FR AN CESC A CECC HIN A T O MALMÖ UNIVERSIT ON MA GNESIUM-MODIFIED TIT ANIUM C O A TIN GS AND MA GNESIUM ALL O Y S FOR OR AL AND ORTHOP AEDIC APPLIC A TIONS: IN VITR O INVES TIG A TION DOCT OR AL DISSERT A TION IN ODONT OL OG Y
FRANCESCA CECCHINATO
ON MAGNESIUM-MODIFIED
TITANIUM COATINGS AND
MAGNESIUM ALLOYS FOR
ORAL AND ORTHOPAEDIC
APPLICATIONS: IN VITRO
INVESTIGATION
O N M A G N E S I U M - M O D I F I E D T I T A N I U M C O A T I N G S A N D M A G N E S I U M A L L O Y S F O R O R A L A N D O R T H O P A E D I C A P P L I C A T I O N S : I N V I T R O I N V E S T I G A T I O N
Malmö University
Faculty of Odontology Doctoral Dissertations 2015
© Francesca Cecchinato, 2015
Photographs and illustrations: Francesca Cecchinato ISBN 978-91-7104-641-3 (print)
ISSN 978-91-7104-642-0 (pdf) Holmbergs, Malmö 2015
FRANCESCA CECCHINATO
ON MAGNESIUM-MODIFIED
TITANIUM COATINGS AND
MAGNESIUM ALLOYS FOR
ORAL AND ORTHOPAEDIC
APPLICATIONS:
IN VITRO
INVESTIGATION
Malmö University, 2015
Department of Prosthodontics
Faculty of Odontology
Malmö, Sweden
This publication is also available in electronic format at: www.mah.se/muep
This thesis is number 47 in a series of investigations on implants, hard tissue, and the locomotor apparatus originating from the Department of Biomaterials, University of Gothenburg and the Department of Prosthodontics/Material Sciences, Malmö University, Sweden.
1. Anders R Eriksson DDS, 1984. Heat-induced Bone Tissue Injury. An in vivo investigation of heat tolerance of bone tissue and temperature rise in the drilling of cortical bone.
Thesis defended 21.2.1984. External examiner: Docent K-G. Thorngren. 2. Magnus Jacobsson MD, 1985. On Bone Behaviour after Irradiation. Thesis defended 29.4.1985. External examiner: Docent A. Nathanson. 3. Fredric Buch MD, 1985. On Electrical Stimulation of Bone Tissue. Thesis defended 28.5.1985. External examiner: Docent T. Ejsing-Jörgensen.
4. Peter Kälebo MD, 1987. On Experimental Bone Regeneration in Titanium Implants. A quantitative microradiographic and histologic investigation using the Bone Harvest Chamber.
Thesis defended 1.10.1987. External examiner: Docent N. Egund.
5. Lars Carlsson MD, 1989. On the Development of a new Concept for Orthopaedic Implant Fixation.
Thesis defended 2.12.1989. External examiner: Docent L-Å Broström. 6. Tord Röstlund MD, 1990. On the Development of a New Arthroplasty. Thesis defended 19.1.1990. External examiner: Docent Å. Carlsson. 7. Carina Johansson Res Tech, 1991. On Tissue Reaction to Metal Implants. Thesis defended 12.4.1991. External examiner: Professor K. Nilner.
8. Lars Sennerby DDS, 1991. On the Bone Tissue Response to Titanium Implants. Thesis defended 24.9.1991. External examiner: Dr J.E. Davies.
9. Per Morberg MD, 1991. On Bone Tissue Reactions to Acrylic Cement. Thesis defended 19.12.1991. External examiner: Docent K. Obrant.
10. Ulla Myhr PT, 1994. On factors of Importance for Sitting in Children with Cerebral Palsy.
Thesis defended 15.4.1994. External examiner: Docent K. Harms-Ringdahl.
11. Magnus Gottlander MD, 1994. On Hard Tissue Reactions to Hydroxyapatite-Coated Titanium Implants.
Thesis defended 25.11.1994. External examiner: Docent P. Aspenberg.
12. Edward Ebramzadeh MScEng, 1995. On Factors Affecting Long-Term Outcome of Total Hip Replacements.
Thesis defended 6.2.1995. External examiner: Docent L. Linder.
13. Patricia Campbell BA, 1995. On Aseptic Loosening in Total Hip Replacement: the Role of UHMWPE Wear Particles.
Thesis defended 7.2.1995. External examiner: Professor D. Howie.
14. Ann Wennerberg, DDS, 1996. On Surface Roughness and Implant Incorporation. Thesis defended 19.4.1996. External examiner: Professor P-O. Glantz.
15. Neil Meredith BDS MSc FDS RCSm, 1997. On the Clinical Measurement of Implant Stability Osseointegration.
Thesis defended 3.6.1997. External examiner: Professor J. Brunski.
16. Lars Rasmusson DDS, 1998. On Implant Integration in Membrane-Induced and Grafter Bone.
Thesis defended 4.12.1998. External examiner: Professor R. Haanaes.
17. Thay Q Lee MSc, 1999. On the Biomechanics of the Patellfemoral Joint and Patellar Resurfacing in Total Knee Arthroplasty.
Thesis defended 19.4.1999. External examiner: Docent G. Nemeth.
18. Anna Karin Lundgren DDS, 1999. On Factors Influencing Guided Regeneration and Augmentation of Intramembraneous Bone.
Thesis defended 7.5.1999. External examiner: Professor B. Klinge.
19. Carl-Johan Ivanoff DDS, 1999. On Surgical and Implant Related Factors Influencing Integration and Function of Titanium Implants. Experimental and Clinical Aspects.
Thesis defended 12.5.1999. External examiner: Professor B. Rosenquist.
20. Bertil Friberg DDS MDS, 1999. On Bone Quality and Implant Stability Measurements.
Thesis defended 12.11.1999. External examiner: Docent P. Åstrand.
21. Åse Allansdotter Johansson MD, 1999. On Implant Integration in Irradiated Bone. An Experimental Study of the Effects of Hyperbaric Oxygeneration and Delayed Implant Placement.
Thesis defended 8.12.1999. External examiner: Docent K. Arvidsson-Fyrberg.
22. Börje Svensson FFS, 2000. On Costochondral Grafts Replacing Mandibular Condyles in Juvenile Chronic Arthritis. A Clinical, Histologic and Experimental Study. Thesis defended 22.5.2000. External examiner: Professor Ch. Lindqvist.
23. Warren Macdonald BEng, MPhil, 2000. On Component Integration on Total Hip Arthroplasties: Pre-Clinical Evaluations.
This thesis is number 47 in a series of investigations on implants, hard tissue, and the locomotor apparatus originating from the Department of Biomaterials, University of Gothenburg and the Department of Prosthodontics/Material Sciences, Malmö University, Sweden.
1. Anders R Eriksson DDS, 1984. Heat-induced Bone Tissue Injury. An in vivo investigation of heat tolerance of bone tissue and temperature rise in the drilling of cortical bone.
Thesis defended 21.2.1984. External examiner: Docent K-G. Thorngren. 2. Magnus Jacobsson MD, 1985. On Bone Behaviour after Irradiation. Thesis defended 29.4.1985. External examiner: Docent A. Nathanson. 3. Fredric Buch MD, 1985. On Electrical Stimulation of Bone Tissue. Thesis defended 28.5.1985. External examiner: Docent T. Ejsing-Jörgensen.
4. Peter Kälebo MD, 1987. On Experimental Bone Regeneration in Titanium Implants. A quantitative microradiographic and histologic investigation using the Bone Harvest Chamber.
Thesis defended 1.10.1987. External examiner: Docent N. Egund.
5. Lars Carlsson MD, 1989. On the Development of a new Concept for Orthopaedic Implant Fixation.
Thesis defended 2.12.1989. External examiner: Docent L-Å Broström. 6. Tord Röstlund MD, 1990. On the Development of a New Arthroplasty. Thesis defended 19.1.1990. External examiner: Docent Å. Carlsson. 7. Carina Johansson Res Tech, 1991. On Tissue Reaction to Metal Implants. Thesis defended 12.4.1991. External examiner: Professor K. Nilner.
8. Lars Sennerby DDS, 1991. On the Bone Tissue Response to Titanium Implants. Thesis defended 24.9.1991. External examiner: Dr J.E. Davies.
9. Per Morberg MD, 1991. On Bone Tissue Reactions to Acrylic Cement. Thesis defended 19.12.1991. External examiner: Docent K. Obrant.
10. Ulla Myhr PT, 1994. On factors of Importance for Sitting in Children with Cerebral Palsy.
Thesis defended 15.4.1994. External examiner: Docent K. Harms-Ringdahl.
