Doct or al D issert a tion in o D ont ol og y a rne Mor D enfelD M al M ö universit
arne MorDenfelD
on tissue reactions to anD
resorPtion of Bone suBstitutes
isbn 978-91-7104-393-1 on tissue rea ctions t o an D resor P tion of B one su B s titutes
O n t i s s u e r e ac t i O n s t O a n d r e s O r p t i O n O f b O n e s u b s t i t u t e s
Malmö University
Faculty of Odontology Doctoral Dissertations 2013
© Arne Mordenfeld 2013
Photographers: Arne Mordenfeld, Eva Larsson, Karin Edkvist Jonsson ISBN 978-91-7104-393-1
arne MOrdenfeld
On tissue reactiOns tO
and resOrptiOn Of bOne
substitutes
Department of Materials Science and Technology,
Malmö University
Department of Oral & Maxillofacial Surgery,
Gävle county hospital
Department of Biomaterials, The Sahlgrenska Academy,
University of Gothenburg, Sweden, 2013
This publication is also available at: www.mah.se/muep
To my mother and father – you would have been proud
“Somewhere, something incredible is waiting to be known”
This thesis represents number 44 in a series of investigations on implants, hard tissue and the locomotor apparatus originating from the Department of Biomaterials, University of Gothenburg and the department of Prosthodontics/Material sciences, Malmö University, Sweden.
1. Anders R Eriksson DDS, 1984. Heat-induced Bone Tissue Injury. An
in vivo investigation of heat tolerance of bone tissue and temperature rise in the drilling of cortical bone. Thesis defended 21.2.1984. External examiner: Docent K-G. Thorngren.
2. Magnus Jacobsson MD, 1985. On Bone Behaviour after Irradiation.
Thesis defended 29.4.1985. External examiner: Docent A. Nathanson.
3. Fredric Buch MD, 1985. On Electrical Stimulation of Bone Tissue.
Thesis defended 28.5.1985. External examiner: Docent T. Ejsing-Jörgensen.
4. Peter Kälebo MD, 1987. On Experimental Bone Regeneration in
Titanium Implants. A quantitative microradiographic and histologic investigation using the Bone Harvest Chamber. Thesis defended 1.10.1987. External examiner: Docent N. Egund.
5. Lars Carlsson MD, 1989. On the Development of a new Concept for
Orthopaedic Implant Fixation. Thesis defended 2.12.1989. External examiner: Docent L-Å Broström.
6. Tord Röstlund MD, 1990. On the Development of a New Arthroplasty.
Thesis defended 19.1.1990. External examiner: Docent Å. Carlsson.
7. Carina Johansson Res Tech, 1991. On Tissue Reaction to Metal
Implants. Thesis defended 12.4.1991. External examiner: Professor K. Nilner.
8. Lars Sennerby DDS, 1991. On the Bone Tissue Response to Titanium
Implants. Thesis defended 24.9.1991. External examiner: Dr J.E. Davies.
9. Per Morberg MD, 1991. On Bone Tissue Reactions to Acrylic Cement.
Thesis defended 19.12.1991. External examiner: Docent K. Obrant.
10. Ulla Myhr PT, 1994. On Factors of Importance for Sitting in Children
with Cerebral Palsy. Thesis defended 15.4.1994. External examiner: Docent K. Harms-Ringdahl.
11. Magnus Gottlander MD, 1994. On Hard Tissue Reactions to
Hydroxyapatite-Coated Titanium Implants. Thesis defended 25.11.1994. External examiner: Docent P. Aspenberg.
12. Edward Ebramzadeh MScEng, 1995. On Factors Affecting Long-Term
Outcome of Total Hip Replacements. Thesis defended 6.2.1995. External examiner: Docent L. Linder.
13. Patricia Campbell BA, 1995. On Aseptic Loosening in Total Hip
Replacement: the Role of UHMWPE Wear Particles. Thesis defended 7.2.1995. External examiner: Professor D. Howie.
14. Ann Wennerberg DDS, 1996. On Surface Roughness and Implant
Incorporation. Thesis defended 19.4.1996. External examiner: Professor P-O. Glantz.
15. Neil Meredith BDS MSc FDS RCSm 1997. On the Clinical
Measurement of Implant Stability Osseointegration. Thesis defended 3.6.1997. External examiner: Professor J. Brunski.
16. Lars Rasmusson DDS, 1998. On Implant Integration in
Membrane-Induced and Grafter Bone. Thesis defended 4.12.1998. External examiner: Professor R. Haanaes.
17. Thay Q Lee MSc, 1999. On the Biomechanics of the Patellfemoral
Joint and Patellar Resurfacing in Total Knee Arthroplasty. Thesis defended 19.4.1999. External examiner: Docent G. Nemeth.
18. Anna Karin Lundgren DDS, 1999. On Factors Influencing Guided
Regeneration and Augmentation of Intramembraneous Bone. Thesis defended 7.5.1999. External examiner: Professor B. Klinge.
19. Carl-Johan Ivanoff DDS, 1999. On Surgical and Impant Related
Factors Influencing Integration and Function of Titanium Implants. Experimental and Clinical Aspects. Thesis defended 12.5.1999. External examiner: Professor B. Rosenquist.
20. Bertil Friberg DDS MDS, 1999. On Bone Quality and Implant
Stability Measurements. Thesis defended 12.11.1999. External examiner: Docent P. Åstrand.
21. Åse Allansdotter Johansson MD, 1999. On Implant Integration in
Irradiated Bone. An Experimental Study of the Effects of Hyperbaric Oxygeneration and Delayed Implant Placement. Thesis defended 8.12.1999. External examiner: Docent K. Arvidsson-Fyrberg.
22. Börje Svensson FFS, 2000. On Costochondral Grafts Replacing
Mandibular Condyles in Juvenile Chronic Arthritis. A Clinical, Histologic and Experimental Study. Thesis defended 22.5.2000. External examiner: Professor Ch. Lindqvist.
23. Warren Macdonald BEng, MPhil, 2000. On Component Integration
on Total Hip Arthroplasties: Pre-Clinical Evaluations. Thesis defended 1.9.2000. External examiner: Dr A.J.C. Lee.
24. Magne Røkkum MD, 2001. On Late Complications with HA Coated
Hip Asthroplasties. Thesis defended 12.10.2001. External examiner: Professor P. Benum.
25. Carin Hallgren Höstner DDS, 2001. On the Bone Response to
Different Implant Textures. A 3D analysis of roughness, wavelength and surface pattern of experi-mental implants. Thesis defended 19.11.2001. External examiner: Professor S. Lundgren.
26. Young-Taeg Sul DDS, 2002. On the Bone Response to Oxidised
Titanium Implants: The role of microporous structure and chemical composition of the surface oxide in enhanced osseointegration. Thesis defended 7.6.2002. External examiner: Professor J.-E. Ellingsen.
27. Victoria Franke Stenport DDS, 2002. On Growth Factors and
Titanium Implant Integration in Bone. Thesis defended 11.6.2002. External examiner: Associate Professor E. Solheim.
28. Mikael Sundfeldt MD, 2002. On the Aetiology of Aseptic Loosening
in Joint Arthroplasties and Routes to Improved cemented Fixation. Thesis defended 14.6.2002. External examiner: Professor N. Dahlén.
29. Christer Slotte CCS, 2003. On Surgical Techniques to Increase Bone
Density and Volume. Studies in the Rat and the Rabbit. Thesis defended 13.6.2003. External examiner: Professor C.H.F. Hämmerle.
30. Anna Arvidsson MSc, 2003. On Surface Mediated Interactions
Related to Chemomechanival Caries Removal. Effects on surrounding tissues and materials. Thesis defended 28.11.2003. External examiner: Professor P. Tengvall.
31. Pia Bolind DDS, 2004. On 606 retrieved oral and cranio-facial
implants. An analysis of consecutively received human specimens. Thesis defended 17.12.2004. External examiner: Professor A. Piattelli.
