Contents lists available atScienceDirect
Water Resources and Industry
journal homepage:www.elsevier.com/locate/wriEnhanced corrosion resistance of metal surfaces by
film forming
amines: A comparative study between cyclohexanamine and
2-(diethylamino)ethanolbased formulations
Erica Pensini
a,⁎, Roy van Lier
a,b, Fabrice Cuoq
a, Wolfgang Hater
c, Tobias Halthur
d,eaSABIC, Geleen, The Netherlands bYara, Zaventem, Belgium cKurita, Düsseldorf, Germany dCR Competence, Lund, Sweden
eMalmö University, Faculty of Health and Society, Department of Biomedical Science, Malmö, Sweden
A R T I C L E I N F O
Keywords: Steam system Corrosion Inhibitor Amine Films Closed loopsA B S T R A C T
The use of recycled process water in steam crackers leads to the accumulation of corrosive im-purities, hence the need for adequate treatment. Two corrosion inhibitor formulations containing N-[(9Z)−9-octadecen-1-yl]−1,3-propanediamine (N-oleyl-1,3-propanediamine) with either cy-clohexanamine (CHA) or 2-(diethylamino)ethanol (DEAE) were compared for their performance. Electrochemical impedance spectroscopy and visual observations showed that the two for-mulations offered comparable protection against corrosion. Bengal Rose testing and experiments conducted using a quartz-crystal microbalance with dissipation monitoring (QCM-D) indicated that the two formulations yielded similar coverage of the metal surfaces, and that the kinetics of mass adsorption were also similar. QCM-D data further suggested that thefilms formed with the two formulations had similar rigidity, and contact angle measurements indicated that they formedfilms with comparable hydrophobicity, which were equally effective in isolating the metal surfaces from water.
1. Introduction
Steam crackers use water as a source of steam to limit coke formation in furnaces, to quench cracked gas exiting the furnaces and as a cooling medium in a great number of heat exchangers, ranging from compressor interstage coolers to distillation column overhead condensers[1]. For economically viable and environmentally responsible operation of a steam cracker it is essential to recycle as much of the water as possible in all of these systems. This is particularly challenging in the high-pressure steam system, where the danger of concentration of impurities to highly corrosive concentrations is greatest. Thus, safe and reliable operation of such a steam system requires the use of suitable corrosion inhibitors.
Film forming amines (FFAs) have been used since the 1960s to inhibit corrosion in steam condensate systems in a number of industrial applications, since they formfilms which can act as a hydrophobic barrier against corrosive species, e.g. oxygen and
https://doi.org/10.1016/j.wri.2017.11.001
Received 28 February 2017; Received in revised form 3 May 2017; Accepted 4 November 2017
⁎Corresponding author.
E-mail address:erica.pensini@gmail.com(E. Pensini).
Abbreviations: AFM, atomic force microscopy; AISI, American Iron and Steel Institute; CHA, cyclohexanamine (cyclohexylamine); CPE, constant phase element; DEAE, 2-(diethylamino)ethanol; EIS, electrochemical impedance spectroscopy; FFA,film forming amine; LUMO, lowest unoccupied molecular orbital; MEA, mono-ethanolamine (2-aminoethan-1-ol); MOPA, 3-methoxypropylamine; PTFE, polytetrafluoroethylene (Teflon®); QCM-D, quartz-crystal microbalance with dissipation monitoring; SCE, saturated calomel electrode; XPS, X-ray photoelectron microscopy
Water Resources and Industry 20 (2018) 93–106
2212-3717/ © 2017 Published by Elsevier B.V. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/BY-NC-ND/4.0/).
carbonic acid[2,3].
Given its effectiveness in inhibiting corrosion while maintaining clean heat transfer surfaces, organic cycle chemistry based on FFAs is being increasingly employed as an alternative to conventional treatment programs. For instance, at the Geleen (petro) chemical site in the Netherlands, FFAs have been successfully utilized in a variety of applications since the mid-1990s[4]including the high-pressure steam system of a naphtha cracker[5].
FFA formulations typically contain neutralizing amines such as cyclohexanamine (cyclohexylamine, CHA), 3-methox-ypropylamine (MOPA), ethylamine, 2-aminoethan-1-ol (monoethanolamine, MEA), morpholine and 2-diethylaminoethanol (DEAE), to control the pH[2]. Some of these amines, and specifically CHA, can also provide the required volatility for alkalization of the steam/condensate part of the steam cycle, and stabilize the amines by acting as hydrotropes for the poorly solublefilming amine(s) in the blend[2]. Research has further shown that CHA can adsorb onto metal surfaces and significantly inhibit its corrosion in the passive region[6]. However, alone, CHA cannot provide adequate protection in the active region, where its adsorption occurs mainly on corrosion reaction intermediates which are only loosely attached to the substrate[6]. Conversely, FFAs adsorb on ferrous ma-terials more strongly in the active than in the passive region[2].
Although CHA has been effectively used in many FFA formulations, CHA is now characterized by hazard phrase 361f, i.e. it is suspected of damaging fertility, hence the search for suitable substitutes, such as DEAE. The transition of CHA to DEAE has already been practised in the industry, notably at the Polish Adamów power plant[7], and research has been conducted to compare the effectiveness of the two formulations containing CHA and DEAE as corrosion inhibitors using electrochemical impedance spectro-scopy (EIS)[8]. However, previous research did not correlate the effectiveness of these formulations to the properties of the films, pinpointing the parameters controlling the ability of FFA formulations to inhibit corrosion[8]. Also, electrochemical methods have been used to probe the effectiveness of different types of corrosion inhibitors[6,9], and their ability to adsorb onto metal surfaces has been investigated through X-ray photoelectron microscopy (XPS)[6,10,11], with atomistic simulations [12], using quartz-crystal microbalance with dissipation monitoring (QCM-D) and via atomic force microscopy (AFM)[13], and with EIS[14]. However, such studies did not compare the performance of CHA and DEAE formulations, investigating the reasons for their similar performance as corrosion inhibitors.
The goal of this research is to: 1) prove that environmentally more benign DEAE-based FFA formulations are an effective al-ternative to CHA-based FFA formulations, and 2) define the parameters controlling the effectiveness of FFA formulations, explaining the reasons for the comparable performance of CHA- and DEAE-based formulations.
Specifically, the research presented here correlates the performance of FFA formulations containing CHA and DEAE with: 1) their ability to adsorb onto metal surfaces and the kinetics of adsorption, as probed using QCM-D, ellipsometry, and adsorption experi-ments (Bengal Rose), and 2) the structure of the adsorbedfilms (as investigated with QCM-D) and their hydrophobicity (as de-termined through contact angle measurements), and thus their ability to isolate and protect metal surfaces against the attack of corrosive species dissolved in water.
