T
HE
E
FFECT OF
L
ANDSCAPE
S
TRUCTURE ON
D
ISTRIBUTION AND
A
BUNDANCE OF
L
OBARIA PULMONARIA
=
Frida Skagerberg
=
bñ~ãÉåë~êÄÉíÉ=á=ÄáçäçÖá=PM=Ü∏ÖëâçäÉéç®åÖI=OMNN=
=
e~åÇäÉÇ~êÉW=_Éêíáä=pí™Üä=çÅÜ=mÉê=gçÜ~åëëçå=JJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJJ=
fåëíáíìíáçåÉå=Ñ∏ê=âìäíìêI=ÉåÉêÖá=çÅÜ=ãáäà∏=Avdelningen för biologi, Högskolan på Gotland, SE-621 67 Visby ïïïKÜÖçKëÉ=
Bilden på framsidan föreställer författaren i fält tillsammans med Lobaria pulmonaria. Fotograf: Micael Söderman.
Denna uppsats är författarens egendom och får inte användas för publicering utan författarens eller dennes rättsinnehavares tillstånd.
CONTENTS
ABSTRACT... 4
INTRODUCTION... 5
MATERIALS AND METHODS ... 8
STUDY ORGANISMS... 8 STUDY AREA... 11 HABITAT MAPPING... 12 FIELDWORK... 12 DATA ANALYSIS... 13 RESULTS ... 14 LICHEN DISTRIBUTION... 14 LICHEN SIZE... 15 POSITION ON TREE... 16
LICHEN FRAGMENTATION AND DAMAGE... 16
LICHEN ASSOCIATION WITH EPIPHYTIC BRYOPHYTES... 17
DISCUSSION ... 18
LICHEN OCCURRENCE AND ABUNDANCE... 18
POSITION ON TREE AND BRYOPHYTE COVER... 19
THALLUS FRAGMENTATION AND THALLUS DAMAGE... 20
THE IMPORTANCE OF OTHER SPECIES... 20
THE IMPORTANCE OF LANDSCAPE HISTORY... 21
IMPLICATIONS FOR MANAGEMENT... 21
FUTURE STUDIES... 23
ACKNOWLEDGEMENTS... 23
REFERENCES... 24
ABSTRACT
The objective of the current study was to analyse the effect of landscape structure (habitat size and exposure to farmland) on the occurrence and abundance of Lobaria pulmonaria, a foliose cyanolichen. Since the agrarian revolution during the 19th century the agricultural landscape
has become increasingly fragmented resulting in isolated meadows and wood-pastures surrounded by farmland. Lobaria pulmonaria is one of the species being affected by this habitat change, much due to their dispersal limitations, specific habitat demands and
susceptibility to air pollution. 36 localities of two different size classes (< 1.5 ha and > 4.5 ha) and two different exposure classes (exposed or unexposed to farmland) were studied. The occurrence, size of lichen thallus and height of lichen patches on tree trunks were significantly positively affected by habitat size and negatively affected by habitat exposure. The
implications of these findings for strategies to manage and conserve L. pulmonaria in a fragmented landscape are discussed.
INTRODUCTION
For centuries, the landscape of Gotland has been shaped by anthropogenic influence and the biological diversity thriving in its footstep is especially visible in the pastures and meadows. Here, hay-making, coppicing of trees and grazing of animals have formed a unique habitat dependent on man. But during the past century these meadows and pastures have suffered greatly from habitat loss and the landscape has been severely fragmented. Over the last 2000 years, the changes in the structure and biodiversity of forests in southern Sweden have mainly been influenced by anthropogenic activity. The most dramatic changes have occurred during the last 500 years (Björse & Bradshaw 1998). The forests in southern Sweden have been transformed from being mainly deciduous to being mainly coniferous (Björse & Bradshaw 1998). During the last century, the landscape changes on the island of Gotland have included an increase of agricultural fields at the expense of meadows, woodland pastures and wetlands. The meadows have been estimated to have covered about 30 000 ha at the beginning of the 18th century (Linné 1997). Today they cover approximately 300 ha. This decrease is much due
to the increase of agricultural fields. The agricultural fields covered 15 000 ha in the 18th century but increased rapidly during the 19th century because of the demand for expansion of agricultural fields. In 1850 it had increased to 25 000 ha and in 1900 to 65 000 ha. Much of this increase was due to the conversion of meadows and clearing of deciduous forest but also due to the major drainage of wetlands. Altogether, 20 000 ha wetlands have disappeared to give way to agricultural fields. Therefore, the increased area of agricultural fields has not only resulted in lost forest habitat, but also in changed humidity and level of subsoil water in the remaining pastures and meadows, as well as an increase in their exposure to agricultural fields. During the 20th century, agricultural fields increased to 85 000 ha in 1950 and to 90 000 ha in 1990 (Linné 1997).
The habitat loss for L. pulmonaria on the island of Gotland is also due to the decreased area of grazed woodland and due to the neglect of remaining meadows. The remaining fragments are today mainly managed by local heritage centres and sports clubs. Hay meadows are traditionally mowed in the summer and then grazed in the autumn, on Gotland often by sheep or heifers. There is a general concern for the biodiversity in the remaining fragments of deciduous forest and pasture-woodland on Gotland (Mebus 2006). How this landscape
structure influences the species living in these fragments is what this thesis will try to answer. To investigate this, the distribution of the lichen Lobaria pulmonaria is investigated.
Because of its susceptibility to environmental change, L. pulmonaria is often used as an indicator in pollution monitoring (Ellis & Coppins 2006, Richardson 1992) and in evaluations of biodiversity-rich forest habitats (Artdatabanken 2005). However, lichens are often
neglected in ecological and biogeographical studies (Ellis & Coppins 2006). This is also true for the meadows and pastures of Gotland, where focus has traditionally been on vascular plants. Its susceptibility to both air pollution and changed microclimate makes L. pulmonaria ideal for this study since the exposure to farmland supposedly makes the microclimate more windy and therefore drier. Adjacent farmlands also increase the amount of air particles. How landscape structure influences the occurrence of L. pulmonaria is important to understand for both theoretical and practical conservation biology. The fragmentation of the landscape, and the edge effects in the fragments, affects the conditions of species living there and, thus, influences the conditions for nature conservation. For example, it could influence the prioritizing of localities for nature reserves and restoration efforts. The landscapes of the world are more and more fragmented and affected by humans and we need to understand its effect on species and ecosystems if we are to save the biodiversity and the ecosystems’ goods and services.
The habitat loss through agricultural conversion on Gotland has created a fragmented landscape with relatively small habitat patches, small populations and a decreased dispersal possibility. In this thesis I hypothesize that there will be more large localities harbouring L.
pulmonaria than small localities, since the extinction probability is lower in larger localities
than in smaller ones. Furthermore, large localities are also hypothesized to harbour a greater abundance of L. pulmonaria than small localities. The likelihood of a species going extinct decreases with increased patch area (Ovaskainen & Hanski 2002). The effect of patch size on biodiversity and ecological functions has been emphasized greatly in landscape ecology ever since the theory of island biogeography was formulated by MacArthur & Wilson in 1967 (MacArthur & Wilson 2001). In small localities, biodiversity is often lost due to isolation, changed microclimate and edge effects. Several researchers have shown a positive
relationship between habitat size and local population persistence and between habitat size and local population size (see Fahrig & Merriam 1994). However, a small patch may be a large patch for some species and vice versa and special scale should be selected with this in mind. It is, as Lindenmayer et al. (2008) points out, species-specific but it is also dependent on which process in the autecology of the species that you would like to investigate. Since L.
pulmonaria is dispersal-limited (Snäll et al. 2005; Gu et al. 2001; Walser et al. 2001;
Scheidegger 1995; Öckinger et al. 2005), I have chosen to focus on the limitations set by the short dispersal distances and the spatial scale that imply for this species.
