TVE 15069
Examensarbete 30 hp
December 2015
Modelling the exfoliation of
graphite for production of graphene
Mehwish Abro
Institutionen för teknikvetenskaper
Teknisk- naturvetenskaplig fakultet UTH-enheten Besöksadress: Ångströmlaboratoriet Lägerhyddsvägen 1 Hus 4, Plan 0 Postadress: Box 536 751 21 Uppsala Telefon: 018 – 471 30 03 Telefax: 018 – 471 30 00 Hemsida: http://www.teknat.uu.se/student
Abstract
Modelling the exfoliation of graphite for production of
graphene
Mehwish Abro
The aim of my thesis is to make a theoretical model of data obtained from liquid-phase exfoliation of graphene. The production of graphene in the liquid phase exfoliation is a cost efficient method One part of this work is devoted to learn the method of production of graphene by the shear mixing technique from the graphite and to estimate some important parameters which are crucial for the process.
Other part of my work is based on studying the liquid-phase exfoliation mechanism of graphene through ultra-sonication technique. This method is time consuming as compared to shear-mixing.
TVE 15069 December Examinator: Nora Masszi
Ämnesgranskare: Dr. Subimal Majee, Dr. Zhibin Zhang Handledare: Dr. Asim Aijaz
Acknowledgements
Firstly, I would like to thank my supervisor Dr. Zhibin Zhang who gives me the opportunity to work his group and complete my master thesis project with his research group.
I would also like to thank my co-supervisor Dr. Subimal Majee for his advisement, encouragement and guidance during my project research as well as going out of his way to help me succeed and pursue my goals during my time at Uppsala University. He helps me in teaching the different experimental methods in the lab. I would also like to thank my other group members.
I would like to express my gratitude to my home country DEAN Prof. Dr. BS Chowdhry , he encouraged and guided me throughout my university education.
A special feeling of gratitude goes to my loving parents, especially my father engr. Abdul Ghani Abro, my mother Rukhsana Abro whose prayers and words of encouragement helped me to reach this point. I am especially thankful to my sister, brothers, my friends, and relatives who encouraged and supported while I was working on this thesis.
I would also like thank to my best friend in Uppsala University; he encouraged me a lot throughout my research work. I cannot conclude these acknowledgments without recognizing the lunch break time chit chat, which also greatly supported the completion of this work.
Finally, I dedicate my work to my supervisors who have supported me throughout my research work process. I will always appreciate all they have done.
Mehwish Abro,
Uppsala University, Sweden January, 2016
Page | 3 Table of Contents: Chapter # 1 Introduction Introduction………..….……….………...…... 8 Structure….………..….……….…….………...…..8 Properties of Graphene………..……….….9
Common Methods for graphene synthesis……….……….…...…10
Common methods, yields and their Applications….……….……….………11
Liquid Phase Exfoliation……….…………...……….…...…12
My Thesis Organization…….……….………..………14
Chapter # 2 Shear Exfoliation of Graphite Introduction of process………...……….…..15
Mechanism of process ……….….16
Experimental Procedure………...………..17
Experimental results and discussions.………..…….….17
Theatrical modelling of the experimental results.………….………...……..19
Viscosity of Solvents………...22
Conclusion of chapter 2……….…..23
Chapter # 3 Sonication of Graphite Introduction of process...………...…24
Experimental procedure ………24
Experimental results and discussions………...……….24
Comparison of both methods………...………..27
Theatrical modelling of the experimental results………...………....28
Conclusion of Chapter 3……….29
Conclusion………...30
Future Work………..……….………..30
Page | 4
List of Figures
1. Graphene.layout ….……….………..…8
2. Synthesis of graphene by two different methods in Liquid based exfoliation…………...……12
3. Shear mixing machine……….………15
4. Attractions in the Layers of graphene………...….……….…16
5. Variation of exfoliated graphene flake thickness and lateral diameters with increasing shear mixing speeds. The variation is for ethanol based exfoliation……...………...18
6. Variation of exfoliated graphene flake thickness and lateral diameters with increasing shear mixing speeds. The variation is for cyclohexanone based exfoliation………18
7. Variation of exfoliated graphene flake thickness and lateral diameters with increasing shear mixing speeds. The variation is for NMP based exfoliation ………...…19
8. Graph of force of friction and applied force………...20
9. Graph of Exfoliation and applied force………...20
10. Graph of force of friction and applied force………...20
11. Ultra-sonication data for Ethanol solvent………..….25
12. Ultra-sonication data for Cyclohexanone solvent………..…26
Page | 5
List of Tables
1. Properties of Graphene………..………..……….9
2. Common Methods for Graphene synthesis……….………….10
3. Common methods yield and their applications……….………..……….…11
4. Viscosity of Solvents………..………...22
5. Comparion of both methods………..………..…………..27
Page | 6
Introduction
Graphene, 1-3 is a two dimensional nanomaterial consist of single atomic layer of sp2-bonded carbon
atoms. 4,5 It is organized in a honeycomb lattice/ benzene like structure, displays amazing electronic,
electrical, mechanical, optical and thermal properties.6,7,8 In the most recent decade graphene has
developed as an energizing new material, with potential to affect numerous areas of science and innovation.9,10 With an innovative method (Scotch-Tape exfoliation) Novoselov and Geim in 2004 have
successfully exfoliated monolayers of graphene and awarded the Nobel prize in Physics.11,12 It is
semimetal material having several outstanding applications. According to the properties for example, electronic devices as micro- and optoelectronics13,14,15,16,17, basic nanocomposites, printed electronics,
conductive coating, biological labeling 18 and batteries and supercapacitors.19 Many other possible
technological applications of monolayer graphene, e.g. in photonics and flexible electronics, ranging from solar cells20, photodetector21,22,23 and light emitting devices24 to touch screens25, ultrafast lasers26, spin
valves 27,28 etc., are additionally being investigated.
Structure
Graphite was derived from the Greek word ` Graphein` which means to write. The term graphene was derived from the graphite, presented by chemists Hanns-Peter Boehm and co-worker in 1986 .29,30
Graphite is the combination of the millions of the graphene layers. Two types of bond are formed among the graphene layers. The bond which holds together the layers of graphene by the weak force called Vander Waal attraction. The Vander Waal bond length between the adjacent graphene layers is 3.41Å (0.341nm).3 Due of this weak attraction between layers, the layers slide each other and the attraction is
strong enough to do the complete exfoliation into individual layers. Another bond known as Covalent bond which present between the carbon-carbon atoms in each layer is 1.42Å (0.142nm) considered as the strong bond .31 Both the hypothetical and experimental research proved that the properties of graphene are
mainly dependent on their geometric structures. (See Figure 1)
Figure 1 : Graphene layout adopted
(https://www.google.se/search?q=graphene+structure&espv=2&biw=1920&bih=935&source=lnms&tbm =isch&sa=X&ved=0CAYQ_AUoAWoVChMI1KXMptTiyAIVpJdyCh11AArw#imgrc=xMbdpfJEXG22 SM%3A)
Page | 7
Properties of Graphene
Graphene has many outstanding properties It is considered as the thinnest possible material in the world and compared to the steel it is 200 times stronger material.32,33 According to Hoorad and their colleagues
the thermal conductivity of graphene is ~33 times greater than the silicon.34 Below given are the
important properties of graphene which are collected from different literature.
Sr.
Property Value References 1.
