Degree project Malmö University
15 credits Faculty of Health and Society
EVALUATION OF
SKIN-FRIENDLY SOLVENTS FOR
INCREASED SOLUBILITY OF A
MODEL NSAID IN TOPICAL
FORMULATIONS
ANNA BERGSTRÖM
EVALUATION OF
SKIN-FRIENDLY SOLVENTS FOR
INCREASED SOLUBILITY OF A
MODEL NSAID IN TOPICAL
FORMULATIONS
ANNA BERGSTRÖM
Bergström, A Evaluation of skin-friendly solvents for increased solubility of a model NSAID in topical formulations. Degree project in Pharmacology 15
credits. Malmö University: Faculty of Health and Society, Department of
Biomedical Science, 2019.
Introduction Pain has been treated with topical or transdermal drugs for many
years, but the concentration of active substance that can be transported over the skin remains low. To be able to substitute the use of opioids in treatment of moderate to severe pain, more efficient formulations of transdermal drugs are needed. The aim of this study was to investigate the possibility to increase the concentration of diclofenac in topical formulations. For this reason, the solubility of diclofenac in different skin-friendly solvents was determined. In addition, the diffusion of diclofenac across silicone membranes from these solvents was investigated using Franz cells.
Methods The solubility of diclofenac was studied at 32ºC in water, phophate
buffered saline solution (PBS), saline solution, polyethylene glycol (PEG) 400, polyethylene glycol (PEG) 1500 (60 wt% in water), glycerol, propylene glycol, 1-propanol and 2-1-propanol. The diffusion of diclofenac over silicon membrane, used as a model for skin membranes, from six formulations, including Voltaren for comparison, was studied using Franz diffusion cells over six hours. Both for the solubility and diffusion experiments, the concentration of diclofenac was determined by an analytical method based on UV-spectroscopy.
Results The solubility of diclofenac was highest in PEG 400 and propylene glycol
with approximately 40 wt. %. The lowest solubility was seen in 2-propanol with approximately 1 %. In the diffusion study, only the formulation containing water as solvent and Voltaren gel resulted in detection of dicofenac in the receptor chamber, i.e. transported over the membrane in the time frame studied.
Discussion The results of the solubility experiments can be rationalized based on
the chemical and physical properties of the solvents and by considering concepts such as the salting-out efffect and appropriate number of hydrogen bond donors and acceptors. The results of the diffusion studies were inconclusive and since only a limited number of experiments could be performed within the scope of this work, the results need to be authenticated.
UTVÄRDERING AV HUDVÄNLIGA
LÖSNINGSMEDEL FÖR ÖKAD
LÖSLIGHET AV NSAID I
TOPIKALA FORMULERINGAR
ANNA BERGSTRÖM
Bergström, A Utvärdering av hudvänliga lösningsmedel för ökad löslighet av NSAID i topikala formuleringar. Examensarbete i Farmakologi 15 poäng. Malmö Universitet: Fakulteten för Hälsa och Samhälle, Institutionen för Biomedicinsk Vetenskap, 2019.
Introduktion Smärta har behandlats med topikala eller transdermala läkemedel i
många år, men det är en utmaning att uppnå höga koncentrationer som kan transporteras över huden. För att kunna ersätta användandet av opioider vid behandling av måttlig till allvarlig smärta behövs mer effektiva formuleringar av transdermala läkemedel. Målet med denna studie var att undersöka möjligheten att öka mängden aktiv substans som transporteras över huden. För detta ändamål, bestämdes lösligheten hos diklofenak i olika lösningsmedel. Vidare bestämdes transporten av diklofenak över silikonmembran från dessa lösningsmedel med hjälp av Franz celler.
Metod Lösligheten hos diklofenak studerades vid 32ºC i vatten, fosfatbuffrad
saltlösning (PBS), saltlösning, polyetylenglykol (PEG) 400, polyetylenglykol (PEG) 1500 (60 vikt % i vatten), glycerol, propylenglykol, 1-propanol och 2-propanol. Diffusion hos diklofenak över silikonmembran från nämnda
formuleringarna, inklusive Voltaren Gel som jämförelse, studerades med hjälp av Franzceller under sex timmar. För både löslighet- och diffusionsexperimenten bestämdes koncentrationen av diklofenak med en analysmetod baserad på UV-spektrofotometri.
Resultat Lösligheten av diklofenak var högst i PEG 400 och propylenglykol med
ungefär 40 vikt %. Den lägsta lösligheten uppmättes i 2-propanol med ca 1 %. I diffusionsstudierna kunde diklofenak enbart detekteras i receptorkammaren, dvs diklofenas transporterades över membranet, hos de formuleringar som innehöll enbart vatten eller Voltaren.
Diskussion Resultatet av löslighetstesterna kunde förklaras genom att ta de
kemiska och fysikaliska egenskaperna hos den olika lösningsmedlen som
användes i beaktande samt genom att använda begrepp som utsaltningseffekt och vätedonatorer/acceptorer. Resultaten av diffusionsstudierna var ofullständiga men då enbart ett begränsat antal experiment kunde utföras inom ramen för detta arbete behöver resultaten vidare styrkas.