11. Magnus Gottlander MD, 1994. On Hard Tissue Reactions to Hydroxyapatite-Coated Titanium Implants.
Thesis defended 25.11.1994. External examiner: Docent P. Aspenberg.
12. Edward Ebramzadeh MScEng, 1995. On Factors Affecting Long-Term Outcome of Total Hip Replacements.
Thesis defended 6.2.1995. External examiner: Docent L. Linder.
13. Patricia Campbell BA, 1995. On Aseptic Loosening in Total Hip Replacement: the Role of UHMWPE Wear Particles.
Thesis defended 7.2.1995. External examiner: Professor D. Howie.
14. Ann Wennerberg, DDS, 1996. On Surface Roughness and Implant Incorporation. Thesis defended 19.4.1996. External examiner: Professor P-O. Glantz.
15. Neil Meredith BDS MSc FDS RCSm, 1997. On the Clinical Measurement of Implant Stability Osseointegration.
Thesis defended 3.6.1997. External examiner: Professor J. Brunski.
16. Lars Rasmusson DDS, 1998. On Implant Integration in Membrane-Induced and Grafter Bone.
Thesis defended 4.12.1998. External examiner: Professor R. Haanaes.
17. Thay Q Lee MSc, 1999. On the Biomechanics of the Patellfemoral Joint and Patellar Resurfacing in Total Knee Arthroplasty.
Thesis defended 19.4.1999. External examiner: Docent G. Nemeth.
18. Anna Karin Lundgren DDS, 1999. On Factors Influencing Guided Regeneration and Augmentation of Intramembraneous Bone.
Thesis defended 7.5.1999. External examiner: Professor B. Klinge.
19. Carl-Johan Ivanoff DDS, 1999. On Surgical and Implant Related Factors Influencing Integration and Function of Titanium Implants. Experimental and Clinical Aspects.
Thesis defended 12.5.1999. External examiner: Professor B. Rosenquist.
20. Bertil Friberg DDS MDS, 1999. On Bone Quality and Implant Stability Measurements.
Thesis defended 12.11.1999. External examiner: Docent P. Åstrand.
21. Åse Allansdotter Johansson MD, 1999. On Implant Integration in Irradiated Bone. An Experimental Study of the Effects of Hyperbaric Oxygeneration and Delayed Implant Placement.
Thesis defended 8.12.1999. External examiner: Docent K. Arvidsson-Fyrberg.
22. Börje Svensson FFS, 2000. On Costochondral Grafts Replacing Mandibular Condyles in Juvenile Chronic Arthritis. A Clinical, Histologic and Experimental Study. Thesis defended 22.5.2000. External examiner: Professor Ch. Lindqvist.
23. Warren Macdonald BEng, MPhil, 2000. On Component Integration on Total Hip Arthroplasties: Pre-Clinical Evaluations.
24. Magne Røkkum MD, 2001. On Late Complications with HA Coated Hip Arthroplasties.
Thesis defended 12.10.2001. External examiner: Professor P. Benum.
25. Carin Hallgren Höstner DDS, 2001. On the Bone Response to Different Implant Textures. A 3D analysis of roughness, wavelength and surface pattern of experimental implants.
Thesis defended 19.11.2001. External examiner: Professor S. Lundgren.
26. Young-Taeg Sul DDS, 2002. On the Bone Response to Oxidised Titanium Implants: The role of microporous structure and chemical composition of the surface oxide in enhanced osseointegration.
Thesis defended 7.6.2002. External examiner: Professor J.E. Ellingsen.
27. Victoria Franke Stenport DDS, 2002. On Growth Factors and Titanium Implant Integration in Bone.
Thesis defended 11.6.2002. External examiner: Associate Professor E. Solheim.
28. Mikael Sundfeldt MD, 2002. On the Aetiology of Aseptic Loosening in Joint Arthroplasties and Routes to Improved cemented Fixation.
Thesis defended 14.6.2002. External examiner: Professor N. Dahlén.
29. Christer Slotte CCS, 2003. On Surgical Techniques to Increase Bone Density and Volume. Studies in Rat and Rabbit.
Thesis defended 13.6.2003. External examiner: Professor C.H.F. Hämmerle.
30. Anna Arvidsson MSc, 2003. On Surface Mediated Interactions Related to Chemomechanical Caries Removal. Effects on surrounding tissues and materials. Thesis defended 28.11.2003. External examiner: Professor P. Tengvall.
31. Pia Bolind DDS, 2004. On 606 retrieved oral and cranio-facial implants. An analysis of consequently received human specimens.
Thesis defended 17.12.2004. External examiner: Professor A. Piattelli.
32. Patricia Miranda Burgos DDS, 2006. On the influence of micro- and macroscopic surface modifications on bone integration of titanium implants.
Thesis defended 1.9.2006. External examiner: Professor A. Piattelli.
33. Jonas P. Becktor DDS, 2006. On factors influencing the outcome of various techniques using endosseous implants for reconstruction of the atrophic edentulous and partially dentate maxilla.
Thesis defended 17.11.2006. External examiner: Professor K.F. Moos. 34. Anna Göransson DDS, 2006. On Possibly Bioactive CP Titanium Surfaces. Thesis defended 8.12.2006. External examiner: Professor B. Melsen.
35. Andreas Thor DDS, 2006. On platelet-rich plasma in reconstructive dental implant surgery.
Thesis defended 8.12.2006. External examiner: Professor E.M. Pinholt.
36. Luiz Meirelles DDS MSc, 2007. On Nano Size Structures for Enhanced Early Bone Formation.
Thesis defended 13.6.2007. External examiner: Professor Lyndon F. Cooper.
37. Pär-Olov Östman DDS, 2007. On various protocols for direct loading of implant-supported fixed prostheses.
Thesis defended 21.12.2007. External examiner: Professor B. Klinge.
38. Kerstin Fischer DDS, 2008. On immediate/early loading of implant supported prostheses in the maxilla.
Thesis defended 8.2.2008. External examiner: Professor K. Arvidsson Fyrberg.
39. Alf Eliasson 2008. On the role of number of fixtures, surgical technique and timing of loading.
Thesis defended 23.5.2008. External examiner: Professor K. Arvidsson Fyrberg. 40. Victoria Fröjd DDS, 2010. On Ca2+ incorporation and nanoporosity of titanium surfaces and the effect on implant performance.
Thesis defended 26.11.2010. External examiner: Professor J.E. Ellingsen.
41. Lory Melin Svanborg DDS, 2011. On the importance of nanometer structures for implant incorporation in bone tissue.
Thesis defended 01.06.2011. External examiner: Associate professor C. Dahlin. 42. Byung-Soo Kang MSc, 2011. On the bone tissue response to surface chemistry modifications of titanium implants.
Thesis defended 30.09.2011. External examiner: Professor J. Pan.
43. Kostas Bougas DDS, 2012. On the influence of biochemical coating on implant bone incorporation.
Thesis defended 12.12.2012. External examiner: Professor T. Berglundh.
44. Arne Mordenfeld DDS, 2013. On tissue reaction to and adsorption of bone substitutes.
Thesis defended 29.5.2013. External examiner: Professor C. Dahlin.
45. Ramesh Chowdhary DDS, 2014. On efficacy of implant thread design for bone stimulation.
Thesis defended 21.05.2014. External examiner: Professor Flemming Isidor.
46. Anders Halldin MSc, 2015. On a biomechanical approach to analysis of stability and load bearing capacity of oral implants.
24. Magne Røkkum MD, 2001. On Late Complications with HA Coated Hip Arthroplasties.
Thesis defended 12.10.2001. External examiner: Professor P. Benum.
25. Carin Hallgren Höstner DDS, 2001. On the Bone Response to Different Implant Textures. A 3D analysis of roughness, wavelength and surface pattern of experimental implants.
Thesis defended 19.11.2001. External examiner: Professor S. Lundgren.
26. Young-Taeg Sul DDS, 2002. On the Bone Response to Oxidised Titanium Implants: The role of microporous structure and chemical composition of the surface oxide in enhanced osseointegration.
Thesis defended 7.6.2002. External examiner: Professor J.E. Ellingsen.
27. Victoria Franke Stenport DDS, 2002. On Growth Factors and Titanium Implant Integration in Bone.
Thesis defended 11.6.2002. External examiner: Associate Professor E. Solheim.
28. Mikael Sundfeldt MD, 2002. On the Aetiology of Aseptic Loosening in Joint Arthroplasties and Routes to Improved cemented Fixation.
Thesis defended 14.6.2002. External examiner: Professor N. Dahlén.
29. Christer Slotte CCS, 2003. On Surgical Techniques to Increase Bone Density and Volume. Studies in Rat and Rabbit.
Thesis defended 13.6.2003. External examiner: Professor C.H.F. Hämmerle.
