Examensarbete i biomedicinsk laboratorievetenskap Malmö universitet
61-90 hp Hälsa och samhälle
Biomedicinska analytikerprogrammet 205 06 Malmö
September 2020
XANTHAN GUM
FERMENTATION OF XANTHOMONAS CAMPESTRIS
1
XANTANGUMMI
FERMENTERING AV XANTHOMONAS CAMPESTRIS
EVGENIA TRUDOVA
Trudova, E Xantangummi: fermentering av Xanthomonas campestris. Examensarbete, 15 högskolepoäng. Malmö Universitet. Fakulteten för hälsa och samhälle, institutionen för Biomedicinsk laboratorievetenskap, 2020.
Abstrakt: Xantangummi är ett av de vanligast förkommande förtjockningsmedlen som används i världen.I den industriella tillverkningsprocessen av xantangummi används billiga, kolhydratrika medier för fermentering av bakterien Xanthomonas campestris. Syftet med denna studie var att jämföra olika fermenteringsmedier innehållande vetemjöl, majsstärkelse, potatisstärkelse, och havremjöl för småskalig fermentation av Xanthomonas campestris. Alla fyra fermentationsmedierna som användes i denna studie visade tecken på förtjockning, vilket indikerar närvaron av levande och växande Xanthamonas campetris. Baserat på tillväxten på MacConkey- och NA-agarplattor visade alla fyra fermenteringprodukterna närvaron av bakterier. Fermenteringsprodukten från odling i medium innehållande både potatisstärkelse och havremjöl uppvisade en högre koncentration av bakterier jämfört med odlingsmedium innehållande vetemjöl eller majsstärkelse. Fermenteringprodukten i närvaro av havremjöl visade en mer än 100 gånger högre bakteriekoncentration jämfört med vetemjöl. Data tyder på att fermentering med potatisstärkelse och havremjöl ger bättre tillväxt än
medium innehållande vetemjöl och majsstärkelse och att mindre allergenbenägna medium kan användas som alternativ för fermentering av Xanthamonas campetris vid produktion av
xantangummi.
2
XANTHAN GUM
FERMENTATION OF XANTHOMONAS CAMPESTRIS.
EVGENIA TRUDOVA
Trudova, E Xanthan gum: fermentation of Xanthomonas Campestris. Examwork, 15 Credits. Malmö University: Faculty of Health and Society, Department of Biomedical Science, 2020. Abstract: Xanthan gum is one of the most common thickening agents used worldwide.The industrial manufacturing process of xanthan gum uses cheap, carbohydrate rich mediums for fermentation of the bacterium Xanthomonas campestris. The objective of this study was to compare different fermentation mediums based on grain powder for small scale fermentation of Xanthomonas campestris.
Culture mediums containing wheat or cornstarch and the less allergen prone medium containing potato starch and oat flour were investigated. All four fermentation mediums of this study showed signs of thickening, indicating the presence of alive and growing Xanthamonas
campetris. Based on the growth on MacConkey and NA-agar plates, all four fermentation
products showed the presence of bacteria. The fermentation product from a culture medium containing both potato starch and oat flour showed a higher concentration of bacteria compared to a culture medium containing wheat flour or cornstarch. The fermentation product in the presence of oat flour showed more than 100 times higher bacterial concentration in the fermentation product compared to wheat flour.
Data suggests that potato starch and oat flour fermentation performed better than wheat flour and cornstarch and these less allergen prone mediums can be used as an alternative for fermentation of Xanthamonas campetris in the production of xanthan gum.
3
ACKNOWLEDGEMENTS
Thank you to my teachers, Gabriela Enggren and Håkan Eriksson for support and beaming enthusiasm. You made this small personal project possible.