Furthermore, I hypothesize that there will be more unexposed than exposed localities harbouring L. pulmonaria, and that unexposed localities will have a greater abundance of L.
pulmonaria than exposed ones. An unexposed locality will be more suitable than an exposed
locality because dispersal of L. pulmonaria will be more likely from other forests surrounding the unexposed localities, than will be possible through the agricultural fields surrounding the exposed localities. Furthermore, an exposed locality will be more affected by changed microclimate and air pollution than an unexposed locality.
The distribution of a species and the ecological processes in a landscape is governed by landscape composition, structure and function. Xu et al. (2006) showed that the dispersion of patches and the distribution of patches in a landscape are important for species, especially for the ones with poor dispersal ability. These two factors may be the most important in
determining species persistence. The landscape structure has an effect on species dispersal (Wagner et al. 2006) and if the species cannot disperse to new habitats then it is most likely to go extinct. This is of course of great importance for epiphytes which are subjected to
deterministic disturbances such as tree mortality.
Landscape connectivity was defined by Taylor et al. (1993) as the degree to which the landscape facilitates or impedes movement among resource patches. There are two types of connectivity; structural and functional. Structural connectivity concerns the spatial
configuration of the physical features of the landscape and the potential they give to species movement in the landscape. This may include patch shape and size. Functional connectivity concerns how species respond to these landscape features and the connectivity that it creates (Wagner et al. 2006). Patches may be connected by a corridor (structural connectivity) but species may still be isolated. Animals may be hindered because of behavioural preferences for not walking in a small corridor and plants and cryptogams may be hindered by inappropriate microclimate in the corridor or an absent vector. Thus, whether species in fragmented patches become isolated or not depends on the surrounding landscape and on the distance to nearby patches, but also on the autecology of the species. Isolated populations in small patches are generally subjected to an increased mortality rate. This is because reproduction success and therefore survival and adaptation (evolution) is dependent on genetic diversity and because small populations often suffer from a changed habitat quality and lost gene flow resulting in
inbreeding and genetic drift (Frankham et al. 2002). The isolation may create an extinction dept; dooming the populations to extinction.
In addition to being more isolated, exposed localities are more likely to have a changed microclimate than unexposed localities. This is probably also true for small localities
compared to large because the probable edge effect will affect a relatively greater area of the locality. The smaller the fragment the greater the proportional area affected by edge effects. Edge effects may include changed microclimate; increased wind speed, decreased air humidity and increased air and water temperature (Pharo & Zartman 2007; Renhorn et al. 1997). Because of the probable occurrence of edge effects I hypothesize that thalli of L.
pulmonaria in my study will be larger and grow higher up on the stems in larger and
unexposed localities because of a more humid microclimate there. Furthermore, bryophytes are expected to be more abundant in localities with a high abundance of the lichen.
Traditionally the only nutrient added by humans to the traditional meadows and pastures is the dung from the cattle. But the exposure to farmland increases the load of nitrogen compounds affecting the localities. The dust spread by the wind from the agricultural fields contains inorganic fertilizers, pesticides, airborne ammonia from farm animals and pollution from farm vehicles (van Haluwyn & van Herk 2002). Since the 1960s there has been a general increase in atmospheric nitrogen deposition throughout Sweden (Berlin et al. 2000). Today, the total amount of nitrogen deposition in Gotland is 9-15 kg per ha/year (SNA 2000). van Herk et al. (2003) point out three different reasons for the observed lichen species
sensitivity to nitrogen compounds: because they react to changes in bark Ph, because they react to increased nitrogen, and because they react to changes in the growth and occurrence of other epiphytes such as other lichens, mosses or algae. The increased growth of algae on the surface of the lichen could inhibit lichen development (van Herk et al. 2003). Increased nitrogen deposition also leads to vegetation changes because of changes in soil chemistry, increased density of the canopy and changes in the mycorrhizal community in the soil (Diekmann & Falkengren-Grerup 2002). Therefore, lichen thalli are expected to be more damaged and fragmented in exposed areas than in unexposed areas.
The questions asked in this study are:
(1) Are there more large and unexposed localities with the occurrence of L. pulmonaria than small and exposed localities?
(2) Is the total area covered by L. pulmonaria per tree larger in large and unexposed localities than in small and exposed ones?
(3) Is L. pulmonaria growing higher up on the tree stems in large and unexposed than in small and exposed localities?
(4) Is L. pulmonaria more fragmented and damaged in small and exposed than in large and unexposed localities?
(5) Is the abundance of L. pulmonaria associated with epiphytic bryophytes?
(6) Is there a larger cover of bryophytes on trees in large and unexposed patches than in small and exposed localities?
MATERIALS AND METHODS Study organisms
Lobaria pulmonaria (L.) Hoffm.
Lobaria pulmonaria is a foliose epiphytic cyanolichen growing on deciduous trees. It is
classified as near threatened in the Red list of Sweden (Artdatabanken 2005; Gärdenfors 2005). It is considered endangered throughout Europe (Zoller et al. 1999). Lobaria
pulmonaria is dependent on habitats with a combination of high and constant air humidity and
moderate light intensity (Kranner et al. 2003; Rai et al. 2002). It is distributed over the temperate and boreal zone of the northern hemisphere and parts of the southern hemisphere (Wagner et al. 2006), for example South Africa (Walser et al. 2004). In Europe, the local frequencies of L. pulmonaria are at their highest along the rainy Atlantic coasts of the British islands, France and Norway (Artdatabanken 2005; Ellis & Coppins 2006). It is found
throughout Sweden, in the south mainly on broad-leaved trees in old-growth forests or pastures, in the north mainly on Salix caprea in old-growth forests. Lobaria pulmonaria is one of the most important indicators of forest biodiversity and forest continuity in Sweden (Artdatabanken 2005) indicating old broad-leaved trees, high humidity, high air quality and medium light conditions. The lichen has several different associated species (Table 1) and in Gotland L. pulmonaria is seen as a good indicator of high lichen diversity (Johansson 2000). Thus, by protecting L. pulmonaria several other species in the same habitat are also protected (Artdatabanken 2005), including many insects, other lichens, bryophytes, and snails. The fact that signal species indicate the presence of certain red list species has seldom been tested (Nordén et al. 2007), although some results have been published. For example, the study of Nilsson et al. (1995) showed that L. pulmonaria can be used as an indicator of forests with many red-listed lichens and Campbell and Fredeen (2004) found a significant correlation between L. pulmonaria abundance and macrolichen diversity. Nilsson et al. (1995) also found a higher number of red-listed wood beetles dependent on hollow trees in stands with L.
pulmonaria, than on trees without the lichen. The distribution of L. pulmonaria in Sweden has
decreased drastically since the beginning of the 20th century (Artdatabanken 2005-07-14). The recent decline in the distribution of the lichen in Sweden is due to the disappearance of old, broad-leaved trees and open forest habitats, as well as increased air pollution. (Artdatabanken 2005-07-14; Zoller et al. 1999).