30. Anna Arvidsson MSc, 2003. On Surface Mediated Interactions Related to Chemomechanical Caries Removal. Effects on surrounding tissues and materials. Thesis defended 28.11.2003. External examiner: Professor P. Tengvall.
31. Pia Bolind DDS, 2004. On 606 retrieved oral and cranio-facial implants. An analysis of consequently received human specimens.
Thesis defended 17.12.2004. External examiner: Professor A. Piattelli.
32. Patricia Miranda Burgos DDS, 2006. On the influence of micro- and macroscopic surface modifications on bone integration of titanium implants.
Thesis defended 1.9.2006. External examiner: Professor A. Piattelli.
33. Jonas P. Becktor DDS, 2006. On factors influencing the outcome of various techniques using endosseous implants for reconstruction of the atrophic edentulous and partially dentate maxilla.
Thesis defended 17.11.2006. External examiner: Professor K.F. Moos. 34. Anna Göransson DDS, 2006. On Possibly Bioactive CP Titanium Surfaces. Thesis defended 8.12.2006. External examiner: Professor B. Melsen.
35. Andreas Thor DDS, 2006. On platelet-rich plasma in reconstructive dental implant surgery.
Thesis defended 8.12.2006. External examiner: Professor E.M. Pinholt.
36. Luiz Meirelles DDS MSc, 2007. On Nano Size Structures for Enhanced Early Bone Formation.
Thesis defended 13.6.2007. External examiner: Professor Lyndon F. Cooper.
37. Pär-Olov Östman DDS, 2007. On various protocols for direct loading of implant-supported fixed prostheses.
Thesis defended 21.12.2007. External examiner: Professor B. Klinge.
38. Kerstin Fischer DDS, 2008. On immediate/early loading of implant supported prostheses in the maxilla.
Thesis defended 8.2.2008. External examiner: Professor K. Arvidsson Fyrberg.
39. Alf Eliasson 2008. On the role of number of fixtures, surgical technique and timing of loading.
Thesis defended 23.5.2008. External examiner: Professor K. Arvidsson Fyrberg. 40. Victoria Fröjd DDS, 2010. On Ca2+ incorporation and nanoporosity of titanium surfaces and the effect on implant performance.
Thesis defended 26.11.2010. External examiner: Professor J.E. Ellingsen.
41. Lory Melin Svanborg DDS, 2011. On the importance of nanometer structures for implant incorporation in bone tissue.
Thesis defended 01.06.2011. External examiner: Associate professor C. Dahlin. 42. Byung-Soo Kang MSc, 2011. On the bone tissue response to surface chemistry modifications of titanium implants.
Thesis defended 30.09.2011. External examiner: Professor J. Pan.
43. Kostas Bougas DDS, 2012. On the influence of biochemical coating on implant bone incorporation.
Thesis defended 12.12.2012. External examiner: Professor T. Berglundh.
44. Arne Mordenfeld DDS, 2013. On tissue reaction to and adsorption of bone substitutes.
Thesis defended 29.5.2013. External examiner: Professor C. Dahlin.
45. Ramesh Chowdhary DDS, 2014. On efficacy of implant thread design for bone stimulation.
Thesis defended 21.05.2014. External examiner: Professor Flemming Isidor.
46. Anders Halldin MSc, 2015. On a biomechanical approach to analysis of stability and load bearing capacity of oral implants.
47. Francesca Cecchinato MSc, 2015. On magnesium-modified titanium coatings and magnesium alloys for oral and orthopaedic applications: in vitro investigation.
Thesis to be defended 20.11.2015. External examiner: Professor C. Stanford. See www.mah.se/muep
TABLE OF CONTENTS
LIST OF PAPERS ... 13
ABSTRACT ... 15
ACRONYMS AND SYMBOLS ... 19
INTRODUCTION ... 22
Bone replacement and repair ...22
Peri-implant bone healing ...27
Magnesium in bone metabolism ...30
Magnesium-based biomaterials ...31
AIMS ... 37
MATERIALS AND METHODS ... 38
Specimen preparation ...38
Surface characterization ...41
Magnesium release...44
Material degradation parameters ...45
Cells ...46
Cell isolation and expansion ...47
Cell seeding and culture ...48
Cell morphology ...49 In vitro cytotoxicity ...50 Cell adhesion ...51 Alkaline phosphatase ...53 Cell mineralization ...53 RNA extraction ...54
Gene expression techniques ...56
Statistics ...59 47. Francesca Cecchinato MSc, 2015. On magnesium-modified titanium coatings and
magnesium alloys for oral and orthopaedic applications: in vitro investigation. Thesis to be defended 20.11.2015. External examiner: Professor C. Stanford. See www.mah.se/muep
RESULTS ... 60
Magnesium as a bioactive substance for mesoporous titania implant coatings ...60
Magnesium alloys as bioresorbable metals for bone applications ...72
DISCUSSION ... 79
Magnesium-modified mesoporous titania films to enhance peri-implant osteogenesis - studies I, II and III ...80
Magnesium alloys as tailored biodegradable implant materials for bone regeneration – study IV ...87
CONCLUSIONS AND FUTURE PERSPECTIVES... 91
Mesoporous titania film as a carrier for magnesium at the peri-implant site ... 91
Magnesium alloys as biodegradable metals for bone tissue regeneration ...92
ACKNOWLEDGEMENTS ... 95
REFERENCES ... 98
LIST OF PAPERS
This thesis is based on the following papers, which are referred to in the text by their Roman numerals.
I. Cecchinato F, Xue Y, Karlsson J, He W, Wennerberg A, Mustafa K, Andersson M, Jimbo R. In vitro evaluation of human foetal osteoblast response to magnesium loaded mesoporous TiO2
coating. J Biomed Mater Res A. 2013; 102(11) 3862-71.
II. Cecchinato F, Karlsson J, Ferroni L, Gardin C, Galli S, Wennerberg A, Zavan B, Andersson M, Jimbo R. Osteogenic potential of human adipose-derived stromal cells on 3-dimensional mesoporous TiO2 coating with magnesium
impregnation. Material Science and Engineering 2015; 225-234. III. Cecchinato F, Atefyekta,Wennerberg A, Andersson M, Jimbo R,
Davies J. Modulation of the nanometer pore size improves magnesium adsorption into mesoporous titania coatings and promotes bone morphogenic protein 4 expression in adhering osteoblasts. Manuscript.
IV. Cecchinato F, Agha NA, Martinez-Sanchez AH, Luthringer BJC, Feyerabend F, Jimbo R, Willumeit-Römer R, Wennerberg A.
Influence of magnesium alloy degradation on undifferentiated human cells. Submitted.
Paper I reprinted by permission from John Wiley and Sons, License Number 3670081040368.
Paper II reprinted by permission from Elsevier, License Number 3670090088607.
Y HYPO THESIS ILLUS TR A TION KEY FINDIN GS evaluation of iO2
Magnesium can be loaded in m
es op or ou s f ilm s, a nd it s r el ea se e nh an ce so st eo b-last activity .
Magnesium adsorbs to mesoporous titania films with a 6-nm average pore size and is released within 24 hours of contact with bone cells, demonstrating a positive effect on initial osteob
-last viability . -iO2 gs w it h m ag ne si um Magnesium exhibitsosteo -conductive potential by
guiding undifferentiated cell differentiation toward the osteoblast phenotype.
Mag nesium rel ease d from 6-nm mes oporou s fil ms
promotes (i) stromal cell differentiation along the osteogenic lineage; and (ii) osteopontin expression in particular
. iz e i m pr ov es m agn e- -morphoge -ote in 4 e xpr es si on in
A 1nanometre increase in pore size increases magnesium content in the meso
po rou s st ruc tur e,
thus improving magnesium release and its osteogenic potential in vitro. The nano-pore dimensions of mesoporous films modulate magnesium adsorption. An increase of 1 nanometre in pore size increases Mg content, which once released, significantly promotes bone morphogenic protein 4 expression at later stages of osteoblast proliferation.
-The degradation behaviour and the surface chemistry and topography of three magnesium alloys dif
-ferently influence early adhesion and spreading of undifferentiated cells. Degradation rate and degradation parameters are high
er fo r M g4 Y 3R E c om pa re d t o M g2 A g a nd Mg10Gd. However
, cellular adhesion structures
are better developed on Mg10Gd, which pos
-sesses homogeneous degradation and, therefore, represents a suitable alternative to biodegradable metal in bone.
T A GL
AN
CE
Figure 4. Schematic illustration of the experimental procedure for HUCPV seeding on pre-incubated Mg alloys for the in vitro investigations (Studies IV).