32. Patricia Miranda Burgos DDS, 2006. On the influence of micro-
and macroscopic surface modifications on bone integration of titanium implants. Thesis defended 1.9.2006. External examiner: Professor A. Piattelli.
33. Jonas P Becktor DDS, 2006. On factors influencing the outcome
of various techniques using, endosseous implants for reconstruction of the atrophic edentulous and partially dentate maxilla. Thesis defended 17.11.2006. External examiner: Professor K.F. Moos.
34. Anna Göransson DDS, 2006. On Possibly Bioactive CP Titanium
Surfaces. Thesis defended 8.12.2006. External examiner: Professor B. Melsen.
35. Andreas Thor DDS, 2006. On platelet-rich plasma in reconstructive
dental implant surgery. Thesis defended 8.12.2006. External examiner: Professor E.M. Pinholt.
36. Luiz Meirelles DDS MSc, 2007. On Nano Size Structures For
Enhanced Early Bone Formation. Thesis defended 13.6.2007. External examiner: Professor Lyndon F. Cooper.
37. Pär-Olov Östman DDS, 2007. On various protocols for direct loading
of implant-supported fixed prostheses. Thesis defended 21.12.2007. External examiner: Professor B. Klinge.
38. Kerstin Fischer DDS, 2008. On immediate/early loading of implant
supported prostheses in the maxilla. Thesis defended 8.2.2008. External examiner: Professor K. Arvidsson Fyrberg.
39. Alf Eliasson 2008. On the role of number of fixtures, surgical
technique and timing of loading. Thesis defended 23.5.2008. External examiner: Professor K. Arvidsson Fyrberg.
40. Victoria Fröjd DDS, 2010. On Ca2+ incorporation and nanoporosity
of titanium surfaces and the effect on implant performance. Thesis defended 26.11.2010. External examiner: Professor J.E. Ellingsen.
41. Lory Melin Svanborg DDS, 2011. On the importance of nanometer
structures for implant incorporation in bone tissue. Thesis defended 01.06.2011. External examiner: Associate professor C. Dahlin.
42. Byung-Soo Kang Msc, 2011. On the bone tissue response to
surface chemistry modifications of titanium implants. Thesis defended 30.09.2011. External examiner: Professor J. Pan.
43. Kostas Bougas DDS, 2012. On the influence of biochemical coating
on implant bone incorporation. Thesis defended 12.12.2012. External examiner: Professor T. Berglundh.
44. Arne Mordenfeld DDS, 2013. On tissue reactions to and resorption
of bone substitutes. Thesis to be defended 29.5.2013. External examiner: Professor C. Dahlin.
table Of cOntents
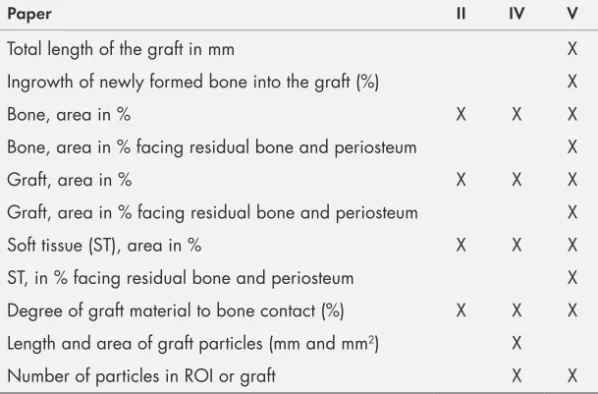
LIST OF PAPERS ... 13 ABSTRACT ... 15 POPULÄRVETENSKAPLIG SAMMANFATTNING ... 19 ABBREVIATIONS ... 21 INTRODUCTION ... 23 Bone biology ...24Bone cells and their functions ...25
Bone formation and remodelling ...27
Different types of graft materials ...29
Graft healing ...34 Surgical techniques ...39 AIMS ... 43 General aim ...43 Specific aims ...43 HYPOTHESES ... 45
MATERIALS AND METHODS ... 47
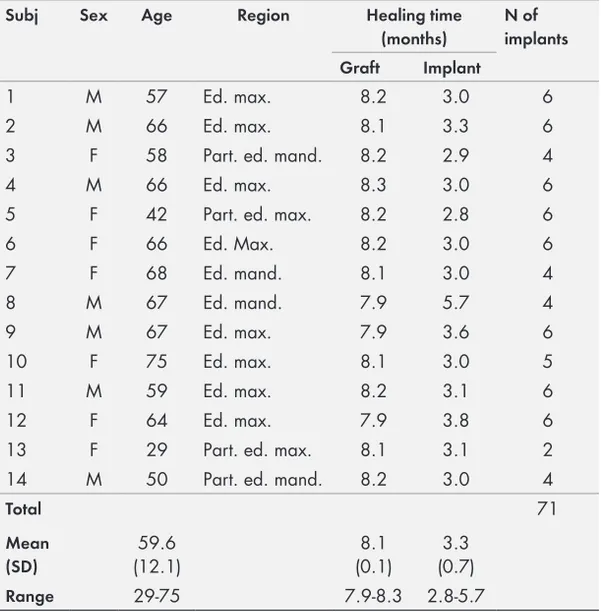
Subjects and experimental outlines ...47
Ethical considerations ...49
Preoperative examinations, inclusion and exclusion criteria ...50
Grafting materials...50
Dental implants ...52
Drop-outs ...52
Clinical follow up ...58
Histology and histomorphometry ...61
Statistics ...63
RESULTS ... 67
Clinical results ...67
Radiographic results ...70
Histology and histomorphometric results ...73
DISCUSSION ... 81
Paper I ...81
Paper II ...84
Papers III and IV ...86
Paper V ...91
Clinical applications and future perspectives ...94
CONCLUSIONS ... 97
ACKNOWLEDGEMENTS ... 99
REFERENCES ...103
list Of papers
This dissertation is based on the following papers, which will be referred to in the text by their Roman numerals (papers reprinted by kind permission of journal editors):
I. Mordenfeld A, Hallman M, Lindskog S. Tissue reactions to subperiosteal onlays of demineralized xenogenous dentin blocks in rats. Dent Traumatol. 2011 Dec;27(6):446-51. II. Lindgren C, Mordenfeld A, Johansson C.B, Hallman M.
A 3-year clinical follow-up of implants placed in two different biomaterials used for sinus augmentation. Int J Oral Maxillofac Implants. 2012 Sep;27(5):1151-62.* III. Mordenfeld A, Albrektsson T, Hallman M. A 10-year clinical
and radiographic study of implants placed after maxillary sinus floor augmentation with an 80:20 mixture of deproteinized bovine bone and autogenous bone. Clin Implant Dent Relat Res. 2012. Epub ahead of print. DOI: 10.1111/cid.12008 IV. Mordenfeld A, Hallman M, Johansson C.B, Albrektsson T.
Histological and histomorphometrical analyses of biopsies harvested 11 years after maxillary sinus floor augmentation with deproteinized bovine and autogenous bone. Clin Oral Implants Res. 2010 Sep;21(9):961-70.
V. Mordenfeld A, Johansson C.B, Albrektsson T, Hallman M. A randomized and controlled clinical trial of two different compositions of deproteinized bovine bone and autogenous bone used for lateral ridge augmentation. Clin Oral Implants Res. 2013. Epub ahead of print. DOI: 10.1111/clr.12143
*This publication has previously been included in a thesis entitled “On healing of titanium implants in biphasic calcium phosphate”, presented for Doctor of medicine degree, defended by Christer Lindgren at Linköping University 2012.
abstract
Background: The increasing need for bone grafting procedures in
implant dentistry and the introduction of a variety of bone substitutes require a deeper understanding of the biological response and short- and long-term behaviour of these materials to choose the adequate graft and surgical procedure for the intended clinical application.