BACKGROUND
As the population of the world and life expectancy increases, the use of pharmaceutical drugs increases at a steady pace. Globally, the pharmaceutical industry has a turnover of close to 7,200 billions annually. The US is dominating with 49% of the total turnover followed by Europe with 22%. In 2017, 200 million unique pharmaceutical drug units were sold in Sweden alone, and of those, 49% were prescription drugs. [1]
To optimize the treatment of patients and to accommodate the need of different patients, a wide variety of formulations has been developed. The far most common dosage form is tablets for oral consumption, but drugs taken orally can also be formulated as capsules, solutions, suspensions or specialty tablets that disintegrates buccally, sub-lingually or orally. Drugs can also be given as eyedrops, eardrops, inhalations or suppositories. When a drug cannot be administered orally, or if extra quick onset is necessary, injections or infusions can be given. Many drugs are also available in different formulations. [2] Another means of administrating drug is via the skin, either topically or
transdermal. Topical drugs give a local effect where they have been applied on the skin whereas the active substance in transdermal drugs is delivered across the skin for systemic or local distribution. Drugs that are given transdermal and/or
topically comes in different formulations and consistencies such as creams, gels, liniments, balms, lotions, ointments etc.
For treatment to be successful, the patient needs to display a high degree of adherence to the prescription. Of all medications prescribed, about 80 % is actually being picked up by the consumer at the pharmacy. From these 80%, only 70 % are used and only 50 % of the 70 % are used correctly. This means that only approximately 30 % of all prescribed drugs in Sweden today are taken according to the prescription or at all. This phenomenon has many reasons, a few being unwanted side effects, a distrust in the treatment, health illiteracy, economical aspects and so on. Poor adherence is a costly problem of the national healthcare system. [3] One way of addressing this problem is by developing more consumer-friendly products that have a high degree of effectiveness without the negative side effects.
The metabolism that an orally administered drug is subjected to before it has passed the liver and entered the systemic circulation for the first time is called first-passage metabolism. This degradation of the drug varies to a high degree between different substances. However, it always influences the amount that is actually biologically available. Despite the fact that many drugs are metabolized before they reach their target, oral drugs often have a high systemic
bioavailability, but this commonly leads to unwanted side effects. Since the orally administered drugs are distributed systemically, they can often cause problems that are unrelated to the disease or syndrome they are meant to treat. Side effects that are commonly seen are, for instance, nausea, vomiting and negative effects on the gastric mucosa. Transdermal administration of drugs has many advantages over other formulations. [4]. Since the surface of the skin is very large, many options are available for applying the drug which makes it easier to target the treatment where it is needed.
When topical products are applied on the area of treatment, the systemic effect that is seen with oral drugs is, in most cases, eliminated, which also means that unwanted side effects are minimized. As the product does not have to pass the liver, the first-passage metabolism is avoided and the pharmacokinetic profile will not be affected by, for instance, food uptake that delays the absorption of the drug. [5] In addition to these advantages, the problems with fear of needles and damage to the skin are also avoided.
The global market for transdermal drugs reached 4,2 billion USD 2016 and is expected to reach 7,4 billion USD by the end of 2024, according to Research and Markets, making transdermal drugs the second largest group of drugs after tablets. [6]
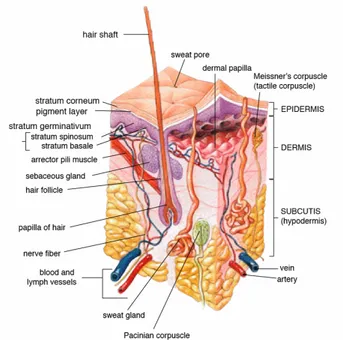
Figure 1 Anatomy of the human skin (source: en.wikipedia.org)
Transdermal delivery is a painless way of administering drugs on intact and healthy skin, but the skin is also a tough barrier to penetrate. The skins characteristics affects what can pass this barrier, and to what extent. [7]
The skin is the largest organ of the body and its main purpose is to protect us from water-loss, entrance of xenobiotics and microbes, regulate body temperature and permit the sensation of touch, heat and cold. The skin consists of three main layers as can be seen in figure 1. The outermost layer of skin is the epidermis that creates a waterproof barrier to the surrounding environment. Epidermis is divided into five sublayers, or strata, containing flattened cells, mainly keratinocytes. The epidermal keratinocytes are tightly linked by cell-cell junctions which gives the epidermis its mechanical strength and provides the body with a physical barrier. Highly organized lipids, acids, hydrolytic enzymes and antimicrobial peptides provides a chemical barrier that inhibits passage of external chemicals and pathogens. As long as the barrier function is intact, the skin provides excellent protection but physiological stress, for instance the use of glucocorticoids, compromises the outermost layer and thereby the barrier function. In addition, sudden and large shifts in the humidity can alter the hydration of the skin and
allow entry of microorganisms and other molecules. [8] Beneath the epidermis is the dermis that contains tough connective tissue, hair follicles, sweat glands, sebaceous glands, apocrine glands, lymphatic vessels, nerves and blood vessels. The dermis also contains mechanoreceptors that provide the sense of touch and thermoreceptors that provide the sense of heat. The deeper subcutaneous tissue is called hypodermis and is made of fat and connective tissue.
