TRITA-LWR Degree Project ISSN 1651-064X
LWR - EX - 2015:16
O
XIDATION OF PHARMACEUTICALS BY
CHLORINE DIOXIDE IN WASTEWATER
EFFLUENT
Raquel Alcala Borao
Raquel Alcala Borao LWR – EX 2015:16
ii © Raquel Alcala Borao 2015
Degree Project for the master program Environmental Engineering and Sustainable Infrastructure
Division of Land and Water Resources Engineering Royal Institute of Technology (KTH)
SE-100 44 STOCKHOLM, Sweden
Reference should be written as: Alcala, R (2015) “Oxidation of pharmaceuticals by chlorine dioxide in wastewater effluent” TRITA-LWR Degree Project 2015:16
Oxidation of pharmaceuticals by chlorine dioxide in wastewater effluent
iii
SUMMARY
The presence of pharmaceuticals in the environment has raised an emerging interest due to the fact that they pose negative environmental impact and health hazards related to long-term toxicity effects. Its removal from wastewater and from important drinking water sources is a big challenge to be faced by wastewater treatment plants (WWTPs) and environmental engineers due to the low concentration that is usually present in the water in addition to its high diversity (chemical structure, mode of action, Kow). Pharmaceuticals end up into the influent
of WWTPs mainly through the sewage system that transports excreted faeces and urine from households but they do not have yet maximum discharge guidelines and standards. Conventional treatments used nowadays in WWTPs are not able to totally remove pharmaceuticals from wastewater and therefore it is necessary to seek for alternative advanced technologies such as oxidation with chlorine dioxide (ClO2). ClO2 needs to be manufactured at the point of use and it is a highly
selective oxidant for several functional groups that are usually present in the chemical structure of pharmaceuticals. The capital cost for a ClO2 generator is
lower than other oxidative technologies such as ozone and therefore it could be a good option for small scale WWTPs (<2000 pe).
Previous studies with ClO2 have mainly tested the disinfection effect of ClO2 in
both drinking water and wastewater, although during the last years, more research has been conducted in ClO2 as oxidant for pharmaceuticals focused on how
(kinetics) and which pharmaceuticals can be removed. However, the objective of this master thesis was to find the most optimal dose – reaction time of ClO2 for
the maximum removal of selected environmentally relevant pharmaceuticals. This was made as a bench-scale study where factorial design and subsequent optimization with MODDE was selected as the best approach to find the optimal dose – time. Batch oxidation tests were conducted on 100 mL aliquots treated with ClO2 using wastewater effluent from Henriksdal WWTP. Solid phase extraction
and quantification of pharmaceuticals was carried out on a high performance liquid chromatography- triple quadrupole mass spectrometry (HPLC-MS/MS).
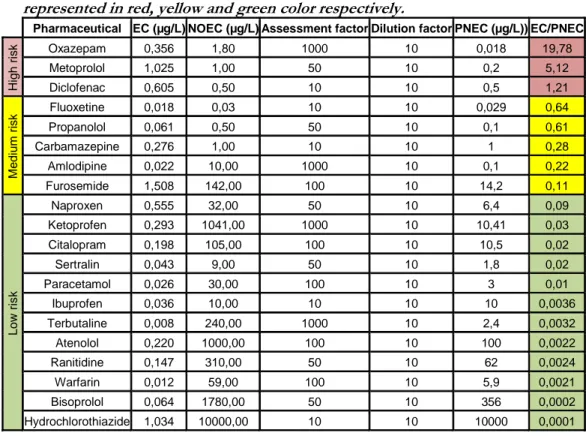
At the beginning of the investigation, a priority list of pharmaceuticals was created based on the risk ratio between the concentration at the effluent and the concentration that has no predicted effect in the environment retrieved from Pharmaceutical Specialties in Sweden (FASS) drug portal data base. 3 of the 23 studied pharmaceuticals (oxazepam, metropolol and diclofenac) were classified as high risk, 5 as moderate risk and the remaining as low risk. Results from the bench scale study states that an optimal dose of 5 g ClO2/m3 and a reaction time of 10
minutes can totally remove more than a half of the studied pharmaceuticals. Furthermore six of the eight pharmaceuticals that posed moderate and high environmental risk before ClO2 treatment, would pose a low environmental risk
after treatment with ClO2. Results indicate also that variations in wastewater
composition (e.g. COD) would affect the oxidant demand and therefore ClO2 –
sensitive absorbance at 254nm and pharmaceuticals dose-response decay curves were measured as possible strategy for ClO2 dosage control in full scale WWTPs.