4
CONTENT
INTRODUCTION
Xanthan gum
Xanthan gum production Xanthamonas campetris
AIMS MATERIALS AND METHODS
Filtration and Sterilisation Fermentation of Xanthamonas Growth on agar plates and CFU Gram staining Oxidase test Catalase test Control experiment ETHICAL APPROVAL RESULTS Observation on thickening
Gram-staining, Oxidase and Catalase tests
Bacteria growth on Mac Conkey and NA-nutrient agar plates DISCUSSION CONCLUDING REMARKS REFERENCES 5 5 6 6 7 7 7 7 8 8 8 8 8 9 9 9 9 10 12 14 15
5
INTRODUCTION
Xanthan gum was discovered by Allene Rosalind Jeanes, an American chemist at the United States Department of Agriculture. She made several biomedical, and chemical polysaccharide discoveries during her active years, 1938-1976; one of which was xanthan gum (Lemenson-MIT 2020).
Xanthan gum
The molecular formula of xanthan gum is [C35H44O29 ]n (Figure 1). It is produced by the
gram-negative bacterium Xanthomonas campestris, and has many commercial uses. It is primarily used as a thickening food additive (Murray et al. 1995).
Xanthan gum presents industrial value due to non toxicity and resistance to enzymatic digestion. It´s molecular weight is estimated to range from 15 to 50×106 g mol−1 which is
considered as high, for a thickening gum. High molecular weight is desirable to obtain an effective thickening agent. The primary structure of xanthan gum, shown in figure 1, is an antiparallel right hand fivefold double helix, consisting of two glucopyranosyl units, two D-mannopyranosyl units and one D-glucopyranosyluronic acid unit. The polymer backbone consists of trisaccharide branches on alternating D-glucopyranosyl unit. The double helix is stabilised by four intermolecular bonds and one intramolecular hydrogen bond (Dumitriu et al. 2005).
Figure 1: Xanthan gum (Vitthal et, al. 2016).
The rigidity of the helical conformation is responsible for the structural stability which is shown by the viscosity-vs.-temperature curve of xanthan gum, where at high temperature, many of the hydrogen bonds break and the hemicellulose structure looses its viscosity. Xanthan gum forms gels that can be crosslinked with other polysaccharides. The biosynthesis starts with an assembly of saccharide repeating units, which are then polymerized to produce a macromolecule. Xanthan is formed by sequential addition of monosaccharides from energy rich sugar nucleotides, involving acetyl-CoA and phosphoenolpyruvate. Given this composition, xanthan is easily dissolved in cold water while giving the aqueous solution thixotropy and viscosity needed for lubricating many simple industrial processes. In later years products utilizing xanthan gum polymerization products have gained biochemical applications, where it has been used in the design of liquid gel systems and carbohydrate based beads that swell upon hydration used in the separation of molecules (Dumitriu et al. 2005).
6 Xanthan gum production
In the production of xanthan gum, microorganisms of the Xanthomonas genus are used in a two step cultivation process, where the first stage is seed fermentation. This step is attained with the purpose of increasing the bacteria concentration in nitrogen containing mediums. The transfer of seed fermentation into a larger scaled cultivation medium for the
fermentation process is referred to as fermentation, which is the second step of cultivation of bacteria. The product of the fermentation is sterilised and precipitated with ethanol or
isopropanol, which breaks bonds between bacteria and polysaccharide structures of the gum. Xanthan gum self-separates from the solution and sinks down as a pellet, which is dried into a powder with thickening properties (Honma et al 1996).
The production of Xanthan gum includes several additives, with the intention of ensuring high productivity and purity of the product. Ammonium nitrate is added as a water soluble inorganic nitrogen source. The carbon source can be glucose or different types of or mixes of starches, while magnesium salts and phosphate ions are added for optimal fermentation and viscosity of the Xanthomonas fermentation. The pH is regulated with a NaOH solution to a preferred range of 5,5-9.0 (Honma et al 1996).
Xanthomonas campestris
Xanthomonas is a subfamily of the Xanthomonadaceae family (order Xanthomonadales).