There are different possible routes of drugs to penetrate the skin. The molecule can pass through either the epidermis, the glands or the hair follicles. Sweat glands and hair follicles make up 0,1-1,0 % of the total skin surface and even though small molecules can pass this route most are absorbed through the
epidermis. The outermost sublayer of the epidermis is called stratum corneum and is the rate-limiting barrier for absorption of a molecule. The stratum corneum is mainly composed of lipids of the following three classes: 1 cholesterol, 2 esters, 3 ceramides. Since the lipids in the skin are in a lamellar structure, both lipophilic and hydrophilic molecules are prevented from entering the body. However, lipophilic molecules with a logP value of around 3 can more easily make it through the outer layer and into the circulation. From the stratum corneum and further down through the skin layers, the route taken can be either through or between the cells. The passing between cells are regulated by different kinds of junctions such as gap junctions, tight junctions among others while molecules pass through cells via pores or diffusion. [9]
There are different categories of pain-relieving medicines where most of them are administered orally. To control severe and very acute pain, different opioids are used. These are all prescription drugs that require careful monitoring since they are highly addictive. Examples of opioids given to treat severe pain are morphine and oxycodone. There are also milder opioids, like codeine or tramadol that are given for moderate to server pain. They are usually combined with other groups of pain medicine. Apart from causing addiction, opioids cause severe unwanted side effects. Another large group of less potent painkillers contains the active
substance paracetamol. They are usually the primary choice for different kinds of pain since they have few side effects and can be used by most people. One disadvantage of paracetamol is the fact that it cannot be used to treat
inflammations. Instead, when experiencing fever and pain in combination with inflammation, so called NSAIDs (Non-Steroid Anti-Inflammatory Drugs) are usually used. Ever since this group of drugs came into use, a few thousand years ago, a large number of products has been developed. However, focus have mainly been on oral drugs. With the increased awareness of the negative effects of orally administered NSAIDs, for instance increased risk of hemorrhaging, negative effect of liver and kidney and increased production of gastric juice, topical
products have been developed to decrease the systemic influence. [10] In Europe, topical painkillers have been on the market for 30 years and in the US since 2007. [11]
On the Swedish market, a number of painkillers that can be applied on the skin as a gel are available. Some of the most well-known are Ipren Gel which contains ibuprofen and Voltaren Gel, where the active substance is diclofenac. Diclofenac works in the body by inhibiting the syntheses of prostaglandins through
cyclooxygenase. [12] In this study, the focus has been on diclofenac as a representative of the NSAIDs.
Diclofenac has the chemical and physical properties listed in Table 1.
Table 1
Chemical and physical properties of diclofenac
Molecular weight 296 g/mol
Solubility in water at 25°C 2,37 mg/l
logP 4,51
logD 1,44
pKa 4,15
Diclofenac (2-(2,6-dichloranilino) phenylacetic acid) (see figure 2) was
developed in the beginning of the 1970s and introduced in Great Britain in 1979. In Voltaren Gel, diclofenac occurs as its salt with a sodium ion to balance the charge of the negatively charged carboxylate group. [13]
a) b)
Figure 2
a diclofenac, b sodium salt of diclofenac divided into ions
Topical formulations contain different excipients to give the drug the properties needed such as stability, texture, chemical and physical properties, scent and aid in the manufacturing process. For example, Voltaren Gel contains carbomer homopolymer type C that works as an emulsifier, polyoxyl 20 cetostearyl ether and liquid paraffin that are ointment bases, cocoyl caprylocaprate and diethyl amine that are both used to regulate the pH in the product, isopropyl alcohol which is used in the manufacturing process propylene glycol that is a humectant, purified water and fragrance.
Voltaren Gel can be bought over the counter in pharmacies and in many
convenient stores. The gel comes in two different concentrations, 11,6 mg/g and 23,2 mg/g. This gives a concentration of active substance of about 1% and 2% respectively. [14]
In a study published in 2017 in Current Medical Research and Opinion it is stated that diclofenac is effective in treating pain and can penetrate the skin to reach the
Cl Cl HN O O Na
problem area in concentrations that are sufficient to help with, for instance, arthrosis but that more studies are needed to better understanding the clinical efficiency of topical drugs. [15]
To avoid side effects and systemic delivery, the treatment of different kinds of pain, for instance muscle pain and rheumatic arthritis, is best performed with a local topical treatment. But, since Voltaren Gel only contain at best 2 % of its active substance, only a relatively small amount will penetrate the skin barrier, stratum corneum, and reach the pain receptors. Therefore, the aim of this study was to increase the concentration of active substance in the solvent as transport over the skin is proportional to the concentration of active substance in a
particular solvent. However, to accomplish this, a suitable solvent must be used. The solvent will have to meet a number of criteria such as moderate in cost, harmless to put on the skin, easy to work with and so on. This study aims at testing different solvents to find a suitable component that can be used in diffusion studies.
Aim
The aim of this study was to investigate the possibility to increase the
concentration of diclofenac in topical formulations, allowing more diclofenac to pass through the skin barrier and create a more efficient topical drug for local treatment of pain.
MATERIALS AND METHODS
Materials
Diclofenac as sodium salt was provided as a gift from a local pharmaceutical company. NaCl, Na2HPO4•2H2O och KH2PO4 were purchased from Merck (Darmstadt, Germany). Polyethylene glycol 1500 Da (PEG 1500), 400 Da (PEG 400), glycerol, propylene glycol and 1-propanol were purchased from Sigma-Aldrich (Saint Louis Missouri, USA). 2-propanol was purchased from VWR Chemicals (Fontenay-sous-Bois, France). A commercial formulation, Voltaren 23,2 mg/g gel (GlaxoSmithKline), was obtain from a local pharmacy. All water used in this work was purified with Purelab Flex Purification Pack LC208.
Preparation of silicone membranes
Membranes from silicone sheeting (Lot no. SM18062662, Speciality
Manufacturing, Inc., Michigan, USA) were washed in distilled water and used for diffusion experiments. The nominal thickness of the membranes was 127µm.
Preparation of skin membranes
Ears from pig were obtained fresh from a local abattoir (Skåne, Sweden) and were store at −80° C until use. After thawing, the ears were rinsed with water and carefully shaved and rinsed again. Finally, tissue from the inner ears was dermatomed (TCM 3000 BL, Nouvag) to a thickness of approximately 500 µm. The skin grafts were then stored at −80° until use.