List of symbols
Mu mass of FFA per unit area adsorbed onto the
cou-pons =
Ct 0 concentration of FFA in solution at time zero =
Ct 12hrs concentration of FFA in solution measured after
12 h
Acoupon surface area of the coupon V test solution's volume
df mean optical thickness
nf refractive index of growingfilm nbuffer refractive index of medium
ψ and Δ measured optical angles
Γ adsorbed mass estimated using the de Feijter for-mula
fdiff i, current frequency of the overtone
τi time decay constant
fi frequency of overtone i
fo,i frequency of overtone i measured during calibra-tion
Di dissipation factor of overtone i
ΔD shift in dissipation factors
Δf shift in overtones
τi time decay constant
ΔM mass adsorbed calculated using Sauerbrey equa-tion
C constant equal to 17.7ng Hz⋅ −1⋅cm−2for 5 MHz AT-cut crystals
N complex refractive index
n real part of the refractive index
i imaginary number
k extinction coefficient
Rt transition resistance between electrode and elec-trolyte
Rf resistance of ion conducting paths in passivating films
Rs resistance of electrode
Y0 capacity of constant phase element
n exponent used to characterize the constant phase element
χ2
parameter used to quantify the quality of thefit of a model electrical circuit with electrical im-pedance spectroscopy data
2. Materials and methods 2.1. Corrosion inhibitors
Two proprietary FFA inhibitor formulations provided by Kurita were used in this study: Cetamine® V211 (referred to as FFA1) and G811 (referred to as FFA2), which contain cyclohexanamine (CHA) or 2-(diethylamino)ethanol (DEAE), respectively. Both contain ca. 2% of N-[(9Z)−9-octadecen-1-yl]−1,3-propanediamine (N-oleyl-1,3-propanediamine) as the filming compound. The con-centrations of DEAE and CHA in the formulations have been chosen considering their different basicity and volatility, so as to ensure that the desired pH values can be attained in all parts of the water-steam cycle.
2.2. Corrosion coupons
Two types of AISI certified coupons were employed for contact angle measurements: C1010 (low carbon steel) and 316L (aus-tenitic stainless steel) coupons. All coupons were cleaned with ethanol and milli-Q water and dried with nitrogen prior to each test.
2.3. Electrochemical Impedance Spectroscopy measurements
EIS measurements were conducted to study the formation of passivatingfilms on carbon steel surfaces upon addition of FFA1 (CHA-based) and FFA2 (DEAE-based), using a Metrohm Autolab frequency response analyzer with an electrochemical interface. EIS measurements allow determining the real and imaginary part of the system's impedance, as well as the changes of the polarization resistance in time.
EIS measurements were performed at room temperature and atmospheric pressure using demineralized water, to which NaCl was added at a concentration of 200 ppm, and the pH of which was adjusted to 9.0 using HCl or NaOH. When no FFAs were added, NaOH was used to increase its pH to 9.0. However, addition of FFA increased the pH of the water, and HCl was thus used to lower it and ensure that the pH was the same in all tests conducted.
The dosage of the formulated products was 200 ppm, volume based. The working electrode was a rotating mild steel disc having a surface area of 0.2 cm2. All experiments were carried out at a speed of 500 rpm to mimic aflowing system representative of water-steam cycles.
A saturated calomel electrode (SCE) was used as reference and a platinum grid as the counter-electrode. The material of the working electrode was XC 38. This is the French equivalent of AISI grade 1035 carbon steel with the following chemical composition in weight %: 0.36% C, 0.66% Mn, 0.27% Si, 0.016% S, 0.020% P 0.20% Ni, 0.21% Cr, 0.02% Mo, 0.20% Cu, 0.060% Al.
The electrochemical systems were mathematically described in terms of electric equivalent circuits comprised of resistances and a constant phase element (CPE). The resistances in the electrical equivalent circuit are as follows: a polarization resistance Rt (re-presenting the transition resistance between the electrodes and the electrolyte) theoretically measured when the frequency ω of the currentflowing through the system is zero, a resistance Rfrepresenting the resistance of the ion conducting paths in the passivating films, and a resistance Rsrepresenting the resistance of the electrolyte. The polarization resistance Rtis a particularly important parameter, in that it correlates to the propensity of a system to undergo corrosion, with high polarization resistances indicating high resistance to corrosion. CPEs model the behavior of a double layer formed in the proximity of the metal surface immersed in liquid. Such elements are described by two parameters: the capacity Y0and an exponent n, which are correlated to the roughness and inhomogeneity of the surface and/or to a non-uniform distribution of the current density on the corroding electrode.
Nyquist plots were utilized to represent the real part of the impedance, which reflects the resistance of the system to current flow, and its imaginary part, which represents the capacitive and inductive character of the system. The rightmost part of the Nyquist plots corresponds to low frequencies and approximates the polarization resistance of the system.
2.4. Film forming amine concentration measurements
The Bengal Rose photometric/colorimetric method detects aliphatic amines with a carbon chain length of 12 and more carbon atoms, and it was used to determine the concentration of FFAs in bulk solutions. This method uses pink Bengal Rose dye to form complexes with the amines, and an acid buffer to lower the pH[15]. At low pH the Bengal Rose-amine complexes are soluble in water and thus increase the absorbance, which can be correlated to the amine concentration in solution. All measurements were conducted at room temperature (25 °C), at a wavelength of 560 nm using a portable DR 900 spectrophotometer (Hach).
2.5. Determination of surface coverage (Bengal Rose test)
Following cleaning with ethanol and water, C010 and 316L coupons were soaked in 200 ppm (volume based) solutions offilming amine formulations (either FFA1 or FFA2) at pH 9.0 (adjusted with NaOH) for 12 h, at room temperature (23 °C)and ambient pressure (1 atm). PTFE containers were used, since amine adsorption onto this material is negligible[16,17].
The mass per unit area of FFA adsorbed onto the coupon surface (Mu) over a 12 h’ period was determined by measuring with the
Bengal Rose method the concentration of FFA amines in a blank (in which no coupons were immersed) and in FFA solutions in which coupons were immersed, and by using the following formula:
= = − = ⋅ M C C A V u t t hrs coupon 0 12 (1) whereCt=0is the concentration at time zero (equal to the concentration in the blank, without coupons immersed),Ct=12hrsis the
concentration measured after 12 h,Acouponis the surface area of the coupon, and V is the test solution's volume.
It is noted that the presence of metal ions dissolved in solution due to the corrosion of the coupons may sequester the FFAs in solution, leading to an overestimate of the FFAs adsorbed onto the coupon's surface when using Eq.(1).
2.6. Quartz-crystal microbalance with dissipation monitoring
The long and short term adsorption of FFA1 and FFA2 to stainless steel was studied with a QCM-D system (Q-Sense, Biolin Scientific). This technique is described in a number of publications, including[18–21]. Briefly, the QCM-D system is equipped with a flow cell, at the bottom of which is a sensor sputter coated with AISI type 316 stainless steel, which acts as the substrate for adsorption. The sensor is intermittently oscillated at the fundamental resonance frequency as well as at its overtones (odd multiples of the resonant frequency). The changes in the resonance frequency and in the overtones are related to the mass of the sensor, and may therefore be used to indicatefilm formation onto the sensor's surface. Moreover, the rate of decay of the waves propagating through the crystal depend are correlated to the dissipation factor as follows:
= ⋅ D t π f τ ( ) 1 i diff i i, (2)
where t is the time, i denotes the overtone considered, D ti( )is the dissipation parameter for each overtone, fdiff i, =fi−f0,iis the
current frequency of the overtone, f0,iis the frequency of the overtone measured during calibration and τiis the time decay constant.