Physiology and habitat requirements
Lobaria pulmonaria is a foliose epiphytic cyanolichen with an ascomycete mycobiont, a
green algal photobiont (Dictyochloropsis reticulata, syn Myrmecia reticulata) and a nitrogen-fixing cyanobacterial photobiont (Nostoc sp.) in cephalodia (Schofield et al. 2003). Nitrogen fixation is generally higher in cyanobacteria in cephalodia than in free-living cyanobacteria of the same species (Hallingbäck 1991). As other cryptogams, L. pulmonaria is poikilohydric and lacks vascular tissue and a protective cortex (Hallingbäck 2007; Renhorn et al. 1997), and as other cyanolichens it needs a constant microclimate with a combination of high air
humidity and moderate light intensity (Kranner et al. 2003; Rai et al. 2002). Cyanobacteria in cephalodia have been found to be more abundant in humid forests than in dry ones (Ellis & Coppins 2006). Thus, L. pulmonaria is highly vulnerable to both desiccation and air pollution and therefore also to forest fragmentation. Desiccation results in oxidative stress because it restricts photosynthesis resulting in light energy being transformed to ground state oxygen. Kranner et al. (2003) experimented with dry L. pulmonaria and found a significant delay in the start of photosynthesis after rehydration implying an inability to rapidly re-establish photosynthesis after desiccation. They also found that the Myrmecia photobiont gradually lost vitality during the desiccation phase. During the experiment L. pulmonaria lost half of its
chlorophyll, either because of active breakdown to avoid damage or because of the damage itself.
Table 1. The associated species of Lobaria pulmonaria.
Latin name English name Swedish name
Collema jelly lichens gelélavar
Degelia plumbea degelia lichen blylav
Gyalecta ulmi elm gyalecta almlav
Leptogium skin lichens skinnlavar
Nephroma kidney lichens njurlavar
Parmeliella triptophylla lead lichen korallblylav
Anomodon anomodon moss baronmossor
Antitrichia curtipendula antitrichia moss fällmossa
Homalothecium sericeum silky wall feather-moss guldlockmossa
Neckera neckera moss fjädermossor
Porella platyphylla wall scalewort trädporella
Ulota crispa crisped pincushion krusig ulota
Cyanolichens have a competitive advantage in humid areas and a disadvantage in dry ones. In wet habitats the frequent wetting and drying of lichen thalli may limit growth because of the lack of nutrients (nitrogen and phosphorous) slowing down growth in green algal lichens lacking N2-fixating cyanobacteria. This gives cyanolichens like L. pulmonaria a competitive
advantage in these habitats. In dry habitats green algal lichens have a competitive advantage because N2-fixation is weakened when water is lacking. This is because cyanobacterial
photobionts need higher thallus water content than green-algal photobionts. In addition, cyanobacterial photobionts are dependent on liquid water for its photosynthesis whereas its green-algal counterpart can survive on water vapour (Ellis & Coppins 2006). In L.
pulmonaria, both types of photobionts can use water vapour for respiration, a fact that,
according to Ellis & Coppins (2006), may be one factor making it difficult for cyanolichens to live in dry habitats since it creates a negative balance in the cyanobacterial cells.
Radiation has been shown to seriously damage L. pulmonaria and this sensitivity is stronger in dry thalli than in wet thalli (Gauslaa & Solhaug 1999; Gauslaa et al. 2006b). At the same time, lichens need a moderate light intensity and most species are disfavoured by dense forests with closed canopies (Renhorn et al. 1997). Pettersson (1990) found a higher abundance of L. pulmonaria in the mowed or grazed areas of the meadows he investigated, indicating it’s need for light.
The importance of large trees for the occurrence of L. pulmonaria has been found by several researchers (Edman et al. 2008; Gu et al. 2001; Öckinger et al. 2005; Snäll et al. 2005). But, as Edman et al. (2008) argue, it is not certain if it is the size of the tree or its age that matters, since these factors are correlated in most tree species. Snäll et al. (2005) mention five
different causes for the association with old trees: time available for colonisation, area of suitable substrate, bark roughness and nutrient and moisture conditions. Old trees have a rougher bark structure and different bark chemistry than younger trees (Hallingbäck 1986). A porous bark has a better water-holding capacity (Gauslaa 1995) and therefore provides a wetter microclimate.
As other lichens, L. pulmonaria lacks vascular tissue and a protective cortex and this makes them vulnerable to air pollution. The photobiont is more susceptible to environmental change than the mycobiont. Lichens especially susceptible to pollution contain Nostoc colonies. The abundance of these cyanobacteria is much lower in soil treated with pesticides and fertilizers than in untreated soils (Hallingbäck 1991). Air pollution may have severe effects on the survival of L. pulmonaria and is one of the reasons why I have hypothesized that the abundance of the lichen will be affected by the exposure to agricultural fields.
The habitat quality may be sufficient for growth in a lichen, but not for reproduction. In addition, habitat requirement might not be the same throughout the life of the lichen
(Öckinger et al. 2005). Therefore, when one talk about habitat requirements of a species one should remember that these do not always include the whole life-cycle (Söderström & During 2005). This is perhaps an important notion for L. pulmonaria in Gotland, where very few individuals are found with apothecia.
Reproduction and dispersal
Lobaria pulmonaria has both sexual and asexual reproduction, but apothecia are found in far
from all populations. It reproduces mainly clonally by soredia, isidia and to a lesser extent by thallus fragments (Wagner et al. 2006). Vegetative diaspores contain the mycobiont and the green algal photobiont and the young thalli need to find the cyanobacterial photobiont. These can be found in other lichens associated with L. pulmonaria (Rikkinen 2002; Zoller et al. 1999). Lobaria pulmonaria thalli start to reproduce (vegetatively or sexually) at the age of approximately 25 years (Werth et al. 2007; Walser 2001; Zoller et al. 1999). Apothecia produce meiospores (ascospores) and persist and individual apothecia continue to produce and discharge ascospores for up to a year (Denison 2003). When the mycobiont is spread to a new environment with potential host trees it has to find new photobionts, preferably both of them. So far, no observations have been made of a formed relationship between young L.
pulmonaria thalli formed by ascospores and free living algae of any sort (Denison 2003).
Denison (2003) argues that the ascospores may instead contribute to genetic diversity by forming heterokaryons in meiotic recombination with the mycobiont in thalli of L.
pulmonaria already containing the two photobionts. As mentioned above, sexual reproduction
in L. pulmonaria does not occur in all populations. The absence of apothecia is widely discussed by a lot of researchers. Edman et al. (2008) found significantly less fertile L.
pulmonaria in selectively cut than in uncut deciduous forest in Canada and suggest that sexual
reproduction becomes too costly when microclimate changes. Furthermore, a trade-off between growth and asexual reproduction by isidia and/or soredia has been shown to exist in
L. pulmonaria (Gauslaa 2006b). Other reasons for the presence or absence of apothecia in
populations of L. pulmonaria may be attributed to exogenous factors like oceanity (Walser et al. 2004) and air pollution, especially SO2 (Öckinger et al. 2005). The suggestion made in
later years is that populations need to be genetically diverse to be able to form apothecia. Walser et al. (2004) found that populations of L. pulmonaria with a high genetic variation in the nuclear ribosomal DNA region (nrDNA) were more likely to reproduce sexually than populations with less variation. This is in agreement with the findings of Zoller et al. (1999) that populations of L. pulmonaria with apothecia have a higher genetic variation than those without apothecia. This may indicate that L. pulmonaria is heterothallic, which means that the formation of apothecia can only be possible if a spermatium of one genotype reaches a
trichogyne of another genotype (Zoller et al. 1999). Thus, sexual reproduction in L.
pulmonaria is probably only possible in genetically variable populations and therefore less
probable in populations in fragmented landscapes affected by loss of genetic diversity. Not much is known about how vegetative propagules and sexual ascospores of L. pulmonaria are spread in the landscape. They may be dispersed by abiotic vectors like wind and water and by animals (Werth et al. 2006). Meier et al. (2002) found living ascomycete and green algal cells in faecal pellets of lichenivorous mites. Hence, as suggested in Gauslaa (2006a) small animals may function as short-distance dispersal vectors of L. pulmonaria
Similar findings were made by Walser et al. (2001) who collected snails (Mollusca) foraging on the lichen and ants (Formicidae) and arachnids (Opiliones and Araneae) on or under the lichen and found DNA of L. pulmonaria in or on most of them. Other possible vectors are
birds (Wagner et al. 2006). It is generally agreed that long-distance dispersal is probably exclusively made by wind and that it probably only involves ascospores. Thallus fragments and propagules are only distributed over shorter distances (Werth et al. 2006). But as Walser (2004) mentions, extreme weather conditions as strong winds may distribute even the heavier propagules (isidia and/or soredia). At shorter distances, propagules are dispersed over longer distances than the much heavier thallus fragments (Gauslaa 1997, Walser et al. 2001).