Preparation of PBS
A phosphate buffered saline solution (PBS) was prepared. NaCl, Na2HPO4·2H2O and KH2PO4 were solubilized at concentrations 130,9 mM, 51 mM and 1,5 mM respectively in water at room temperature. pH was set to 7,4 using 1M NaOH.
Preparation of PEG 1500
A solution containing 60 wt % PEG 1500 was prepared. PEG 1500 crystals were weighed up and transferred to a vial. The vial was filled with water up to 6 ml and placed on the magnetic stirrer in the oven at 32ºC over night before use.
Preparation of saline solution
A saline solution was prepared to match the salt concentration of PBS buffer. NaCl was solubilized in double distilled water to a concentration of 130,9 mM. All preparations were made at room temperature.
Calibration curve
From a stock solution containing 10 mg/ml diclofenac sodium in water, seven dilutions were made to obtain concentrations of 0,1 mg/ml, 0,08 mg/ml, 0,06 mg/ml, 0,04 mg/ml, 0,02 mg/ml, 0,01 mg/ml and 0,005 mg/ml, respectively. All dilutions were made and stored at room temperature. The samples were analyzed using UV-spectrophotometry over a spectrum between 800 nm and 250 nm on a Shimadzu UV-1800. The data was collected using the software UVProbe Version 2.43. The absorbance was plotted as a function of concentration in Excel and a calibration curve was obtained by using the absorbance at 279 nm wavelength from each calibration solution.
Solubility of diclofenac
2 ml of water, PBS, saline solution, 1-propanol and 2-propanol was transferred to vials in triplicates and a magnetic bar was added. Propylene glycol, glycerol, PEG 400 and PEG 1500 (60 wt % in water) was also transferred to vials in triplicates but the volume was weighed instead of pipetted due to the viscosity of the
solutions. Magnetic bars were added. Diclofenac sodium was added to all 27 vials and they were placed on the magnetic stirrer at 32 ºC. As the diclofenac dissolved, more was added to eventually obtain a saturated solution as indicated by excess of diclofenac crystals.
When diclofenac was added to the non-viscous solutions it disolved readily and no more diclofenac was added after the solution was opaque or a precipitate was observed on the bottom. To ensure that the solutions were saturated, all vials were moved to a shaking table and heated to 70 ºC for approximately 10 minutes before they were transferred back to 32 ºC. During the heating all crystals and
precipitations disappeared and reappeared when the temperature was lowered again.
For the viscous solvents, i.e. glycerol, propylene glycol, PEG 400 and PEG 1500, diclofenac was added for a period of two weeks until saturation was obtained. Due to the viscous properties of these solvents it was more complicated to determine whether saturation was obtained or not. The solutions were, after two weeks so viscous that the magnetic stirrer bars could not move. Therefore, all vials were moved to the shaking table at 32 ºC. All vials were also heated to 70 ºC for approximately 10 minutes after the addition of more diclofenac. Eventually propylene glycol, PEG 400 and PEG 1500 displayed an opaque precipitation which did not dissolve even after agitation for several days, indicating that
saturation was obtained. Upon adding diclofenac to glycerol, the formulation first displayed an opaque precipitation but after aggitation quickly solified, creating an solid-like product that could not be used for further experiments. Thus, the
experiments with glycerol had to be repeated twice to make sure that the solution stayed fluid. The reason why the diclofenac-glycerol mixture solified was not further studied. It should also be noted that during these experiments, one of the PEG 1500 vials was lost and only two vials were used in the results.
The saturated solutions were then filtered through a 0,2 µm membrane filter (Titan 2 Black PVDF), discarding approximately the first 0,5 ml of the filtrate. A small amount of each filtrate was weighed, transferred to a measuring flask and
diluted with water. The samples were then diluted further and analyzed using
UV-spectrophotometry by recording a spectrum between 800 nm and 250 nm on a Shimadzu UV-1800. The data was collected using the software UVProbe Version 2.43. From the concentrations obtained using the calibrations curve, the average weight percent was calculated together with standard deviation and relative standard deviation. Since the formulations containing glycerol, in particular, but also the other viscous solutions, were difficult to work with when completely saturated, it was decided to continue the diffusion study at a concentration of 80 % below the saturation limit, instead of 100%.
Diffusion studies
Based on the solubility results, new formulations containing glycerol, PEG 400, PEG 1500, propylene glycol or water, with 80% saturation of diclofenac, were prepared. The solutions were prepared the day before the study and left on the magnetic stirrer over night at 32 ºC. On the day of the diffusion study the
waterbath surrounding the ten Franz cells used was heated to 32 ºC approximately 1 hour before the experminent started. Fresh PBS was prepared and degassed for 1 hour per liter of solution. The membranes were rinsed in PBS buffer before
mounted in the Franz cells.
The cells were set up according to the manufacturer’s instructions. The cells were filled with PBS (approximately 6 ml) and the membranes were placed over the reciever chambers after a magnetic stirrer bar had been added. The doner chamber was secured on each cell and the cell was topped up with more PBS. When all ten cells were prepared, samples were added to the doner chambers and the first fractions were collected (0,8 ml). The cells were refilled with 0,8 ml PBS and allowed to run for 30 minutes before the next fraction was collected. Fractions were then collected every hour for six hours before the experiment was ended. All fractions were analyzed using UV-spectrophotometry over a spectrum between 800 nm and 250 nm on a Shimadzu UV-1800. The data was collected using the software UVProbe Version 2.43.