Cell morphology
Scanning electron microscopy - Studies I, II, and IV
Scanning electron microscopy (SEM) is the most commonly used form of electron microscopy for visualizing cells adhering to a sub-strate. After culturing, cells were immobilized in a fixation proce-dure before dehydration in a graded series of ethanol. Dehydration was necessary to replace the water inside the cells with 100% ethanol. Cells then underwent critical-point drying. This method replaces the liquid inside biological structures with a suitable inert fluid, such as CO2, whose critical temperature is just above ambient temperature. Thus, no surface tension effect occurs, and this allows cells to maintain their morphology without distortion. Samples were then mounted and covered them with a thin layer of conductive powder to make the cell monolayer electrically conduc-tive prior to imaging.
In Study I, hFOB were fixed after for 1 and 24 hours of cell culture into specimens, dehydrated the cells in graded ethanol, and then dried them at the critical point in a Balzers CPD 010 critical point dryer (BalTec, Pfäffikon ZH, Switzerland). Last, micrographs of the cultured cells were taken with SEM (JSM-7400F; JEOL Ltd., Tokyo, Japan).
In Study II, ADSC were fixed after 5 and 24 hours of culture, de-hydrated the samples in ethanol, and dried them at the critical point in a Balzers CPD 030 (Leica Microsystem AB, Wetzlar,
ABSTRACT
In dentistry and orthopaedic surgery, research to find and develop improved biomaterials is progressing rapidly.
Of specific interest is to accelerate bone formation around the implant surface, which could improve the reliability of the implant even in patients with compromised situations. Although the surface modification of the implant has been proven to certain extent to promote osseointegration, the lack of bone in the patient remains a major issue and bone augmentation is commonly conducted prior to implant insertion. Synthetic and naturally derived resorbable materials are commonly used. However, problems such as the lack of optimal mechanical properties or the undesirable material resorption kinetics still exist and there still remain possibility for improvement.
Clinical approaches for orthopaedic trauma require the use of non-resorbable screws, plates and pins made of metallic materials such as titanium, cobalt-chrome and stainless steel alloys. The major drawback of these materials is the need of implant removal at re-entry. Therefore, the research of bioresorbable materials that could withstand the mechanical stresses is an ongoing topic.
Based on this clinical reality, the aim of this thesis was to investigate the suitability of magnesium (Mg) as a biomaterial for regenerative bone applications. Namely, Mg as a doping material for engineered mesoporous titanium implant surfaces (Studies I, II and III), and as a bioresorbable metal alloy for bone regeneration in bone trauma and bone defects conditions (Study IV).
Study I, II, III
Mesoporous titania films produced with evaporation-induced self-assembly (EISA) technique and applied as implant surface coatings are under investigation as a release system for the controlled administration of several substances, such as osteoporotic drugs, to enhance early bone anchorage to the implant. Modulating the pore size of such films though the selection of EISA parameters permits to control the adsorption of such substances into the mesoporous matrix and their subsequent release into the peri-implant region. Studies I, II and III analysed the effect of Mg incorporation into mesoporous titania coatings towards two cellular models during early and later stages of cell activity.
Study I characterized the morphology, chemistry, and topography
of mesoporous titania coatings and the effects of Mg-loading on surface micro- and nano-structures. Mg release was determined and its effect was evaluated on human foetal osteoblast popu-lations. It was shown that mesoporous films possessed a smooth surface with pores that faced outward. Mg adsorption did not substantially alter the mesoporous surface roughness both at micro- and nano- levels. Mg was released within 24 hours of incubation in cell culture conditions, thus its bioactive effect only occurred during initial osteoblasts activity.
Study II evaluated the ability of Mg-loaded mesoporous coatings to
modulate multipotent adipose-derived stromal cell differentiation toward the osteoblast phenotype. The results demonstrated that Mg release had a strong impact on this cellular model, promoting osteoblast marker expression in standard cell culture conditions. Interestingly, Mg significantly promoted the expression of osteo-pontin, a protein that is essential for early biomaterial-cell osteogenic interaction.
In study III, the reagents and EISA parameters in the mesoporous
deposition were varied to generate three mesoporous titania coatings with 2-, 6- and 7-nm average pore size, to increase Mg content in the interconnected porosity of the films. The effect of various Mg contents released from the three mesoporous structures
was tested on human foetal osteoblasts populations with pre-de-signed osteogenic PCR arrays and real-time polymerase chain reaction. It was shown that Mg release affected osteogenesis and was controlled by tuning the pore dimensions of the mesoporous films. Increasing pore size by 1 nm (from 6 nm to 7 nm) significantly enhanced the bioactivity of the film without altering the surface roughness.
Study IV
In orthopaedics Mg alloys has received increasing attention as bioresorbable metals for bone regeneration. However, localized material degradation is too fast and provokes the premature loss of mechanical properties, preventing correct cellular development and bone healing in vivo . For this reason, various alloying elements are combined with high-purity Mg to modulate and optimize degrada-tion behaviour.
Study IV of this thesis investigated the degradation parameters of
Mg2Ag, Mg10Gd, and Mg4Y3RE alloys and how the alloys differently affect human umbilical cord perivascular cell adhesion and spreading. Mg4Y3RE showed the highest degradation rate and, thereby, the highest trend in increases in pH and osmolality of the surrounding fluid. However, both Mg4Y3RE and Mg10Gd allowed cells to better adhere and spread across their degraded surfaces; in comparison, surface degradation of Mg2Ag was more aggressive with weak or no visible cellular structures on it.
Conclusions
In summary, the results of the present thesis explored the potential of Mg for its application in bone tissue regeneration. Titanium implant surfaces coated with mesoporous TiO2 thin films and
further loaded with Mg enhanced bone cell activity and osteo-progenitor development into mature osteoblasts. Thus, mesopor-ous deposition followed by Mg loading may be a suitable alternative to existing implant surface treatments.
Bioresorbable materials must degrade slowly and uniformly in order to simulate the tissue healing process. Mg10Gd possesses reduced content of alloying element and a suitable homogenous degradation pattern in vitro that allows proper adhesion of undifferentiated cells. Mg10Gd thus represents a biodegradable Mg-based material with promising mechanical and biological properties for use in dental and orthopaedic fields.
ACRONYMS AND SYMBOLS
ACRONYMS 3-D ADSC AFM ASTM ATP cDNA CO2 Co-Cr CPD CP Ti DAPI DMEM/MEM DNA ECM EDTA EDX EISA FBS FITC HA hFOB HUCPV MAO Mg2Ag Mg4Y3RE Mg10Gd MP MPMg Three-dimensionalAdipose-derived stromal cells Atomic force microscopy
American Society for Testing and Materials Adenosine triphosphate
Complementary deoxyribonucleic acid Carbon dioxide
Cobalt-chromium Critical point drying
Commercially pure titanium
2-(4-Amidinophenyl)-1H-indole-6-carboxamidine Dulbecco’s modified eagle medium
Deoxyribonucleic acid Extracellular matrix
Ethylenediaminetetraacetic acid Energy-dispersive X-ray spectroscopy Evaporation-induced self-assembly Foetal bovine serum
Fluorescein isothiocyanate Hydroxyapatite
Human foetal osteoblasts
Human umbilical cord perivascular Micro-arc oxidation Magnesium-silver Magnesium-yttrium-rare-earth Magnesium-gadolinium Mesoporous Mg-loaded mesoporous
MSCs MTT NaCl NP PIIID pNPP PTFE PTH QCM-D RNA RT-PCR SEM TEOT TiO2 TRITC-phalloidin ISO 10993 WJ XPS
Mesenchymal stem cells
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide Sodium chloride
Non-porous
Plasma immersion ion implantation and deposition p-nitrophenyl phosphate
Polytetrafluoroethylene Parathyroid hormone
Quartz crystal microbalance with dissipation monitoring Ribonucleic acid
Real-time polymerase chain reaction Scanning electron microscopy Titanium (IV) ethoxide Titanium dioxide, or titania
Tetramethylrhodamine isothiocyanate-conjugated phalloidin International Standardization Organization, Biological Evaluation of Medical Devices
Wharton’s jelly
X-ray photoelectron spectroscopy
ELEMENT SYMBOLS Ag Al Ar C Ca Ce F Gd La Li Mg Mn N O P S Ti Silver Aluminium Argon Carbon Calcium Cerium Fluor Gadolinium Lanthanum Lithium Magnesium Manganese Nitrogen Oxygen Phosphorus Sulphur Titanium
V Y Zn Zr Vanadium Yttrium Zinc Zirconium GENE SYMBOLS ALP B2M BMP BMP2 BMP4 BSP COL1 GAPDH MMP MMP8 OCN (BGLAP) ONC OPN OSX RUNX2 TGFB1 Alkaline phosphatase Beta-2-microglobulin Bone morphogenic protein Bone morphogenic protein 2 Bone morphogenic protein 4 Bone sialoprotein
Collagen, type 1
Glyceraldehyde 3-phosphate dehydrogenase Matrix metalloproteinase
Matrix metalloproteinase 8
Osteocalcin (bone gamma-carboxyglutamate [gla] protein) Osteonectin
Osteopontin Osterix
Runt-related transcription factor 2 Transforming growth factor, beta 1
INTRODUCTION
Bone replacement and repair
Overview
Worldwide, the annual number of prostheses utilized in patients has risen dramatically in recent decades. Increasing life expec-tancies, which have generated more patients in need of treatment, is one factor in this trend [1-3]. About 90% of the population over age 40 years suffers from degenerative bone and joint diseases, such as osteoporosis and osteoarthritis [4]. In osteoporosis, reduced bone mineral density weakens bone structure, potentially causing severe fractures. Approximately 30% of postmenopausal women suffer from osteoporosis in the United States and Europe [5, 6]. Thus, all populations around the world are expected to experience increasing incidences of degenerative bone diseases due to aging.