The bacterium is named after the word Xanthos (lat. yellow), referring to the pigmented colonies growing on agar plates. Xanthomonas campestris bacteria are oxidase-negative and gram-negative and has the morphology of a polar rod with a flagellum 0,4-- 1,0 µm wide and 1,2-3,0 µm long (Schaad et al 2001).
The Xanthomonas produces a viscous xanthan gum-based biofilm matrix, which protects bacteria from abiotic stress on the surface of a plant and the bacteria blocks the vascular system of the plant causing an area of wilting (Schaad et al 2001).
The reaction of the plant to the microbial attack is to produce hydrogen peroxide.
Xanthomonas campestris has several protection strategies, two of the most common are
encoded by the genes; katA, encoding a weak monofunctional catalase, and katG, that encodes a catalase-peroxidase giving the bacteria a more efficient protection against hydrogen peroxide (Charoenlap et al. 2011).
There are 27 known species of Xanthomonas and 400 known plant hosts and the numbers are growing. Xanthomonas is an agricultural pest and causes black rot which is a common plant disease (Schaad et al 2001).
Commonly available xanthan gun powders also contain co-precipitated Xanthomonas
campestris and no seed fermentation was done in this project to isolate the bacteria before
fermentation. Instead fermentation was performed by directly re-suspend a commercial preparation of Xanthan gum powder in fermentation medium in order to propagate and culture the bacteria present in a commercial preparation of Xanthan gum powder.
7
AIM
The aim of this study was to compare the growth of Xanthomonas campestris on fermentation mediums of wheat and cornstarch as energy sources, and on less allergen prone mediums, such as mediums from potato starch and oat flour sources.
MATERIALS AND METHODS
The experiment was designed with available materials, using Xanthan gum (Xantangummi Glutenfritt LindROOS Örebro Sverige.) and four fermentation ingredients. These being: wheat flour (Vetemjöl, Kungsörnen), cornstarch (Majsmjöl; Sverige ICA Solna.), potato starch (Potatismjöl; Sverige ICA Solna.) and oat flour (Havremjöl; RISENTA AB Sollentuna Sverige).Chemicals that have been used are gram staining kit (Merck chemicals.Germany. Darmstadt), oxidase test (Sigma Aldrich; Germany) and 10% hydrogen peroxide provided by Malmö University.
Filtration and Sterilisation
Working with bacteria required maintenance of a sterile environment to prevent contamination and growth of unknown microorganisms. Flat work surfaces were disinfected with alcoholbased surface disinfectant. Only deionized water was used in this experiment.
Stock solution of large volumes not containing glucose were sterilized by autoclave chamber; 120 oC at 20 min in 3 cycles. All empty containers were sterilised by autoclave chamber, 120 oC at 20 min in 3 cycles.Smaller volumes of sensitive glucose containing solutions were
sterilized through filtering through a FilterpurV50 and PES 0,2 µm (SARSTEDT, USA. New York.).
Fermentation of Xanthomonas
This experiment modified the early recipe published in the patent “Method of fermentation and production of Xanthan Gum” patented Dec 3, 1996. The modification included removal of all industrial additives described in the original recipe, as the aim of this study was a simple comparison of the quality of bacterial growth following fermentation of different medium. Seed fermentation was initiated by adding 2 grams of the xanthan gum powder to 0,3 L of deionized water containing 1.5 g glucose and the sample was incubated at room temperature for 2 hours. Then, the gelatinous seed fermentation product was transferred to 1 L of deionized water containing 18.5 g glucose and the various grain products.
One of the modifications to the patented recipe was removal of inorganic nitrogen source from the fermentation. The original recipe recommends a proportion of 1: 3 of xantan gum to nitrogen source and suggests several plant based alternatives to inorganic nitrogen salts.This experiment uses wheat flour, corn starch, potato starch and oat flour as plant alternative to nitrogen sources. Two grams of of xanthan gum was added to the seed fermentation. Seed fermentation was then transferred to 1 L of water containing 6 g of grain medium; adding up to 1: 3 proportions of gum to grain; containing among other components needed, carbohydrates and nitrogen required in final solution.The final fermentation recipe contained: the seed fermentation gel (0,3 L water, 1,5 g of glucose and 2 grams of xantan gum), 1 L of deionized water containing 18,5 g of glucose and 6 g of wheat flour, corn starch, potato starch or oat flour. The fermentation process was then continued for 7 days at room temperature and kept under stirring. The fermentation process utilizing the four different fermentation mediums was repeated twice.