Large Surface Area
~ (3000 m2g-1) 30,36 2. Stretch elasticity ~ 20% 51 3. Optical Transparency ~ 97.7% 37,38 4. Tensile Strength ~ (130GPa) 30,39 5. Thermal Conductivity ~ ( (3000-5000)WmK-1) 30,40,41,42 6. Breaking Strength ~ ( 42Nm-1 ) 10,40
7. High Carrier Mobility ~ (10,000cm2 V-1 S-1 ) 36,39,41,39,42,48
8. Young’s Modulus
~ (1.0TPa ) 30,39,41,41
9. Large Spring Constant ~ (1-5Nm-1) 30,39,49
10.
Page | 8
Common Methods for Graphene synthesis
There are tremendous efforts have been done to develop synthesis methods for graphene. These different methods are used to achieve high yield of graphene for different application. Generally the synthesis methods can be classified as the Top-Down and Bottom-Up approaches .51 All the methods have their
own pros and cons depend on final applications. Many researchers proved that the graphene production by bottom-up methods have high quality but unfortunately suffers from low scalability. On the other hand the graphene produced by Top-down methods have high quantity but poor quality. The production of graphene in the large scale and at low cost as explained by the top-down techniques.30 Precise control
over graphene synthesis is therefore required for testing their fundamental physical properties and then introduce them in promising applications.52
Top -Down Bottom-Up
Mechanical Exfoliation4,42,53
(1) Apply the adhesive tape on graphite block and peeled back
(2) Join the two pieces of tape together to reduce layers
(3) Finally press the tape on the smooth silicon substrate & peel back leaving atomic single layer thick graphene.
Liquid Phase Exfoliation
Chemical Vapor Deposition (CVD)37,42,53,56,58,59,60 (1)A substrate usually copper (sometimes Ni) is heated in furnace about ~1000Ċ at low pressure (2) CH4 and H2
gasses are added through furnace
(3) Carbon atoms are deposited on copper
substrate and
continuous graphene sheets are formed.
Epitaxial Growth on Silicon Carbide 42,53,59,60 (1)Small amount of SiC is placed into box with small hole in it
(2)Seal the box in non-reactive environment and heated at about ~ 1500Ċ (3)Because of heating, Si molecule from the surface of SiC evaporates, leaving high quality monolayer graphene. /Chemical Exfoliation53,54,55 (1) In this process ultrasound is used to break graphite into flakes into organic solvents. (2) After some duration, large quantity of flakes are produced (3) Centrifuge process is helpful for enriching the graphene quality.
Chemical Exfoliation Via Graphene Oxide
56,57
(1) In this process the graphite is first oxidized and then exposed in chemical exfoliation to produce graphene oxide flakes
(2) Centrifuge process is used for further enriching of graphene sheets
(3) The solution is deposited on substrates and reduced thermally and chemically to get graphene.
Page | 9
Common Methods Yield and their Applications
Methods flake size
(Top view)
Cost Throughput Flake thickness (cross section view) Number of layers Applications
Mechanical process Greater
than 1 mm
low low 10 nm Single &
multiple Research purpose Chemical Exfoliation 1 μm low μm to few nm μm to few nm Single and multiple
Inkjet printer ink, polymers fillers, coating, paint, composites, transparent electrode, sensors, energy storage and bio applications
Chemical
Exfoliation Via
Graphene Oxide μm to few nm low high nm Single and multiple
Inkjet printer ink, polymers fillers, energy storage, Battery electrodes, supercapacitors
CVD cm high Moderate < 1nm Monolayer to multilayer Touch screen, small windows, flexible LCDs & LEDs Epitaxial Growth on Silicon Carbide
100 mm high low < 1nm Monolayer to multilayer
Transistor circuits, interconnect, memory
Page | 10
Liquid Phase Exfoliation:
The exfoliation of graphite in the liquid environments can be effectively done by exploiting shear mixing and ultrasound to extract individual layers. The liquid phase exfoliation process normally includes three stages.(see figure: 2)
1. Dissolve graphite in a solvent, 2. Exfoliation, and
3. Purification.
Shear Mixing Exfoliation
Ultra-sonication Exfoliation
Figure 2: Synthesis of graphene by two different methods in Liquid based exfoliation
Liquid phase exfoliation is a method to exfoliate graphite into liquid solution. It is a feasible way to obtain colloidal suspension of graphene layers in the solution. The quality and quantity of graphene layers are higher than those produced from graphite oxide due to the absence of oxygen functionalities which disturb the properties like electrical conductivity and carrier mobility of graphene layers. This method is more efficient when the applied force to the graphene can overcome the graphene–graphene interlayer van der Waal interaction force. According to Loh et.al, the exfoliation is better when the surface energy of the solvent is close to the graphene.61 By the mechanical force, which is sufficiently greater than
Vander Waals force, we can separate graphene layers. Ethanol
Cyclohexanone
N-Methyl-2-pyrrolidone
Page | 11
The mechanical force is achieved by either sonication or shear-mixing. The long term sonication prompts to undesirable fragmentation intoexfoliated graphene layers which brings about small size graphene layers. Generally, sonication is a process to transfer the sound energies to fragment the particles.62 It has
been found that the surface energies play important role when graphite surface is immersed in the liquid.30
Liquid phase exfoliation is one of the effective and straightforward method to decrease the strength of the Vander Waals attractions. 63
Meanwhile in shear mixing and ultra-sonication, the growth and the breakdown of the micrometer-sized bubbles because of pressure fluctuations, work over the bulk material and induce exfoliation.62 After
exfoliation, the interaction of solvent–graphene needs to adjust the attractive forces in between the inter-sheets. Ideal solvents to disperse graphene are those that actually minimize the interfacial surface tension [mN m−1] between the solvent and graphene flakes. For example, the forces that reduce the area of the surfaces in contact.30,64
Many groups have worked in this method and made it possible to produce large scale of graphene. Stankovich and their followers oxidized the graphite and produced the graphite oxide layer. During the oxidation process, the functional groups like hydroxyl and epoxide attached covalently to the graphite oxide reducing the interlayer interactions, which causes complete exfoliation and produce the single layer GO. The presence of oxygen functional group on the GO causes sheet hydrophilic and shows less thermal, electrical and mechanical properties as compared to pure graphene.3,65,66,67 So reduction of GO into
graphene has become main area of research.68 Recently, some groups demonstrated the exfoliation of GO
into the organic solvent like dimethyl foraminde (DMF), N-methyl-2-pyrrolidone (NMP), ethylene glycol and tetrahydrofuran (THF) with moderate sonication. 35,48,69,70
Hernandez and their fellows in 2008 explained the first successful exfoliation of graphite in the organic solvent such as NMP and DMF by sonication based technique.71 After centrifugation, they confirmed
their characterization through Atomic Force Microscopy (AFM) and Transmission Electron Microscopy (TEM), that they obtained pristine graphene which were chemically unmodified. Surprisingly they achieved closely 100% graphene nanosheet and atomic thickness was less than 6 layers and samples was consisting of 28% monolayers. 35,48,71,72,73,74,75,76,77 But NMP and DMF liquid have a few drawbacks e.g.