High ratios of the changes in the dissipation factor over the changes of the overtone to which it relates are indicative of softfilms with visco-elastic properties, whereas when this ratio is smallfilms are considered rigid and predominantly elastic.
In the case of rigidfilms the mass adsorbed on the sensor surface (ΔM) can be estimated using Sauerbrey's equation[22]: = −
ΔM Cf
i diff i,
(3) where C is a constant equal to 17.7ng Hz⋅ −1⋅cm−2for 5 MHz AT-cut crystals and the meaning of the other symbols is as outlined above. It must be noted that the Sauerbrey model was developed under simplifying assumptions, but has been suggested to be a good approximation when the change in dissipation is less than 10−6per 5 Hz ofΔf[23].
Short term adsorption experiments were conducted using concentrated FFA solutions (2500 ppm of the formulations in milli-Q water, volume based) pumped in the cell at a constantflowrate of 0.1 mL/min, whereas long term adsorption experiments were conducted using dilute FFA solutions (25 ppm of the formulations in milli-Q water, volume based) pumped in the cell at a constant flowrate of 0.04 mL/min. After injecting FFA solutions, the cell was flushed with either DEAE or CHA solutions, or with milli-Q water, to verify that FFAs were irreversibly adsorbed onto the sensor's surface. All experiments were conducted at room temperature (23 °C) and ambient pressure (1 atm), and the pH was adjusted to approximately 9.6 using NaOH and HCl. Theflow regime was laminar in all tests conducted.
2.7. Ellipsometry
Ellipsometry was used to determine the mass of FFA adsorbed on metal surfaces underflow conditions.
Ellipsometry is an optical method that measures the changes in polarization of light upon reflection at a planar surface[24]. The instrument used was a type 436 thinfilm ellipsometer, (Rudolph Research), equipped with a xenon arc lamp and high-precision step motors, computer controlled.
Measurements were performed at room temperature (25 °C), at a wavelength of 4015 Å (401.5 nm) and an angle of incidence of 67.7°. A more detailed description of the setup of the instrument can be found elsewhere[25]. A stainless steel coated QCM sensor was used as substrate, and cleaned as in QCM-D measurements. Prior to multilayer adsorption, a four-zone measurement was per-formed in milli-Q water, to determine the effective complex refractive index N of the substrate, as well as to reduce effects of optical component imperfections. The complex refractive index N can be expressed as follows:
= +
N n ik (4)
where n is the real part of the refractive index and indicates the phase velocity,iis the imaginary number and k is the extinction coefficient, which represents the amount of attenuation when the electromagnetic wave propagates through the material.
A baseline was then recorded in milli-Q water, and the FFA2 formulation diluted to a dosage of 2500 ppm was then pumped into the 5 mL cuvette and the ellipsometric anglesψ and Δ were recorded in situ during the adsorption, followed by rinsing with the pure DEAE additive at the same dilution.
Numerical methods can be used to estimate the mean optical thickness (df ) and refractive index (nf) of the growingfilm[26], and
the thickness (df ) and the refractive index of thefilm (nf) and of the medium (nbuffer) can be used to calculate the adsorbed amount,Γ
= ⋅ −
Γ df n n
dn dc/
f buffer
(5) In the calculations conducted adn dc/ value of 0.15 has been used for the FFAs. This value was previously used for polyelectrolyte multilayers of poly(L-glutamic acid) and poly(L-lysine) with an initial layer of polyethyleneimine[28]. Since this value was not experimentally determined for FFAs, a sensitivity analysis was conducted to estimate changes in the mass estimated using different values ofdn dc/ .
During the measurements the solutions were continuously stirred, simulatingflow conditions. 2.8. Contact angle measurements
Contact angle measurements were conducted to assess the ability of the amines present in the FFA1 and FFA2 formulations to form hydrophobicfilms on C1010 and 316L coupons, thus repelling water and inhibiting corrosion. They were carried out using a ThetaLite optical tensiometer (Biolin Scientific) in combination with OneAttension software (Biolin Scientific).
Contact angles were measured on the coupons immediately after cleaning and after exposure of the coupons to either FFA solutions at 200 ppm and pH 9.0, or to milli-Q water at pH 9.0, for either 17 or 24 h. All coupons were rinsed with milli-Q after soaking, and the contact angles measured thus reflect the hydrophobic properties of films irreversibly adsorbed at the coupon surface. At least twenty droplets were analyzed for each system, and the standard deviation was 5.6° or less for all systems analyzed.
3. Results and discussion
3.1. Electrochemical Impedance Spectroscopy measurements
EIS measurements were conducted to quantitatively assess the effectiveness of FFA1 and FFA2 in inhibiting corrosion of carbon steel in NaCl solutions at pH 9.0. It is noted that salts different from NaCl can be present in water. However, in water-steam cycles much emphasis is placed on the purification of the make-up water. Residual calcium and magnesium concentrations are in the low ppb range and there is no risk of scale formation. Problematic ions are especially chloride and sulfate since these may concentrate to highly corrosive levels underneath boiler deposits as well as in the phase transition zone in condensing turbines. The test water represents a worst case condition of very high chloride levels in a similar way as in other studies[29]. It is also noted that under the temperature conditions (up to more than 500 °C) in the water-steam cycle microbiological growth is not an issue.
The EIS spectra obtained with blank samples (without amines added) were distinctively different from those obtained in the presence of FFAs, clearly demonstrating the formation of passivatingfilms on the steel surface[9](Figs. 1–3). The EIS spectra were comparable with FFA1 and FFA2 added, indicating that the two formulations formed similar passivatingfilms on the electrode surface. The EIS spectra for the blank could be modelled by a single asymmetrical semi-circle andfitted by a single circuit with two resistances in series and a CPE representing the depletion layer at the electrode's surface. Conversely, the EIS spectra of the systems containing FFA1 and FFA2 were characterized by a shoulder at high frequencies (Figs. 2–3) and could not be modelled by a single circuit: rather, two circuits each consisting of a resistance parallel to a CPE were used to model them (Supplementary Material). The parameters describing the circuits are given inTable 1. Among these parameters, the polarization resistance Rthas a particular significance, because it relates to the propensity of a system to undergo corrosion. After 20 min the value of the resistance Rtwas similar for FFA1 and FFA2, and significantly greater with either formulation than for the blank, showing the effectiveness of both formulations as corrosion inhibitors.
The polarization resistance was further determined at different times, and the data are shown inFig. 4. The comparison of the time evolution of the polarization resistances of the blank and the samples exposed to the amine formulations further highlights marked differences between the two systems (Fig. 4). The polarization resistance increased with time in a similar fashion for the systems containing either FFA1 or FFA2, whereas the Rtvalue of the blank decreased from 2.436 kΩ cm2to 1.652 kΩ cm2(Fig. 4).