Caluculations of data
When calculating the results of the data received in the diffusions studies an equation was used to take into account the dilution of the reciever solution due to the fact that fractions were taken out and replaced with fresh buffer. The
cumulative drug permeation (Qt) was calculated as follows:
(Qt) = VrCt + Ʃ VsCi (1)
where Ct is the drug concentration of the reciever solution at each sampling time, Ci is the drug concentration of the ith sample, and Vr and Vs are the volumes of the reciever solution and the sample, respectively. Data were expressed as the cumulative drug permeation per unit of skin surface area, Qt/S (S=0,64 cm2). The steady-state flux (Jss) was calculated by linear regression interpolation of the experimental data at a steady state:
Jss = ΔQt/(Δt*S) (2)
Analyzes of the membranes after diffusion
To investigate if the properties of the silicone membranes could give any help in interpreting the results from the diffusion studies, the membranes that were used in the Franz cells were analyzed using a scanning electron microscope (SEM). A unused membrane and one that had been used with diclofenac dissolved in PEG 400 in one of the Franz cells were mounted on an adhesive surface and a small amount of silver paint was added to facilitate conduction. The samples were left to dry and then coated with gold in an Agar Auto Sputter Coater. The coated
membranes were then analyzed in an EVO LS10 scanning electron microscope at magnifications 100x, 500x, 2500x and 10000x.
Diffusion test with pig skin
One Franz cell was set up at room temperature to test the method with the skin grafts that hade been prepared and stored. The cell was set up accordning to the manufacturers manual and the skin was mounted on the cell. A solutions of PEG 400 solubilized to 80% with diclofenac was added and fractions were taken at 0 minutes, 30 minutes and then every hour for 6 hours. All fractions were analyzed using UV-spectrophotometry over a spectrum between 800 nm and 250 nm.
RESULTS
Analytic method
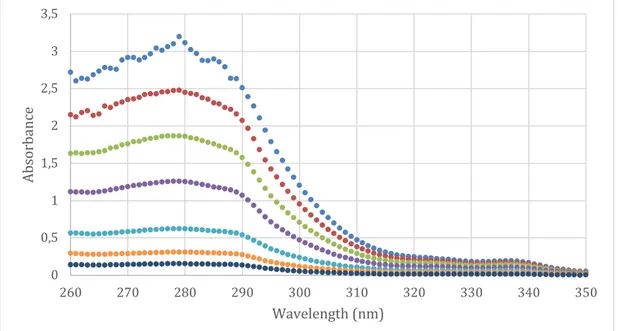
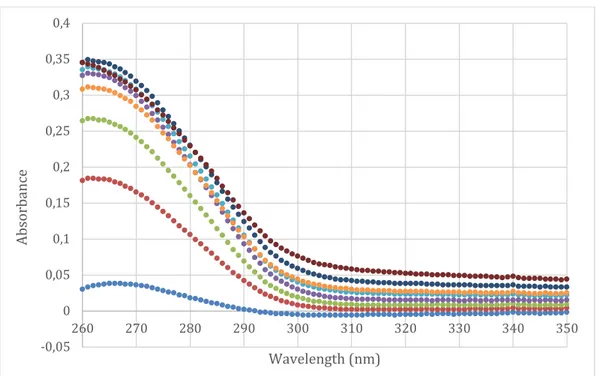
From the stock solution a calibration curve was constructed. A representative curve is presented in figure 4b. A new calibration curve was constructed each day from the same dilutions analyzed with UV-light. The slope of the curve showed little or no difference between measurements.
Figure 4a Raw data from UV-spectrophotometry analyzes of seven concentrations.
Figure 4b Calibration curve of diclofenac disolved in water.
0 0,5 1 1,5 2 2,5 3 3,5 260 270 280 290 300 310 320 330 340 350 Ab so rb an ce Wavelength (nm)
Dilution 1 Dilution 2 Dilution 3 Dilution 4 Dilution 5 Dilution 6 Dilution 7
y = 31,503x - 0,0041 R² = 0,9997 0 0,5 1 1,5 2 2,5 3 3,5 0 0,02 0,04 0,06 0,08 0,1 0,12 Ab so rb an ce Concentration (mg/ml)
Solubility of diclofenac
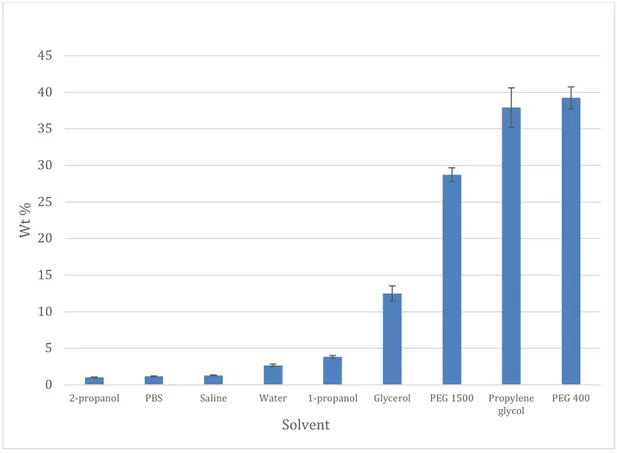
The results of the solubility tests, where diclofenac was dissolved in different solvents until saturation, are presented in table 2 and figure 5. The solubility was highest in propylene glycol and PEG 400 with an average weight percent of almost 40%. The lowest solubility was seen in 2-propanol with an approximate weight percent of 1%.