According to the 2014 Annual Report of the Activities of the Bone and Joint Decade, the huge impact of musculoskeletal conditions on global health represents the second greatest cause of disability, increasing 45% between 1990 and 2010 [7]. Development of new and modified biomaterials is thus needed to improve quality of life, particularly in bone replacement and repair. Use of non-resorbable pins, screws, plates, and rods of metallic materials is common in fracture management, whilst joint replacement includes permanent devices for the hip, knee, shoulders, ankle, and elbow. The non-resorbable characteristic of these materials can be a drawback if re-entry into the surgical site, which is invasive and costly, is needed [8, 9].
The pursuit of alternative bioresorbable materials that function as well as traditional non-resorbable materials has been of some inter-est in recent decades. For instance, polymers and ceramics have replaced some types of metal implants. These newer materials pos-sess excellent biofunctionality, but they do not pospos-sess the strength and mechanical properties that load-bearing implants require [10]. Metallic devices have been widely used to rehabilitate edentulism in the past 50 years [11]. One of the factors proposed by Albrektsson and co-workers that strongly influences the integration of an implant in bone is the implant surface quality [12]. Wennerberg et al. demonstrated that surfaces with moderate microroughness yielded the highest implant success rates in terms of direct bone-to-implant contact [13, 14]. Excessively rough surfaces, such as thermal spray implant surfaces, have been largely discussed, and their use is hesitant due to the risk of negative biological reactions around the implant [15-18].
Cell recruitment and initial interaction seem to occur more rapidly on nano-structured surfaces, which appear to enhance surface bio-activity [19, 20]. Nano-scale features, such as surface nano-pits or isotropic nano-porosity, reportedly influence initial attachment of osteoprogenitor cells positively [21-23]. Modification of an implant surface at the nano level generally includes modulation of the topography and also the chemistry, providing a synergistic effect that promotes new bone formation [24, 25]. Thus, improving the bioactive properties of the implant surface is a clever strategy for overcoming negative clinical outcomes and improving bone-implant surface adherence in the early stages of bone formation, which is essential for early loading of the implant.
Common metals for orthopaedic applications
The fundamental requirements of orthopaedic materials are optimal wear and corrosion resistance and a combination of mechanical and physical properties that allow them to withstand constant loading during function. Currently, the best materials for bone replacement and fixation are considered to be metals: stainless steel (316L stainless steel, ASTM F-593),
cobalt-chromium alloys (ASTM F-75), commercially pure (CP) titanium (Ti) (ASTM F-76), and Ti alloys (ASTM F-136). Stainless steel is still used in applications ranging from cardiovascular to otorhinology. Cobalt-chromium (Co-Cr) alloys have demonstrated excellent wear resistance and, thereby, better functionality in total joint and femoral head replacement. These applications are subjected to continuous load during function, thus accurate transfer of the loading pressure to the host bone is necessary. Another concern is the release of metallic non-physiological ions and nano-particles from conventional metals, which may occur at the peri-implant site and lead to inflammatory progression, thus reducing implant stability [26]. The high difference in elastic modulus of these materials compared to bone results in stress shielding events caused by the transfer of normal stresses through the implant to the bone [27]. CP Ti and Ti alloys are the materials of choice in failed joint replacement or fractured bone fixation, due to their light weight, lower density, and better ductility. Moreover, Ti and its alloys have widely demonstrated to be the most compatible materials for tissue healing when inserted into the human body [28]. The bioinert properties of Titanium make the metal ideally suitable for use in dentistry for tooth replacement, orthodontics, and maxillofacial surgery applications.
Materials for oral and maxillofacial applications
Surgical repair of maxillofacial bones requires that the plates and screws are able to withstand varying stresses, especially in the mandibular and maxillary regions. In 1976, Champy introduced mini-plates and mini-screws in facial surgery [29]. These components are produced in various sizes and lengths for use in cortical bone of varying thicknesses in the orofacial regions.
Another challenge is the treatment of large bony defects. Localized lack of bone in the jaw is generally caused by infection, trauma, tumours, and skeletal abnormalities [30]. Nowadays, the common strategy for regenerating bone in the oral and maxillofacial region, often involves barrier membranes. The advantage of barrier membranes is their ability to generate space that allows bone
forming cells to repopulate the damaged site and thus enhance the healing. Currently, a large range of membranes is available on the market. They are made of various materials, such as non-resorbable synthetic polymers or metals, or naturally-derived materials like collagen. Initially, non-resorbable membranes made of polytetrafluoroethylene (PTFE) and reinforced with a Ti frame were adapted to the defect margins to provide a rigid space-keeping effect [32]. The use of Ti meshes was subsequently introduced. These non-resorbable applications provided more stable space maintenance and, thereby, generated more adequate bone volume in the defect than PTFE membranes [33-35]. Regenerative treatment with non-resorbable membranes requires fixation of the device with Ti mini-pins. The entire system must be removed once its role is completed. Therefore, other materials have been used to produce membranes that degrade upon the healing of the injured site, eliminating the need of a second surgery. For instance, pure porcine collagen membranes completely and safely resorb, promoting the natural healing of the bone [36-38]. The problematic relies on its low rigidity and, thereby, poor space-keeping effect. For this reason collagen membranes are normally used together with allogeneic or xenogeneic bone [39].
Dental implants: current trends of implant surface
modifications
Ti implants were introduced in the 1960s when Brånemark and his colleagues accidentally discovered that Ti successfully integrated in bone, and they are currently used in tooth restoration with a clinical success rate greater than 95%. However, stimulation of initial bone apposition at the peri-implant interface during healing to achieve firm implant stability remains a prominent goal. One of the factors proposed by Albrektsson and co-workers that strongly influenced the integration of implant in bone is implant surface quality [12]. The topographical, chemical, physical, and mechanical characteristics of implant surfaces have been extensively modified to develop implants with predictable tissue-integrative properties. Wennerberg and Albrektsson have proposed guidelines for developing and analysing new Ti implant surfaces with optimal topography [40]. Experimental evidence
demonstrated that optimal bone response occurred with a moderately roughened implant surface (Sa about 1.5 µm) [13, 14].
Many studies, utilizing different techniques, have attempted to modify the chemical composition of implant surfaces. The principle is based on the assumption that a specific surface chemistry would induce faster bonding of the surrounding cells and proteins to the implant, converting the inert nature of Ti into a bioactive one. Techniques for modifying the surface of dental implants during manufacture include etching with various acid solutions to obtain a surface roughness of specific dimensions. Ellingsen’s group first introduced this technique using hydrofluoric acid (HF) to etch Ti implants, which they then placed in rabbit bone; after 4 and 8 week of healing, implant retention was increased four-fold compared with non-etched Ti surfaces [41]. Acid treatment produces defined surface textures, with controlled micro- and spontaneously formed nano-scale structures. In some cases, the surface chemistry can be modified by choice of acid solution [41-44]. Such treatment generates surfaces that incorporate specific electrolytes and hydroxyl groups, thereby changing the surface chemistry. This occurs also in case of electrochemical oxidation treatments, which generates porous structures on the implant surface [43].
Ti implant surfaces can also be modified by grit-blasting. This technique generates defined micro- and nano-structures when metallic or hard ceramic particles are projected at high speeds onto the implant surface. Even here, surface chemistry may be modified due to the incorporation of residual particles [45]. Another strategy for modifying implant surfaces is the deposition of sol-gel coatings, which have been shown to yield good results in vitro and in vivo. Sol-gel coating is the deposition of a gel onto the implant surface that generates various topographical and chemical properties depending on choice of sol-gel solution, period of immersion, and heat treatment parameters [46, 47].