8 Growth on agar plates and CFU
The finished fermentation product was plated on nutrient agar (NA) and MacConkey agar plates using the Copacabana method, method when glass beads are rolled over bacteria spreading it on the agar.
The fermentation solutions were thoroughly mixed by Vortex (Genie 2; LaborA Scientific Industries N. Y. USA) and 100 ml of a serial dilution from 10-1 to 10-5 using an automatic
pipette was applied to the middle of the agar plates. The mixture was spread with the Copacabana method and left to grow for 24h at a temperature of 37 oC under aerobic
conditions. Viable count data was provided by assuming that each of the future colonies is growing from individual bacteria.
Gram staining
Gram staining is a differentiation method between gram positive and gram-negative bacteria with a series of coloration steps. The sample of bacteria was fixed with fire to glass (Menzel Glaser Thermo Scientific, USA, Massachusetts.) and coated with a primary stain of crystal violet. The stain was washed off with deionised water. The second coat is a Lugol solution which contains iodine, a trapping agent. The stain was washed off with deionised water. An acetone coat was used to decolorize the stain. The finished sample was coated with counterstain of Safranin. The gram staining solutions were from a kit by Merck chemicals (Germany. Darmstadt).
Gram positive bacteria remain blue/purple due to trapped purple violet. Gram negative bacteria turns pink/red from decolourisation. Bacteria was observed with a light microscope (Olympus CH30, Germany).
Oxidase test
The bacterial culture was applied directly onto the oxidase test plates (Sigma Aldrich; Germany).
A positive oxidase reaction, i.e the presence of indophenol, was shown as blue colouration upon addition of the bacterial culture, while a negative oxidase reaction was colourless.
Catalase test
The Catalase test is a common differential method of examination of bacterial colonies. Catalase is an enzyme present in many organisms which facilitates decomposition of hydrogen peroxide into oxygen and water. This method is performed in room temperature, by applying a single drop of 10% hydrogen peroxide solution to bacterial colonies and observing the production of oxygen, visible as rapid or slow production based on the creation of bubbles (Murray et al. 1995).
Positive reaction creates bubbles of O2 since H2O2 is digested by catalase into oxygen and water.
A negative reaction does not create bubbles. Control experiment
Escherichia coli was grown as control to Xanthamonas campetris. Sample of bacteria was
plated on the NA and MacConkey agar plates using plastic loops (SARSTEDT Nümbrecht SARSTEDT Nümbrecht). Agar plates were grown at 37 oC for 12 hours in an anerobic
9
ETHICAL APPROVAL
This fermentation study did not need ethic approval as it was performed with safe and commercially available materials. Xanthomonas was extracted from Xantangummi - glutenfritt (LindROOS Överbro Sverige.). A control sample of Escherichia coli was provided by Malmö University.
RESULTS
Bacteria were not isolated as a pure culture of Xanthamona campetris before the fermentation process was initiated. The industrial production of xanthan gum powder is done by addition of alcohol directly to fermentation product of metabolically active Xanthamona campetris, followed by direct dehydration of the precipitate into a powder. Presence of dehydrated
Xanthamonas campetris can then be anticipated in dehydrated xanthan gum powder.
All four fermentation mediums in this study gave bacterial growth on agar plates and showed signs of thickening indicating the presence of xanthan gum and Xanthamonas campetris. To verify the presence of Xanthamonas in the fermentation medium, gram staining, oxidase and catalase tests were performed. As a control. Escherichia coli was cultured, and the colonies were used as control against colonies of Xanthamonas..