Two different types of species distributions occur in a fragmented landscape: those limited by habitat and those limited by dispersal, or a combination of the two (Hallingbäck 2007;
Söderström & During 2005). Öckinger et al. (2005) found that the local distribution of L.
pulmonaria in a Picea abies forest in the Swedish province of Småland was limited by
dispersal capacity rather than habitat quality, but that habitat quality mattered on a smaller spatial scale. Several other researchers have come to the conclusion that L. pulmonaria is dispersal limited (Snäll et al. 2005; Gu et al. 2001; Walser et al. 2001; Scheidegger, C. 1995). Consequently, L. pulmonaria is often not randomly distributed (Söderström & During 2005) and populations are expected to be genetically well differentiated (Werth et al. 2007). Researchers have come up with different dispersal distances; Walser et al. 2001 found diaspores trapped in snow cover 50 m away from the source, Walser (2004) found identical genotypes at a maximum distance of 230 m and Werth (unpublished data, see Kalwij et al. 2005) found thalli up to 2 km away. Öckinger et al. (2005) found the maximum distance between an occupied tree and a colonized tree to be 35 m over a study-period of 9 years. Trees
The host trees of L. pulmonaria included in the study are ash Fraxinus excelsior, pedunculate oak Quercus robur, small-leaved elm Ulmus minor and small-leaved lime Tilia cordata. A mixed forest type including old ash-trees and young pendunculate oak-trees dominate the deciduous forests of Gotland, covering 93 % of the total area, but only 3 % of the trees are oaks (Johansson 2000). In the beginning of the 19th century almost no oak trees existed on the island. Thus, there are very few ancient oaks on the island. The oak population is a very young one favoured by the disappearance of wood pastures (Johansson 2000). The highest biodiversity value among the trees of Gotland is found on the old pollarded ashes and limes. Sixty-nine red-listed lichens have been found in the deciduous forests of Gotland. Especially the pollarded ashes are of great importance for lichens (Johansson 2000). Ash has
traditionally been pollarded and their leaves used for fodder and has therefore been favoured and left in the pastures and meadows. As Johansson (2000) points out, the late leafing of ash makes the tree less vulnerable to grazing early in spring than are other broad-leaved trees and this could have strengthened their survival.
Bryophytes
Epiphytic bryophytes can be of importance for the establishment of macrolichens. They create a special microhabitat by storing water and humus creating a rough surface texture compared to smooth bark (Sillett et al. 2000). Therefore moss mats could enhance the substrate quality of trees. Several lichens associated with old-growth forest have been found strongly
associated with bryophytes (Sillett et al. 2000). Öckinger et al. (2005) found a larger cover of bryophytes on trees occupied by L. pulmonaria than on trees of similar size without this lichen and both Ellis & Coppins (2006) and Öckinger et al. (2005) found bryophytes and cyanolichens to be positively correlated.
Study area
This study was conducted on the island of Gotland in the Baltic Sea. Gotland is situated in the boreonemoral vegetation zone (Gustafsson & Ahlén 1996) and 41 % of the island is covered by forest: 87 % of this forest is made up of coniferous trees and 13 % of broad-leaved trees
(Linné 1997). The mean air temperature in July is 16° C and -1° C to -2,5° C in January; the lowest figure being the temperature in the inland and the highest the temperature on the coast (SMHI 2003). Precipitation ranges between 600 and 700 mm/yr (Alexandersson & Andersson 1995). Most of Gotland consists of calcareous rock and the terrain is flat with the highest point 82 m above sea level. Study localities were chosen randomly over the island, excluding the most southern and most northern parts because of different soil properties compared to the central parts of the island (Johansson pers comm. 2006).
Habitat mapping
Study localities were selected to be as homogeneous as possible using criteria shown in Table 2. The structure and the compositional values of the landscape surrounding the meadows were divided into different classes. The selection for the right size, forest type and canopy closure was made in Excel and the selection for the right exposure was made using ArcView.
Exposure was defined as the percentage of the habitat edge that is bordering agricultural fields or open pastures. localities with more than 75 % of the edge bordering open land were defined as exposed, whereas localities with less than 25 % of the edge bordering open land were defined as unexposed. The rest of the edge is bordering forest or woodland pastures of any kind.
To avoid population overlap all localities were checked in ArcView. The size of these landscapes was determined on the basis of the longest dispersal capacity of L. pulmonaria found so far, 2 km (S. Werth, unpublished data, see Kalwij et al. 2005).
Table 2. Criteria for selected localities
Criteria for selected localities
• mowed or grazed
• presence of broad-leaved trees • canopy closure 20-70 %
• no recent (past 10 years) restoration through clearing • either smaller than 1,5 ha or larger than 4,5 ha
• either more than 75 % or less than 25 % of the edge bordering agricultural fields or open pastures • non-overlapping with a 2 km buffer zone
Fieldwork
In total, 76 localities were chosen for further investigation in the field. Of these, 40 were excluded during the fieldwork because of factors not seen in the GIS-files or in the files for canopy closure, such as overgrowth, recent clearing, or wrong type of exposure or size. At each locality, thalli of L. pulmonaria were searched for 0.5-3 h. Sampling of trees at localities where the lichen was found was set according to locality size and amount of broad-leaved trees large enough to, at least hypothetically, have L. pulmonaria. Twenty trees per locality were set as a minimum requirement. For each tree, diameter at breast height (hereafter called dbh) was recorded. All trees included in the study had a dbh greater than 30 cm. Both trees with and without L. pulmonaria were mapped using a portable GPS-devise. Unfortunately, the exact location could not be plotted on a map for exact positioning and future research since the portable GPS-devise worked poorly during cloudy days and in more dense forest stands. On trees having L. pulmonaria, lichen size (in dm²) and position on tree (in cm) were measured. Lichen size was defined as the total area of L. pulmonaria, irrespective of the number of thalli, on one single tree (hereafter called lichen size). Position on tree was defined as the medium height at which the lichen was found on one single tree (hereafter called position on tree). When necessary, binoculars were used. Presence of damaged and
fragmented thalli as well as occurrence of apothecia was noted. In addition, bryophyte cover on the trunk below 2 m was estimated.
Data analysis
Data from different tree species were aggregated for all procedures since separate datasets were too small for statistical analyses. Also, data on lichen damage and fragmentation as well as on lichen association with epiphytic bryophytes were not analysed statistically.
Distribution
The effect of habitat size and habitat exposure (both class variables) on the presence of L.
pulmonaria was analysed using a generalised logits model (CATMOD PROC; SAS Inst. Inc.
2002).
Lichen size
The effect of habitat size, habitat exposure and tree dbh on lichen size was analysed using an ANOVA model (GLM procedure within SAS Inst. Inc. 2002). Habitat size and habitat exposure were defined as class variables and tree dbh was defined as a continuous variable. Because data did not meet the assumption of normality data were log-transformed prior to testing.