NMP is an eye irritant and may be dangerous to the reproductive organs, while DMF may have toxic effects on various organs. 78,79 It is considered that the solvents having the surface energy close to the
graphene are appropriate for the direct exfoliation of graphene .30
Mustafa Lotya et.al in 2009 demonstrated that the mechanism of liquid phase production of graphene depends on utilizing the specific solvents whose surface energies are around that of graphene.80 These
organic solvents require unique consideration when handling. However, it is considered that the solvents which have low boiling point is good for exfoliation of graphene but that graphene has limit application, while on the other hand the solvents which have high boiling point like (NMP 203
℃
, cyclohexanone 156℃
and DMF 154℃
) are suitable for the exfoliation of graphene but the high boiling points of these solvents restrict their use for the real manipulation, specifically in organic electronics.30 Manyindependent group explained unfortunately that water has a surface energy (72.7 mJm-2) too much high to
use it as for exfoliation of graphene .71, 80,81
In 2010 a group from Ireland which observed the dispersion of graphene in NMP solvent by using ultra sonication exfoliation method at low power 23W for long times up to 460hours.82 They have achieved
Page | 12
the concentration of 1mg mL−1. The size and thickness of flakes decrease with sonication time. For long sonication times the average flakes dimension still remains above 1μm . 82
The graphene application´s market is basically driven by progress in the synthesis of graphene with properties fitting for the specific application, what's more, this circumstance is liable to proceed for the next decade or if nothing else until each of graphene's many potential applications meets its own particular requirements. Currently there are most likely a dozen of techniques being used and developed to synthesis graphene of different dimensions, quality and shapes. 83,84
My thesis organization:
In the next chapter of my thesis, one can find the exfoliation of graphene in the liquid phase exfoliation using the shear mixing method. During my stay in the Uppsala University Sweden, I was involved in the experiments with the liquid phase exfoliation method of graphene and modelling the production mechanism of graphene. In this chapter I have presented the detail of the protocols involved in the synthesis of graphene and the modelling of the surface energies of Ethanol, Cyclohexanone, NMP w.r.t to graphene and the graphical representation of reduction of the graphene flake thickness with some shear mixing parameters.
In the third chapter, I have explained the exfoliation of graphite by using the sound waves in the liquid solution and finally the advantages and disadvantages of both the exfoliation methods. And I have calculated the time for exfoliation of single layer. Finally, the conclusion is summarized for both methods and some future outlook is presented.
Page | 13 Chapter # 2
Shear exfoliation of Graphene
Introduction of the process
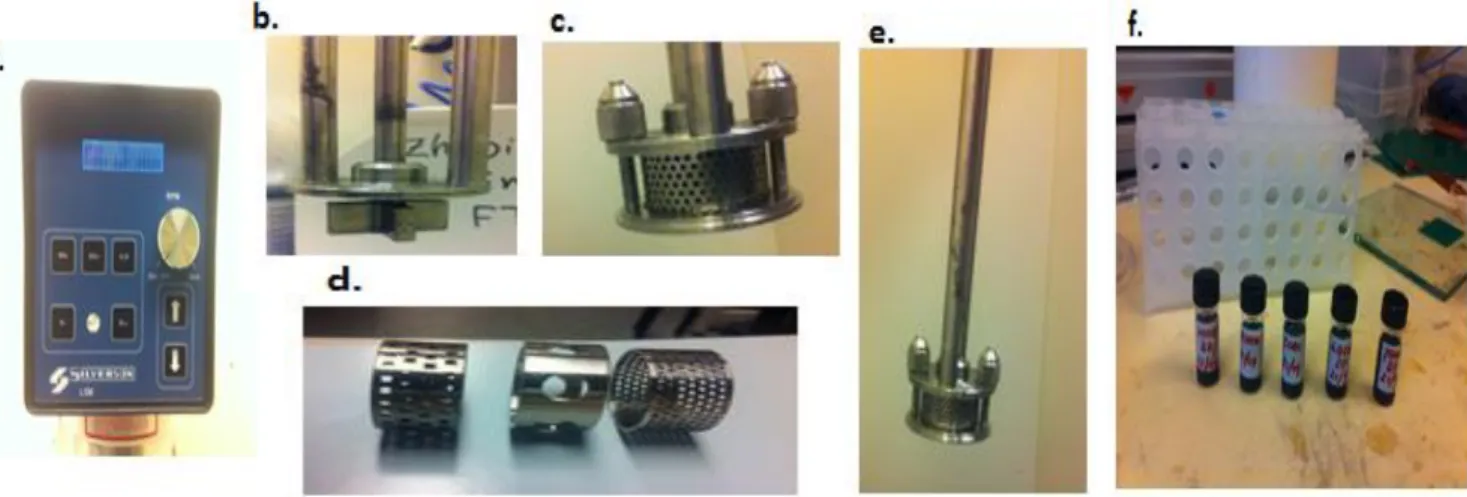
Shear mixing is a process of exfoliation of the graphite in the liquid phase by using the shear force mechanism85. In this process, a machine (Silverson model L5M mixer) is used to generate high shear
force. The Silverson model consists of manual control system, rotor and stator part (see figure 1) and the purpose of this machine is to mix the solution of solvent and graphite and shear the graphite. In shear mixing process, the graphite is mixed with the organic solvent like ethanol, Cyclohexanone and NMP.
Figure 1: Shear mixing machine. a, Image of shear mixing operator. b, Rotor. c, clear view of functioning part. d, different shape of stator. e, combination of Rotor and stator. f, exfoliated samples
Here, we can say that high-shear mixing is a scalable alternative to the sonication process for the peeling of layered crystal of graphite. Shear mixing is already generally used to disperse nanoparticles in solvents. It includes separating of microparticle agglomerates that are weakly attached compare with the intersheet binding strength in graphite. The exfoliation of graphite or layered material that incorporates shear mixing as a part of the procedure is reported in different articles 3. In shear mixing, the layered material is initially
swelled by intercalation, significantly reducing the interlayer binding strength.3,35,71 The shear mixing
exfoliated the crystal material graphite to give dispersed nanosheets. However, shear mixing method just limiting the rate step (Time) for intercalation and exfoliation, increases the potential for scale-up. The shear mixing process has much more ability to exfoliate the untreated graphite crystal material into liquid solution. In addition, Shear mixing process utilizes less power densities as compared to Ultra-sonication method.
Page | 14
Mechanism of the process
The shear mixing exfoliation method works for the exfoliation of graphene in liquid phase. To understand the mechanism of exfoliation in liquid we have to first understand some term like cohesive force, surface energy and viscosity. The cohesive force is defined as the attraction of one molecule toward other molecule which held together the molecules of the atom in the bulk material in the layers. Surface energy is defined as the imbalance energies at the top surfaces of the materials such as liquid or solid. In the bulk material,
Figure 2: Attractions in the Layers of graphene
the energies in centre remain same so every molecule get force exerted equally from surrounded molecules. The molecules present in the top layer of material have different energies because top layer remain in the contact of the atmosphere and experience the imbalance energies in the surface. In term of graphite, when solid graphite mixed with the solvents, they attach into the layers of graphite to reduce the Vander Waal force in between layers (see figure 2). Actually, the total energy (ET) required to exfoliate
the graphite in the liquid is the combination of the Vander Waal forces (Evwf) and the change of the
surface energies (Es).