Before a pilot or full scale implementation of this technology can be performed, supplementary and deeper ecotoxicologial studies needs to be done regarding the formation of byproducts after oxidation with ClO2 that can be more toxic than the
Raquel Alcala Borao LWR – EX – 2015:16
Oxidation of pharmaceuticals by chlorine dioxide in wastewater effluent
v
SAMMANFATTNING
Läkemedel i miljön har rönt ett växande intresse på grund av att de har negativ miljöpåverkan och utgör en hälsorisk till följd av toxiska effekter på lång sikt. Deras avlägsnande från avloppsvatten och från viktiga dricksvattenkällor är en stor utmaning för reningsverk och miljöingenjörer. Anledningen till detta är de låga koncentrationerna som vanligtvis finns i vattnet samt likheten mellan läkemedlen (olika kemisk struktur, verkningsmekanism, KOW). Läkemedelsubstanser hamnar i
inflödet till reningsverk främst genom avloppssystem som transporterar urin och fekalier från hushåll. I dagsläget finns det dock inga riktlinjer och standarder för maximalt utsläpp.
Konventionella behandlingar som används i reningsverk idag kan inte avlägsna läkemedelsubstanser helt från avloppsvatten och därför är det nödvändigt att identifiera avancerade tekniker för uppgradering såsom oxidation med klordioxid (ClO2). ClO2 tillverkas vid användningsstället och det är en mycket selektiv oxidant
för olika funktionella grupper som vanligtvis förekommer i den kemiska strukturen av läkemedelsubstanser (fenoler, tertiära/sekundära aminer, organiska svavelgrupper). Kapitalkostnaden för ClO2 är lägre jämfört med andra oxidativa
tekniker som ozon och skulle därför vara ett bra alternativ för mindre reningsverk (<2000 pe). Tidigare studier med ClO2 har främst testat desinfektion effekten i
både dricksvatten och avloppsvatten, men under de senaste åren har mer forskning fokuserat på ClO2 som oxidationsmedel om hur (kinetik) och vilka
läkemedelsubstanser som kan tas bort. Syftet med detta examensarbete var dock att hitta den mest optimala dos – reaktionstid av ClO2 för maximalt avlägsnande av
utvalda miljörelevanta läkemedel. Detta utfördes med försök i laboratorieskala studie med faktor försök. För den efterföljande optimering utsågs MODDE som det bästa sättet att hitta den optimala dosen – tid. Oxidations tester genomfördes på 100 mL prover med avloppsvatten från Henriksdal reningsverk som behandlades med ClO2. Därefter genomfördes fastfasextraktion och kvantifiering
av läkemedelsubstanser på en högupplösande vätskekromatografi – trippelkvadrupolmasspektrometeri (HPLC-MS/MS).
I början av studien skapades en prioriteringslista över läkemedel baserad på riskkvot mellan koncentration vid utflödet och koncentration som inte har någon förutspådde effekt i miljön (PNEC). Dessa data hämtades från Pharmaceutical Specialities in Sweden (FASS) databas. 3 av de 23 studerade läkemedel (oxazepam, metoprolol och diklofenak) klassificerades som hög risk ämnen, 5 att utgöra måttlig risk och resterande som låg risk ämnen. Resultat från laboratorieförsöken visade på att en optimal dos av 5 g ClO2/m3 och reaktionstid av 10 minuter kan ta
bort mer än hälften av de studerade läkemedel. Sex av de åtta läkemedelsubstanser som hade måttlig och hög miljörisk innan ClO2 behandling skulle medföra en låg
miljörisk efter behandling med optimal ClO2 dos -tid. Resultat visar också att
variationer i avloppsvattensammansättning (t.ex. COD) påverkar efterfrågan av oxidationsmedel och därför mättes ClO2 – känslig absorbans vid 254nm och
läkemedels dos-respons kurvor som möjlig strategi för ClO2 doseringskontroll i
fullskala på reningsverk.