Aims: The overall aim was to clinically and histologically study
the short- and long-term tissue reactions to and resorption of bone substitutes after bone augmentation.
Material and methods: In paper I, dentin blocks with different
demi-neralization times were placed subperiostally in 40 rat skulls. After a healing period of 4 weeks the rats were sacrificed and the healing of the dentin blocks were evaluated. In paper II, eleven patients were treated with bilateral sinus floor augmentation using biphasic calcium phosphate (BCP) on one side and deproteinized bovine bone (DPBB) on the contralateral side, acting as control. After 3 years, biopsies were retrieved from the grafted area for histological evaluation and histomorphometry and 62 dental implants, placed 8 months after graft healing, were clinically evaluated. In paper III and IV, fourteen (22 sinuses) of the included 20 patients (30 sinuses) treated with sinus floor augmentation with a mixture of 80% DPBB and 20% autogenous bone (AB) from the chin were followed throughout the 10 years study period. These patients had 53 implants placed in grafted sites and 15 implants placed in non-grafted bone. Clinical and radiographic examinations were performed. Biopsies were retrieved from the grafted sinuses after 11 years of graft healing for
histological evaluation and histomorphometry. The particle sizes were compared with samples retrieved after 6 months from the same patients and pristine particles from the manufacturer. In paper V, 13 patients (14 jaws) were treated with lateral ridge augmentation using 2 different mixtures of DPBB:AB (90:10 and 60:40) in a randomized and controlled trial, designed as a split mouth study. The width and volume changes were evaluated after 7.5 months by means of cone beam computed tomography. After 8 months of graft healing, at the time of implant placement, biopsies were retrieved for histological evaluation and histomorphometry.
Results: Resorption increased with increasing degree of
demineraliza-tion of dentin blocks while bone formation increased with increasing
degree of demineralization, in the latter case provided inflammation was compensated for (paper I). After 3 years of healing the BCP particles showed different levels of dissolution, in contrast to DPBB particles that showed no signs of resorption. The overall implant survival rate was 96.8% and the success rate for implants placed in BCP and DPBB was 91.7% and 95.7% respectively (paper II). The cumulative survival rate of the implants after 10 years was 86% and the marginal bone loss was 1.6 mm. There was only a reduction in graft height between 3 months and 2 years but no further reduction up to 10 years (paper III). There was no difference between the size of DPBB particles after 11 years compared to those measured after 6 months or to particles from the manufacturer (paper IV). The gain in width of the alveolar crest was 3.5 mm and 2.9 mm and the reduction of the grafts were 37% and 47% for the 60:40 mixture and 90:10 mixture respectively (significant differences). There were no histomorphometrical differences between the groups (paper V).
Conclusions: Partial demineralization may provide a method for
optimizing the integration of dentin onlays. A similar degree of bone formation and bone-to-graft contact for BCP and DBB was found 3 years after maxillary sinus augmentation with similar success rates for implants placed in both grafting materials. At 10 years follow-up after sinus floor augmentation with 80:20 (DPBB:AB) graft, the remaining implants presented good clinical and radiological results and there seems to be no further graft resorption after 2 years of
graft healing. DPBB particles were found to be well integrated in lamellar bone, showing no apparent signs of resorption after 11 years in humans. Despite a small difference in width changes after lateral ridge augmentation, the amount of AB added to DPBB did not seem to have a major impact on the graft healing and graft reduction, thus making it possible to install implants in all grafted sites.
pOpulÄrVetensKapliG
saMManfattninG
Tandlöshet och tandprotesbärande innebär ofta ett stort socialt handikapp. Idag är tandimplantat en väl etablerad metod för att ersätta tandförluster. I vissa fall saknas tillräcklig benmängd för att kunna installera implantat på ett adekvat sätt eller på rätt position för att uppnå ett fullgott estetiskt resultat. Transplantat med ben från annan del av patientens käkar eller från höften har varit den mest accepterade metoden för att bygga upp det förlorade benet efter tandförluster. Detta ingrepp innebär ytterligare kirurgi, som inte sällan måste utföras i narkos, med ökade besvär för patienten. För att minska patientens besvär och sjukvårdens kostnader har benersättningsmaterial blivit allt vanligare både inom käkkirurgin och i den ortopediska verksamheten. Det ökade behovet av bentransplantationer på senare år och introduktionen av nya material på marknaden ökar vikten av kunskap om hur materialen fungerar kortsiktigt och långsiktigt. Det är angeläget att minimera ogynnsamma vävnadsreaktioner och att välja rätt material i det individuella fallet för ett optimalt slutresultat.
Denna avhandlings övergripande syfte var att kliniskt och histo-logiskt utvärdera vävnadsreaktioner efter transplantation med dentin (tandben), ett syntetiskt benersättningsmaterial (keramiskt material) och speciellt behandlat kalvben, både kortsiktigt och långsiktigt, samt att bedöma materialens eventuella resorption.
I den första studien utvärderades möjligheten att påverka benin-läkningen vid transplantation (till råttor) av dentin med olika urkalkningsgrad.
I studie II utvärderades vävnadsreaktionerna och materialens resorption 3 år efter transplantation av syntetiskt benersättnings-material och kalvben till bihålorna för att öka benmängden inför behandling med tandimplantat. Dessutom utvärderades tandimplan-tatens 3-års överlevnad och status.
I studie III och IV utvärderades vävnadsreaktionerna och materialets resorption 10-11 år efter transplantation med kalvben till bihålorna och tandimplantatens överlevnad samt status.
I studie V utvärderades betydelsen av olika mängd tillsatt eget ben till kalvben för transplantatets inläkning vid uppbyggnad av extremt tunna käkben och volymstabilitet inför installation av tandimplantat. Studierna visar att:
• Partiell urkalkning kan vara en fungerande metod för att optimera beninläkningen av dentin.
• Det var ingen skillnad i beninläkning efter 3 år i bihålan mellan kalvben och syntetiskt benersättningsmaterial. Det senare uppvisade dock en högre grad av inflammation. Lyckande- graden för tandimplantat var lika hög i båda transplantaten (96%).
• Det tycks inte föreligga några volymsförändringar av
kalvben mellan 2 och 10 år efter transplantation till bihålan. Tandimplantaten uppvisade goda kliniska och radiologiska 10-års resultat oavsett om de var placerade i transplanterat eller eget ben.
• Kalvbenet var mycket väl inläkt 11 år efter transplantation till bihålan och kalvbenspartiklarna uppvisade inga uppenbara tecken på resorption.
• Det var lika god inläkning av transplantaten om det hade tillsatts 40% eller 10% eget ben till kalvbenet vid uppbyggnad av tunna käkar. Det var något mindre breddvinst med 10% eget ben, men i samtliga 14 fall lyckades man installera tandimplantat 8 månader efter benuppbyggnaden oavsett vilken transplantatblandning som använts.