Observation on thickening
All of the fermentation solutions showed signs of rapid thickening after addition of 2 grams of xanthan gum powder and 6 grams of fermentation medium in a total of 1.3 liter. Various thickening behaviour was observed from the different fermentation mediums. Powders of corn starch and potato starch quickly dissolved and maintained a homogeneous and gelatinous appearance through-out the whole experiment. Fermentation mediums containing wheat flour and oat flour became not fully homogeneous and gelatinous, some particles and fragments of flours were observed at the bottom of the container.
Gram staining, Oxidase and Catalase tests
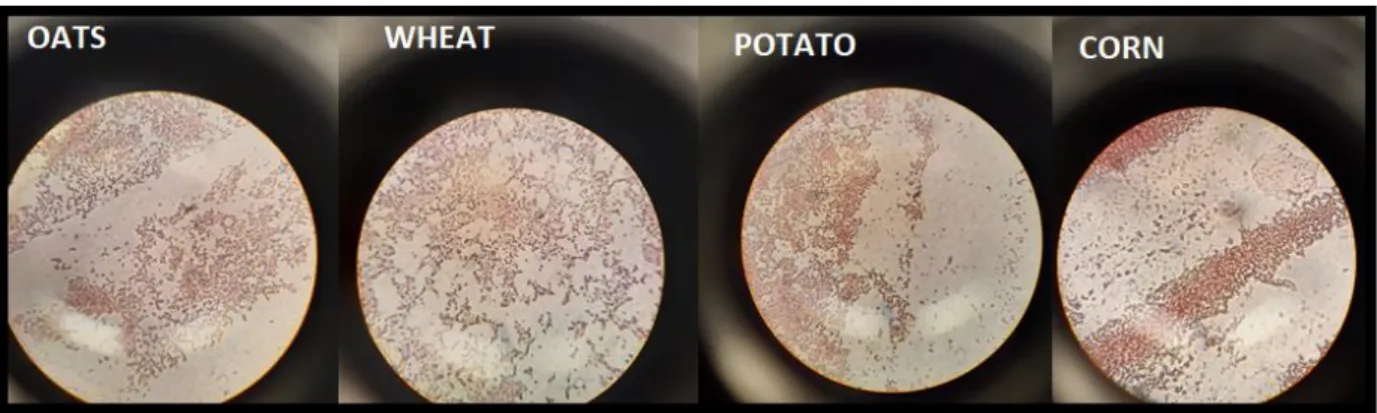
Bacteria grown on agar plates were examined using gram staining, oxidase and catalase tests and the results are shown in figure 2 and summarized in table 1.
Figure 2: Light microscopy photographs of gram staining of colonies from oat, wheat, potato starch, and corn
10 As a control, Escherichia coli, a gram negative, oxidase negative and catalase positive bacterium was used and gram staining of Escherichia coli is shown in figure 3.
Figure 3: Light microscopy photograph of gram staining of E.coli.
Table 1: Gram staining, oxidase and catalase examination of colonies of E.coli, and wheat, corn starch, potato
starch, and oat flour fermentation products.
E. coli Wheat Corn Potato Oats
Gram Neg. Neg. Neg Neg. Neg.
Oxidase Neg. Neg. Neg. Neg. Neg.
Catalase Pos. Pos. Pos. Pos. Pos.
Bacteria growth on Mac Conkey and NA-nutrient agar plates
Colony growth of the various fermentation products and the control E.coli was investigated on Mac Conkey and NA-nutrient agar plates and colony colour, appearance, scent, and numbers were examined. The results are summarized in tables 2 and 3.
Table 2: Comparison of agar plate growth on Mac Conkey and NA-agar of control bacteria E.coli and the
fermentation products after fermentation on the presence of wheat flour, corn starch, potato starch and oat flour.