Innan ett fullskaligt genomförande av denna teknik kan utföras behövs dock vidare och fördjupade toxikologiska tester om biprodukter eller mellanliggande substanser som kan vara mer toxiska än moderföreningarna.
Raquel Alcala Borao LWR – EX – 2015:16
Oxidation of pharmaceuticals by chlorine dioxide in wastewater effluent
vii
ACKNOWLEDGEMENTS
First of all I would like to thank both my adviser at KTH, Maritha Hörsing, and my supervisor at IVL Swedish Environmental Institute, Jörgen Magnér, for their supervision, advices and inspiration during the project. I would also like to thank Christian Baresel for his support and people from Hammarby Sjöstadsverk and IVL for helping me in the laboratory and making these months more enjoyable. Finally, I would like to thank the company Xinix AB for providing their product AquaCare to perform all the experiments.
Last but not least I would like to thank my parents, friends and boyfriend for all their support throughout these two years as master student at KTH and during the work of this master thesis.
Stockholm, June 2015 Raquel Alcala Borao
Raquel Alcala Borao LWR – EX – 2015:16
Oxidation of pharmaceuticals by chlorine dioxide in wastewater effluent ix
TABLE OF CONTENTS
Summary ... iii Sammanfattning ... v Acknowledgements ... vii Table of contents ... ix Abbreviations ... xi Abstract ... 1 1. Introduction ... 11.1. Pharmaceuticals in the environment ... 1
1.2.Pharmaceuticals and legislation ... 3
1.3.Removal of pharmaceuticals in wastewater treatment plants ... 4
1.3.1.Conventional treatments ... 4
1.3.2.Advanced treatment alternatives for pharmaceutical removal ... 6
2. Aim of the study ... 8
3. Background ... 8
3.1.Chemical aspects of chlorine dioxide ... 8
3.2.By-products formation with ClO2 as oxidative agent ... 9
3.3.Previous studies with ClO2 ... 10
3.4.Motivation and possibilities for ClO2 treatment in WWTPs ... 11
4.Materials and methods ... 13
4.1.Chemicals ... 13
4.2.Analytical methods ... 13
4.2.1.Chlorine dioxide analysis ... 13
4.2.2.Ultraviolet absorbance measurement ... 13
4.2.3.Microtox test ... 13
4.2.4.Pharmaceuticals analysis ... 13
4.3.Experimental setup ... 16
4.3.1.Wastewater effluent ... 16
4.3.2.Methodology to find optimal ClO2 dose- reaction time ... 16
4.3.3.Relation between pharmaceuticals removal and ultraviolet absorbance (UVA254nm) ... 19
4.4.Ranking of pharmaceuticals according to their environmental impact indicator19 5.Results ... 20
5.1.Priority list of pharmaceuticals ... 20
5.2.Optimal ClO2 dose- reaction time ... 21
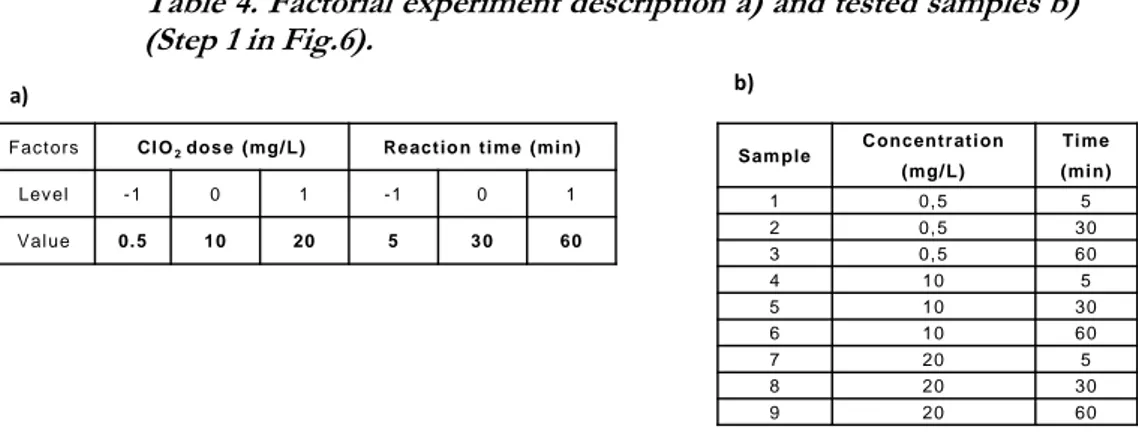
5.2.1.First factorial experiment ... 22
5.2.2.Second factorial experiment ... 24
5.2.3.Optimum for most relevant pharmaceuticals ... 26
5.2.4. Verification of pharmaceutical removal after treatment with common optimal ClO2 dose-time ... .26
Raquel Alcala Borao LWR – EX – 2015:16
x
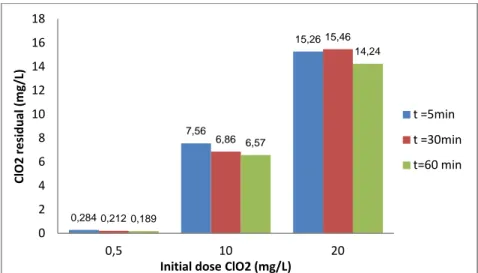