Another interesting surface coating is mesoporous (MP) titania coating, which is generated using the evaporation-induced self-assembly (EISA) technique. This advanced surface modification combines the benefits of an engineered three-dimensional (3-D) matrix characterized by homogeneous porosity and a nano-struc-tured surface topography [48]. The presence of interconnected nano-porosity that is evenly distributed along the thickness of the coating allows this surface modification to act as a drug delivery system [49, 50]. Although this implant surface is not yet com-mercialized, recent research has demonstrated that the MP matrix can be loaded with drugs, such as Raloxifene and Alendronate, which can be released in a controlled and sustained manner immediately after implant placement into animal tissues. Clearly, this coating may be a suitable alternative for improving the bioactivity of implant surfaces; loading it with bioactive substances or molecules should enhance integration of the implant with peri-implant bone.
Peri-implant bone healing
When inserted into host tissue, a biomedical device first makes contact with blood, which excites a series of biological events at the peri-implant site, such as protein adsorption, coagulum formation, inflammation, and new tissue formation. In peri-implant bone healing, osteogenesis occurs as contact osteogenesis and distance osteogenesis. Osborn and Newesly, who defined these two phenomena, described contact osteogenesis as the process by which bone cells build newly formed bone on the implant surface, and distance osteogenesis as the process by which new bone is formed on the surface of the host bone in apposition to the implant. In contact osteogenesis, the adsorption of proteins such as fibrinogen, fibronectin, and vitronectin to the implant surface facilitates cell adhesion to the device [51-54]. Mesenchymal stem cells (MSCs) migrate to the damage site after platelet and specific protein adhesion [55]. MSCs are unspecialized cells (no specific function) that are capable of renewing themselves through cell division. They can differentiate into tissue-specific cells, such as adipocytes, chondrocytes, osteoblasts, neurons, and muscle cells under certain physiological or experimental conditions [56]. MSCs
are classified according to their origin as embryonic, foetal, or adult: embryonic stem cells derive from the early embryo, such as human umbilical cord stem cells; foetal stem cells derive from the developing foetus; and adult stem cells derive from adult mature organs, such as bone marrow, adipose tissue, the heart, and dental pulp [57].
One of the prerequisites for successful osseointegration around an implant is the correct adhesion and proliferation of MSCs onto the implant surface (Figure 1), which concludes with the complete maturation of an osteoblast population, producing a mineralized matrix and thus the desired conditions for osteoid formation [55, 58]. Biodegradable implants that gradually degrade from the surface must be manufactured from a material that does not shed overly large fragments and products from the outer layer but instead possesses a surface that initially degrades slowly and homo-geneously in order to achieve the desired cell adhesion [59]. Thus, choice of material – and its topographical and chemical, but also physical and mechanical properties – has been proven to be crucial for guiding cell colonization and differentiation [19, 20, 60-62].
Figure 1. Schematic illustration of MSC morphology during adhesion, proliferation, and differentiation on a material surface [63].
Cell adhesion, proliferation, and differentiation
MSCs adhere to a surface through focal adhesions and cytoskeleton bonding [64]. Once the cells adhere to the implant surface, a specialized group of factors initiates commitment of the MSCs to a specific cellular phenotype (Figure 2). Runt-related transcription factor 2 (RUNX2) plays a central role in the
differentiation of MSCs into osteoblasts, in part by inhibiting differentiation into adipocytes [65, 66]. After MSC commitment to the osteoblast lineage, MSCs evolve into preosteoblasts prior to developing into immature osteoblasts. Early bone markers that are weakly expressed by preosteoblasts are abundantly produced by immature osteoblasts, thus functioning to indicate the progression of osteogenic differentiation [66]. Immature osteoblasts are spindle-shaped cells that produce bone-related proteins such as osteocalcin (OCN), osteopontin (OPN), and bone sialoprotein (BSP); these non-collagenous proteins are further produced throughout osteoblast development. Mature osteoblasts possess a cuboidal shape and are capable of forming a mineralized bone matrix, due to the expression of collagen type I (COL I); its synthesis is one of the primary activities of differentiated bone cells [67]. Non-collagenous proteins and COL I are major components of extracellular matrix deposition [67]. This matrix stores factors that are involved in bone remodelling, and thus in the activation of bone resorption. Transforming growth factor beta 1 (TGFBI) is a growth factor produced by osteoblasts, but it is also involved in osteoclast-activated bone resorption. Metalloproteinases (MMP) are another class of proteins that plays a crucial role in later stages of bone remodelling. These proteins are collagenases and gelatin-ases that degrade collagen structures in mature bone [55, 68, 69].
Figure 2. Schematic illustration of mesenchymal stem cell (MSC) morphology and gene expression during differentiation into mature osteoblasts. Runt-related transcription factor 2 (RUNX2) initiates MSC osteogenic differentiation and inhibits adipogenesis. After commitment, MSCs develop into preosteoblasts that express osteogenic factors such as distal-less homeobox 5 (Dlx5). Immature osteoblasts express bone morphogenic protein 2 (BMP2), osterix (OSX), bone sialoprotein, and osteopontin. Mature osteoblasts are characterized by the expression of collagen type I [70].
Magnesium in bone metabolism
Magnesium (Mg) is an essential component of the human body. The Mg ion (Mg2+) is a cofactor in more than 300 enzymatic reactions: it
is a determinant in the synthesis of proteins and of DNA and RNA; in ion transportation; in cell migration and function; and in intracellular energy production via the adenosine triphosphate (ATP) system [71-73]. About 60% of the body’s Mg is located in bone; Mg is thus highly influential in mineral and matrix bone metabolism, affecting both bone cell function and hydroxyapatite (HA) crystal formation [74]. Mg is involved in the mineralization process of bone formation, particularly in immature stages, and is suggested to be responsible for the regulation of osteoblast and osteoclast activation [75]. Many studies have demonstrated a correlation between Mg and bone mineral density. Mg deficiency in various animal models leads to impaired bone growth, induced osteopenia, and increased
skeletal fragility [76, 77]. Reduced Mg content was observed in women suffering from osteoporosis, while larger HA crystals formed in trabecular bone becoming brittle, thus reducing bone stiffness [78, 79]. Low Mg intake also affects cartilage and bone differentiation [80]. Mg deficiency is associated with a reduction in parathyroid hormone (PTH) secretion and, consequently, with a decrease in vitamin D levels [81]. Indeed, Sahota et al. found lower levels of PTH and Mg in post-menopausal women who were vitamin-D defi-cient than in post-menopausal women who were not [82]. This strong correlation between Mg and bone health has led researchers to consider the element as a potential material for strengthening newly formed bone or fastening its apposition around a medical device.
Magnesium-based biomaterials
Clinical applications of Mg and Mg alloys are most probably in the reconstruction of bone defects and the repair of bone fractures, or possibly as a bioactive incorporated substance for oral implant surfaces.
Magnesium and magnesium alloys for medical applications
Metals that can degrade in the physiological environment have been recently introduced as potential materials for oral maxillofacial and orthopaedic applications. As previously described, biodegradable polymer devices are generally considered to have insufficient me-chanical properties, and non-biodegradable metal implants must often be removed when they are no longer necessary.
Mg and Mg alloys have shown promising potential as tailored bio-degradable metals for medical implants, changing the paradigm of metal implant use in medical conditions [83]. In 1907, Lambotte was the first to attempt to use a pure Mg plate in lower leg fracture healing, comparing its performance to that of a gold-plated steel nail – the standard treatment at that time. The Mg plate attempts were unsuccessful due to rapid degradation in vivo, disappearing only 8 days after implantation and generating a high amount of hydrogen gas. However, Lambotte demonstrated that pure Mg within cortical bone constituted a biocompatible system [84]. Mg
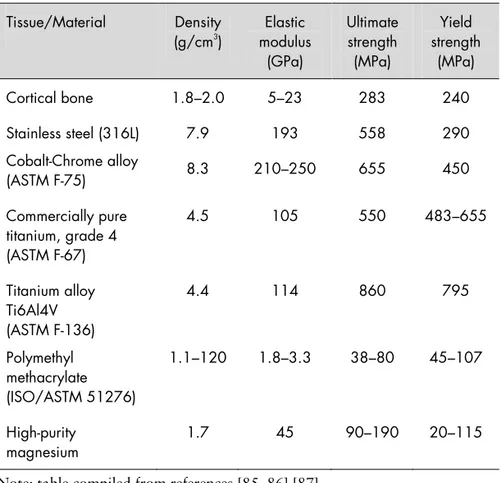
and its alloys are attractive because they possess the biodegradable nature of polymers, which were the earliest and most commonly used materials for such applications, and mechanical properties similar to cortical bone, such as density and elastic modulus. Table 1 lists the mechanical properties of some of the materials being used for orthopaedic implants today, according to ASTM specifications.
Table 1. Mechanical properties of cortical bone and some of the materials being used in orthopaedic applications today. (ASTM = American Society for Testing and Materials).
Note: table compiled from references [85, 86] [87].