• Det finns idag benersättningsmaterial på marknaden som fungerar väl vid uppbyggnad av käkben inför
implantatbehandling och sålunda minskar både patientens lidande och kostnaderna för sjukvården.
abbreViatiOns
AB Autogenous bone
BCP Biphasic calcium phosphate
BMP Bone morphogenetic protein
BMU Basic multicellular unit
BoP Bleeding on probing
CaP Calcium phosphate
CB/CT Cone beam computed tomography
CSR Cumulative survival rate
DPBB Deproteinized bovine bone
EDTA Ethylene-diamine-tetra-acetic acid
GBR Guided bone regeneration
HA Hydroxyapatite
IGF Insulin-like growth factor
IL Interleukin
ISQ Implant stability quotient
M-CSF Macrophage colony-stimulating factor
MGC Multinucleated giant cells
MMP Matrix metalloproteinase OB Osteoblast OC Osteocyte OPG Osteoprotegerin OPN Osteopontin PD Pocket depth PI Plaque index PMN Polymorphonuclear neutrophil PTH Parathyroid hormone
RANKL Receptor activator of NF-κβ ligand
RFA Resonance frequency analysis
RGD Arginine-glycine-aspartic acid
SBI Sulcus bleeding index
TBV Total bone volume
TCP Tricalcium phosphate
TGF Transforming growth factor
intrOductiOn
The loss of teeth is commonly caused by caries, periodontal disease, trauma and infection. Edentulous or partially edentulous people may have aesthetical, social and functional problems. Implant supported prosthesis is a procedure that successfully has been used as an alternative treatment to fixed dental supported bridges and
removable dentures in the past 30 years with good results.1 As a
consequence of tooth loss the alveolar bone is resorbed in various
degrees,2-4 often resulting in inadequate bone volume to allow dental
implant placement or restricting ideal positioning of the dental implants. In addition to resorption of the alveolar crest, inadequate bone volume is also caused by pneumatised sinuses of the posterior
maxilla.5 When there is a lack of bone height or bone width, bone
grafts may be harvested from the jaws or the iliac crest and grafted to the area in need of bone reconstruction. After a period of graft healing, implants can be successfully installed in the reconstructed area. Autogenous (from the same person) bone (AB) has been considered the “gold standard” in bone reconstruction. However,
this procedure is associated with morbidity at the donor site,6
prolonged operating time, higher costs, sometimes a need of general anaesthesia and the subsequent degree of graft resorption may be
difficult to predict.7-9
In order to reduce morbidity and to simplify the procedure bone substitutes have been used instead of or in combination with AB. Due to higher aesthetical demands combined with an increase of dental implant treatment, the need for bone grafts and bone substitutes has dramatically increased in recent years.
Some authors suggest that the ideal bone substitute should be
biocom-patible, resorbed and replaced by new bone,10 and should possess
properties that may enhance bone graft incorporation (i.e.
osteoinduc-tive and osteoconducosteoinduc-tive characteristics)11 without inducing unwanted
side effects such as excess inflammation-induced resorption.
As a result of tooth avulsion and injury to the periodontal membrane the root may be replaced by bone (ankylosis) after
replantation.12 Furthermore, it has been suggested that dentin
possesses both osteoinductive (contains BMP) and osteoconductive properties. These facts may indicate that dentin might function as a bone substitute even if some results from animal studies are
cont-radictory.13-15 Further development of the pre-operative preparation
of the dentin material and optimizing suitable osteoconductive environment may enhance graft integration.
One of the most documented bone substitutes for intra oral
augmentation is Bio-Oss® (Geistlich, Wolhusen, Switzerland), a
deproteinized and sterilized bovine bone.16-18 Since all proteins are
removed from the material, AB is often combined with BioOss®
when grafted to the jaws to add osteoinductive properties. Further clinical and histological studies are necessary to investigate the fate
of Bio-Oss® grafts in the long-term. In addition, it is not known if
the combination of Bio-Oss® and AB contributes to a better healing
and less resorption in lateral ridge augmentation.19
Since biological bone substitutes have a potential risk of disease
transmission.20, 21 there has been a growing market for synthetic
bone substitutes. Several synthetic bone substitutes have been used
for sinus floor augmentation.22, 23 Recently a novel biphasic calcium
phosphate (BCP), (BoneCeramic®, Straumann, Basel, Switzerland),
was introduced to the market, however long term clinical and
histo-logical studies of this graft material are missing.18
The increasing need for bone grafting procedures in the recent years and the introduction of a variety of bone substitutes requires a greater understanding of the biology of bone and bone grafts to choose the adequate graft and surgical procedure for the intended clinical application.
bOne biOlOGy
Bone biology developments in recent years have nicely been
human body is composed of 213 bones and make up about 20% of
the body mass.24 The bones can be categorized as long bones (i.e.
humerus, femur and tibia), short bones (i.e. carpals and tarsals), irregular bones (i.e. mandible, vertebrae and sacrum) and flat bones (i.e. scapula, sternum and the scull). Bones have several functions including support and protection of vital organs; attachments for muscles, ligaments and tendons; locomotion; support of haemato-poiesis in bone marrow; reservoir for and regulation of minerals
(in particular calcium); and repair of bony defects and fractures.25,
26 The bones are composed of cortical or compact bone (80%) and
trabecular bone (20%). Cortical bone is mainly found in the surface of flat bones and in the shaft of long bones. The cortical bone has an outer layer called periosteum, consisting of an outer and inner fibrous layer, whose osteogenic potential is important for apposi-tional growth and fracture healing. The inner surface of the cortical bone and the surfaces of the trabecular bone are covered by the endosteum. The trabecular bone accounts for about 70% of the bone
metabolism and ensures the stability and elasticity of the skeleton.24,
27 Fifty to 70% of the bone is composed of mineral, mainly
hydroxy-apatite, Ca10(PO4)6(OH)2), and small parts of magnesium, carbonate
and acid phosphate. The bone matrix (20% to 40% of the bone) consists of type I collagen (90%) and non-structural fibres like osteocalcin, bone sialoprotein, osteopontin (OPN), osteonektin and a great number of growth factors e.g. bone morphogenetic proteins (BMPs), insulin-like growth factors (IGFs) and transforming growth
factor-β (TGF-β). The remaining components consist of 5% to 10%
water and < 3% of lipids.24
bOne cells and their functiOns
There are three types of bone cells defined which are responsible for the bone turnover and healing of fractures: osteoblasts, osteocytes and osteoclasts.
Osteoblasts (OB)
The primary function of the OBs is bone formation. They are derived from the multipotential mesenchymal stem cells alternatively capable of differentiating into fibroblasts, chondrocytes, myoblasts and adipocytes. In osteoblast differentiation, 4 stages of maturation
have been identified according to Kular and co-workers28;
preosteob-last, osteobpreosteob-last, osteocyte and bone-lining cells (non-active flattened osteoblasts). OBs secrete type I collagen and produce non-collage-nous proteins including osteocalcin and alkaline phosphatase, which is responsible for mineral deposition. Approximately 50% to 70% of OBs undergo apoptosis and the rest become bone-lining cells or osteocytes. Macrophage colony-stimulating factor (M-CSF),
osteo-protegerin (OPG) and cytokine receptor activator of NFκβ ligand
(RANKL), factors essential for osteoclast formation and function,
are produced by OBs.28
Osteocytes
An osteocyte is a cell located in the bone matrix. The osteocytes represent 95% of all bone cells. They are often regarded as terminally differentiated osteoblasts entrapped and incorporated initially in osteoid (the non-mineralized protein layer between the osteoblasts and the mineralized bone) and then, after mineralization, in more mature, mainly cortical bone. The osteocytes are smaller than osteoblasts and lie in lacunae, spatially separated from each other; however, dendritic processes that extend from their surfaces and traverse the bone within canaliculi connect them with each other
as well as bone-lining cells and osteoblasts on the bone surface.28,
29 There seem to be an evidence based fact that osteocytes act as
mechano-sensors in bone and control bone formation. In addition, recent research suggest that osteocytes also may play an important role in bone resorption by providing the majority of RANKL that
controls osteoclast formation in cancellous bone,30, 31 i.e. osteoblasts/
osteocytes share a combined action mechanism with osteoclasts.