E
T= Evwf + ∆ E
E
T= Evwf + [ Eg - Es]
Where,
ET is the total energy requires for exfoliation,
Evwf is the VanderWaal force between the layers of graphite,
E is the changes in surface energies,
Page | 15
The exfoliation of graphite requires to overcoming these energies. Generally, the graphene layers are attached with each other by weak Vander Waal attraction and that attractions are strong enough to exfoliate the graphene single layer from the graphite.
Experimental procedure
We use the shear mixing process to exfoliate the graphite for production of graphene. For this we mix the graphite powder and organic solvent in the beaker with a specified weight ratio. We also add some polymer stabilizer along with them, in order to keep the exfoliated graphene flakes stable inside the dispersion. The beaker is placed at the bottom stage of the shear mixer. By controlling the setup, the rotor (outside diameter 32 mm) is inserted into the beaker. The speed of the rotor can be varied using the setup monitor. The maximum rotor speed that can be obtained with full load is 8000 rpm. In order to see the changes of thickness and the lateral diameters of the exfoliated graphene flakes on the types of solvents generally used, a wide range of solvents with different surface energies are used in our study. The speed of the rotor is varied from 1000 rpm to 6000 rpm. The process was performed for a fixed duration of 20 min. After each shear mixing completion, the supernatant obtained from the top part of the dispersion is spin coated on Si/SiO2 substrates and the morphology of the films are characterized through atomic force
microscopy technique using non-contact mode. The thickness and the lateral diameters of the flakes are evaluated through this process.
Experimental results and discussions
The shear mixing is first performed in ethanol based exfoliation. Initially the thickness of graphite flakes was 1μm. When the shear speed is increases from 0 to 1000 rpm; the thickness and the diameter of graphene flakes get affected and the graphene flake thickness is reduced from 1000 nm to 740 nm. When the shear speed varies from 1000 to 5000 rpm, the thickness is reduced sharply with the increasing speed and reduced down to ~100 nm. The diameter is also reduced from 20,000 nm to 2900 nm. After that the shear speed doesn’t affect the exfoliation process and the thickness as well as the diameters remain constant (see figure 4).
In Cyclohexanone based exfoliation, when the shear speed is increase from 0 to 1000 rpm, the thickness and the diameter are reduced. The thickness is reduced from 1000 nm to 500 nm. When the shear speed is increased from 1000 to 5000 rpm, the thickness is sharply reduced down to 70 nm. The diameter is also reduced from 20,000 nm to 1000 nm. After that the shear rate don’t not affect the thickness and diameter (see figure 5).
Similarly, when the shear mixing process is used with the NMP, the shear speed affects the thickness and the diameter. When shear speed increases from 0 to 1000 rpm, the thickness is reduced from 1000 nm to 400 nm. When the shear speed is increased gradually from the 1000 to 5000 rpm, the thickness and diameter are sharply reduced down. The thickness is reduced down and the minimum reduced thickness obtained is 10 nm for 5000 rpm speed. On the other hand the diameter is also reduced from 20,000 to 250 nm After that the shear speed doesn’t affect the exfoliation process and the thickness as well as the diameter remains constant. The NMP is greatly affecting the shear mixing process, and the thickness and diameter reduce more as compared to the ethanol and cyclohexanone (see figure 6).
Page | 16
When the shear rate is increased gradually, the thicknesses as well as the diameters of the graphene flakes are reduced for all the above mentioned organic solvents. There is a clear trend of sharp reduction of the thickness and the diameters after 1000 rpm and we found a critical speed of 5000 rpm above which there is no further exfoliation. If we compare three organic solvents, we can see through graph that the ethanol shows the less exfoliation (see Figure 4). The cyclohexanone shows the moderate exfoliation as compared to ethanol and NMP (see figure 5). NMP based exfoliation shows better exfoliation rate as compared to the ethanol and cyclohexanone (see Figure 6).
1000 2000 3000 4000 5000 6000 0 100 200 300 400 500 600 700 800 Flake thickness Flake diameter Shear speed (rpm) Fla ke t hickne ss (n m) 2000 4000 6000 8000 10000 12000 Fla ke d iame ter (n m)
Figure: 4 Variation of exfoliated graphene flake thickness and lateral diameters with increasing shear mixing speeds. The variation is for ethanol based exfoliation.
1000 2000 3000 4000 5000 6000 0 100 200 300 400 500 Flake thickness Flake diameter Shear rate (r.p.m) Fla ke t hickne ss (n m) 0 2000 4000 6000 8000 Fla ke d iame ter (n m)
Figure: 5 Variation of exfoliated graphene flake thickness and lateral diameters with increasing shear mixing speeds. The variation is for cyclohenanone based exfoliation.
Page | 17 1000 2000 3000 4000 5000 6000 0 100 200 300 400 Flake thickness Flake diameter Shear speed (rpm) Fla ke t hickne ss (n m) 0 1000 2000 3000 4000 Fla ke d iame ter (n m)
Figure: 6 Variation of exfoliated graphene flake thickness and lateral diameters with increasing shear mixing speeds. The variation is for NMP based exfoliation.
Theoretical modelling of the experimental results
The shear mixing mechanism works on the force of friction and applied force. When we use the shear mixing process, rotor rotates and applies force into the solution. Initially, the static friction occurs when the applied force through the rotor exerted into liquid solution (solid graphite and organic solvent), the friction produced into the solution. With the increase of the shear mixing speeds, the applied rotational force faced by the flakes increases as well, this allows the sliding of the graphene layers between themselves. As a results, the friction between the two adjacent graphene layers increases linearly as shown in Figure 3.1, which is termed as the static friction. Due to increase of the friction with the applied force, it is hard to separate the individual layers form each other and almost no exfoliation occurs in this region (from F0 to F1 as shown in Figure 3.2). In our practical experiments, we did not see any exfoliation when the shear mixing speeds are increased slowly from 0 to1000 rpm. We suppose the frictional force is so huge in this region, therefore basically it is impossible to separate the layers of graphene. As a result, there is no reduction of either thickness or size of the flakes as shown in figure 3.3. When further increase of the applied force, the frictional force starts to reduce and therefore the exfoliate occurs. We can see than exponential decay of the frictional force with the applied force in the region between F1 and F2 (Figure 3.1). In this period the graphene layers start to slide between each other and with the increase of the applied force they separate from each other. We can say that the exfoliation starts from this region and from our practical measurements, it occurs after 1000 rpm. As a result, both the thickness and the size of the flakes reduce exponentially as we have shown in Figure 4, 5 and 6.
With further increase of the applied force, the frictional force becomes constant, which means easy exfoliation of the layers, however, there is a limit of the exfoliation of the layers which depends on the surface energies of the solvents used, we can see a stable thickness and the size of the flakes after a certain speed of 5000 rpm (corresponds to F2 in the Figure 3.2).
Page | 18
Figure 3.1: Graph of force of friction and applied force http://www.sciencehq.com/physics/frictional-force.html
Exfoliation
Figure 3.2: Graph of Exfoliation and applied force
Thickness
Page | 19
Figure 3.3: Graph of force of friction and applied force The surface energies are calculated through the following calculation,
ṙmin = [
√Egraphene – √Esolventŋ∗𝑙
]
2
ṙmin = [
√Egrapheneŋ – √Esolvent]
2
∗
1𝑙2
𝑙 = [
√Egraphene – √Esolventŋ∗𝑙
]
2
∗
1ṙ𝑚𝑖𝑛
𝑙 =
√𝐸graphene – √Esolventŋ
∗
1 √ṙ𝑚𝑖𝑛
𝑙 =
√𝐸graphene – √Esolventŋ∗√ṙ𝑚𝑖𝑛
---(1)
Where,
ṙmin ₌ Shear rate (s-1)
Eg ₌ Surface energy of graphene, (N/m)
Es ₌ Surface energy of solvent, (N/m) ŋ ₌ solvent viscosity, (Pa.s)
Page | 20
Viscosity of Solvents
The viscosity of solvent is defines as the thickness of the solvents which resist to flow. It is also important term for the exfoliation of graphite in the liquid. The viscosity of the liquids which we are using in our experiments is given below.