Position on tree
The effects of habitat size, habitat exposure to farmland, and tree dbh on the lichen’s position on tree were analysed in the same manor as in previous analyses, using an ANOVA model. Habitat size and habitat exposure were defined as a class variable and tree dbh was defined as a continuous variable.
RESULTS
Localities had different compositions of tree species. Some are dominated by pendunculate oaks whereas others are dominated by ash and small-leaved elm. Small-leaved lime was found in only a few localities. Oaks had the largest dbh, both with regards to mean (Figure 1A) and maximum sizes (Figure 1B). The average tree dbh differed in the different types of habitat size classes and habitat exposure classes (Figure 1C). The largest trees were found at large and exposed localities.
A B 0 20 40 60 80 100 120 140 Fraxinus excelsior Fraxinus excelsior p Quercus robur Ulmus minor Ulmus minor p Tilia cordata p m ax im um d bh ( cm ) 0 10 20 30 40 50 60 Fraxinus excelsior Fraxinus excelsior p Quercus robur Ulmus minor Ulmus minor p Tilia cordata p m ea n db h (c m ) C
Figure 1. Descriptive tree statistics for the trees measured in the study. (A) Maximum dbh (cm) of the different tree species found in all localities, (B) mean dbh (cm) (+ 1 SE) of the different tree species found in all localities, (C) mean dbh (cm) (+ 1 SE) of all trees in the different types of habitat. Tree species: Fraxinus excelsior (n=188);
Fraxinus excelsior p (n=121); Quercus robur (n=376); Ulmus minor
(n=154); Ulmus minor p (n=2); Tilia cordata (n=11), (p = pollarded).
Lichen distribution
Lobaria pulmonaria was found in 18 of the 36 study localities (Figure 2). It was found in 6
out of 10 of the small and unexposed localities. In small and exposed localities L. pulmonaria was found in only 3 out of 15 localities. In large and exposed localities L. pulmonaria was found in 6 out of 8 localities and in large and unexposed localities L. pulmonaria was found in all localities visited. The size and exposure of the localities found with and without L.
pulmonaria can be seen in Table 3. When the presence and absence were analysed, the
generalised logits model showed that the presence of lichen was significantly positively affected by habitat size (p< 0.01) and significantly negatively affected by habitat exposure to farmland ().
Table 3. Study localities with and without Lobaria pulmonaria.
Small (<1.5 ha) Large (>4.5 ha)
with without with without
Unexposed (<25 %) 6 4 3 0 Exposed (>75 %) 3 12 6 2 0 10 20 30 40 50 60 < 1,5 ha >75 % < 1,5 ha <25 % > 4,5 ha >75 % > 4,5 ha <25 % m ea n db h (c m )
# # # # # # # # # # # # # # # # # # # # ## # # # # # # # # # # # # #
Figure 2. Study localities. Occurrence of Lobaria pulmonaria indicated by black dots, absence by grey dots.
Lichen size
An analysis of variance showed that, even when considering tree dbh, lichen size per tree was significantly affected positively by habitat size (p < 0.05) and significantly negatively by habitat exposure (Table 4; Figure 3A). No interaction effect was found between the two class variables. Tree dbh was included in the analysis since the largest trees were found in large (> 4.5 ha) and exposed (>75 %) habitats (Figure 1C). If tree dbh would have had an effect on lichen size the analysis of habitat size and exposure would have been misleading.
Table 4. Ananlysis of variance for lichen size.
Source df MS F P
Habitat size 1 2,35 4,74 0,031
Habitat exposure 1 2,75 5,56 0,020
Tree dbh 1 1,91 3,85 0,051
Habitat size X Habitat exposure 1 0,15 0,30 0,586
Position on tree
An analysis of variance showed that, even when considering tree dbh, the position on tree was significantly positively affected by habitat size and significantly negatively affected by habitat exposure (Table 5). No interaction effect was found between the two class variables. Just like in the lichen size analysis, tree dbh was included in the analysis since the largest trees were found in large (> 4.5 ha) and exposed (>75 %) habitats (Figure 1C). If tree dbh would have had an effect on lichen size the analysis of habitat size and exposure would have been misleading.
Table 5. Ananlysis of variance for lichen position on tree.
Source df MS F P
Habitat size 1 4,33 4,88 0,021
Habitat exposure 1 20,65 24,30 <0,0001
Tree dbh 1 1,71 2,16 0,143
Habitat size X Habitat exposure 1 0,61 0,77 0,381
error 170 0,79 A B 0 0,1 0,2 0,3 0,4 0,5 0,6 0,7 0,8 0,9 1 < 1,5 ha >75 % < 1,5 ha <25 % > 4,5 ha >75 % > 4,5 ha < 25 % lo g lic he n si ze 0 0,2 0,4 0,6 0,8 1 1,2 1,4 1,6 1,8 2 < 1,5 ha >75 % < 1,5 ha <25 % > 4,5 ha >75 % > 4,5 ha < 25 % m ea n st em h ei gh t ( m )
Figure 3. Lichen size and position on tree. (A) Mean size (+ 1 SE) of Lobaria pulmonaria in the different habitats with SE. (B) Mean stem height (m) (+ 1 SE) at which Lobaria pulmonaria was found in the different habitats.
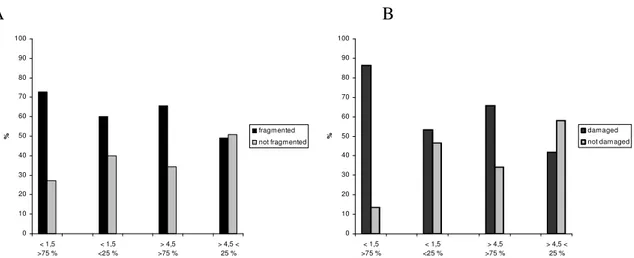
Lichen fragmentation and damage
The occurrence of fragmented thalli of L. pulmonaria in this study had a tendency to be more frequent in small and exposed localities than in large and unexposed localities, irrespectively (Figure 4A). Furthermore, there seems to be a tendency of a more frequent occurrence of damaged thalli of L. pulmonaria in small and exposed localities than in large and unexposed localities (Figure 4B).
A B 0 10 20 30 40 50 60 70 80 90 100 < 1,5 >75 % < 1,5 <25 % > 4,5 >75 % > 4,5 < 25 % % fragmented not fragmented 0 10 20 30 40 50 60 70 80 90 100 < 1,5 >75 % < 1,5 <25 % > 4,5 >75 % > 4,5 < 25 % % damaged
not dam aged
Figure 4. (A) Percentage of thalli of Lobaria pulmonaria fragmented and not fragmented in the different
localities. (B) Percentage of thalli of Lobaria pulmonaria damaged and not damaged in the different localities
Lichen association with epiphytic bryophytes
Average bryophyte cover on tree stems below 2 m had a tendency to differ between the different types of habitats (Figure 5A). There seems to be a larger cover of bryophytes in particularly unexposed localities. In addition, there is a tendency of bryophyte cover to be larger in large than in small localities although I found a more abundant bryophyte cover in small but unexposed than in large and exposed areas. Furthermore, there is a tendency of bryophytes being associated with trees with L. pulmonaria (Figure 5B).
0 2 4 6 8 10 12 14 16 18 20 < 1,5 ha >75 % < 1,5 ha <25 % > 4,5 ha >75 % > 4,5 ha < 25 % % 0 2 4 6 8 10 12 14 16 18 20
trees without Lobaria pulmonaria trees with Lobaria pulmonaria
%
Figure 5. Bryophyte cover. (A) Mean bryophyte cover (%) (+ 1 SE) in the different localities. (B) Mean
DISCUSSION
My results show that occurrence and abundance of L. pulmonaria probably is affected by landscape fragmentation. The occurrence of L. pulmonaria and the absence of damaged and fragmented thalli are more probable in large and/or unexposed localities than in small and/or exposed. Furthermore, in large and/or unexposed sites the lichen is found higher up on the trees and cover larger areas on the tree stems.