In aqueous environments, however, one concern in the biomedical use of Mg and its alloys is the evolution of hydrogen gas that accompanies degradation, according to the following reaction:
𝑀𝑔 + 2𝐻2𝑂 → 𝑀𝑔(𝑂𝐻)2+ 𝐻2 Tissue/Material Density (g/cm3 ) modulus Elastic (GPa) Ultimate strength (MPa) Yield strength (MPa) Cortical bone 1.8–2.0 5–23 283 240 Stainless steel (316L) 7.9 193 558 290 Cobalt-Chrome alloy (ASTM F-75) 8.3 210–250 655 450 Commercially pure titanium, grade 4 (ASTM F-67) 4.5 105 550 483–655 Titanium alloy Ti6Al4V (ASTM F-136) 4.4 114 860 795 Polymethyl methacrylate (ISO/ASTM 51276) 1.1–120 1.8–3.3 38–80 45–107 High-purity magnesium 1.7 45 90–190 20–115
Mg degradation alters the surface chemistry of the implant by pro-ducing degradation products of varying natures that dissolve and increase the pH of the peri-implant region, but also protect the material from further degradation [88].
Use of biodegradable Mg implants for osteosynthesis requires ade-quate maintenance of the device during the healing period. If the implant degrades too rapidly, hydrogen bubbles interpose between the implant surface and the surrounding tissues, impeding attach-ment of proteins and cells to the material surface and, thereby, pro-voking premature failure of the implant. The ideal course of degra-dation begins slowly and increases with time once the damaged tissue has healed sufficiently.
Several alloying elements have been combined with pure Mg to achieve moderate and homogeneous degradation behaviour. Witte and co-workers demonstrated that implant degradation depends on alloy composition and, in Guinea pig bones, the device should be present for at least 12 weeks to allow the healing [89]. The researchers alloyed pure Mg with cadmium to produce plates and screws for securing fractures. Of 34 cases, only 9 failed, possibly due to infection, while the rest were successfully tolerated by the host, preserving higher mechanical integrity than pure Mg. McBride et al. reported positive bone tissue reactions to magnesium-aluminium-manganese (Mg-Al-Mn) screws and pegs in the repair of fractures and bone grafts [90].
Aluminium (Al) has been considered to improve the strength and wear resistance of Mg, however, Al leads to the depletion of phosphate in tissues, a factor in the progression of senile dementia [89, 91]. Essential physiological elements, such as calcium (Ca) and zinc (Zn) have also been used in Mg alloys, but their effect on reducing the degradation rate and improving mechanical strength is mild. Nanda and Saravanan designed and tested binary Mg-silver [Ag] alloys with varying percentages of
Ag in vitro and in vivo [92]. Ag proved to tailor the degradation
process and the mechanical properties of Mg, showing no cytotoxic effect on mammalian cells [93, 94]. Other elements,
such as lithium (Li) and zirconium (Zr) have been combined with Mg in attempts to improve the mechanical properties of Mg [95]. The best outcomes in terms of bone response were obtained from
in vivo applications of Mg implants containing rare earth elements
(REE) [89]. REEs are a group of 17 elements which are mainly used to improve ductility, degradation resistance, and grain boundary strength in Mg. Johnson et al. reported that bone marrow-derived mesenchymal stem cells adhered to magnesium-yttrium (Mg-Y) surfaces in a homogenous and healthy state [96]. Feyerabend and co-workers elucidated the effect of some REEs on viability, apoptosis, and inflammatory cytokine expression of four cell types in vitro. The results revealed that lanthanum (La) and cerium (Ce) induced the highest cytotoxicity in the group while gadolinium (Gd) and Y seemed to be suitable for promoting cell growth [97]. Witte et al. explored the performances of two Mg-REE rods, comparing them to two Mg-Al-Zn rods inserted in Guinea pig femur and retrieved at 6 and 18 weeks of healing. Analyses of the degradation layer within the bone found that the REEs were localized in the degradation layer and had not diffused into the surrounding tissue. Moreover, high levels of Ca and P were detected at the interface between the degradation layer and the newly formed bone, indicating the possible formation of amor-phous apatite [89].
The initial degradation rate of Mg alloys, however, remained too high and localized in vivo, even in cases of Mg4Y3RE which, up to now, represents the most promising Mg-based biodegradable material for bone applications. Thus, demand for new Mg alloy compositions continues to increase, along with a need for a fundamental understanding of their degradation process in physiological conditions, of their in vitro cytocompatibility, and of their in vivo tissue response.
Some Mg alloys have been tested as possible open-porous scaffolds for load-bearing applications in tissue engineering. Witte et al. cast one Mg-Al-Zn alloy and one Mg-Al-Zn-Mn alloy as porous metallic scaffolds, which they tested in vivo; however, degradation
was still too rapid, and traces of Al and Zn triggered inflammatory reactions [98]. In addition, Mg alloys have been produced in particle form for use as matrix composites in musculoskeletal and dental treatments. Some examples are an Mg matrix composite reinforced with hydroxyapatite (HA) [99] and injectable Mg-phosphorous [P] cements [100].
Magnesium-modified titanium dental implants
Chemical surface modification of Ti dental implants is a promising strategy for improving the biochemical bonding of implants with bone [41, 101, 102]. Bioactive ions, such as Ca, Mg, P, and fluorine (F), have been incorporated into Ti surfaces with different techniques [103-106]. In particular, Ca and Mg proved to be crucial in many biochemical mechanisms that occur at the bone-implant interface.
Several research groups have focused on the bioactivity of Mg-incorporated surfaces to elucidate the biochemical role of Mg ions in bone generation. Zreiquat et al. reported that osteoblast adhesion to biomaterials depends on an integrin-mediated mechanism, and that Mg supplementation to bioceramic substrates appeared to be involved in this molecular process [107]. It was shown in another report that Ti alloy modification with Mg contributed to human osteoblast function and differentiation [108].
A few years later, Revell et al. analysed interfacial bonding be-tween bone and Mg-ion embedded HA coatings deposited on Ti implants, revealing the positive effect of Mg in bone cell activity compared with non-enriched HA surfaces [109]. Sul and co-workers examined in depth the influence of Mg on bone-cell responses, incorporating it into Ti implants via micro-arc oxidation (MAO) and, subsequently, with plasma immersion ion implantation and deposition (PIIID). In vivo experimental evidence demonstrated that Mg-incorporated implants promoted significantly higher bonding strength and faster osseointegration compared to non-incorporated CP Ti [110-112].
Park et al. investigated the impact of Mg-incorporated nano-porous Ti oxide surfaces on osteoblasts in vitro. The results showed that Mg-incorporated surfaces increased not only initial cellular adhesion but also subsequent cellular events such as alkaline phosphatase (ALP) activity and RNA expression of integrins and transcription factors [113].
AIMS
The overall aim of Studies I, II, and III was to assess Mg as a bioactive substance when loaded into a MP titania thin films produced via EISA, and considered as a novel surface modification for Ti implants. The effect of Mg release was tested on osteoprogenitors and osteoblast populations in vitro, with the aim of enhancing initial cell apposition, proliferation, and differentiation.
Specific aims (Studies I, II, and III):
To estimate Mg release profile in cell culture conditions (Study I).
To evaluate if Mg loading alters the surface morphology and topography of native MP coatings (Study I and III) To investigate the osteoconductive potential of Mg release
toward human foetal osteoblasts (hFOB) at early and late stages of growth (Studies I).
To investigate the ability of Mg to promote adipose-derived stromal cell (ADSC) differentiation toward the osteogenic lineage (Study II).
To investigate if increasing MP pore dimensions improves Mg loading and release and, thus, enhances hFOB develop-ment at even later stages of their proliferation (Study III). The aim of Study IV was:
To evaluated the degradation behaviour of Mg2Ag, Mg10Gd, and Mg4Y3RE alloys as well as high purity Mg in controlled cell culture conditions. The short-term cell response of human umbilical cord perivascular cells (HUCPV) was investigated in terms of cell viability and adhesion structures, providing a preliminary indication of the suitability of these alloys as biodegradable metals for medical applications.
MATERIALS AND METHODS
Specimen preparation
Magnesium-loaded mesoporous TiO
2coatings
(Studies I, II, and III)
CP Ti (grade 4; Zimmer Holdings, Warsaw, IN, USA) discs were used in Studies I, II, and III. In Studies I and II, the discs were 15 mm in diameter and 1 mm in thickness; and in Study III, 12 mm and 1 mm. All specimens were soaked in absolute ethanol
(≥ 99.8% )and rinsed in an ultrasonic bath before coating
deposition. After each coating and Mg loading procedure, the discs underwent gamma radiation for sterilization.