E. coli Wheat Corn Potato Oats
Mac Conkey Growth Growth Growth Growth Growth
Colour Pink Pink Pink Pink Pink
Colonies Dry Soft &
Wet
Soft & Wet Soft & Wet Soft & Wet Scent Bad, richly sour
and rotting floral sweetness characteristic of E. coli Weak Sour Weak Sour Weak Sour Weak Sour Nutrient (NA)
Growth Growth Growth Growth Growth
Colour White White White White White
Colonies Dry Soft &
Wet
Soft & Wet Soft & Wet Soft & Wet Scent Characteristic of
E. coli
11 Colony Forming Units, CFU, from serial dilutions of the fermentation products were calculated upon growth on the two types of agar plates (Table 3). If the agar plates contained too many colonies for visual calculation, the symbol “-” is shown in table 3.
Table 3: CFU upon serial dilutions of the fermentation products of wheat flour, corn starch, potato starch and
oat flour.
Serial Dilution
10 -1 10 -2 10-3 10-4 10-5
Agar M.Con NA M.Con. NA M.Con. NA M.Con NA M.Con NA
Wheat - - - - 232 312 32 43 5 1
Corn - - - 323 94 67 10 3
Potato - - - 536 178 288 23 134
Oats - - - 280 162 148
CFU presented in table 3 was evaluated and the dilution of 10-5 for both Maconkey and
NA-agar plates was used to calculate the concentration of bacteria in the various fermentation products (Figure 4).
Figure 4: Concentration of bacteria in the various fermentation products calculated from CFU obtained upon
growth on Mac Conkey and NA agar plates.
Wheat flour Corn starch Potato starch Oat flour
Mac Conkey 5.00E+05 1.00E+06 2.30E+06 1.62E+07
NA 1.00E+05 3.00E+05 1.34E+07 1.48E+07
0.00E+00 5.00E+06 1.00E+07 1.50E+07 2.00E+07 N U M B ER O F B A C TE R IA/ M L FERMENTATION MEDIUM
Concentration of bacteria from fermentation of wheat flour, corn starch, potatostarch and oatflour with xanthan gum assumed to
contain a sample of Xantamonas campetris.
12
DISCUSSION
Xanthan gum is a common additive in food and hygiene articles and products containing xanthan gum are often used by individuals allergic to grain proteins. Xanthan gum is produced from the bacteria Xanthomonas campestris, however, the fermentation medium of
Xanthomonas campestris often contains low cost carbohydrates orginating from wheat or corn
and thereby will the fermentation product, the xanthan gum, contain trace amounts of allergens. The first objective was to obtain a sample of isolated Xanthamonas campetris. Even though this bacterium is commonly found on leafy vegetables, isolation and identification of Xanthamonas
campetris is quite difficult from a mixed bacterial flora that coats the surface of vegetables.
Commercially available Xanthan gum powder is claimed to contain the bacteria Xanthamonas
campetris co-precipitated in the isolation process of xanthan gum. Instead of searching for a
wild sample of the bacteria this project aimed to hydrate and extract bacteria from a cheap and accessible dehydrated product of xanthan gum. The industrial production of xanthan gum powder is done by addition of alcohol directly to fermentation product of metabolically active
Xanthamona campetris, followed by direct dehydration of the precipitate into a powder.
Presence of dehydrated Xanthamonas campetris can then be anticipated in the dehydrated xanthan gum powder. This study attempted to reverse this process and directly fermentate bacteria from the xanthan gum powder by dissolving the powder in a solution of water, glucose and carbohydrate rich grain-based fermentation mediums
The fermentation process used in this study was based upon a recipe published in “Method for fermentation production of xantan gum” (Honma et al.1996). This recipe starts with an isolated pure culture of Xanthomonas and a seed fermentation. If the seed fermentation step is ignored;
Xanthamonas metabolism might be disturbed and production of xanthan gum ceases
completely. In this study no isolated culture of Xanthomonas was used. Instead bacteria present in a commercial preparation of xanthan gum powder was used. Xanthamonas campetris in the dried powder requires rehydration and this was done in small volume of water with dissolved glucose and incubated for 2 hours. This step replaced the seed fermentation described by Honma et al.,1996, and at the end of the incubation the preparation showed a visible gel like texture, indicating the presence of thickening properties and theoretically the presence of alive bacteria. Then the initial Xanthamonas fermentation obtained by re-suspenion of xanthan gum powder was added directly to a large volume of a fermentation medium containing grain carbohydrates and glucose for a longer fermentation process.