Lichen occurrence and abundance
The fact that both habitat exposure and habitat size were found to have an effect on the occurrence and abundance of L. pulmonaria is all in agreement with my hypotheses. One interesting finding is that habitat size had a larger effect on the occurrence of L. pulmonaria than habitat exposure. This could imply that it is easier for the lichen to survive in large habitats irrespective of the exposure to farmland. The fact that habitat exposure is not as important as habitat size could mean that the occurrence of the lichen is not governed by the dispersal possibilities, since unexposed areas are supposed to be more easily re-colonized in case of extinction. Therefore, one could argue that the populations found in the localities today are not the result of dispersal from other localities after the fragmentation of the landscape. Because of their longevity, the time interval that had passed since the fragmentation of the landscape begun may only have involved a few generations of L.
pulmonaria (Scheidegger & Goward 2002). One could also argue that this is a result of the
exposed localities not being isolated.
What constitutes connectivity for L. pulmonaria is not fully understood. Through which matrix can its propagules survive and disperse? How long can it disperse? Is it possible for the diaspores to survive in dry matrix like agricultural fields? Can they survive the influence of air pollution from pesticides and fertilizers in these areas? As Walser (2004) mentions, extreme weather conditions, such as strong winds, may distribute not only the lighter ascospores but also the heavier diaspores. Perhaps this type of long-distance dispersal is enough for the survival of L. pulmonaria in a landscape for longer periods of time since this species probably can become quite old. Somehow L. pulmonaria managed to colonize the island of Gotland. However, considering the knowledge about the species so far, it seems to be dispersal limited (Gu et al. 2001; Öckinger et al. 2005; Snäll et al. 2005; Walser et al. 2001; Scheidegger 1995) and thus probably isolated in the small fragments in the agricultural landscape.
Dupré and Ehrlén (2002) found that the importance of habitat configuration to species of vascular plants varied with life history. Habitat specialists, clonal perennials and species producing fewer seeds tended to be more negatively affected by patch isolation than other species. Lobaria pulmonaria could be compared to all of these three classes. If localities are indeed isolated, populations found here could be suffering from an extinction dept because of a genetic bottleneck caused by this isolation. This is especially true for lichens associated with old-growth forests, such as L. pulmonaria (Scheidegger & Goward 2002).
As discussed above, a habitat may be sufficient for survival but not for reproduction.
Individuals of L. pulmonaria found here today may perhaps survive for a few more years, but cannot spread to nearby trees as they are sterile. As Berglund and Jonsson (2005) point out, the extinction dept may complicate examination of the distribution of a species in recently isolated remnants. In my study, apothecia were never found in any of the localities. However, I only visited a portion of the trees in every locality and apothecia have been reported from localities where my investigation found none. Still, apothecia are seldom seen on L.
dispersal by the heavier diaspores could be important, but they are probably only spread at short distances. The occurrence of isidia and/or soredia was not noted in this study and should be in future studies. As mentioned before, little is known about the possible dispersal
distances for this lichen. Another reason for a possible extinction dept is the absence of suitable trees. Even if the lichen dispersal is functioning, there are rarely any new suitable trees to disperse to, especially not in the near future. If the populations of L. pulmonaria are suffering from an extinction dept, this may also affect associate species. If that is the case, action must be taken now to restore these localities before it is too late.
The occurrence of L. pulmonaria seems to be more affected by habitat size than habitat exposure, which could signify that large localities may be large enough for the edge effect not to be affecting the area as a whole. In this way, exposed and large areas may still have an intact area inside with a microclimate unaffected by the exposure to farmland. Edge effects could be both positive and negative, depending on the studied species. For example, L.
pulmonaria could be positively affected by an edge effect in a closed forest creating enhanced
light conditions. Furthermore, dispersal could be enhanced at the edges bordering agricultural fields because of stronger winds here. However, negative edge effects are expected to
outnumber the positive effects. For example, the negative effects of changed humidity almost certainly outweigh the gain in light intensity at exposed edges. However, this should be investigated further.
The degree of microclimate change due to edge effects depends on weather conditions, time of day, edge orientation, edge form and the surrounding landscape (Renhorn et al. 1997). The hay-meadows and wood-pastures on Gotland could be subjected to temporal edge effects; for example edge effects only obvious during the dry summer (as opposed to wet winters) or during dry days (as opposed to more humid nights). The edge effects on Gotland will
probably also be affected by closeness to other landscape features such as the sea, lakes, rivers and wetlands. Therefore, the drainage of wetlands resulting in died rivers in the summer is probably having a severe effect on the lichen. As mentioned above, the edge effects are of course also gradual; from the centre of the locality and to the edge. Edman et al. (2008) used a 120 m buffer zone from the edge when choosing their localities to avoid edge effects in their study on L. pulmonaria in Canada. Whether edge effects exist and to what extent they affect
L. pulmonaria in the fragments is something I had planned to study in this thesis. But a
malfunctioning GPS-device made this impossible.
Nilsson et al. (1995) point out that L. pulmonaria may be absent in old-growth forest affected by pollution. My findings, that L. pulmonaria occurrence and size is negatively affected by habitat exposure to farmland and that thallus fragmentation and damage are positively affected by the same variable, is in accordance with a study made by Mitchell et al. (2005). They analysed the occurrence of L. pulmonaria against N levels in different oak woods in Scotland and found the lichen to be most abundant in the locality with the lowest level of nitrogen.
Position on tree and bryophyte cover
Thalli of L. pulmonaria were growing significantly higher up on the tree stems in large and unexposed localities. In addition, habitat exposure had a greater effect on position on tree than habitat size. This could perhaps imply edge effects in the exposed localities. Since L.
pulmonaria is dependent on a stable microclimate (Kranner et al. 2003; Rai et al. 2002) it is
susceptive to changed humidity conditions. My findings are in agreement with the findings of Campbell and Fredeen (2004). They found that the height, to which the cyanolichens they investigated grew, increased significantly with stand age. They conclude that the height in the stand may represent the upper limit of suitable humid microclimate. In young stands they
found the distribution of the lichen to be restricted to coarse woody debris and low branches because of the forest-floor water creating a humid microclimate there, a microclimate that is otherwise only present in old-growth forest (Campbell & Fredeen 2004). In addition to explaining the position on tree, this upper limit may well explain the increase of the abundance of L. pulmonaria in protected and/or large habitats found in my investigation, since its distribution is three dimensional; not only limited by a vertical area but also by a horizontal one.
On dry substrates, cyanobacteria are most likely to be restricted to areas with high humidity (Hallingbäck 1991). Ellis & Coppins (2006) found an effect of both tree age and humidity on the distribution of epiphytes (lichens and bryophytes) in Scotland. They also found old trees in dry habitats to have an epiphytic community similar to that of younger trees in more humid habitats. They concluded that in drier forests cyanolichens may be found in comparably wet microhabitats such as on old tree bark and in wet moss mats, microhabitats that are not needed in wet forests. This is in accordance with my finding that L. pulmonaria seem to be positively associated with epiphytic bryophytes. Bryophytes are probably important in lichen establishment, facilitating attachment (Öckinger et al. 2005). Furthermore, epiphytic
bryophytes could imply and create a humid microclimate. There seem to be a larger cover of bryophytes in especially unexposed localities. In addition, there is a tendency of bryophyte cover to be larger in large areas although I found a more abundant bryophyte cover in small but unexposed than in large and exposed areas. But caution must be taken, since all
bryophytes do not have the same habitat requirement.