Fig. 2. EIS spectra. Comparison between the measured andfitted EIS spectra after 20 min at pH 9.0 for a system to which FFA1 was added: overview (top) and detail (bottom).
Fig. 3. EIS spectra. Comparison between the measured andfitted EIS spectra after 20 min at pH 9.0 for a system to which FFA2 was added: overview (top) and detail (bottom).
The decrease of the polarization resistance observed for the blank at pH 9.0 is not visible in the plot due to the scale used. These data suggest that in the presence of a passivating aminefilm, the carbon steel surface became progressively more resilient to corrosion, whereas it became more prone to corrosion with time in the absence of FFAs.
In conclusion, EIS data suggest that the performance of the formulations was satisfactory and similar, in agreement with the visual observations of coupons soaked in the aqueous solutions (Supplementary material).
3.2. Determination of surface coverage
Bengal Rose analyses were performed to assess the adsorption of CHA- (FFA1) and DEAE-based (FFA2) FFA formulations onto C1010 and 316L coupons under no-flow conditions at room temperature (23 °C), and to correlate it to their effectiveness in inhibiting corrosion. The mass of FFAs adsorbed onto the coupons from 200 ppm (volume based) solutions of either FFA1 or FFA2 over 12 h is given inTable 2.
The data reveal that the mass of FFA adsorbed over 12 h from either FFA2 or FFA1 solutions, onto either 316L or C1010 coupons, was similar and in good agreement with previously published data[8].
The similar mass adsorbed with FFA1 and FFA2 suggests that the two formulations yielded comparable coverage. These data may explain the similar increase in the polarization resistance of carbon steel coupons with FFA2 and FFA1, as shown by EIS measure-ments, and thus the comparable corrosion inhibition achieved with the two formulations, as assessed by visual inspection of the
Table 1
Parameters obtained after 20 min for the equivalent circuit shown inFigs. 1–3for systems containing formulation FFA2 and FFA1 and for the blank system (no amines added).χ2indicates how accurately the modelfits the data. The other symbols are defined in the Materials and Method section as well as on the first footnote of the
manuscript.
Parameter Unit Blank FFA1 FFA2
Rs [kΩ•cm2] 0.380 0.269 0.279 Y0(CPEf)- circuit 1 [F/cm2] 3.88•10−11 8.27•10−10 n (CPEf)– circuit 1 0.384 0.453 Rf [kΩ•cm2] 0.74 0.88 Y0(CPEf)– circuit 2 [F/cm2] 1.69•10−6 1.58•10−5 1.77•10−5 n (CPEf)– circuit 2 0.662 0.872 0.884 Rt [kΩ•cm2] 2.436 92.12 117.01 χ2 4.70•10−2 1.92•10−2 6.59•10−3
Fig. 4. Polarization resistance. Comparison of the polarization resistance Rtderived from spectrafitting of test solutions containing formulation FFA2 and FFA1 and for
the blank system (no amines added) at pH 9.0. Let it be noted that Rtof the blank decreased from 2.436 kΩ cm2after 20 min to 1.652 kΩ cm2after 100 min.
Table 2
FFA concentration in the blank (no coupons immersed) and in test solutions containing formulation FFA1 and FFA2 into which stainless steel (316L) and carbon steel (C1010) coupons had been immersed, and FFA mass adsorbed per unit area. The maximum standard deviation in the FFA concentrations measured in solution was 0.2 ppm (leading to a maximum standard deviation of approximately 50% in the amine mass adsorbed per unit area).
FFA1 FFA2
FFA concentration in the bulk (ppm)
blank 2.5 2.4
316L 2.2 1.9
C1010 2.1 1.8
Amine mass adsorbed per unit area (g/m2)
316L 0.05 0.09
coupons (Supplementary material). In addition to good coverage, thefilm structure and their hydrophobicity may also affect the ability of FFAfilms to impede corrosion, as will be further discussed in the following sections.
It is noted that the concentration of FFA in the blank measured with the Bengal Rose test (2.5 ppm) was lower compared to the expected concentration (4 ppm) based on the nominal FFA concentration in the formulation (2%) and the dilution used. This dis-crepancy is likely due to the fact that FFAs can form micelles in the highly-concentrated FFA1 and FFA2 formulation, possibly leading to statistical inhomogeneities of the FFA concentration in samples of microliter volume. However, because the comparison between the blank and the solution in which coupons were soaked was done with the same solution, the observed changes in FFA con-centration with time were truly related to FFA sorption onto the metal coupons. It is further noted that the 2.5 ppm concon-centration detected with the Bengal Rose test refers to the effective concentration of FFA, which differs from the concentration of the for-mulation added to the solution, because the forfor-mulation used contained 2% (and not 100%) FFA.
In this study the sorption behavior of FFA was investigated at ambient temperature (23 °C) only, yet sorption is known to be temperature dependent[30–33]. The effect of temperature on adsorption onto stainless steel has been specifically investigated for FFA N-oleyl-1,3-propanediamine, the adsorption of which was found to be greater at 60 °C than at ambient temperature[33]. Although the adsorption behavior of the FFA used should be temperature-dependent, it is speculated that the effect of temperature on sorption would likely be similar for both FFA1 and FFA2, because: 1) the two formulations contained the same FFA (although the alkalizing amines differed) and 2) they showed similar adsorption behavior at room temperature. However, since the temperature in water-steam cycles (for which these formulations are intended) can exceed 500 °C, the effect of temperature on the sorption of FFA1 and FFA2 will be the object of future research.
3.3. Quartz-crystal microbalance with dissipation monitoring and ellipsometry
QCM-D measurements were conducted to study amine adsorption onto stainless steel and to probe the physical properties of the aminefilm formed under laminar flow conditions, using different amine concentrations and different sequences of exposure to FFA1/ FFA2 and CHA/DEAE solutions. Adsorption of amines onto stainless steel was further investigated using ellipsometry. Such
Fig. 5. Overtones and dissipation factors normalized relative to the overtones. Change in the resonance frequency normalized by the overtone number (a) and dissipation (b) for the 3rd to 11th overtone, for an experiment where the stainless steel coated sensor has been exposed to amine formulations at high concentrations (2500 ppm), according to the following sequence of test solutions: Phase I: milli-Q water (not shown), Phase II: FFA1, Phase III: CHA. Phase I was used as reference relative to which the shifts in the overtones (Δf) and the dissipation factors (ΔD) are given: therefore Δf and ΔD are zero in this phase.
measurements were conducted using a QCM-D sensor in a stirred cuvette. The adsorbed mass estimated using ellipsometry thus correlates with the mass estimated using QCM-D, because of the substrate used and since measurements were conducted under similarflow conditions.
Raw QCM-D data (resonance frequency and dissipation) and the adsorbed Sauerbrey mass for the fast and slow QCM-D ex-periments at high and low amine concentration are shown inFigs. 5–8.