Table 2 Results of solubility tests with diclofenac in different solvents
Figure 5 Solubility of diclofenac in nine different solvents presented as weight percent.
0 5 10 15 20 25 30 35 40 45
2-propanol PBS Saline Water 1-propanol Glycerol PEG 1500 Propylene
glycol PEG 400 Wt % Solvent Replicates (n) Average weight
percent (%) Standard deviation Relative standard deviation
2-propanol 3 1,04 0,05 4,74 PBS 3 1,16 0,06 4,95 Saline 3 1,29 0,07 5,34 Water 3 2,68 0,17 6,30 1-propanol 3 3,84 0,18 4,76 Glycerol 3 12,48 1,05 8,42 PEG 1500 2 28,73 0,93 3,25 Propylene glycol 3 37,91 2,70 7,11 PEG 400 3 39,23 1,50 3,83
Diffusion studies with silicon membranes
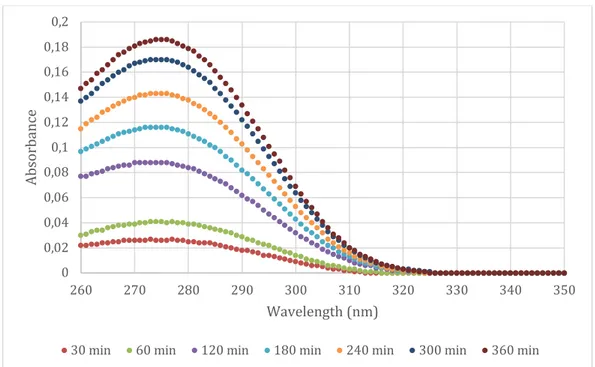
Several attempts at running Franz cells with glycerol, propylene glycol, PEG 400, PEG 1500 and water was performed with 80% of saturation with diclofenac. However, none of the experiments where diclofenac was dissolved in either glycerol, propylene glycol, PEG 400 or PEG 1500 showed any measurable permeability to diclofenac across the silicone membranes. Only the samples were diclofenac was dissolved in water gave any data that could be analyzed. For comparison, Voltaren Gel was also applied and the results were similar to those of water. The results are presented in figure 6 a and b and figure 7.
Figure 6a Raw data from Franz cell run at 32 °C with diclofenac dissolved in water
Figure 6b Raw data from Franz cell run at 32 °C with Voltaren Gel
0 0,05 0,1 0,15 0,2 0,25 0,3 0,35 0,4 260 270 280 290 300 310 320 330 340 350 Ab so rb an ce Wavelength (nm)
30 min 60 min 120 min 180 min 240 min 300 min 20 h
0 0,02 0,04 0,06 0,08 0,1 0,12 0,14 0,16 0,18 0,2 260 270 280 290 300 310 320 330 340 350 Ab so rb an ce Wavelength (nm)
Figure 7 A comparison of cumulative penetration of diclofenac through silicon after application of an aqueous solution saturated to 80% with diclofenac (blue squares) and Voltaren Gel (gray triangles).
Analyzes of the silicone membrane
The silicon membranes analyzed using scanning electron microscopy showed no difference between the membrane that was unused and the membrane that had been used in the Franz cells. The results are shown in figure 8.
Figure 8 Scanning electron microscope images of silicon membranes before run (l) and after run (r) in Franz cell with PEG 400 containing 80% diclofenac.
Diffusion studies on skin
Due to lack of time, only one experiment with skin was performed using a formulation containing PEG 400. The raw data is shown in figure 9. The
wavelength at which diclofenac was detected, 279 nm, could not be distinguished in the data, possibly due to skin debris. The samples would need to be further purified and analyzed using high-performance liquid chromatography (HPLC) and UV-detection but due to time restraints, this remains to be done.
0 10 20 30 40 50 60 70 80 0 1 2 3 4 5 6 7 Cu m ul at iv e dr ug p er m ea ti on p er u ni t o f ski n su rf ace area ( µg/cm 2) Time (h)
Figure 9 Data collected over six hours from a Franz cell in room temperature using a skin membrane and PEG 400.
DISCUSSION
One of the largest challange with transdermal or topical drug formulations is getting as much of the drug across the skin as possible. In the gels that are available today, over the counter at pharmacies, there is only a very low
concentration of active substance and of that substance, an even smaller amount actually penetrates the skin. The aim of this study was to experimentally study the solubility of diclofenac in different solvents and test if any of the formulations could be used to achieve elevated concentrations of diclofenac, with the final aim to increase the transport over the skin membrane. For this, we used silicone
membranes as an initial, simplified, model for the skin membrane. When choosing a suitable solvent, many different aspcts must be considered. For instance, the cost of the solvent must be low to keep the cost of the finished product at a resonable level. Also, the solvent must be harmless as it is to be applied to skin and
unwanted reactions must be avoided. Furthermore, the solvent must be manageble to work with at conditions suitable for use. The physical and chemical properties of the mixtures of diclofenac and the different solvents that were used in this study played a significant role when determining how efficient and suitable the formulation was and if it acutually could be possible to use in a commercial pharmacutical product.