Lobaria pulmonaria is more susceptible to a decrease in air humidity if the precipitation is
low (Kalwij et al. 2005). Thus, since Gotland has a low annual amount of precipitation, changes in humidity must be of great importance for the L. pulmonaria living on the island. As mentioned before, the disappearance of many wetlands and streams must have had a great impact on the populations in the meadows and forests, especially in the dry summers. This is something that should be investigated further.
Thallus fragmentation and thallus damage
Large and unexposed localities were the only localities with a higher percentage of non-damaged thalli than non-damaged thalli. This is also true for thallus fragmentation. Both fragmentation and damage could be a cause of changed microclimate (decreased humidity and/or increased radiance) and air pollution, and could be a sign that the lichens are slowly dying. Damaged thalli were bleached, probably as a result of loss of chlorophyll (Gauslaa & Solhaug 2000), though it could be an effect of snail grazing. Damages and fragmentation could therefore mean different things in different habitats. Fragmentation could also be a sign of newly established thalli, although this was not the impression I got in the field. Thalli fragments looked as if they were disintegrating, not as if they were newly established. As thallus fragmentation and damage were more common in small and exposed localities, this is probably true. This should however be investigated further before any conclusions can be drawn.
The importance of other species
In conservation biology it is very important not only to take into consideration the autecology of the focus species, but also the autecology of the species interacting with it, be it through competition, predation or by any other interaction affecting the existence of the species. Some of these will be threats, others will be fundamental to its existence. Species interactions may be strongly affected by the fragmentation of the landscape and therefore play an important role in the survival of the species in focus. For L. pulmonaria, snails are not only important as potential dispersal vectors but are also important in shaping the lower distribution limit of
epiphytic lichen communities on trees (Gauslaa et al. 2006a). They probably also keep away algae from growing on the surface of the thallus and keep away lichen competitors. This may be especially crucial for survival in nitrogen-influenced habitats where cyanolichens have a disadvantage. Therefore it may be important to understand the ecology of the snails and how they react to fragmentation if we are to save L. pulmonaria on the island. If the snails are affected by the drier microclimate, then L. pulmonaria may be affected by their absence - positively by the lack of grazing, destroying the thallus, and negatively by the absence of grazers controlling lichen competitors and by the absence of snail dispersal.
It is also important to understand how different types of grazing animals affect the lichen and the habitat it lives in. For example, different animals have different grazing preferences and affect the trees (by scratching etc) and habitat differently. Different grazing regimes could result in different light and humidity levels affecting L. pulmonaria differently. Furthermore, grazing animals may decrease the amount of host-trees by eating the young shoots. At the same time L. pulmonaria is dependent on the gap-dynamics created by the grazers; by the continuous possibility of new host trees because of enhanced light conditions and by the light conditions themselves creating a suitable microclimate for the lichen.
The importance of landscape history
This study cannot reveal if the results depend on isolation, or habitat degradation through a changed microclimate or nitrogen deposition. Furthermore, one cannot know if the occurrence of L. pulmonaria in this study reflects the present or the historic landscape. But forest
continuity could have a stronger influence on its distribution than present landscape structure. As pointed out earlier, the distribution of patch-tracking species should reflect the past
landscape even for a long time after landscape change (Dupré & Ehrlén 2002, Snäll et al. 2004). Snäll et al. (2004) suggest that the distribution of the epiphytic bryophyte Neckera
pennata is explained by the age of the patches and by the connectivity to past dispersal
sources. This could be true for L. pulmonaria in this study. The localities were chosen so that none of them could be the same population because of long distances between them. This is based on the landscape as we see it today, and not on a historical landscape. Since Lobaria
pulmonaria has a long life-span, the populations we see today could represent fragments of
one single population, once living in a more or less continuous deciduous forest landscape. As Dupré and Ehrlén (2002) points out presently isolated habitats may up until recently have been part of a large forest making the species patterns of the present population represent a subpopulation of a former large metapopulation. On the other hand presently intact forests may have been fragmented in the near past making the present species distribution represent historically isolated small populations. This will of course affect the species response to habitat change and is an important factor to take into consideration when analysing responses to landscape change. Species do not always respond to habitat change in a linear way
(Hallingbäck 2007) and populations should be followed in a long-term study to evaluate the causes of negative trends (Hylander & Jonsson 2007).
Implications for management
If an extinction dept do occur in a fragmented landscape and the remaining habitat is not restored or recreated, this could lead to extinctions starting at the stand level and then
continuing at the landscape level. Therefore, restoration and creation of connectivity might be crucial for the survival of L. pulmonaria and many of its associated species.
Large and well-connected patches with tree continuity are important for the future existence of L. pulmonaria on the island of Gotland. For a habitat-tracking organism like L. pulmonaria it is important to enlarge existing patches and improve the quality of them (Thomas 1994). For species living in dynamic habitats it is especially important that larger networks of
connected habitats are managed as to create a natural dynamic that will provide new habitat, in the case of L. pulmonaria, new host trees. Adding to this, new habitat should be created close enough for the species to colonize.
In the fragmented agricultural landscape, the survival of L. pulmonaria is not only affected by isolation, changed microclimate and air pollution. It is also affected by the dynamics of the tree population of the trees they live on (Werth et al. 2007). Therefore, the classic
metapopulation model does not apply to L. pulmonaria since the model does not take into account within-patch dynamics, only between-patch dynamics in terms of immigration and extinction rates. But as stated by Thomas (1994) in his habitat-tracking model, habitat patches are dynamic and this is evident for epiphytes on trees, since trees themselves are dynamic. Since L. pulmonaria often live on a host tree, its vulnerability is not only dependent on its own demographic and environmental stochasticity, but also on the stochasticity of the host population. Therefore the epiphytes have to follow the patch-tracking dynamics of their host trees (Snäll et al. 2005, Snäll et al. 2003, Keymer et al. 2000). As Söderström and During (2005) point out, metapopulation studies should take into account the life strategies of the species. At a large-scale, landscapes themselves are dynamic and change over time; new patches are created and old ones disappear (Xu et al. 2006). In addition, disturbances in the landscape are not only stochastic but also deterministic. For example, host trees have only a limited life time and therefore the epiphytes that inhabit them are subjected to deterministic disturbance. Therefore, large localities with a larger amount of trees will have a more viable epiphytic population than small localities. This is in accordance with the findings of this study.
Since L. pulmonaria is dependent on old and ancient trees, it is important to always have trees of different ages in the same habitat so that the lichen can disperse from dying trees to new ones in order to sustain a viable population. On Gotland, most trees in the meadows and pastures are evenly-aged and newly pollarded young trees and re-pollarded old ones are scarce. The only tree species reforested here is the oak. Therefore, young trees need to be continuously created in order to retain new microhabitats for the lichen. But since the Dutch elm disease and ash dieback has reached the island of Gotland, it could be difficult to grow elm and ash trees. Possible substitutes are the Norway maple Acer platanoides or the
sycamore maple Acer pseudoplatanus. In Switzerland, sycamore maple Acer pseudoplatanus is the most important host-tree for L. pulmonaria (Kalwij et al. 2005) although they also grow on beech Fagus sylvatica (Werth et al. 2006).