The data reveal that when concentrated FFA1 or FFA2 solutions (2500 ppm, volume based) were injected in the cell the overtones and the dissipation factors changed rapidly (Figs. 5–6, phase II). The shifts of the overtones upon switching from milli-Q water to filming amine solutions may be due to a combination of diverse effects, including bulk effects, conformation of the molecules adsorbed onto the sensor, mass adsorption onto the sensor, formation of electric charge at the sensor surface as well as changes in the slip at the sensor surface[18,34,35]. To verify that FFAs were adsorbed at the sensor surface and that the observed changes were not solely due to bulk effects, the cell was rinsed with either CHA or DEAE solution at the same concentration as in the formulation used, but withoutfilming amines added (Figs. 5–6, phase III). The data show that upon rinsing with either CHA or DEAE solutions (Figs. 5–6) or with milli-Q water (Fig. 8) the overtones and the dissipation factors underwent minor changes and were markedly different from those measured before FFA1 or FFA2 were injected into the cell. These results indicate that under the experimental conditions considered FFAs from either FFA1 or FFA2 were irreversibly adsorbed onto the sensor surface after rinsing with CHA and DEAE solutions.
In the long term experiments performed using low concentrations of the FFA formulations (25 ppm, volume based), the kinetics of adsorption were extremely slow. Equilibrium was not attained even after 24 h, and the sensor needed to be exposed to a second injection of fresh formulation before an adsorption plateau was reached after 48 h (data only shown for the calculated Sauerbrey mass inFig. 8). The comparison between the equilibration time in slow and fast experiments shows that the kinetics of adsorption of FFAs onto stainless steel displayed a marked dependence on the concentration. Long term experiments confirmed the irreversible adsorption of amines on stainless steel, corroborating the results of fast QCM-D experiments. Small changes in the overtones were detected upon rinsing with CHA or DEAE solution, and then with milli-Q water at pH 5.5, possibly due to bulk effects, partial desorption, or to a slight de-swelling of thefilm as the pH was decreased. However, the overtones and the dissipation factors
Fig. 6. Overtones and dissipation factors normalized relative to the overtones. Change in the overtones normalized by the overtone number (a) and dissipation (b) for the 3rd to 11th overtone, for an experiment where the stainless steel coated sensor has been exposed to amine formulations at high concentrations (2500 ppm), according to the following sequence of test solutions: Phase I: milli-Q water (not shown), Phase II: FFA2, Phase III: DEAE. Phase I was used as reference relative to which the shifts in the overtones (Δf) and the dissipation factors (ΔD) are given: therefore Δf and ΔD are zero in this phase.
remained significantly different from the ones measured in milli-Q water before FFA1 or FFA2 were injected in the cell, indicating irreversible adsorption. The irreversible adsorption of FFA onto metal surfaces after rinsing with milli-Q was further probed with contact angle measurements, as discussed in the following paragraph. The comparison of the QCM-D response after rinsing with the samefluid (milli-Q water) allows eliminating confounding factors such as bulk effects and differences in the FFA conformation at the surface in different water chemistries (CHA vs. DEAE solutions), and making an accurate comparison of the mass irreversibly ad-sorbed onto the sensor surface after injection of either FFA1 or FFA2. While differences in the mass adsorbed with FFA1 and FFA2 before rinsing with milli-Q cannot be discounted, the differences in the overtones (and the Sauerbrey mass) after rinsing with milli-Q were negligible (phase V,Fig. 8), strongly suggesting that the mass irreversibly adsorbed onto the sensor surface was similar with either FFA1 or FFA2.
Importantly, the overtones measured after rinsing in tests conducted at high and low FFA concentrations were comparable, suggesting that a similar mass was irreversibly adsorbed onto the stainless steel surface with either 25 ppm or 2500 ppm of for-mulation injected into the QCM-D cell, with either FFA1 or FFA2. The similarity of the mass irreversibly adsorbed onto stainless steel with FFA1 and FFA2 may explain the comparable corrosion inhibition achieved with the two formulations, as assessed by visual inspection of the coupons, as discussed earlier. The irreversible adsorption of FFAs suggested by QCM-D experiments is in agreement with published literature reporting that amines can adsorb onto metal surfaces due to electrostatic attraction between the charged molecules and the charged metal, as well as interactions of unshared electron pairs and ofπ-electrons with the metal surface[30,31].
Fig. 7. Sauerbrey total mass calculated for the 7th overtone for the two experiments conducted using an FFA formulation dosage of 2500 ppm (seeFig. 6). The stainless steel sensors in the graph have been exposed to the following sequence of solutions: (blue): Phase II: FFA1, Phase III: CHA; (red): Phase II, FFA2, Phase III: DEAE. A stable baseline wasfirst established for both experiments in pure milli-Q water in phase I (omitted from the graph).
Fig. 8. Sauerbrey total mass calculated for the 7th overtone for two experiments, where the stainless steel sensors in the graph have been exposed to the following sequence of solutions: (dark grey): Phase II: FFA1, Phase III: FFA1, Phase IV: CHA, Phase V: milli-Q; (light grey): Phase II, FFA2, Phase III: FFA2, Phase IV: DEAE, Phase V: milli-Q. A stable baseline wasfirst established for both experiments in pure milli-Q in phase I (omitted from the graph). The concentration of the formulations was 25 ppm, volume based.
It is reported that the effectiveness of corrosion inhibitors is correlated to the strength of the attraction between the amine and the surface[36], and the irreversible adsorption of either FFA1 and FFA2 therefore suggests that these inhibitors are effective in pro-tecting the metal surface against corrosion.
In addition to good coverage, thefilm structure and their hydrophobicity may also affect the ability of FFA films to impede corrosion. QCM-D data provided insights regarding the rigidity of thefilms. The dissipation factor is correlated to the softness and the visco-elastic properties of thefilms deposited on the sensor surface: high ratios between the dissipation factor and the overtone suggest that thefilms formed are soft and visco-elastic, whereas small ratios are typical of relatively rigid and elastic films. In this study the ratio between the shift in the dissipation factors and the shift in the overtones was small, suggesting thatfilms were fairly rigid and elastic for all the amine concentrations tested[37].
Given the rigidity of thefilms adsorbed at the sensor surface, the Sauerbrey equation was used to obtain an estimate of the mass adsorbed. The irreversible mass adsorbed estimated using this method was 340–380 ng/cm2
, (approximately ⋅4 10−3g m/ 2), after rinsing with CHA or DEAE in either the experiments conducted using low or high FFA formulation dosages (seeFigs. 7–8). The Sauerbrey mass is in agreement with a previous study[31], in which the authors investigated adsorption of oleyldiamine (N-oleyl-1,3-propanediamine) on stainless steel and reported coverages of ⋅5 10−3g m/ 2– ⋅2 10−3g m/ 2after a 3 h’ adsorption period. Moreover, the Sauerbrey mass closely resembles results for lipid bilayers[38]. However, the Sauerbrey mass is greatly different from the one estimated using the Bengal-Rose test, possibly due to six main reasons. First of all, QCM-D experiments were conducted under laminar flow conditions, whereas Bengal Rose tests were conducted under no-flow/stagnant conditions. The different flow regime could have led to deposition of multilayers in Bengal Rose tests, as opposed to monolayers in QCM-D experiments. Secondly, the porosity and roughness of the stainless steel sputter coated onto the QCM-D sensor could differ from those of the metal coupons used in Bengal Rose tests. Thirdly, in the Bengal Rose test the mass adsorbed on the coupon was estimated by measuring changes in the bulk concentrations, rather than directly measuring the mass adsorbed onto the coupon. Fourthly, deviations may be introduced when not allfilming amine is available for photometric analysis. For instance, it is possible that in Bengal Rose tests FFA was sequestered by metal ions (e.g. iron) dissolved in solution due to the corrosion of the coupons, leading to an overestimate of the FFA molecules adsorbed onto the surface of the coupons. Moreover, the Sauerbrey model was developed for perfectly rigidfilms uniformly adsorbed on the sensor in air, whereas thefilms studies here were adsorbed in aqueous environments and were possibly inhomogeneous. Differences in the solution volume to steel area ratios used in the adsorption experiments may also have played a role.