Viscosity of solvants (Pa.S)
Ethanol 0.00107
Cyclohexanone 0.00202
NMP 0.00167
The surface energies of the three solvents e.g. Ethanol, Cyclohexanone and NMP are very important when we talk about the exfoliation of graphite. We have taken the model reference from the literature 85
(Scalable production of large quantities of defect-free few-layers graphene by shear exfoliation in liquids) and calculated the surface energies of the solvents.
From equation (1) , the shear rate is the mixing rate of the solution and it is given as,
ṙ
min=
2 𝜋 ND
∆R
where,N ₌ number of revolution per min, D ₌ 0.0320 m, ∆R ₌ gap of rotor-stator, So,
ṙ
min=
2 ∗3.14∗83.3334∗ 0.0320 0.0001
ṙ
min₌ 167467 s
-1Page | 21
this is the value of the shear rate of all solvents for 5000 rpm, Now for the calculation of surface energy of Ethanol √E Ethonal ,by rearranging the equation (1), we get
√E
Ethanol= √E
graphene–
√ r
min * ŋ *𝑙
= √ 0.0763 – √ 167467 * 0.00107 * 0.000007
= 0.2645 – √ 0.001254
= 0.2645 – 0.03541
√E
Ethanol= 0.22909
Take the squaring on both sides,
(√E
Ethonal ) 2=
(0.22909)
2E
Ethanol= 0.0524 N/m
E
Ethanol= 52mJ/m
2By using the similar calculation and procedure, the surface energies of the cyclohexanone is calculated as 62 mJ/m2 and for the NMP is 69 mJ/m2
.
Conclusion of chapter 2
1. We have exfoliated graphene flakes out of graphite from a novel method which is shear exfoliation technique. In this process, the applied shear force helps to separate the graphene layers by overcoming the vander Waal’s force of attraction.
2. We have shown the variation of the thickness and the lateral diameters of the flakes with shear mixing speeds.
3. We have defined a critical shear mixing speed beyond which there is no more exfoliation.
4. Using an available shear mixing mechanism, we have extracted the surface energy values of some well known solents usually used for exfoliation of graphene.
Page | 22 Chapter # 3
Sonication of graphite
Introduction of the process
Sonication is another method for exfoliation of graphite layers into the solution. It is based on the sound waves in which the solution is placed in the ultra-sonication bath. The solution kept in the beaker, and placed in the sonication bath. The sonication bath exerted the pressure in the beaker solution and then the solution in which the organic solvent and the graphite are present experiences the force from the sonication bath which causes reduction of the flake thickness. It is slow process for the exfoliation. The result from the sonication process for three organic solvents ethanol, cyclohexanone and NMP are given below.
Experimental procedure
We use the sonication mechanism in the liquid phase to exfoliate the graphite into the solution for production of graphene. For this process, we mix the graphite powder, organic solvent and polymer stabilizer with specific quantity into the beaker and keep the beaker into the sonication bath. We add the polymer stabilizer into solution because to keep the graphene flakes stable into the solution. The sonication bath works on different frequencies. We used 20 KHz frequency to disperse and exfoliation of the solution. The sonication bath transfers the energy into the water present into the bath then the water exerted the pressure to the breaker. The pressure then transmits in to the solution, disperse and exfoliate the graphite into the solution. This process is time consuming and produces the graphene into the liquid. The process was performed at different times. After the completion of ultra-sonication, we take the small amount of supernatant from the top part of the solution to spin coat it on the substrate of Si/ SiO2. Then
we characterize the sample through the atomic force microscopy technique.
Experimental results and discussions
The ultra-sonication technique in the liquid phase exfoliation is first accomplished in ethanol based exfoliation. In this process, initial thickness of flakes of graphite was 1μm (1000 nm). In order to increase the sonication time (hour), the thickness of graphite flakes is decrease. Briefly, when the ultra-sonication time is increased from 0 to 300 hour, the thickness of the graphite flakes is reduced down from 1000 nm to 80 nm for ethanol. After that the ultra-sonication time doesn’t affect the exfoliation process and the thickness remains constant (see Figure 1).
For the cyclohexanone, when the ultra-sonication time increases from 0 to 300 hour, the thickness of graphite flakes is get affected through the sonication time. The thickness is reduced down from 1000 nm to 40 nm. After this thickness, there is no further reduction of the thickness (see figure 2) .
Page | 23
In NMP based exfoliation, the affect is much severe. When ultra-sonication time increases from 0 to 300 hour, the thickness is reduced down from 1000 nm to 10 nm. After this point, it doesn’t affect the exfoliation process and the thickness remains constant. (see figure 3).
-100 0 100 200 300 400 500 600 700 800 60 80 100 120 140 160 180 200 Fla ke t hickne ss (n m)
Sonication time (hour)
Figure 1: Ultra-sonication data for Ethanol solvent
Page | 24 -100 0 100 200 300 400 500 600 700 800 20 40 60 80 100 120 140 160 Fla ke t hickne ss (n m)
Sonication time (hour)
Figure 2: Ultra-sonication data for Cyclohexanone solvent
-100 0 100 200 300 400 500 600 700 800 0 10 20 30 40 50 60 70 80 90 Fla ke t hickne ss (n m)
Sonication time (hour)
Page | 25
Comparison of the both methods
We have used to different methods in liquid phase exfoliation for production of graphene. But methods have some advantages and disadvantages. If we compare both methods, according to some parameters, we have some conclusion given below.
Parameter Shear Mixing Exfoliation Ultra-sonication Exfoliation
Time
Less time Longer time
Thickness of final product same same Scalability Yes No Concentration of final product same same
Page | 26
Theoretical modelling of the experimental results
Here is the calculation of one layer exfoliation.The distance between the graphite layers is 0.34 nm. and initially the thickness of grahite is 1000 nm and we notice that the linear exfoliation happens upto 300 hours and the thickness reduces at 80 nm for ethonol.
The exfoliated thickness at 300 hour is = 1000 - 80 = 920 nm
We know that the distance between the layers is 0.34 nm. The number of layers exfoliated at 300 hour is given as Number of layers exfoliated = 920
0.34
Number of layers exfoliated of ethanol = 2705 So,
One layers exfoliation time (min) is = 300 ∗ 60
2705
One layers exfoliation time is = 6.65 min for ethanol
Now the remaining layers at 80 nm = 80
0.34
Remaining layers = 235
So now we can know that the total number of layers (LT) at initial 1000 nm thickness is
LT = 2705 + 235
LT = 2940
On the other hand, if we calculate the exfoliation of single layer time with same method for Cyclohexanone and NMP, it is given as,
Solvent Time(min) Reduced
thickness(nm) # of layers exfoliated 1 layer exfoliated time Remaining layers Cyclohexanone NMP 300 300 960 990 2823 2911 6.37 6.1 117 29
Page | 27
We can see the exfoliation time for the one layer from the graphite flakes is reduces when the surface energy value of the corresponding solvent is close to that of the graphene. Since, from our previous chapter, we concluded that the surface energy of NMP is much close to graphene and from this chapter we concluded that the time required for one layer exfoliation is minimum for NMP, we can correlate both conclusions.