Osteoclasts (OC)
OC is the only cell type that can resorb bone. They are members of the monocyte macrophage lineage, derived from the hematopoietic stem cells, and are formed via multiple cellular fusions from the mononuclear precursors. A differentiated human osteoclast contains approximately five to eight nuclei. After starting resorption, the osteoclasts have a limited life-span, however the exact mean age
is not known, and finally they die via apoptosis.32 Therefore, they
area. OCs have a unique capacity to dissolve bone mineral and in the end of the resorption process they also perform an enzymatic
degradation of the organic bone matrix.32 The osteoclast precursors
express receptor activator of NFκβ (RANK). Osteoclast formation,
activation and resorption are regulated by the ratio of RANKL (which binds to RANK and activates osteoclastogenesis) to OPG (which inhibits osteoclastogenesis), Interleukin-1 (IL-1) and Interleukin 6 (IL-6), M-CSF, parathyroid hormone,
1,25-dihydroxy-vitamin D (1,25(OH)2-D3), and calcitonin.33, 34 A prerequisite for
the OCs to start the resorption process is the attachment to the
bone matrix and formation of a sealing zone.35 Osteoblasts secrete
matrix metalloproteinases (MMPs) which degrades the osteoid, lining the bone surface, and expose matrix proteins containing arginine-glycine-aspartic acid (RGD) motifs. These adhesion sites
are necessary to facilitate osteoclast attachment.36 OCs bind to bone
matrix peptides via integrin receptors in the osteoclast membrane.29
The main integrin receptor mediating this osteoclast binding is ανβ3
integrin. The ligands for ανβ3 integrin include osteopontin and bone
sialoprotein.24, 36 Once attached to the bone surface, the OC forms
a ruffled boarder on the surface of the cytoplasm facing the bone. Secretion of hydrochloride acid starts in the compartment beneath the OCs called resorption lacuna, creating a low pH and subse-quently dissolves the hydroxyapatite. In addition, matrix degrading proteases, mainly cathepsin K, TRAP and MMPs are secreted to
digest the bone matrix.32
The induction of bone resorption is mainly regulated by indirect mechanisms. These involve up regulation of the expression of M-CSF and RANKL by osteoblasts, osteocytes and other cells. In addition to the capacity of bone resorption, OCs also regulate osteoblast
functions.24
bOne fOrMatiOn and reMOdellinG
Two types of bone formation have been described: endochondral ossi-fication (the most common mechanism of primary bone formation) and intramembranous ossification. The endochondral ossification occurs in the long bones, skull base, vertebral column and pelvis. The ossification is preceded by a cartilage template. The cartilage is matured involving hypertrophy of the chondrocytes leading to
matrix erosion. The remaining cartilage matrix mineralizes, the chondrocytes regress and dies, and the calcified cartilage model is invaded by blood vessels carrying primitive mesenchymal stem cells which may be differentiated into osteoblasts and subsequently start
bone formation.37
In intramembranous ossification, bone is synthesized initially
without the mediation of a cartilage phase.38 The flat bones of
the skull, the clavicle and the mandible are formed through intra-membranous ossification. The primitive mesenchymal cells initially condense into a rich vascularized connective tissue layer. The diffe-rentiation of primitive mesenchymal cells into osteoblasts starts and a trabecular pattern of early bone matrix is produced. Bone matrix is matured through cellular synthesis and secretion of bone matrix components. Thereafter, calcium phosphate in the form of
hydroxy-apatite (HA) crystals is deposited at the bone matrix site.37
During adult life old bone, with a high prevalence of micro fractures, is continuously replaced by new bone. Under normal physiological conditions equal amount of bone is formed as the amount of bone resorbed, keeping the total bone mass unchanged. This process is called bone remodelling and is aiming at the maintenance of skeletal mechanical properties and support mineral
homeostasis.39 The duration of the resorption process is
approxima-tely 3 to 4 weeks and the duration of the following bone formation is approximately 2 to 4 months. Every year approximately 10% of the skeleton (25% of the trabecular and 3% of the cortical bone) is replaced by new bone, thus after 10 years the whole skeleton
is renewed.39 Three calcium-regulation hormones control serum
calcium: 1,25(OH)2-D3 and; parathyroid hormone (PTH), which stimulates bone resorption, and; an inhibitor of osteoclast resorption,
calcitonin.34, 39 Bone remodelling is a complex process starting with
the creation of bone resorption cavities by osteoclasts and continues with bone formation by osteoblasts tightly coupled within a basic multicellular unit (BMU). Concentric lamellae of bone in the walls of the cavities are produced by the osteoblasts forming osteons. Four distinct phases can be distinguished in this process: activation,
different types Of Graft Materials
The terminology of different types of bone grafts is based on the genome of the donor and the recipient’s immune-surveillance as
described by Urist.40 The term graft applies to viable bone and
implant applies to non-viable bone or synthetic bone substitute
materials.40
Autogenous bone (AB) graft
AB grafts originates from the same individual and is considered “gold standard” in reconstruction of defects in the jaws due to the
osteoinductive and osteoconductive properties41 in conjunction with
the absent risk of disease transmission and minimal cost. The most common donor sites in pre-implant reconstruction of the alveolar crest and the sinus floor are the mandibular retromolar area, the
mandibular ramus, the chin and the iliac crest.16, 42-45 AB has been
used extensively for horizontal and vertical augmentation of the
alveolar crest as well as for sinus floor augmentation.45-49 AB can be
grafted as a bone block or in particulated form using either cortical, cancellous or cortico-cancellous grafts depending on the mechanical stability and volume needed. Particulated bone grafts have mainly been used in small peri-implant defects such as dehiscenses and
fene-strations associated with guided bone regeneration,50, 51 but also in
larger defects and larger edentulous areas.48, 52 Gordh and Alberius53
reported some basic factors essential to block graft persistence: (i) Embryonic origin, membranous bone seem to be superior over enchondral bone probably due to difference in revascularization and biological difference; (ii) revascularization, incorporation, remodelling and maintenance of the onlay bone graft is related to the rate of revascularization; (iii) graft structure, the cortico-cancellous ratio and the anatomical structure of the cancellous part of the graft seem to influence the rate of revascularization and subsequently determine graft volume persistence; (iv) rigid fixation, is believed to
decrease the rate of graft resorption and enhance graft integration.54
Bone substitutes
In this context, bone substitutes are defined as non-autogenous bone grafts including allogeneic grafts/implants, xenogeneic grafts/ implants and alloplastic grafts. It has been suggested that the optimal
bone substitute should possess osteoinductive, osteoconductive and osteogenetic properties in combination with no risk of transferring infectious diseases. Furthermore, bone substitutes ought to undergo remodelling, be readily available at an acceptable cost, manageable,
biocompatible and bioresorbable.55
Allogeneic graft/implant
Allogeneic bone originates from another individual of the same species. All of the components in bone, i.e. cells, collagen, ground substances and inorganic minerals, are potentially immunogenic. Bone minerals do not seem immunogenic and collagen is only weakly antigenic. During resorption of the old matrix, allogeneic implants are revascularized by invasion of capillary sprouts from the host bed. Transplantation of allogeneic cortical and cancellous bone leads to an immunologic response, which delays graft incorporation,
revascularization, resorption and appositional bone formation.56 In
order to sterilize and minimize the immunogenic properties, the graft may be processed by freeze-drying, demineralization, deep freezing (< -70°C), chemo sterilization or radiation. Cortical bone is preferable due to its high content of collagen compared to cancellous bone, resulting in a weaker immunologic reaction. Puros® (MCBA; Zimmer Dental GmbH), a solvent-dehydrated, limited-dose,
gamma-irradiated source of allogeneic human bone57 is an example of an
allogeneic implant available on the market.
Xenogeneic graft/implant
Xenogeneic bone grafts originate from another species than the host. Furthermore, bone-like minerals derived from corals or algae
may be included among the xenogeneic bone substitutes58. The
organic portion is removed to obtain safety of disease transmission
and to avoid immunologic rejection. Algipore® (Dentsply Friadent,
Mannheim, Germany) is a biologic HA derived from calcified maritime algae and the organic components are completely removed by hydrothermal conversion of the calcium carbonate in the presence
of ammonium phosphate at about 700°C.59 Algipore® has been used
in periodontal treatment60 and for sinus floor augmentation.61
Deproteinized bovine bone (DPBB) is a natural bone mineral, characterized by its high structural and chemical similarity to bone.
The most documented DPBB used for augmentation in the
maxillo-facial region is Bio-Oss® (Geistlich, Wolhusen, Switzerland).