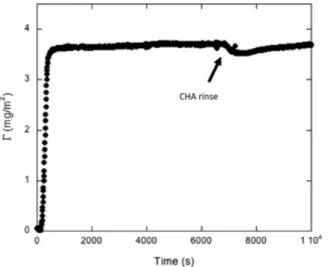
To further understand the reason for the discrepancy in surface coverage, ellipsometry measurements were conducted using FFA2. Based on ellipsometry tests, the dry adsorbed mass adsorbed from FFA2 formulation diluted to a dosage of 2500 ppm was 360 ng/ cm2, using a value ofdn dc/ of 0.15 (Fig. 9). Changing the dn/dc value between 0.13 (valid for the non-ionic surfactant pentaethylene glycol monododecyl ether C12E5) and 0.18 (valid for most globular proteins), the adsorbed mass would only change by 4 ng/cm2and 3.2 ng/cm2, respectively. Minimal changes in the adsorbed mass were observed upon rinsing the sensor with CHA, indicating that FFAfilms were irreversibly adsorbed at the sensor surface, in agreement with QCM-D and Bengal Rose data.
The mass estimated using ellipsometry measurements matches closely the Sauerbrey mass estimated using QCM-D data. The comparison between the QCM-D/Sauerbrey mass and ellipsometry data, obtained using QCM-D sensors underflow conditions, and Bengal Rose results, obtained with coupons under stagnant conditions, suggests that theflow regime and the characteristics of the material used (e.g. roughness) have a major impact on the FFA mass adsorbed at the metal surface.
Although there are fundamental differences between the Sauerbrey mass and the mass estimated with the Bengal Rose test, all datasets do indicate that FFAs can yield comparable coverage of metal surfaces in the presence of either CHA or DEAE, thus pro-tecting them against corrosion in a similar manner. These data thus substantiate the use of formulation FFA2 as an environmentally more benign alternative to CHA-bearing FFA1, and are also in agreement with EIS results and the visual observations of coupons
Fig. 9. Dry mass of FFA adsorbed from FFA2 formulation diluted to a dosage of 2500 ppm estimated from ellipsometry measurements before and after rinsing with CHA. The moment when pure CHA was introduced is indicated by the arrow.
soaked in solution for up to 24 h (Supplementary material).
3.4. Contact angle measurements
In addition to adequate coverage andfilm structure, the hydrophobicity of the amine films and their ability to shield metal surfaces from corrodents in the water also plays a role in their effectiveness in inhibiting corrosion. It is reported that hydrophobicity is an important parameter in controlling the performance of corrosion inhibitors in the case of amines[36]as well as of different types of corrosion inhibitors. For instance, it is reported that super-hydrophobicfilms prepared by myristic acid significantly de-creased the corrosion currents densities, corrosion rates and double layer capacitance, and simultaneously inde-creased the values of polarization resistance of aluminium in sterile seawater[39]. Hydrophobicity was also reported to be an important parameter in determining the effectiveness of 12-aminododecanoic acid[40]and diquaternary ammonium surfactants[41]in protecting carbon steel surfaces against corrosion.
Contact angle measurements were conducted to verify the formation of hydrophobic aminefilms, able to repel water and inhibit corrosion of steel coupons. All coupons were rinsed with unbuffered milli-Q water (pH 5.5) and dried with nitrogen prior to contact angle measurements, and the difference of the contact angles before and after immersion in amine solutions thus reflects the presence of irreversibly adsorbed aminefilms. These results complement QCM-D experiments, in which the irreversible adsorption of FFA onto metal surfaces was verified after rinsing with CHA and DEAE solutions, as well as with milli-Q water.
The results of the contact angle measurements are graphically summarized inFig. 10.
Te contact angles before immersion in solution were approximately 40° and 57° for the 316L and C1010 coupons, respectively. Stainless steel (316L) coupons did not corrode in milli-Q water at pH 9.0, with or without amines added. However, the changes in the contact angles measured for 316L coupons with time displayed a marked dependence on the water chemistry. When amines were not added to milli-Q water at pH 9.0 the contact angles decreased with time. Conversely, when amines were added, the contact angles of stainless steel (316L) coupons increased from 40° to 79° and 74° after 18 h, with FFA2 and FFA1, respectively. These results indicate that the FFAs had irreversibly adsorbed onto the coupon surface, providing a hydrophobic barrier and rendering the metal surface only partially wettable, thus inhibiting the progressive hydration of stainless steel. These data are in agreement with QCM-D results, which showed that both FFA1 and FFA2 formedfilms that were irreversibly adsorbed at the stainless steel surface of the sensor, under the experimental conditions considered. After 24 h’ soaking, the contact angle of 316L coupons remained fairly con-stant and significantly higher than those measured without amines added.
Without amines added, carbon steel (C1010) coupons corroded in milli-Q water at pH 9.0 and their contact angles were equal to approximately 4.5° and 0° after 18 h and 24 h, respectively. In the presence of either FFA1 or FFA2 corrosion of C1010 coupons was
Fig. 10. Contact angles. Comparison between the contact angles measured for 316L (top) and C1010 (bottom) coupons, with formulation FFA2 and FFA1 and without amines added (Blank). Note: 316L coupons did not corrode in any experiments conducted. The standard deviation was at most 5.6° in all measurements.
less marked than in the blank, and the contact angles remained approximately constant for up to 24 h. The data show a correlation between contact angles and the degree of corrosion of C1010 coupons, with the lowest contact angles measured for the most corroded metal surfaces.
QCM-D measurements indicated that progressive adsorption occurred even after 18 h, whereas contact angles remained ap-proximately constant from 18 to 24 h. These results may indicate that although the density of hydrophobic amine molecules on the steel surface increased, steel surfaces progressively hydrated, possibly due to partial permeability of the aminefilms to water, to imperfections in the FFAfilms or to incomplete coverage. The hypothesis of imperfections in the amine films and/or of incomplete coverage may explain the differences in the contact angles measured for carbon steel and stainless steel coupons after exposure to FFA solutions for 18 and 24 h.