5.3.Residual ClO2 after oxidation treatment ... 28
5.3.1.Influence of reaction time ... 28
5.3.2.Influence of COD ... 28
5.4.Relationship between ClO2 sensitive-UVA 254 nm and pharmaceuticals……29
6.Discussion ... 31
6.1.Methodology and analytical errors ... 31
6.1.1.Factorial experiment and MODDE ... 31
6.1.2.Matrix effects in the quantification of pharmaceuticals ... 31
6.2.Priority list of pharmaceuticals ... 32
6.3.Optimal ClO2 dose – time –response ... 33
6.4.ClO2 dosage control ... 34
6.5.Final evaluation ... 34
6.5.1.Efficiency of ClO2 treatment ... 34
6.5.2.Cost ... 35
6.5.3.Ecotoxicological by-products ... 35
7.Future studies ... 37
8.Conclusion ... 37
9.References ... 38
Appendix I - Characteristics of 23 analyzed pharmaceuticals ... 42
Appendix II - Results from analysis of pharmaceuticals ... 47
Oxidation of pharmaceuticals by chlorine dioxide in wastewater effluent
xi
ABBREVIATIONS
API = Atmospheric pressure ionization CCF = face centered central composite design COD = Chemical Oxygen demand
ClO2 =Chlorine dioxide
DBPs = Disinfection by-products DOC = Dissolved organic matter E2 = 17 beta-estradiol
EC = Environmental concentration
EC50 = Half maximal effective concentration
EDTA = Ethylenediaminetetraacetic acid EE2 = 17 alpha-ethinylestradiol
FASS = Pharmaceutical Specialties in Sweden drug portal GAC = granular activated carbon
HAAs = haloacetic acids
HPLC-MS/MS = High performance liquid chromatography coupled to tandem mass spectrometry
IS = Internal Standard
KOw = octanol/water partition coefficient
LOD = Limit of detection LOQ = Limit of quantification NOEC = No observed effect level
NSAIDs = nonsteroidal anti-inflammatory drugs PAC = powdered activated carbon
PE = population equivalent
PNEC = Predicted no effect concentration RMS = Response surface modeling
RO = Reverse osmosis
SAC = Spectral absorption coefficient SPE = Solid phase extraction
THM = Trihalomethane
UVA254nm = Ultraviolet absorbance at 254nm WWTPs = Wastewater treatment plants
Raquel Alcala Borao LWR – EX – 2015:16
Oxidation of pharmaceuticals by chlorine dioxide in wastewater effluent
1
ABSTRACT
The presence of pharmaceuticals in the environment has raised an emerging interest due to the fact that they pose negative environmental impact and health hazards related to long-term toxicity effects. As conventional treatments are not able to totally remove these substances it is necessary to seek for alternative advanced technologies such as oxidation with chlorine dioxide (ClO2). The
objective of this master thesis is thus to find the most optimal dose – reaction time of ClO2 for the oxidation and maximum removal of selected
environmentally relevant pharmaceuticals. Factorial design and subsequent optimization with MODDE was selected as the best approach to find the optimal dose – time. Batch oxidation tests were conducted on 100mL aliquots treated with ClO2 using wastewater effluent from Henriksdal WWTP.