Bio-Oss® has been investigated extensively in both experimental62-65
and clinical studies.18, 22, 66-75 Since all proteins are claimed to have
been extracted, Bio-Oss® would not possess any osteoinductive
properties and osseous integration of the graft is only achieved by
osteoconduction.76 In order to enhance graft integration by
osteoin-duction, AB has been added to the graft, in different ratios, in sinus floor augmentation procedures and in alveolar ridge augmentation
procedures.71, 77-80 In a recently published systematic review assessing
implant treatment outcome when Bio-Oss® alone or Bio-Oss® was
mixed with AB used as graft for sinus floor augmentation, Jensen et
al71 concluded that the hypothesis of no differences between the two
modalities could neither be confirmed nor rejected. The researchers
also summarized that addition of AB to Bio-Oss® did not seem to
influence the biodegradation of Bio-Oss®, although long-term studies
were not available. However, in a recent animal study the same authors concluded that the graft volume was better preserved when DPBB was added to AB grafts and the volumetric reduction of the graft was significantly influenced by the ratio of DPBB and AB after
sinus floor augmentation.63 In addition, it appears that the ratio of
DPBB and AB may influence the bone remodelling patterns.69 On the
other hand, in a randomized controlled trial, it was demonstrated that addition of AB to DPBB in a ratio 1:4 did not lead to enhanced new bone formation in comparison to 100% DPBB, 4 months after sinus
floor augmentation.81 Hence, there is a need to further investigate the
influence of the AB to DPBB ratio on graft integration and volumetric resistance in clinical, randomized and controlled trials.
There is a controversy in the literature whether DPBB is resorbable or not. Some authors have claimed that DPBB probably is non
resorbable,58, 77, 82, 83 however several authors have observed signs of
resorption i.e. resorption lacunas and the presence of osteoclasts on the particle surface, or a decrease over time of the fraction of DPBB, in the biopsy, compared to the initial fraction of bone and soft
tissues in the graft.17, 65, 84-93 It is of great interest for the clinician to
understand the long term outcome of the grafting material, especially when used in aesthetically demanding areas, hence, further
It has been claimed by some researchers that Bio-Oss® after all
contains considerable amounts of protein94 and even transforming
growth factor β (TGF-β),95 whilst others have questioned these results
and found no evidence for the presence of protein in Bio-Oss®96.
However, a recent systematic review indicates that bovine derived
bone substitutes may carry a risk of prion transmission to patients20
even though no clinical reports of this complication have been published.
Alloplastic grafts
Alloplastic bone grafts are chemically produced materials such as calcium phosphate ceramics (CaPs) including HA and tricalcium phosphate (TCP), calcium-sulphate, bioactive glasses and polymers. CaPs are defined as an inorganic phase solid prepared by thermal
treatment (sintering) and subsequent cooling.55 The materials can be
manufactured in a variety of shapes, forms, structures and chemical compositions. They possess different mechanical and biological properties and the time of degradation may vary. Their similarity in composition to bone mineral, their biodegradability and
osteo-conductivity were the rationales for development of CaPs.97 HA is
the least soluble of the naturally occurring calcium phosphate salts and resistant to physiologic resorption, whilst TCP has a higher degradation rate. However, the chemical impurity in the contents of
the TCP ceramics results in varying degradation times 98 which may
lead to unpredictable clinical results. β -TCP has been developed
to achieve a more organized crystal structure, still with extensive degradation, and is now frequently used in bone reconstructive surgery. The dissolution process results in high extracellular concen-trations of calcium and phosphate, resulting in precipitation of apatites on a substrate ceramic, forming a carbonate-apatite-crystal layer. This layer is believed to influence the very strong interface
between the material and bone99. Cerasorb® (Curasan,
Kleinost-heim, Germany) is an example of a β-TCP, having 45% to 50%
porosity. The material is osteoconductive and biocompatible, but
not osteoinductive.100
Straumann BoneCeramic® is a novel, fully synthetic bone substitute
on the market, consisting of 60% HA and 40% β-TCP. The material
The TCP component is supposed to dissolve relatively quickly and the calcium and phosphate ions are suggested to stimulate bone formation. The HA component is less degradable and will protect the augmented area from resorption and subsequently maintain
the volume of the graft.101 Other examples of alloplastic grafts are
calcium sulphate and Bioglass with different tissue response to their
resorption and dissolution.58
Dentin
Dentin is composed of approximately 70% mineral, 20% organic matrix and 10% water by weight. The mineral part mainly consists of carbonate-substituted hydroxyapatite. The organic part contains 90% fibrous proteins (mainly type I collagen and a small percentage
of type III collagen), lipids and non-collagenous matrix proteins102
similar, although not identical, with those of bone. Dentin contains BMP and possesses both osteoconductive and osteoinductive
properties103-106 that might enhance bone integration compared to
strictly osteoconductive bone substitutes. Demineralized dentin induces formation of new bone when implanted in muscle pouches
in rats, rabbits and pigs.104, 107-109 However, allogeneic
deminera-lized dentin blocks implanted in the palatal connective tissue in rats appear not to induce any bone formation up to 4 weeks after
implantation.13 Furthermore, non-demineralized dentin may resorb
fast with no signs of bone formation when implanted in animal
muscle.104, 106, 109 Due to instability of the graft and/or ruptures in
the periosteum, particulated demineralized dentin or hydroxyapa-tite ceramics applied as bone onlays are frequently encapsulated in
fibrous tissue.15, 110-113 Hence, there is reason to believe that for dentin,
besides the surgical technique, the demineralization procedure, and the implant environment play important roles in osteoinduction and osteoconduction.
Fibrin glue
Fibrin sealants are biological adhesives that have been used extensively in most surgical specialties due to the haemostatic and adhesive
properties.114 The activity of the material is based on the natural
clotting cascade. Fibrinogen is converted to fibrin by thrombin in the final step and cross-linking of fibrin monomers into an insoluble
complex. When applied in contaminated sites an increased risk of infection may occur by providing a growth medium of nutrient
proteins.115 Fibrin glue has been used frequently in combination with
bone substitutes to make the grafting material easier to handle.16, 116,
117 However, there is a controversy in the literature whether fibrin
glue has a positive or negative impact on bone formation.114
Graft healinG
The biological properties of bone grafts and bone substitutes are often described by the terms osteoinduction, osteoconduction and
osteogenesis.55
Osteoconduction – is a process where biological or non-biological
materials may function as a three-dimensional structure, allowing ingrowth of sprouting capillaries, perivascular tissue and
osteopro-genitor cells from the recipient bed.118 This basically depends on the
number and size of the channels through the graft, affecting both
the rate of graft replacement and stabilization.119 The process starts
within a few days after grafting.
Osteoinduction – is the process of recruitment of host mesenchymal
type cells from the surrounding tissue e.g. periosteum and bone
marrow, under the influence of a soluble protein called BMP.120
The BMP belongs to the family of transforming growth factors,
(TGF)-β.121 The major phases of osteoinduction are: i) chemotaxis
– defined as a directed migration of cells in response to a chemical gradient e.g. BMP; ii) mitosis and proliferation of newly attached mesenchymal cells; and finally iii) differentiation of the cells into chondroblasts with subsequent cartilage formation. After vascular invasion, mineralization of cartilage take place, osteoblasts are
diffe-rentiated and bone formation starts,122 similar to endochondral
ossi-fication.