Evaporation-induced self-assembly
MP TiO2 thin films were synthesized via EISA. Various
structure-directing agents, amphiphilic surfactants or block-copolymers with defined chain lengths, tuned the pore size of the films. Together with an inorganic precursor, the agents assembled into micelles to generate ordered structures. The nature and ratio of each compo-nent as well as the self-assembly parameters, such as aging time and calcination, are fundamental to guiding the periodicity of pore distribution as well as their size.
In Studies I and II, MP films with an average pore size of 6.0 nm were generated onto non-porous (NP) Ti discs. To do this, a struc-ture-directing agent, the amphiphilic block copolymer Pluronic® P123 (triblock copolymer EO20PO70EO20; Sigma-Aldrich, St.
Louis, MO, USA), were mixed with an inorganic precursor, Ti (IV) ethoxide (TEOT). A drop of final solution was deposited onto the Ti discs and spin-coated them at 7000 rpm for 1 minute. After spin-coating, the discs were stored at room temperature to allow
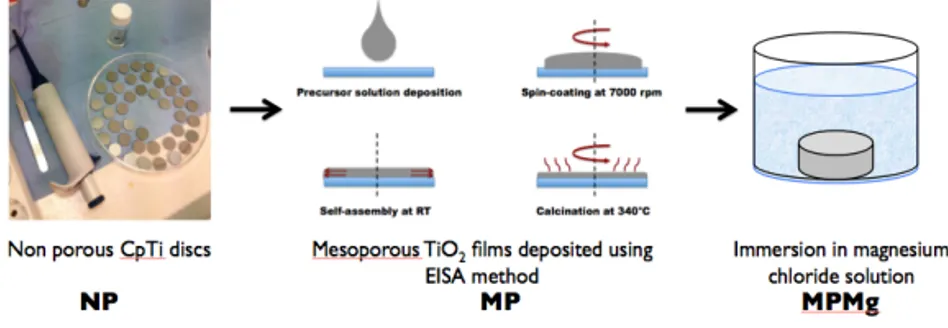
self-assembly to complete. Then the structure-directing agent was removed through calcination, which also increased condensation and the crosslinking density of the TiO2 matrix. Figure 3 illustrates
the mesoporous TiO2 films deposited via evaporation-induced
self-assembly and subsequently loaded with Mg.
Figure 3. Illustration of evaporation-induced self-assembly to form
mesoporous TiO2 films (MP) and magnesium (Mg) loading through immersion
in a magnesium chloride solution to yield the final magnesium-loaded
mesoporous TiO2 film (MPMg).
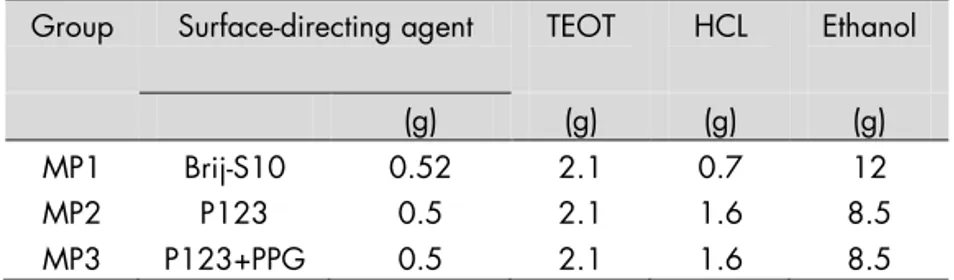
In Study III, the synthesis parameters in EISA were varied to obtain
average pore sizes of 2 nm (MP1) and 7 nm (MP3) in the MP films; this modification was done based on the results of Studies I and II for MP films with average pore sizes of 6 nm (MP2; see Results). For all films, the precursor was a TEOT solution, but the structure-directing agent that was mixed with the precursor solution varied. Briefly: MP1 films were generated using Brij® S10 C18H
37-(OCH2CH2)nOH; Sigma-Aldrich); MP2 films using Pluronic P123
(Sigma-Aldrich); and MP3 films in a mixture of P123 with an organic additive, polypropylene glycol (PPG), which increased pore size. Table 2 describes the recipes for each group in detail, including component volumes. To achieve uniformity, all films were spin-coated at 7000 rpm for 1 minute. MP1 and MP2 films were than aged at room temperature, followed by calcination at 350°C; MP3 films were aged for 1 day prior to calcination in a sealed chamber containing saturated NaCl solution.
Table 2. Types and amounts of components used to synthesize the mesoporous (MP) titania films in Study III.
Brij® S10 = C18H37(OCH2CH2)nOH; P123 = Pluronic® P123; PPG =
polypropylene glycol; TEOT = Ti (IV) ethoxide; HCL = hydrochloric acid
Magnesium loading
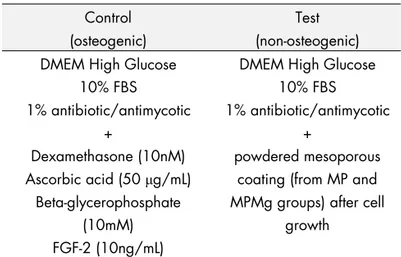
Half of the coated discs of each group were soaked for 1 hour in a magnesium chloride (MgCl2) solution to load the discs with Mg
and then dried in the oven at 100°C to evaporate the liquid. Therefore, Studies I and II discuss MPMg (the magnesium-loaded MP TiO2 films of 6-nm pore size), whilst Study III discusses
MP1Mg, MP2Mg, and MP3Mg (the magnesium-loaded MP TiO2
films of 2-, 6-, and 7-nm pore size, respectively).
Magnesium alloys (Study IV)
Three Mg-based materials were selected as test materials in Study IV: Mg2Ag (1.89% Ag, the remainder was Mg), Mg10Gd (8.4% Gd, the remainder was Mg), and Mg4Y3RE (3.45% Y, 2.03% Nd, 0.84% Ce, the remainder was Mg). High-purity Mg (99.97%) was used as a control. The discs had a diameter of 1 cm, thicknesses of 1.5 mm, and average weights of 0.2 g; the production steps were casting, homogenizing heat treatment, extrusion, turning, and cutting.
Casting and heat treatment
Permanent mould gravity casting was used to produce Mg alloys (Mg2Ag, Mg10Gd, and Mg4Y3RE). After melting pure Mg, Mg was kept at 720°C and the preheated alloying elements were added under continuous stirring. The melt was poured into a pre-heated (550°C) permanent steel mould treated with boron nitride and T4 was selected as heat treatment to homogenize the alloys prior to
Group Surface-directing agent TEOT HCL Ethanol
(g) (g) (g) (g)
MP1 Brij-S10 0.52 2.1 0.7 12
MP2 P123 0.5 2.1 1.6 8.5
extrusion in an argon (Ar) atmosphere at 550°C (Mg10Gd and Mg4Y3RE) or 420°C (Mg2Ag) for 6 hours. High-purity Mg was cast by permanent mould direct chill cast.
Extrusion, turning, and cutting
The alloys were extruded indirectly with an extrusion ratio of 4:25 (Strangpreßzentrum Berlin, Germany). The extrusion chamber was set to 370°C and the billets (diameter = 30 mm) were pre-heated for 1 hour at 370°C (Mg2Ag), 390°C (Mg4Y3RE) or 430°C (Mg10Gd). Extrusion speed was between 3 and 4.5 mm/sec.
The cast billet (diameter = 110 mm) of high-purity Mg was extruded indirectly with an extrusion ratio 1/84. The temperature of the billet was 340°C and extrusion speed was 0.7 mm/sec. Discs (10 mm in diameter and 1.5-mm thickness) were machined from the extruded bars (Henschel KG, Munich, Germany) and sterilized with gamma radiation.
Surface characterization
Scanning electron microscopy (Studies I and III)
In scanning electron microscopy (SEM), an electron source generates an electron beam that then scans the sample surface. When the electrons penetrate the outer layer of the material, they transfer energy to electrons inside the sample (secondary electrons) that scatter in various directions depending on the topography of the sample. Detection of the emitted electrons generates a topogra-phical image of the material surface.
In the present thesis, high-resolution SEM images (LEO Ultra 55 FEG scanning electron microscope; Zeiss, Oberkochen, Germany) under an accelerating voltage of 5 KV were obtained to evaluate the thickness and pore direction of the MP TiO2 coatings in Studies
I and III.
Optical interferometry – Study I
Optical interferometry uses a light beam (λ 550 nm) with detection accuracy within a few millimetres. The beam is split into two separate beams: one beam hits the material surface and reflects; the

![Figure 1. Schematic illustration of MSC morphology during adhesion, proliferation, and differentiation on a material surface [63]](https://thumb-eu.123doks.com/thumbv2/5dokorg/3972693.77938/30.743.122.580.614.750/figure-schematic-illustration-morphology-adhesion-proliferation-differentiation-material.webp)