The progressive hydration of C1010 in the absence of amines is in agreement with previous research showing that metal surfaces are strongly hydrated at alkaline pH[42]. The partial permeability to water of aminefilms explains the presence of some corrosion product on the C1010 coupons soaked in water for 17 and 24 h with either FFA1 or FFA2. However, the extent of rusting in the presence of amines was markedly less than in milli-Q water without amines added.
The data reveal that both FFA formulations could adsorb onto either stainless steel (316L) or carbon steel (C1010) surfaces, and that corrosion was inhibited by the formation of hydrophobicfilms with limited permeability to water. The data further suggest that the hydrophobicity of the aminefilms obtained with either FFA1 or FFA2 was comparable, indicating that the DEAE-based FFA formulation can be a suitable substitute for its CHA-based counterpart, in agreement with the EIS, QCM-D and Bengal Rose test data discussed in the previous paragraphs.
It is noted that, in addition to hydrophobicity and good coverage, the LUMO energy (ELUMO)[43]and the electron affinity[44]of corrosion inhibitors is also correlated to their performance. Specifically, it was found that the performance of hydroxybenzaldehyde Schiff bases increased with increasing ELUMO[43]. It was further found that Schiff bases synthesized by the condensation of iso-nicotinohydrazide and an appropriate aldehyde in methanolic solutions were most effective when their electron affinity was low [44]. Finally, theflexibility of the corrosion inhibitor molecules can further affect the adsorption process and hence the effectiveness of the inhibitors[32]. These aspects were not investigated in this study, and will be the object of future research.
4. Conclusions
The need to minimize the environmental impact of steam crackers by reusing water and treating it with environmentally benign chemicals has motivated this study, which compares two commercially available corrosion inhibitingfilm forming amine (FFA) formulations to treat water-steam cycles. One formulation was cyclohexanamine (CHA)-based (FFA1), whereas the other, FFA2, contained 2-(diethylamino)ethanol (DEAE) as the neutralizing amine. The data show that the two formulations increased the po-larization resistance of metal surfaces in similar ways, thus inhibiting their corrosion, as demonstrated based on visual inspection of metal coupons.
The comparable performance of the two formulations was explained based on the coverage of the metal surface attained under flow and no-flow conditions, as well as on the structure and hydrophobicity of the FFA films. Bengal Rose and quartz-crystal mi-crobalance with dissipation monitoring (QCM-D) data indicated that the mass adsorbed on stainless steel and carbon steel surfaces was similar with either FFA1 or FFA2 under eitherflow or no-flow conditions, suggesting that a similar coverage was attained with both formulations. QCM-D data and contact angle measurements further demonstrated that thefilms had similar rigidity and hy-drophobicity, shielding metal surfaces against water and providing equally good protection against corrosive water-soluble species. The combined results strongly suggest that in plant practice no notable difference in protection against corrosion is expected when CHA-based FFA formulations are substituted with environmentally more benign DEAE-based alternatives.
Acknowledgments
The authors wish to warmly thank Kirsten Zimmer, Christoph Weyn, and Julia Jasper (all of Kurita) for their support and their helpful contributions to this work. The contributions of Maria Huffman during the initial stages of the QCM-D work are also gratefully acknowledged. The authors further wish to extend their appreciation to SABIC's management for allowing publication of the present work.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version athttp://dx.doi.org/10.1016/j.wri.2017.11. 001.
References
[2] I. Betova, M. Bojinov, T. Saario, Film-Forming Amines in Steam/Water Cycles–Structure, Properties, and Influence on Corrosion and Deposition Processes, Technical Research Centre of Finland (VTT), Espoo, 2014.
[3] C. Forêt, G. Stoianovici, P. Bleriot, W. Hater, J. Matheis, Film forming amines for closed cooling/heating water systems. AWT, 2013, Uncasville (CT). [4] P. Janssen, J. Savelkoul, In search of an alternative high pressure boiler treatment program, Powerplant Chem. 14 (7) (2012) 440–448.
[5] R. van Lier, F. Cuoq, R. Peters, J. Savelkoul, Ten years of experience with polyamines in the high-pressure steam system of a naphtha cracker, Powerplant Chem. 17 (6) (2015) 356–363.
[6] P. Li, J.Y. Lin, K.L. Tan, J.Y. Lee, Electrochemical impedance and X-ray photoelectron spectroscopic studies of the inhibition of mild steel corrosion in acids by cyclohexylamine, Electrochim. Acta 42 (4) (1997) 605–615.
[7] E. Sylwestrzak, W. Moszczynski, W. Hater, T. Dembowski, A. de Bache, Experiences with the Treatment of the Water/steam Cycle of the Adamów Power Plant with Film Forming Amines, 8 VGB Powertech, 2016, pp. 69–74.
[8] W. Hater, P. Kraft, C. Forêt, The influence of alkalising amines on the film formation by oleyldiamine, Int. J. Corros. Scale Inhib. 4 (4) (2015) 353–364. [9] P. Bommersbach, C. Alemany-Dumont, J. Millet, B. Normand, Formation and behaviour study of an environment-friendly corrosion inhibitor by electrochemical
methods, Electrochim. Acta 51 (6) (2005) 1076–1084.
[10]F. Bentiss, F. Gassama, D. Barbry, L. Gengembre, H. Vezin, M. Lagrenée, M. Traisnel, Enhanced corrosion resistance of mild steel in molar hydrochloric acid solution by 1,4-bis(2-pyridyl)-5H-pyridazino[4,5-b]indole: electrochemical, theoretical and XPS studies, Appl. Surf. Sci. 252 (2006) 2684–2691.
[11]N. Ochoa, F. Moran, N. Pébère, B. Tribollet, Influence of flow on the corrosion inhibition of carbon steel by fatty amines in association with phosphonocarboxylic acid salts, Corr. Sci. 47 (3) (2005) 593–604.
[12]S. Ramachandran, B.L. Tsai, M. Blanco, H. Chen, Y. Tang, W.A. Goddard, Atomistic simulations of sleic imidazolines bound to ferric clusters, J. Phys. Chem. A 101 (1997) 83–89.
[13]K. Magne, J. Sjöblom, G. Øye, E. Gulbrandsen, A quartz crystal microbalance study of the adsorption of quaternary ammonium derivates on iron and cementite, Colloids Surf. A: Physicochem. Eng. Asp. 250 (1) (2004) 269–278.
[14]M. Duprat, M.-C. Lafont, F. Dabosi, F. Moran, Study of the corrosion and inhibition processes of a carbon steel in a low conductivity medium by electrochemical methods, Electrochim. Acta 30 (3) (1985) 353–365.
[15]K. Stiller, T. Wittig, M. Urschey, The analysis offilm forming amines - methods, possibilities, limits and recommendations, Powerplant Chem. 13 (10) (2011) 602–613.
[16]W. Hater, A. de Bache, T. Petrick, Dry lay-up of steam generators withfilm forming amines: studies and field experiences, Powerplant Chem. 16 (5) (2014) 284–292.