Osteogenesis – is defined as formation of new bone from
progenitor cells. Osteogenesis can be divided into spontaneous
osteo-genesis, when new bone is formed by osteprogenitor cells within
the wound defect, i.e. a bone fracture and transplanted osteogenesis when the formation of new bone is related to the presence of bone-forming cells within the bone-graft. In autogenous bone graft the living osteoprogenitor cells may survive and potentially differentiate to osteoblasts and eventually to osteocytes. These cells represent the
Healing of autogenous bone grafts
The most important factors in the mechanism of AB block integration are the amount of cellular marrow in the graft, the vascularity of the
recipient site and the stability of the grafted bone achieved.124 Cells
of the bone marrow, periosteum, endosteum and the outermost of bone surfaces may survive but the majority of the graft undergoes
necrosis.56 Revascularization of a free bone graft may partly occur
through micro anastomoses with pre-existing host vessels.125
A non-infected bone graft may be: i) incorporated in the surrounding bone and after a while sharing the same biological, mechanical och esthetical characteristics; ii) partly or totally resorbed ; or iii) sequestrated, encapsulated in fibrous tissue as a result of foreign body reaction. Incorporation is the process of envelopment and interdigitation of the donor bone or bone substitute with new bone deposited by the recipient which occurs during resorption of the old, dead grafted bone and by remodelling of the new living bone
structure.120 Three phases of incorporation may be distinguished by
the histologic appearance:126
Phase 1 – Inflammation (minutes to hours). The graft is
surrounded by a hematoma. Platelets are attracted by the fibrin clot. Leucocytes and macrophages invade the graft after vasodilatation and exudation of plasma.
Phase 2 - Consolidation (days). Fibrovascular ingrowth proceed,
osteoclasts are differentiated and starts resorption of the graft. Mesenchymal cells are recruited from the vicinity as a response to growth factors and BMPs (osteoinduction) and they differentiate into osteoblasts, which deposit osteoid on graft trabeculae, the latter serving as scaffold for the osteoblasts (osteoconduction)
Phase 3 – Remodelling (weeks-years). The graft is continuously
replaced by the remodelling process from the recipient bone.
The bone block graft is replaced by creeping substitution, however cortical bone may be extremely slowly replaced and parts may be unresorbed for as long as 20 years. In children, bone resorption and new tissue remodelling is much more rapid than in adults and bone
grafts may not be microscopically recognizable after 2 years.40
Cortical bone hardly survives after grafting and incorporation is mainly dependent upon the tissues at the recipient site at least from a cellular point of view. In contrast, cancellous bone grafting, if performed under good conditions (e.g. minimally traumatizing
and rapid grafting to a vascular bed without any infection) actively contribute to osteogenesis by providing large numbers of osteogenic
and potentially osteogenic cells.127 In addition, the preparation of
the recipient bed seems to have an important effect on bone graft integration and graft volume resistance. In an experimental study
in rats, Alberius et al128 removed the cortical bone by grinding and
noted that marrow exposure resulted in increased graft integration, however, some grafts became partly submerged into the recipient site. Instead, perforation of the recipient bone seemed to improve bone graft integration without submersion of the graft into the host
bone.129-131
Healing of Bio-Oss
In an experimental study on dogs, Araujo et al132 clarified the
dynamics of Bio-Oss® collagen incorporation in the host tissue.
Different phases were described; first, the biomaterial was trapped in the fibrin network of the coagulum and polymorphous nuclear (PMN) cells migrated to the surface of the particles. In a second phase the PMN cells were replaced by osteoclasts. The osteoclasts resided on OPN-positive cement lines on the surface of the particles, indicating that the cells were properly attached and apparently removed material from the surface of the xenogenic material. After 1-2 weeks the osteoclasts disappeared and were followed by osteoblasts, laying down bone mineral in the collagen bundles of the
provisional matrix. The Bio-Oss® particles became integrated in bone
in this third phase. Tapety et al133 investigated the response of bone
cells to Bio-Oss®, which was grafted to bone defects in rat femur.
After 3-5 days osteoblasts were recognized starting bone formation on the surface of the particles. TRAPase-reactive osteoclasts were not found on the trabeculae until day 7. The authors speculated
that Bio-Oss® might be subjected to resorption by osteoclasts due to
physiologic bone remodelling. In these studies it was assumed that proteins may cover the particles produced by different mesenchymal
or hematopoietic cells132 or may have been released from the
periphery of the host bone during drilling.133 The protein layer
may attract the bone cells and allow them to attach to the surface of the particles. It is unknown whether the osteoclasts are able to actually resorb the deproteinized bovine bone and if the findings are
applicable to humans. It has been suggested by some researchers that
the osteoclasts presented on the graft surfaces are indeed active,88, 90,
92, 133 however, it has also been suggested that the function of the
osteoclast is impaired.91 Although Bio-Oss® has excellent
osteo-conductive properties, several animal studies have demonstrated delayed healing when the material has been grafted to bony defects
or used with guided bone regeneration (GBR) technique.62, 134-137 For
ethical reasons the process of early healing of grafting materials in humans is not possible to investigate. However, since the grafting procedure and implant insertion is often performed in 2 stages, biopsies can be harvested at the second stage without any additional surgical intervention. After sinus floor augmentation, similar tissue responses have been reported after 3 to 8 months healing periods of the graft. Inflammation and foreign body reactions are rarely present. Newly formed bone, mainly woven in character if in some areas consisting of more mature, lamellar bone, is frequently seen in direct contact with the DPBB particles. In addition the particles are often interconnected by trabecular formation. Occasionally it was possible to observe osteoclasts in close contact with DPBB
particles and newly formed bone.16, 88, 138-145 In studies where AB had
been added to DPBB, residual AB particles were seldom present or
difficult to detect.77 In biopsies retrieved after a healing period of 3
years following sinus floor augmentation, Hallman et al77 reported
that lamellar bone filled the space between the particles resulting in an overall dense appearance with the majority of the DPBB particles (>90% ) in direct contact with lamellar bone. Long-term evaluation of the fate of DPBB particles is sparse and published articles are mainly case reports or case series with a limited number of patients.
After a healing period of 4.5 years,146 6 years,82 7 years,147 9 years148
and 14 years,149 histological evaluations have demonstrated the
presence of DPBB particles embedded in mature lamellar bone without any inflammatory reactions. In some cases osteoclasts were evident on the surfaces of the DPBB particles and the presence of lacunae that might indicate slow resorption. It has been speculated that the slow resorption rate by osteoclasts may be caused by the high calcium concentration on the biomaterial surface. The authors also speculated that long-lasting presence of DPBB particles could be explained by a bonding mechanism that maintains the biomechanical
integrity of the bone-biomaterial interface during the remodelling
process, although not further explained in the paper.148
In a recent review on DPBB in periodontal and implant surgery it was stated that comparative studies are needed to improve evidence on clinical outcomes of DPBB used for bone augmentation
procedures.76
Healing of BoneCeramic
®Ceramics do not possess any osteogenic or osteoinductive properties. The osteoconductive properties of these materials are dependent on the pore size, porosity and degradation potential. Even though the literature is not conclusive, the optimal macro pore size for tissue
ingrowth seems to be between 150 µm and 500 µm.55 A definition of
thresholds regarding macro pore characterization of bone substitutes has been suggested considering to part divergent literature data, namely; pore size < 60 µm for the penetration of individual cells and formation of non-mineralized tissue and pore size > 250 µm
for the ingrowth of fully mineralized bone.150 Ideally, the ratio of
degradation and new bone formation is 1:1, but if the degradation is faster than the bone formation, the result of the grafting procedure will end up in inadequate bone volume. The degradation in vivo may be achieved by dissolution or is cell mediated (multinuclear cells, osteoclasts and macrophages). Evidence concerning osteoclastic response to HA substrates is controversial and although osteoclasts are able to adhere to the surfaces of ceramics, this does not
necessarily mean that the cells have any ability to resorb them.151-153
However, osteoclastic resorption has been demonstrated in biphasic
calcium phosphate (BCP) with different HA/TCP ratios.99, 154 The
cells involved and speed of resorption are determined by the local
biological environment (e.g. pH, presence of cells and H2O content)
and material properties (e.g. Ca/P ratio, crystallinity, particle size,
surface area and porosity).97 The concept of BCP is based on the
balance between the more stable phase of HA and the more soluble TCP. During its dissolution in the body, calcium and phosphate ions are released into the biological medium, which may stimulate bone
formation155 or at least slow down osteoclastic activity.99, 154 Jensen at
al156 evaluated the short-, mid- and long-term influence of different
in an experimental study in minipigs. There seemed to be a delayed healing for all bone substitutes compared to AB. Bone formation and degradation of the ceramics seemed to be inversely propor-tional to the HA/TCP ratio. This is in accordance with other animal
studies.152 The amount of newly formed bone was similar for BCP
80/20, BCP 60/40 and DPBB but lower compared to AB and BCP 20/80. However, whilst in DPBB defects woven bone amalgamated and connected the particles, abundant bone formation around the BCP particles (BCP 80/20 and BCP 60/40), only near the defect walls, was the dominating feature. The degradation of BCP 80/20 and BCP 60/40 was limited, probably due to the high crystallinity.