Solubility in water, PBS and saline
Diclofenac was dissolved in water, PBS and a saline solution with the same concentration of NaCl as PBS but without the buffering system. The results showed that the solubility was lowest in the saline solution and highst in pure water, even though none of the media showed a strong ability to dissolve
-0,05 0 0,05 0,1 0,15 0,2 0,25 0,3 0,35 0,4 260 270 280 290 300 310 320 330 340 350 Ab so rb an ce Wavelength (nm)
diclofenac (see figure 10). A likely reason for this is due to the salting out effect that may occur in the PBS and saline solutions. Salting out, or "screening of charges", is an effect based on the interactions between electrolyte, such as Na+ and Cl-, and charged molecules, such as diclofenac in this case, in a solution. Diclofenac salt has a negatively charged carboxylic group and in the presence of a high concentration of ions, the cations from the salt/buffer will interact with, or shield, the negtive charge and thereby decrease the net charge of diclofenac. In effect, this will result in a lower solubility of diclofenac. In pure water diclofenac can form hydrogen bonds with water molecules, with less hindrance of sodium ions, giving a higher degree of solubility.
Figure 10 Solubility of diclofenac in water, saline solution and PBS.
Solubility in 1-propanol and 2-propanol, glycerol and propylene glycol
To be able to compare the solubility of diclofenac in solvents that contain a different number of hydroxyl groups experiments were performed with glycerol, propylene glycol and the two forms of propanol were tested. 1-propanol and 2-propanol each have one hydrogen donor, while propylene glycol have two and glycerol three (i.e. equivalent to the number of -OH groups). The hydrogen donors on the molecules can be expected to interact with the hydrogen accoptors on diclofenac, according to figure 11.
Figure 11 Interaction between diclofenac and propylene glycol. Diclofenac acts as a hydrogen acceptor to the hydrogens that are donated by propylene glycol.
0 0,5 1 1,5 2 2,5 3 PBS Saline Water wt % Solvent Cl Cl HN O O Na + +
-The two forms of propanol, 1-propanol with the hydroxylgroup on the first carbon and 2-propanol with the hydroxyl group on the second carbon, showed a relatively low ability to dissolve diclofenac in general, but the solubility was higher in 1-propanol (see figure 12).
Figure 12 Solubility of diclofenac in 2-propanol, 1-propanol, glycerol and propylene glycol
The reason for this is likely due to the steric hindrance that prevents 2-propanol from binding to diclofenac quite as efficient as 1-propanol does. Glycerol, on the other hand, has three hydroxyl groups, one on each of the carbons. Here, there are more possibilies for diclofenac to form hydrogen bonds with the solvent.
However, this also means that the glycerol molecule, that interacts with diclofenac, is less available to interact with other glycerol molecules. This is perhaps less favorable and a probable reason for lower solubility, as compared to propylene glycol. Another issue related to glycerol occured when trying to
dissolve diclofenac in glycerol until saturation was achieved. Instead of displaying a white precipitation evenly distributed throughout the media, diclofenac clustered in “sticky” flakes that were difficult to dissolve. Upon adding diclofenac to the vials, glycerol easily became solid and very difficult to work with. Preparations with glycerol were also very sensitive to contact with water and readily formed clusters when even the slightest amount of water came into contact with it (for example during the diffusion experiments).
The highest solubility for diclofenac was displayed in propylene glycol, which, as opposed to glycerol that is very viscous, is a fluid solvent similar to water. No clusters or flakes were formed when diclofenac was added to the media and upon reaching saturation an evenly distributed white precipitation was shown. The high solubility of diclofenac in propylene glycol is perhaps due to that propylene glycol has two hydroxyl groups with positivly charges that fits with the resonance
0 5 10 15 20 25 30 35 40 45
2-propanol 1-propanol Propylene glycol Glycerol
Wt
%
structure that is formed by the oxygen atoms of the negatively charged carboxyl group of diclofenac, which may result in a good molecular match with favorable interactions (see figure 11). Probably, this arrangment is beneficial for the system and gives increases the solubility of diclofenac.
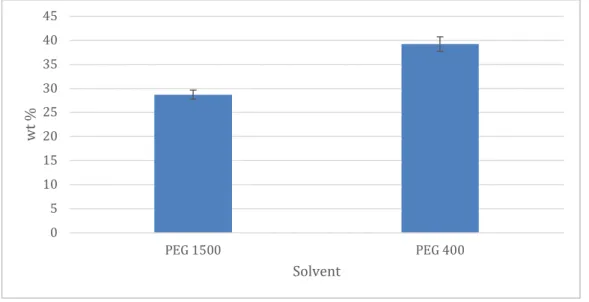
Solubility in PEG 400 and PEG 1500
Both PEG 400 and 1500 are very good solvents of diclofenac. In PEG 400, the weight percent dissolved was close to 40%. PEG 400 is a relatively large molecule, as compared to for example glycerol, with approximatelly 9 repeting sections. This gives a lot of possibilities for diclofenac to bind to the large PEG 400 molecule. Eventhough, larger molecules generally tend to lower the solubility many advantageous interactions can be formed between PEG 400 and diclofenac.
Figure 13 The general structur of polyethylene glycol (PEG)
In PEG 1500, which is a bigger molecule with approximately 34 repeating
sections, the solubility was lower but since PEG 1500 is solid in form. It had to be dissolved in water and the ratio 60% PEG 1500 to 40% water was choosen. Since water is a relatively bad solvent of diclofenac as seen in figure 10, and PEG 1500 only is present in the solution at 60 %, the ability to disolve diclofenac is lower than for PEG 400as can be seen in figure 14. If PEG 1500 had been liquid instead of solid the solubility would probably not have differed as much but PEG 1500 might still have showed a slightly lesser ability of dissolve diclofenac since the larger molecules of PEG 1500 is less mobile and binding is thereby reduced.
