi s pr oc e dur e proved t o be s u f f i c i e n t t o p r e v e n t o x i d a t i o n a t t he t e mp e r a t u r e s ment i oned.
Ni t r og e n coul d be f l u s h e d from t h e o p p o s i t e s i d e of t h e r e a c t i o n t ube i n f u r nace F3 by means of two s t o pc o c k s (T2 and S4 in Fi g. 9) so as t o make s u r e t h a t a i r was e v a c u a t e d as c o mp l e t e l y as p o s s i b l e .
The second gas p u r i f i c a t i o n syst em was used f o r t h e hydronen. Thi s gas was a l s o pas s e d over c opper t u r n i n g s a t 500^0 in f u r n a c e F2 t o remove oxygen i f p r e s e n t . From t h e r e , i t pass ed t hrough a c o n d u i t c o n t a i n i n g s i l i c a gel t o remove mo i s t u r e i f s t i l l p r e s e n t . Si nc e llg gas had t o be mixed 'with HgS gas t o a c t as t h e c a r r i e r gas a t d i f f e r e n t H2S/H2 r a t i o s , and in o r d e r t o p r e v e n t p o s s i b l e s e g r e g a t i o n due t o d i f f e r e n c e s in t e mp e r a t u r e and mo l e c u l a r we i g ht s d u r i n g t he mixing o p e r a t i o n , hydrogen was pass ed through a pyrex g l a s s s p i r a l (SF) a f t e r l e a v i n g t he p u r i f i c a t i o n f u r n a c e F2 and t h e d r y i n g t u b e . Thi s al l owed t he hydrogen t o cool do\/n t o room t e mp e r a t u r e b e f o r e e n t e r i n g flo' wmeter F;12.
CM f—f Li-