Glucose was used in this study as second fermentation medium beside the four grains that were used in the fermentation medium. The addition of glucose was done to ensure the growth of bacteria in all experiments since the culture properties of the grain containing fermentation medium was unknown. With glucose present in the fermentation medium at least some bacteria would survive through the fermentation process in all examined solutions.
Another component that is important in fermentation of Xathamonas is nitrogen. Industrial production of xantan gum uses organic and and inorganic sources of nitrogen. Inorganic sources are water-soluble of ammonium salts, ammonium bromate and ammonium phosphate salts e.g. One type of mediums contains up to a dozen of different ammonium salts. These mediums are cost effective in production, however, the final product might contain residues of ammonia, a chemical that is difficult to remove. Industrial xantan gum is consumption safe, but residues of ammonia is unessesary and undesirable in common additives such as xantham gum.
13 Organic sources that can be used are aqueous organic components of biologically active yeasts, extracts of peptone, solutions of gelatine and amino acids as well as protein rich grain brooths such as soybean. These are often byproduct of many of the Asian based fermented products, such as soy sause, rice and bean paste products. American production of xantan gum uses byproducts such as potato peels and corn plant leaves for the same industrial purporse. This is not a preferred fermentation technique; due to high cost of processing, involving enzymes, high labour and energy costs, and the handling of a highly viscousous solution.
Soybeans, rice, corn, and potato are plant based mediums that have been used as nitrogen source; similar result was expected from plant based finely ground flour of the four grains that were chosen in this experiment. The four grains acted primarily as source of carbohydrate; but also as source of nitrogen for the small scale fermentation applied in this study. The aim of this study was to compare CFU and maintain bacteria alive in different fermentation mediums, not the full optimisation of a fast, cheap and large scale industrial xantan gum production line.
The second objective of this study was identification of fermented bacteria as Xanthamonas. This bacterium is known for environmental adaption with several strategies and without knowing which genetic variant that was used to produce the xanthan gum powder, the identification was a challenge. Genetic variants of the bacteria show high to very weak catalase activity and the catalase activity of Xanthamonas campetris colonies is correlated with virulence of the bacteria. Catalase activity was chosen as an identification marker in this study, however, without knowing the virulence or the exact genetic variant of bacteria, a range of catalase activity was expected in the colonies grown from the fermentation product, from very weak/negative to strong/positive, depending on the catalase strategies coded by the genetic forms (Charoenlap et al. 2011).
In an attempt to identify the bacteria in the fermentation product of the four grain mediums isolated on agar plates, gram staining, appearance, catalase activity, colony colour, scent, and numbers were examined.
One of challenges with this study was the possible contamination of bacteria in the fermentation product other than Xanthamonas and the growth of and appearance of E.coli was chosen as control. Both Xanthomonas and E.coli are facultatively anaerobic and gram negative bacteria.
E.coli is catalase positive and this study was designed with the hope that the Xanthomonas campestris species cultured from xanthan gum powder would be a catalase negative variety.
This would have been a tool of identification and differentiation of Xanthamonas from the catalase positive E.coli. However, the bacteria grown from the fermentation products were all shown to be catalase positive upon examination of colonies (Gritsenko & Bukharin 1996). These results left fewer differentiation possibilities, however, careful analysis of the agar plates and the scent of the bacteria, enough differentiation data was found to suggest that bacteria grown from fermentation products were not from E.coli contamination or any other contamination but from Xanthomonas campestris. Visually, the colonies of the grain fermentation products were larger, wetter, and more slimy in appearances while E.coli had dried, firm, and slightly smaller colonies. The characteristic scent of E. coli was present on the control plate with E.coli, but lacking on any of the fermentation medium agar plates
Comparison of the data obtained by CFU calculations and thereby the concentration of bacteria/ ml in the fermentation product, was indicated by MacConkey and NA agar plates that potato starch and oat flour performs better than wheat flour and corn starch in terms of growth of gram negative, oxidase, and catalase negative bacteria.