Conclusion of chapter 3:
We can see the exfoliation time for the one layer from the graphite flakes is reduces when the surface energy value of the corresponding solvent is close to that of the graphene. Since, from our previous chapter, we concluded that the surface energy of NMP is much close to graphene and from this chapter we concluded that the time required for one layer exfoliation is minimum for NMP, we can correlate both conclusions.
1. We have exfoliated graphene flakes out of graphite through the ultra-sonication technique. In this process, the applied sound energy helps to separate the graphene layers by overcoming the vander Waal’s force of attraction.
2. We have shown the variation of the thickness with ultrasonication time
3. We have defined a critical ultrasonication time, above which there is no more exfoliation.
Page | 28
Conclusion
In this project, we have used to different methods for the production of graphene from graphite. The methods are based on the liquid phase exfoliation. Through these two methods we have come to know that the surface energy is one of the main parameters for the exfoliation. We used three different organic solvents for the exfoliation. These were Ethanol, Cyclohexanone and NMP. The liquid which has surface energy close to the graphene is better for the exfoliation. These two methods produced graphene in which the shear mixing exfoliation takes less times for the production of graphene on the other hand, the ultra-sonication consumes more time.
If we compare both methods, the shear mixing exfoliation is more scalable method because it can produce large quantity of flakes compared to the ultra-sonication based exfoliation.
Future work
Our work gave a solid indication of the choice of solvent requires for the efficient exfoliation. In future, efforts should be devoted to use the track presented here to exfoliate the graphene flakes efficiently with further optimization of the methods.
Finally a way should found to produce large amount of high quality graphene through both methods in the liquid phase exfoliation so that we can use it in our daily life application.
Page | 29
Bibliography
(1) K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Electric field effect in atomically thin carbon films. Science, 2004, 306, 666
(2) A. K. Geim and K. S. Novoselov, The rise of graphene, Nat. Mater., 2007, 6, 183–191
(3) Minzhen Cai a, Daniel Thorpe a, Douglas H. Adamson b and Hannes C. Schniepp *a , Methods of graphite exfoliation, J. Mater. Chem., 2012, 22, 24992-25002
(4) K. S. Novoselov*, D. Jiang*, F. Schedin*, T. J. Booth*, V. V. Khotkevich*, S. V. Morozov†, and
A. K. Geim*‡ , Two-dimensional atomic crystals, Proc. Natl. Acad. Sci. U. S. A., 2005, 102,
10451
(5) K. S. Novoselov1, A. K. Geim1, S. V. Morozov2, D. Jiang1, M. I. Katsnelson3, I. V. Grigorieva1, S.
V. Dubonos2 & A. A. Firsov2, Two-dimensional gas of massless Dirac fermions in
graphene, Nature, 2005, 438, 197
(6) M. J. Allen, V. C. Tung and R. B. Kaner, Honeycomb carbon: a review of graphene , Chem.
Rev., 2010, 110, 132–145
(7) A. A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, F. Miao and C. N. Lau, Superior thermal conductivity of single-layer graphene, Nano Lett., 2008, 8, 902–907
(8) M. S. Dresselhaus and G. Dresselhaus, Intercalation compounds of graphite, Adv. Phys.,2002, 51, 1–186
(9) C. N. R. Rao, A. K. Sood, K. S. Subrahmanyam and A. Govindaraj, Angew, Graphene: The New Two-Dimensional Nanomaterial, Chem., Int. Ed., 2009, 48, 7752–7777
(10) D. R. Dreyer, R. S. Ruoff and C. W. Bielawski, Angew, From Conception to Realization: An Historial Account of Graphene and Some Perspectives for Its Future, Chem., Int. Ed., 2010, 49, 9336–9344
(11) A. K. Geim, Science, 2009, 324, 1530–1534, Graphene: Status and Prospects, Science, 2009, 324, 1530–1534
(12) E. P. Randviir, D. A. C. Brownson and C. E. Banks, A decade of graphene research: production, applications and outlook, Mater. Today, 2014, 17, 426–432
Page | 30
(13) P. Avouris, Z. H. Chen and V. Perebeinos, Carbon-based electronics, Nat. Nanotechnol., 2007, 2, 605–615
(14) J. S. Wu, W. Pisula and K. Müllen, Graphenes as Potential Material for Electronics, Chem. Rev., 2007, 107, 718–747
(15) K. Müllen and J. P. Rabe, Nanographenes as Active Components of Single-Molecule Electronics and How a Scanning Tunneling Microscope Puts Them To Work, Acc. Chem. Res., 2008, 41, 511–520
(16) G. Eda and M. Chhowalla, Graphene-based Composite Thin Films for Electronics, Nano Lett., 2009, 9, 814–818
(17) Yan Q1, Huang B, Yu J, Zheng F, Zang J, Wu J, Gu BL, Liu F, Duan W., Intrinsic
current-voltage characteristics of graphene nanoribbon transistors and effect of edge doping, Nano Lett. 2007 Jun;7(6):1469-73.