Figure 14 Solubility of diclofenac in PEG 400 and PEG 1500 (60 wt%)
0 5 10 15 20 25 30 35 40 45 PEG 1500 PEG 400 wt % Solvent
Diffusion
In the diffusion experiments, the results showed that only in the formulation with pure water and Voltaren, diclofenac was able to pass through the silicone
membrane. This was unexpected and we can only hypotizise as to why this is. As to the question why glycerol would not allow diclofenac to pass a possible answer is that glycerol reacted with water that leaked through the membrane because of the osmotic pressure and made a white precipitation that could only be described as solid. That this phenomena prevents the formulation with glycerol to diffuse is clear. As to PEG 400, PEG 1500 and propylene glycol the answer is not as clear. One possible reason is the lack of water in the formulations and therefore an extreme gradient of the osmotic pressure. This would then also explain why diclofenac can pass through the membrane when water is present on both sides of the membrane, which is a situation with a lower gradient in osmotic pressure.
Analyzes of the silicon membranes
Even if the hypothesis that the water activity and the osmotic pressure is responsible for the lack of results in the Franz cells with all other formulations besides water and Voltaren is correct, we wanted to investigate if the physical properties of the membrane could sheed any further light on why the penetration was so low. When the membranes were anayzed with SEM, there was no
appearent difference between the unused and the used membrane. This would indicate that diclofenac has not been absorbed in the membrane but rather not penetrated at all. There were also no distinguishable pores in the membrane that could have allowed for diclofenac to pass the membrane without help from, for instance, osmosis. This would then support the hypothesis that diclofenac can not pass silicone membranes and that the effect seen with water and Voltaren is due to water activity (aw). The term water activity refers to the amount of free water and the more particles that are dissolved in water, the lower the value of aw. Because of the osmotic pressure, water is transported from a solution with a higher
concentration of particles to a solution with a lower concentration i.e. to a solution with a higher aw.
One of the aims with this study was to also study diffusion over skin membranes but time only allowed for one trial run. This is of course the next logical step in finding a suitable formulation that can be used to increase the concetration of diclofenac that penetrates the skin and can be even more useful in treatment of pain.
CONCLUSIONS
In conclusion, there is several solvents that can be tested in the pursuit of developing a more potent topical drug for treatment of pain but only a limited number was included in this work. Substances such as PEG and propylene glycol proved to be good solvents of diclofenac but did not give saticfactory results in our diffusion studies. On the other hand, water that is a less effective solvent of diclofenac gave low but measurable results in the diffusion study. Further work is needed in order to better understand how a more efficicent product can be
ACKNOWLEDGEMENTS
I would like to extend a sincere thank you to Krister Thuresson for invaluble discussions and insights. Also, a thank you to Peter Falkman for professional help with the electronmicroscope and the staff and students of the Department of Biomedical Science for allowing me to work at their facility.
REFERENCES
1. TLV (2018) Läkemedelsmarknaden.
>https://www.tlv.se/lakemedel/lakemedelsmarknaden.html< HTML (2019-03-20)
2. Anselmo A, Mitragotri S, (2014) An overview of clinical and commercial
impact of drug delivery systems. J Control. Release 190, 15-28
3. Eriksson T, (2014) Apotekaren, receptarien och klinisk farmaci –
Utbildning, utveckling, utmaningar och behov av kraftsamling för att bli samhällets läkemedelsexperter. Lund MedCul
4. Hadgraft J, Lane M, (2005) Skin permeation: The years of enlightment, International Journal of Pharmaceutics 305, 2-12
5. Willis J, Kendall M, Jack D (1981) The Influence of food on the
absorption of diclofenac after single and multiple oral doses
Eur. J. Clin. Pharmacol., 19, 33-37
6. Reasearch and Markets (2018) Transdermal Drug Delivery Market:
Global Demand Analysis & Opportunity Outlook 2024 Research Nester
Pvt. Ltd
7. Naik A, Kalia Y, Guy R, (2000) Transdermal drug delivery: overcoming
the skin’s barrier function, Pharmaceutical Science & Technology Today,
3(9), 318-326
8. Prescott, S. L., Larcombe, D. L., Logan, A. C., West, C., Burks, W.,
Caraballo, L., Levin, M., Etten, E. V., Horwitz, P., Kozyrskyj, A., … Campbell, D. E. (2017). The skin microbiome: impact of modern
environments on skin ecology, barrier integrity, and systemic immune programming. The World Allergy Organization journal, 10(1), 1-16
9. Alkilani A, McCrudden M, Donnelly R, (2015) Transdermal drug
delivery: innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum, Pharmaceutics 7(4), 438–
470.
10. Goldstein JL, Cryer B. (2015) Gastrointestinal injury associated with
NSAID use: a case study and review of risk factors and preventative strategies. Drug Healthc Patient Saf. 2015;7, 31-41.
11. McCarberg BH, Argoff CE, (2010) Topical diclofenac epolamine patch
1.3% for treatment of acute pain caused by soft tissue injury, Int. J. Clin.
Pract. 64(11), 1546-53
12. Tong J. Gan (2010) Diclofenac: an update on its mechanism of action and
safety profile, Current Medical Research and Opinion 26:7, 1715-1731
13. National Center for Biotechnology Information (2018), PubChem Compound Database; CID=3033
>https://pubchem.ncbi.nlm.nih.gov/compound/3033< (2019-03-20) 14. Fass Vårdpersonal (2018) Produktresumé Voltaren Gel
>https://www.fass.se/LIF/product?userType=0&nplId=20040607005149& docType=6&scrollPosition=340< (2019-03-20)
15. Hagen M, Baker M, (2017) Skin penetration and tissue permeation after
topical administration of diclofenac, Curr Med Res Opin. 33(9),