This study purposefully excluded ingredients from the original recipe for fermentation of xanthan gum to be able to compare the fermentation solution containing just three basic
14 ingredients: filtered glucose solution, xanthan gum powder, and grain containing fermentation medium. Four different fermentation mediums were used and an interesting observation regarding the growth of bacteria from the potato starch fermentation product on yeast containing NA agar plates which gave a higher number of colonies/plate compared to growth on MacConkey agar plates. This unexpected growth might have connections to the properties of potato starch and the experiment needs to be repeated to verify the results.
CONCLUDING REMARKS
In conclusion, exclusion of allergenic substances like wheat and corn proteins from industrial products will provide a relief to allergic individuals attempting to avoid allergens. The results of this project suggest that bacteria of the subfamily Xanthomonas can be fermented and produce xanthan gum in a fermentation medium containing potato starch or oat flour instead of wheat flour or corn starch.
Inspite of the small size of this study, the experimental data suggests that oat flour and potato starch might be an industrial and economical alternative to traditional fermentation mediums during xanthan gum production. Good performance of oat and potato grain fermentation; without additional nitrogen salts gives hope for production of Xantan gum with fewer ingredients and as result less ammonia and allergens in common household products. The study can be repeated with genetically confirmed Xanthomonas spieces, selective agar blends and a Maldi-TOF examination of the fermentation product in order to identify the exact species of
15
REFERENCES
Charoenlap N, Buranajitpakorn S, Duang-Nkern J, Namichaiw P, Vattanaviboon P, Mongkolsuk S (2011) Evaluation of the virulence of Xanthomonas campestris pv. campestris
mutant strains lacking functional genes in the OxyR regulon. Curr Microbiology;
Aug;63(2):232-7. doi: 10.1007/s00284-011-9970-9.
Dumitriu S. (red.). (2005) Polysaccharides. Structural diversity and functional versatility. New York: Marcel Dekker.
Gritesenko VA, Bukharin OV. (2000) The ecological and medical aspects of the symbiosis
between Escherichia coli and man.Zh Mikrobiol Epidemiol Immunobiol. May-June;(3):
92-9. PMID:10925888
Honma T, Shigehiro J, Murofushi K. (1996) Method for fermentation production of
xanthan gum. United States. Patent nr: 5580763/ US005580763A Filed Aug. 25, 1994.
Appl.No: 296115.Japan. Patent nr: 5-277997. Filed Nov. 8,1993. Shin Etsu Chemical Co Ltd. Shin Etsu Bio Inc.
https://patents.google.com/patent/US5580763A/en?inventor=Taira+Honma (Hämtad 2020-03-27 )
Lemenson-MIT. (2020) Historical inventors: Allene Rosalinde Jeanes.
https://lemelson.mit.edu/resources/allene-rosalinde-jeanes (Hämtad 2020-03-27).
Murray R P, Baron J E, Pfaller A M, Tenover C F, Yolken H R. (1995) Manual of Clinical
Microbiology. Washington: American Society for Microbiology.
Schaad NW, Jones JB, Chun W (2001). Laboratory Guide for Identification of Plant
Pathogenic Bacteria. USA: American Phytopathological Society Press.
Vitthal S Kulkarni, Shaw C. (2016) Essential Chemestry for Formulators of Semisolid and
Liquid Dosages: Use of Polymers and Thickeners in Semisolid and Liquid Formulations.
Academic Press: Elsevier Inc. doi:10.1016/C2013-0-18871-X.