BoneCeramic® (consisting of 60% HA and 40% TCP) has been
used clinically for sinus floor augmentation18, 157 and for lateral ridge
augmentation.158 In 3 clinical studies histological and
histomorp-hometrical outcomes were studied comparing BoneCeramic® and
Bio-Oss® 6 to 8 monthsafter sinus floor augmentation.101, 157, 159 In
all studies there was no difference in new bone formation between the materials and no inflammation or pathologic finding were reported. However, most of the bone formation took place between
the BoneCeramic® particles,159 whilst the new formed bone was more
intimately associated with the Bio-Oss® particles, which might reflect
different osteoconductivity of the materials.101 On the other hand,
the histological results of biopsies retrieved 5 months after sinus floor augmentation revealed god tissue integration and direct bone
to particle contact in both sites augmented with BoneCeramic® and
Bio-Oss®. 143 In the same study there was significantly more residual
bone substitute material in the Bio-Oss® group but no difference
in new bone formation between the groups. In all studies it was concluded that there is a need for further investigations regarding
the clinical relevance of the properties of BoneCeramic®.
surGical techniques
Sinus floor augmentation
Due to resorption of the alveolar crest following tooth extraction in combination with pneumatization of the maxillary sinus, there is often lack of adequate bone volume for implant treatment and a need for bone grafting in the posterior maxilla. Sinus floor
a surgical technique, where the sinus floor was grafted with parti-culated cancellous bone from the iliac crest after the lateral sinus wall had been fenestrated and the Schneiderian membrane had been elevated. The procedure has been frequently used since, with excellent
clinical results.74 Due to the drawbacks of AB grafting, several
different bone substitutes have been used alone or in combination with AB. A consensus on the most appropriate material in sinus floor augmentation has not been reached despite several systematic
reviews and meta-analyses have been conducted on the topic.21, 23, 74,
161 The most frequently used bone substitutes include demineralized
freeze-dried bone, hydroxyapatite (HA), β-tricalcium phosphate
(β-TCP), deproteinized bovine bone (DPBB) and combinations of
these materials.23 The percentage of total bone volume (TBV) in a
histologic section of the graft has been a frequently used parameter to assess the performance of a bone graft or bone substitute in an
augmented area.17, 161-163 Recently two meta-analyses were performed
to analyse the TBV after sinus floor augmentation with different
grafting materials.23, 161 Handschel et al23 reported that AB shows the
highest TBV values in a relatively early phase after grafting, i.e. 4 to 9 months, but after 9 months there were no significant difference between the different grafting materials. Additionally, only DPBB, β-TCP and AB (and the combinations of AB and these materials) presented evaluable data for meta-analyses. In contrast, Klijn et
al161 reported that TBV was significantly higher before 4.5 months
and after 9 months of AB graft healing compared to the period in between. For all other bone substitutes no significant effect on TBV in time could be proven. The authors stated that AB still has to be considered to be the gold standard. However, in an interven-tion review to determine which are the most effective augmentainterven-tion
procedures of the maxillary sinus, it was concluded that Bio-Oss®
and Ceracorb® (Curasan AG, Kleinostheim, Germany) (β-TCP)
appear to be as effective as AB and can be used as replacement
for AB grafting.22 Furthermore, it has yet to be proven if TBV is
an adequate variable to evaluate the clinical outcome of graft and implant success.
Lundgren et al164 described a sinus membrane elevation technique
using a lateral approach with a replaceable bone window, showing
and simultaneous implant installation, a space filled with blood is created allowing for bone formation according the principles of
tissue generation,166 without the use of any filling material.165 The
sinus becomes a self-contained defect, promoting space maintenance and coagulum stability, allowing continuous blood supply from the bony wall and cell migration, resulting in favourable healing
conditions.139 Although the surgical technique seems demanding and
operator sensitive, the reduced morbidity and lower costs for the patient makes the procedure a highly interesting alternative to using grafting materials. Apart from TBV, the amount of newly formed bone is also considered an accurate indicator to assess and compare the healing potential of graft or bone replacement graft materials
after sinus floor augmentation.159 According to the results of
Lundgren et al, it could be questioned whether sinus floor augmen-tation is a suitable environment or not to test the osteoinductive and osteoconductive properties of different grafting materials per se. Furthermore, when future studies are conducted to test the performance of grafting materials the described technique should be considered used as control.
Lateral ridge augmentation
Lateral ridge augmentation procedures are necessary when the width of the recipient alveolar crest presents with inadequate dimensions for implant placement. A number of surgical procedures have been performed to create adequate bone width either in combination with implant placement (associated with dehiscenses or fenestration bone
defects) or following a period of graft healing.167 These procedures
include guided bone regeneration (GBR) alone or in combination with grafting materials, osteodistraction, “split-ridge” osteotomy for lateral expansion and bone grafting with different grafting materials (AB, allograft, xenograft and alloplastic materials). The grafting materials may be used as blocks (onlay) or particulates. In recent reviews, it was not possible to demonstrate the superiority of
one augmentation technique over another.43, 66
The principal feature of GBR is to create a secluded space to allow presumably slower moving osteoprecursor cells to migrate from the surrounding bone rims and simultaneously prevent cellular ingrowth from the surrounding soft tissue, so that the
osteoge-nesis may occur unhindered. This situation is provided by a semi-permeable membrane allowing free diffusion of tissue-fluids and
nutrients.53 In addition, to excluding unwanted cells, the membrane
also acts to stabilize the blood clot.168 The technique was first
described by Dahlin et al,166, 169 who tested the ability of generating
new bone around titanium implants with GBR in a rabbit model. GBR is frequently combined with a filler material (i.e. AB or a bone substitute) to support the membrane, stabilize the blood clot and
reduce its volume and shrinkage.170 The membranes can be resorbable
or non-resorbable. If a particulated graft is used, a membrane to
cover the graft is advocated.171 One common complication in GBR is
soft tissue dehiscenses and exposure of the membrane which might lead to bacterial infection and compromised graft healing, especially in non-resorbable membrane sites. In addition, another disadvan-tage with non-resorbable membranes is that it must be removed in a second surgical intervention.
In larger edentulous defects, autogenous bone used as onlay grafts
have been advocated,172 however; the morbidity from the donor site
and the higher costs compared to for example GBR 173 in combination
with filling material, must be considered in the choice of surgical
procedure to be utilized. Lateral augmentation with Bio-Oss® has
been performed in both animal studies174-176 and human studies.79, 80,
177 It appears that Bio-Oss® can be used with a slightly higher risk of
having an implant failure.44 Since Bio-Oss® only possesses
ostecon-ductive properties, AB has been added to DPBB in different ratios, in grafting procedures, to add osteoinductive properties and
subse-quently enhance bone formation.22, 77, 79, 80, 90, 173, 178 However, the
optimal ratio of Bio-Oss® and AB, and its effect on bone formation is
not known.63 To the best of the author´s knowledge, different ratios
of Bio-Oss® and AB have not been compared for lateral