[17]W. Hater, A. de Bache, T. Petrick, Dry lay-up of steam generators withfilm forming amines-studies and field experiences, Cah. l'Assoc. Sci. Eur. l'Eau St. 19 (2014) 5.
[18]E. Pensini, B. Sleep, C. Yip, D. O'Carrol, Carboxymethyl cellulose binding to mineral substrates: characterization by atomic force microscopy-based force spectroscopy and quartz-crystal microbalance with dissipation monitoring, J. Colloid Interf. Sci. 402 (15) (2013) 58–67.
[19]M. Rodahl, F. Höök, B. Kasemo, QCM operation in liquids: an explanation of measured variations in frequency and Q factor with liquid conductivity, Anal. Chem. 68 (13) (1996) 2219–2227.
[20]L. Alagha, S. Wang, Z. Xu, J. Masliyah, Adsorption kinetics of a novel organic–inorganic hybrid polymer on silica and alumina studied by quartz crystal microbalance, J. Phys. Chem. C 115 (31) (2011) 15390–15402.
[21]T.J. Halthur, T. Arnebrant, L. Macakova, A. Feiler, Sequential adsorption of bovine mucin and lactoperoxidase to various substrates studied with quartz crystal microbalance with dissipation, Langmuir 26 (7) (2010) 4901–4908.
[22]G. Sauerbrey, Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung, Z. Phys. 155 (2) (1959) 206–222.
[23]F. Höök, B. Kasemo, T. Nylander, C. Fant, K. Sott, H. Elwing, Variations in coupled water, viscoelastic properties, andfilm thickness of a Mefp−1 protein film during adsorption and cross-linking: a quartz crystal microbalance with dissipation monitoring, ellipsometry, and surface plasmon resonance study, Anal. Chem. 73 (24) (2001) 5796–5804.
[24]R.M.A. Azzam, N.M. Bashara, Ellipsometry and Polarized Light, North-Holland Publisher Company, 1977.
[25]M. Landgren, J. Bengt, Determination of the optical properties of silicon/silica surfaces by means of ellipsometry, using different ambient media, J. Phys. Chem.
97 (8) (1993) 1656–1660.
[26]F.L. McCrackin, E. Passaglia, R.R. Stromberg, H.L.J. Steinberg, Measurement of the thickness and refractive index of very thinfilms and the optical properties of surfaces by ellipsometry, J. Res. Natl. Bur. Stand. 67A (1963) 363–377.
[27]J. De Feijter, D.J. Benjamins, F.A. Veer, Ellipsometry as a tool to study the adsorption behavior of synthetic and biopolymers at the air–water interface, Biopolymers 17 (7) (1978) 1759–1772.
[28]T.J. Halthur, P.M. Claesson, U.M. Elofsson, Stability of polypeptide multilayers as studied by in situ ellipsometry: effects of drying and post-buildup changes in temperature and pH, J. Am. Chem. Soc. 126 (51) (2004) 17009–17015.
[29]F. Lijuan, H. Yang, F. Wang, Experimental and theoretical studies for corrosion inhibition of carbon steel by imidazoline derivative in 5% NaCl saturated Ca(OH) 2 solution, Electrochim. Acta 58 (2011) 427–436.
[30]O. Ghasemi, I. Danaee, G.R. Rashed, M. Rashvand Avei, M.H. Maddahy, Inhibition effect of a synthesized N,N′-bis(2-hydroxybenzaldehyde)-1, 3-propanediimine on corrosion of mild steel in HCl, J. Cent. South Univ. 20 (2013) 301–311.
[31]A.K. Singh, Inhibition of mild steel corrosion in hydrochloric acid solution by 3-(4-((Z)-indolin-3-ylideneamino)phenylimino)indolin-2-one, Ind. Eng. Chem. Res. 51 (2012) 3215–3223.
[32]L. Herrag, B. Hammouti, S. Elkadiri, A. Aouniti, C. Jama, H. Vezin, F. Bentiss, Adsorption properties and inhibition of mild steel corrosion in hydrochloric solution by some newly synthesized diamine derivatives: experimental and theoretical investigations, Corros. Sci. 52 (9) (2010) 3042–3051.
[33] M. Jack, S. Weerakul, D.H. Lister. The Interaction of a Film Forming Amine with Surfaces of a Recirculating Experimental Water Loop. Proceedings of International Conference on Fouling and Cleaning, 2015 Enfield, Ireland.
[34]T. Tekla, T. Saarinen, M. Österberg, J. Laine, Preparation of Langmuir/Blodgett-cellulose surfaces by using horizontal dipping procedure. application for polyelectrolyte adsorption studies performed with QCM-D, Cellulose 13 (5) (2006) 519–535.
[35]A. Tsortos, G. Papadakis, E. Gizeli, Shear acoustic wave biosensor for detecting DNA intrinsic viscosity and conformation: a study with QCM-D, Biosens. Bioelectron. 24 (4) (2008) 836–841.
[36]R.D. Braun, E.E. Lopez, D.P. Vollmer, Low molecular weight straight-chain amines as corrosion inhibitors, Corros. Sci. 34 (8) (1993) 1251–1257. [37]G. Bohnsack, Korrosionsinhibierung durch das im Helamin enthaltenefilmbildende Amin, VGB Kraftw. 77 (10) (1997) 841–847.
[38]R.P. Richter, A.R. Brisson, Following the formation of supported lipid bilayers on mica: a study combining AFM, QCM-D, and ellipsometry, Biophys. J. 88 (2005) 3422–3433.
[39]Y. Yin, T. Liu, S. Chen, T. Liu, S. Cheng, Structure stability and corrosion inhibition of super-hydrophobicfilm on aluminum in seawater, Appl. Surf. Sci. 255 (2008) 2978–2984.
[40]S. Ghareba, S. Omanovic, Interaction of 12-aminododecanoic acid with a carbon steel surface: towards the development of‘green’ corrosion inhibitors, Corros. Sci. 52 (6) (2010) 2104–2113.
[41]N.A. Negm, A.M. Al Sabagh, M.A. Migahed, H.M. Abdel Bary, H.M. El Din, Effectiveness of some diquaternary ammonium surfactants as corrosion inhibitors for carbon steel in 0.5 M HCl solution, Corros. Sci. 52 (6) (2010) 2122–2132.
[42]E. Pensini, B.E. Sleep, C.M. Yip, D. O’Carroll, Forces of interaction between fresh iron particles and iron oxide (magnetite): effect of water chemistry and polymer coatings, Colloids Surf. A 433 (2013) 104–110.
[43]I. Danaee, O. Ghasemi, G.R. Rashed, M. Rashvand Avei, M.H. Maddahy, Effect of hydroxyl group position on adsorption behavior and corrosion inhibition of hydroxybenzaldehyde Schiff bases: electrochemical and quantum calculations, J. Mol. Struct. 1035 (2013) 247–259.
[44]I. Ahamad, R. Prasad, M.A. Quraishi, Thermodynamic, electrochemical and quantum chemical investigation of some Schiff bases as corrosion inhibitors for mild steel in hydrochloric acid solutions, Corros. Sci. 52 (3) (2010) 933–942.