(18) W. R. Yang, K. R. Ratinac, S. P. Ringer, P. Thordarson, J. J. Gooding and F. Braet, Angew., Carbon Nanomaterials in Biosensors: Should You Use Nanotubes or Graphene?, Chem., Int. Ed., 2010, 49, 2114–2138
(19) M. D. Stoller, S. J. Park, Y. W. Zhu, J. H. An and R. S. Ruoff, Graphene-Based Ultracapacitors,
Nano Lett., 2008, 8, 3498–3502
(20) X. Wang, L. J. Zhi and K. Müllen, Transparent, Conductive Graphene Electrodes for Dye-Sensitized Solar Cells , Nano Lett., 2008, 8, 323–327
(21) F. N. Xia, T. Mueller, Y. M. Lin, A. Valdes-Garcia and P. Avouris, Ultrafast graphene photodetector ,Nat. Nanotechnol., 2009, 4, 839–843
(22) T. Mueller, F. N. A. Xia and P. Avouris, Graphene photodetectors for high-speed optical communications, Nat. Photonics, 2010, 4, 297–301
(23) T. J. Echtermeyer, L. Britnell, P. K. Jasnos, A. Lombardo, R. V. Gorbachev, A. N. Grigorenko, A. K. Geim, A. C. Ferrari and K. S. Novoselov, Strong plasmonic enhancement of photovoltage in graphene, Nat. Commun., 2011, 2, 458–462
Page | 31
(24) J. B. Wu, M. Agrawal, H. A. Becerril, Z. N. Bao, Z. F. Liu, Y. S. Chen and P. Peumans, Organic Light-Emitting Diodes on Solution-Processed Graphene Transparent Electrodes, ACS Nano, 2010, 4, 43–48
(25) S. Bae, H. Kim, Y. Lee, X. F. Xu, J. S. Park, Y. Zheng, J. Balakrishnan, T. Lei, H. R. Kim, Y. I. Song, Y. J. Kim, K. S. Kim, B. Ozyilmaz, J. H. Ahn, B. H. Hong and S. Iijima, Roll-to-roll production of 30-inch graphene films for transparent electrodes, Nat. Nanotechnol., 2010, 5, 574–578
(26) T. Hasan, F. Torrisi, Z. Sun, D. Popa, V. Nicolosi, G. Privitera, F. Bonaccorso and A. C. Ferrari, Solution-phase exfoliation of graphite for ultrafast photonics,Phys. Status Solidi B, 2010, 247, 2953–2957
(27) E. W. Hill, A. K. Geim, K. Novoselov, F. Schedin and P. Blake, Graphene Spin Valve Devices,IEEE Trans. Magn., 2006, 42, 2694–2696
(28) N. Tombros, C. Jozsa, M. Popinciuc, H. T. Jonkman and B. J. van Wees, Electronic spin transport and spin precession in single graphene layers at room temperature, Nature, 2007, 448, 571–574
(29) Yu Bin Tan and Jong-Min Lee * , Graphene for supercapacitor applications, J. Mater. Chem. A, 2013, 1, 14814-14843
(30) Artur Ciesielskia and Paolo Samorì*, Graphene via sonication assisted liquid-phase exfoliation, Chem. Soc. Rev, 2014,43, 381-398
(31) P. L. de Andres, R. Ramírez, and J. A. Vergés, Strong covalent bonding between two graphene layers, Phys. Rev. B 77, 045403
(32) Diana Berman1, Ali Erdemir2, Anirudha V. Sumant1,Graphene: a new emerging lubricant,
Materials Today, voulume 17,Numb 1, January/Febraury 2014
(33) Changgu Lee1,*, Qunyang Li2,*, William Kalb1, Xin-Zhou Liu3, Helmuth Berger4, Robert W. Carpick2,†, James Hone1, Frictional Characteristics of Atomically Thin Sheets, Science ,2010, 328(5974)76-80
(34) H Pourzand, P Pai, M Tabib-Azar, Thickness dependent adhesion force and its correlation to surface roughness in multilayered graphene, SENSORS, 2013 IEEE, page no.1 – 4
Page | 32
(35) U. Khan, A. O'Neill, H. Porwal, P. May, K. Nawaz and J. N. Coleman, Size selection of dispersed, exfoliated graphene flakes by controlled centrifugation, Carbon, 2012, 50, 470–475
(36) H. K. Chae, D. Y. Siberio-Perez, J. Kim, Y. Go, M. Eddaoudi, A. J. Matzger, M. O'Keeffe and O. M. Yaghi, A route to high surface area, porosity and inclusion of large molecules in crystals, Nature, 2004, 427, 523–527
(37) Xiao Huang a, Xiaoying Qi a, Freddy Boey ab and Hua Zhang *ab, Graphene-based composites,
Chem. Soc. Rev., 2012, 41, 666-686
(38) R. R. Nair, P. Blake, A. N. Grigorenko, K. S. Novoselov, T. J. Booth, T. Stauber, N. M. R. Peres and A. K. Geim, Fine Structure Constant Defines Visual Transparency of Graphene, Science, 2008, 320, 1308–1308
(39) R. Faccio, P. A. Denis, H. Pardo, C. Goyenola and A. W. Mombru, Mechanical properties of suspended graphene sheets, J. Phys.: Condens. Matter, 2009, 21, 285304–285310
(40) A. A. Balandin, Thermal properties of graphene and nanostructured carbon materials, Nat. Mater., 2011, 10, 569–581
(41) F. Scarpa, S. Adhikari and A. S. Phani, Effective elastic mechanical properties of single layer graphene sheets, Nanotechnology, 2009, 20, 065709
(42) Sungjin Park1 & Rodney S. Ruoff1, Chemical methods for the production of graphenes, Nature Nanotechnology, 4, 217 - 224 (2009)
(43) C. Lee, X. Wei, J. W. Kysar and J. Hone, Measurement of the elastic properties and intrinsic strength of monolayer graphene, Science, 2008, 321, 385
(44) S. V. Morozov, K. S. Novoselov, M. I. Katsnelson, F. Schedin, D. C. Elias, J. A. Jaszczak and A. K. Geim, Giant Intrinsic Carrier Mobilities in Graphene and Its Bilayer ,Phys. Rev. Lett., 2008, 100, 016602–016605
(45) K. I. Bolotin, K. J. Sikes, Z. Jiang, M. Klima, G. Fudenberg, J. Hone, P. Kim and H. L. Stormer,
Ultrahigh electron mobility in suspended graphene,Solid State Commun, 2008, 146, 351–355
(46) X. Du, I. Skachko, A. Barker and E. Y. Andrei, Approaching ballistic transport in suspended graphene, , Nat. Nanotechno, 2008, 3, 491–495
Page | 33
(47) S. Unarunotai, Y. Murata, C. E. Chialvo, N. Mason, I. Petrov, R. G. Nuzzo, J. S. Moore and J. A. Rogers, , Conjugated Carbon Monolayer Membranes: Methods for Synthesis and Integration, Adv.
Mater., 2010, 22, 1072–1077
(48) Christopher E. Hamilton, Jay R. Lomeda, Zhengzong Sun, James M. Tour* and Andrew R. Barron*,High-Yield Organic Dispersions of Unfunctionalized Graphene, Nano Lett., 2009, 9 (10), pp 3460–3462
(49) I. W. Frank, D. M. Tanenbaum, A. M. Van der Zande and P. L. McEuen, Mechanical properties of suspended graphene sheets, J. Vac. Sci. Technol., B, 2007, 25, 2558–2561
(50) Mahato, Neelima; Parveen, Nazish; Cho, Moo Hwan, Graphene nanodiscs from electrochemical
assisted micromechanical exfoliation of graphite: Morphology and supramolecular
behavior, Materials Express, Volume 5, Number 6
(51) Wonbong Choi, Jo-won Lee, Graphene: Synthesis and Applications, Nanomaterials and their Applications, CRC Press, 2011 , 394 Pages - 190 B/W Illustrations
(52) Qingbin Zheng, , Jang-Kyo Kim, Graphene for Transparent Conductors, 2015, pp 29-94
(53) F. Bonaccorso, A. Lombardo, T. Hasan, Z. P. Sun, L. Colombo and A. C. Ferrari, Production and processing of graphene and 2d crystals, Mater. Today, 2012, 15, 564–589
(54) J. N. Coleman, Liquid-Phase Exfoliation of Nanotubes and Graphene, Adv. Funct. Mater., 2009, 19, 3680–3695
(55) J. N. Coleman, Liquid Exfoliation of Defect-Free Graphene, Acc. Chem. Res., 2013, 46, 14–22
(56) B. C. Brodie, Ann., Production methods of graphene and resulting material properties, Chim.