Because of the host tree dynamics, the lichen have a short period of time (seeing it through the timescale of L. pulmonaria) before it has to disperse to another tree. The total amount of time on one tree depends on habitat humidity and bark structure (when the tree has the right microclimate) and on the lifetime of the particular tree (when it dies). This time span becomes much shorter in a fragmented landscape since the small patches of forest probably have a considerably drier microclimate than continuous forests. Therefore, the trees must have a rougher tree bark and therefore become much older before they have the right microclimate to be able to host cyanolichens (Sillett et al. 2000). On Gotland, this time span for lichen
establishment is probably affected by the traditional coppicing of deciduous trees for fodder. Pollarded trees usually get older and are more often bent and has a rougher bark structure than un-pollarded trees. Gauslaa (1995) found L. pulmonaria inhabiting drainage channels below old and large wounds on the trunks of different deciduous trees. These places where richer in minerals and had higher pH. There is also the possibility that L. pulmonaria could be spread by the action of coppicing, like vascular plants spread by the hay-making. The Swedish Environmental Protection Agency (2004) has produced an Action Plan for Trees with High Conservation values in the Cultural and Urban Landscape which includes the old and
pollarded trees in the meadows.
Vascular plants are often the main target when managing or restoring a traditional hay-meadow or woodland pastures and lichens are often neglected. In some of the most famous hay-meadows in Gotland tree stems were covered with small branches probably making it impossibly dark for L. pulmonaria to survive.
My results have shown the negative effect of exposure on lichen occurrence, abundance and height. Therefore exposed habitats should be protected by a buffer zone of intact forest to prevent a possible edge effect. By doing this, even small habitats could be saved, at least temporarily. Since L. pulmonaria needs a constant microclimate with high humidity, other restoration efforts should concentrate on enhancing the humidity levels in the landscape. This could be done by recreation of wetlands, meandering rivers and small ponds traditionally found in the meadows and wood-pastures. A more humid landscape, with water flowing through the streams and rivers even in the summer, could be crucial in the effort to save the remaining populations of L. pulmonaria on Gotland. In their checklist for ecological
management of landscapes for conservation, Lindenmayer et al. (2008) stress the need for integration of terrestrial and aquatic areas in conservation management. The importance of aquatic areas for species living in land habitats is often forgotten in conservation biology.
Future studies
A genetic survey of the populations of L. pulmonaria could perhaps trace population isolation and explain the rarity of apothecia on the island. But, as Walser et al. (2005) suggests, the longevity of L. pulmonaria individuals makes it difficult to trace genetic isolation in the species caused by changes in the landscape during the last hundred years. On the other hand; this makes it possible to check the different populations on the island and see where gene flow has existed during the past centuries. If there is a low genetic diversity in the population on Gotland this may of course be a result of a historic founder effect. Therefore a possible low genetic diversity does not necessarily have to be an effect of landscape change and population isolation.
As Lindenmayer et al. (2008) and other researchers in landscape ecology have pointed out, there is a risk in concentrating on the patch-matrix theory and not pay attention to the differences in the matrix. This is something that should be observed in future studies; do the different types of agricultural fields pose different problems to the remaining fragmented wood-pastures and meadows? The problem should also be looked at a larger scale. Do the areas of deciduous forest in the landscape surrounding the localities under investigation affect the microhabitat in it and therefore the habitat for L. pulmonaria? This is obviously
constrained by the difficulties in finding replicate landscapes with identical disturbance history (Wagner et al. 2006), but should at least be considered.
Last but not least, it is crucial to continuously monitor the populations of L. pulmonaria on Gotland to see how the species respond to further landscape changes, be it positive or negative ones. Only by monitoring the species can we find out more about the health of the ecosystem it lives in and learn more about this lichen and its associated species.
ACKNOWLEDGEMENTS
I am thankful to Bertil Ståhl for all the advise on academic writing and thinking and for making it possible, Per Johansson for giving me the idea for this thesis and for inspirational talks, Oskar Kullingsjö for making it possible technically and financially, Kjell Larsson for providing helpful statistical advice and for help with the statistical analyses, Micael Söderman for always being there and for all the landowners and for all my friends for giving me support and encouragement.
REFERENCES
Alexandersson, H. and Andersson, T. 1995. Nederbörd och åska. In: Raab, B & Vedin, H (eds), Klimat, sjöar och vattendrag. Sveriges Nationalatlas. SNA-förlag, Stockholm. Artdatabanken 2005-07-14. Faktablad: Lobaria pulmonaria – lunglav. Author: Svante Hultengren 2005
Berglund, H. and Jonsson, B.G. 2005. Verifying an extinction dept among lichens and fungi in northern Swedish boreal forests. Conservation Biology 19(2): 338-348.
Berlin, G.A.I., Linusson, A. and Olsson, E.G. 2000. Vegetation changes in semi-natural meadows with unchanged management in southern Sweden, 1965-1990. Acta Oecologica 21(2): 125-138.
Björse, G. and Bradshaw, R. 1998. 2000 years of forest dynamics in southern Sweden: suggestions for forest management. Forest Ecology and Management 104(1-3): 15-26. Campbell, J. and Fredeen, A.L. 2004. Lobaria pulmonaria abundance as an indicator of macrolichen diversity in Interior Cedar-Hemlock forests of east-central British Columbia. Canadian Journal of Botany 82: 970-982
Denison W.C. 2003. Apothecia and ascospores of Lobaria oregana and Lobaria pulmonaria investigated. Mycologia 95(3): 513-518
Diekmann, M. and Falkengren-Grerup, U. 2002 Prediction of species response to atmospheric nitrogen deposition by means of ecological measures and life history traits. Journal of
Ecology 90(1): 108-120.
Dupré, C. and Ehrlén, J. 2002. Habitat configuration, species traits and plant distributions. Journal of Ecology 90(5): 796-805
Edman, M., Eriksson, A-M. and Villard, M-A. 2008. Effects of selection cutting on the abundance and fertility of indicator lichens Lobaria pulmonaria and Lobaria quercizans. Journal of Applied Ecology 45 (1):26-33.
Ellis, C.J. and Coppins, B.J. 2006. Contrasting functional traits maintain lichen epiphyte diversity in response to climate and autogenic succession. Journal of Biogeography. 33(9): 1643-1656.
Fahrig, L. and Merriam, G. 1994. Conservation of fragmented populations. Conservation
Biology 8(1): 50-59.
Frankham, R., Briscoe, D.A., Ballou, J.D. and Mcinness, K.H.. 2002. Introduction to
Conservation Genetics. 1 ed. Cambridge University press. Cambridge, UK
Gärdenfors, U. (Ed.). 2005. Rödlistade arter i Sverige 2005 – The 2006 Red List of Swedish
Species. ArtDatabanken, SLU, Uppsala.
Gauslaa, Y. 1995. The Lobarion, an epiphytic community of ancient forests threatened by acid rain. The Lichenologist 27(1):59-76.
Gauslaa, Y. 1997. Population structure of the epiphytic lichen Usnea longissima in a boreal Picea abies canopy. The Lichenologist 29:455-469
Gauslaa, Y., Holien, H., Ohlson, M., and Solhøy, T. 2006a. Does snail grazing affect growth of the old forest lichen Lobaria pulmonaria? The Lichenologist 38(6): 587-593
Gauslaa Y. 2006. Trade-off between reproduction and growth in the foliose old forest lichen Lobaria pulmonaria. Basic and Applied Ecology 7:455-560
Gauslaa, Y and Solhaug, K.A. 2000. High-light-intensity damage to the foliose lichen Lobaria pulmonaria within a natural forest: the applicability of chlorophyll fluorescence methods. The Lichenologist 32(3): 271-289
Gauslaa, Y and Solhaug, K.A 1999. High-light damage in air-dry thalli of the old forest lichen Lobaria pulmonaria: interactions of irradiance, exposure duration and high temperature. Journal of Experimental Botany 50(334): 697-705.