Phys., 1860, 59, 466–472
(57) W. S. Hummers and R. E. Offeman, Preparation of Graphitic Oxide,J. Am. Chem. Soc., 1958, 80, 1339
(58) X. S. Li, W. W. Cai, J. H. An, S. Kim, J. Nah, D. X. Yang, R. Piner, A. Velamakanni, I. Jung, E. Tutuc, S. K. Banerjee, L. Colombo and R. S. Ruoff, Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils, Science, 2009, 324, 1312–1314
Page | 34
(59) C. Berger, Z. M. Song, X. B. Li, X. S. Wu, N. Brown, C. Naud, D. Mayou, T. B. Li, J. Hass, A. N. Marchenkov, E. H. Conrad, P. N. First and W. A. de Heer, Electronic Confinement and Coherence in Patterned Epitaxial Graphene, Science, 2006, 312, 1191–1196
(60) L. Chen, Y. Hernandez, X. L. Feng and K. Müllen, Angew., From Nanographene and Graphene
Nanoribbons to Graphene Sheets: Chemical Synthesis, Chem., Int. Ed., 2012, 51, 7640–7654
(61) Kian Ping Loh *, Qiaoliang Bao , Priscilla Kailian Ang and Jiaxiang Yang , The chemistry of graphene, J. Mater. Chem., 2010, 20, 2277-2289
(62) Hugo Miguel Santos, Carlos Lodeiro and Prof. José-Luis Capelo-Martínez, The Power of Ultrasound, Published Online: 9 NOV 2009, DOI: 10.1002/9783527623501.ch1
(63) Wencheng Du,a Xiaoqing Jiang*a and Lihua Zhub , From graphite to graphene: direct
liquid-phase exfoliation of graphite to produce single- and few-layered pristine graphene, J. Mater. Chem.
A, 2013,1, 10592-10606
(64) J. N. Israelachvili, Intermolecular and surface forces, Academic press, revised 3rd edn, 2011 (65) B. C. Brodie, Philos. Trans , On the Atomic Weight of Graphite, R. Soc. London, 1859, 149,
249–259
(66) L. Staudenmaier, Ber. Dtsch, The structure of graphite oxide: Investigation of its surface
chemical groups, Chem. Ges, 1898, 31, 1481–1487,
(67) W. S. Hummers Jr and R. E. Offeman, Preparation of graphitic oxide, J. Am. Chem. Soc, 1958, 80, 1339
(68) S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide, Carbon, 2007, 45, 1558–1565
(69) J. I. Paredes, S. Villar-Rodil, A. Martinez-Alonso and J. M. D. Tascon, Graphene oxide dispersions in organic solvents , Langmuir, 2008, 24, 10560–10564
(70) Hannes C. Schniepp,† Je-Luen Li,‡ Michael J. McAllister,† Hiroaki Sai,† Margarita Herrera-Alonso,† Douglas H. Adamson,§ Robert K. Prud’homme,† Roberto Car,‡ Dudley A. Saville,† and Ilhan A. Aksay*,†, Functionalized Single Graphene Sheets Derived from Splitting Graphite Oxide,
Page | 35
(71) Y. Hernandez, V. Nicolosi, M. Lotya, F. M. Blighe, Z. Y. Sun, S. De, I. T. McGovern, B. Holland, M. Byrne, Y. K. Gun'ko, J. J. Boland, P. Niraj, G. Duesberg, S. Krishnamurthy, R. Goodhue, J. Hutchison, V. Scardaci, A. C. Ferrari and J. N. Coleman, High-yield production of graphene by liquid-phase exfoliation of graphite , Nat. Nanotechnology., 2008, 3, 563–568
(72) Athanasios B. Bourlinos,Vasilios Georgakilas,Radek Zboril,Theodore A. Steriotis,Athanasios K. Stubos, Liquid-Phase Exfoliation of Graphite Towards Solubilized Graphenes†, SMALL, Volume 5,2009, Pages 1841–1845
(73) Ming Zhou,1 Tian Tian,1 Xuanfu Li,1,2 Xudong Sun,1 Juan Zhang,1,2 Ping Cui,1 Jie Tang,3 and
Lu-Chang Qin1,4 ,Production of Graphene by Liquid-Phase Exfoliation of Intercalated Graphite, Int. J.
Electrochem. Sci., 9 (2014) 810 – 820
(74) X. Y. Zhang, A. C. Coleman, N. Katsonis, W. R. Browne, B. J. van Wees and B. L. Feringa, Dispersion of graphene in ethanol using a simple solvent exchange method, Chem.
Commun., 2010, 46, 7539–7541
(75) D. Nuvoli, L. Valentini, V. Alzari, S. Scognamillo, S. B. Bon, M. Piccinini, J. Illescas and A. Mariani, High concentration few-layer graphene sheets obtained by liquid phase exfoliation of graphite in ionic liquid ,J. Mater. Chem., 2011, 21, 3428–3431
(76) A. O'Neill, U. Khan, P. N. Nirmalraj, J. Boland and J. N. Coleman, Graphene Dispersion and Exfoliation in Low Boiling Point Solvents, J. Phys. Chem. C, 2011, 115, 5422–5428
(77) U. Khan, H. Porwal, A. O'Neill, K. Nawaz, P. May and J. N. Coleman, Solvent-Exfoliated Graphene at Extremely High Concentration, Langmuir, 2011, 27, 9077–9082
(78) H. M. Solomon, B. A. Burgess, G. L. Kennedy and R. E. Staples, 1-methyl-2-pyrrolidone (nmp): reproductive and developmental toxicity study by inhalation in the rat, Drug Chem. Toxicol., 1995, 18, 271–293
(79) G. L. Kennedy and H. Sherman, Acute and Subchronic Toxicity of Dimethylformam Ide and
Dimethylacetamide Following Various Routes of Administration, Drug Chem. Toxicol.,1986, 9,
147–170
(80) Mustafa Lotya†, Yenny Hernandez†, Paul J. King†, Ronan J. Smith†, Valeria Nicolosi‡, Lisa S. Karlsson‡,Fiona M. Blighe†, Sukanta De†§, Zhiming Wang†, I. T. McGovern†, Georg S. Duesberg§∥ and Jonathan N. Coleman*†§, Liquid Phase Production of Graphene by Exfoliation of
Page | 36
(81) Zhen Yuan Xia1, Sergio Pezzini2,Emanuele Treossi1,3, Giuliano Giambastiani4, Franco
Corticelli5, Vittorio Morandi5,Alberto Zanelli1, Vittorio Bellani2and Vincenzo Palermo1,* The
Exfoliation of Graphene in Liquids by Electrochemical, Chemical, and Sonication-Assisted Techniques: A Nanoscale Study, Advanced Functional Materials, Volume 23, Issue 37, pages 4684–4693
(82) U. Khan, A. O'Neill, M. Lotya, S. De and J. N. Coleman, High-Concentration Solvent Exfoliation of Graphene, Small, 2010, 6, 864–871
(83) K. S. Novoselov, V. I. Fal'ko, L. Colombo, P. R. Gellert, M. G. Schwab and K. Kim, A road map for graphene , Nature, 2012, 490, 192–200
(84) K. S. Kim, Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, J. H. Ahn, P. Kim, J. Y. Choi and B. H. Hong, Large-scale pattern growth of graphene films for stretchable transparent electrodes, Nature, 2009, 457, 706–710
(85) Keith R. Paton, Eswaraiah Varrla, Claudia Backes, Ronan J. Smith, Umar Khan, Arlene O’Neill, Conor Boland, Mustafa Lotya, Oana M. Istrate, Paul King, Tom Higgins, Sebastian Barwich, Peter May, Pawel Puczkarski, Iftikhar Ahmed, Matthias Moebius, Henrik Pettersson, Edmund Long, João Coelho, Sean E. O’Brien, Eva K. McGuire, Beatriz Mendoza Sanchez, Georg S. Duesberg, Niall McEvoy, Timothy J. Pennycook et al., Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids, Nature Materials, 2014, 13,624–630