Thereafter solid phase extraction and final determination of pharmaceuticals was carried out on a high performance liquid chromatography- triple quadrupole mass spectrometry (HPLC-MS/MS). Results showed that applying a dose of 5 mg ClO2/L and a reaction time of 10 minutes, it is possible to
remove more than a half of the 17 analyzed substances. Besides most of the pharmaceuticals with high and moderate environmental risk, would pose a low risk for the environment after treatment with the optimal ClO2 dose – reaction
time. Despite the fact that ClO2 could successfully degrade most
environmentally relevant pharmaceuticals, deeper research concerning the formation of toxic by-products after oxidative treatment needs to be done before upscaling this technology to pilot or full scale as a suitable end of pipe technology for pharmaceuticals removal.
Key words: pharmaceuticals; chlorine dioxide; wastewater effluent; environmental risk; factorial design; MODDE
1.
INTRODUCTION
In recent years, the existence of micropollutants in the aquatic environment has become an emerging issue because of their recognized negative environmental and human health effects. These micropollutans, also called emerging pollutants, integrate a broad range of both anthropogenic and natural substances such as pharmaceuticals, pesticides, biocides, flame retardants, etc. (Luo et al., 2014). The term “emerging” does not mean that the presence of these substances in the environment is new but it refers to the emerging interest by the scientific community in the presence of these contaminants in the environment as well as the analytical procedures required to their detection (Aga, 2008). Another issue that have raised the interest in these substances is that even though they are usually present in trace concentrations (few ng/l to several µg/l) they may cause negative environmental impact and health hazards related to short-term and long-term toxicity, endocrine disrupting effects and rise of antibiotic-resistant genes in bacteria (Klavarioti et al., 2009). This last fact is very relevant since the increase in antibiotic resistance is a serious threat to our availability to face infection diseases.
1.1. Pharmaceuticals in the environment
The removal of pharmaceuticals from wastewater and from important drinking water sources, such as rivers, lakes or groundwater is a big challenge to be faced by wastewater treatment plants (WWTPs) and environmental engineers due to the low concentration that is usually present in the water in addition to its high diversity. There are several
Raquel Alcala Borao
groups of pharmaceuticals that have been found in the water such as antibiotics, antidepressants, analgesics, anti
anticoagulants, stimulants and antihypertensives among others ( Utrilla et al.
low levels in our microbial degradation. concentrations
advanced analyt
concentration (e.g liquid chromatography spectrometry [LC
The most relevant characteristics of and chemically complex structures with high and molecular weight; ii)
certain degree of
partition coefficient (Log K KOW higher than 3
and those with Log K
be attached to sludge and suspended particles in water ; iii) pharmaceuticals are
on the pH of the medium; iv)
differs depending on the pharmaceutical paracetamol
meanwhile others
(Rivera-values higher than 3
According to previous studies about t pharmaceuticals
the influent of excreted
pharmaceuticals come into the aquatic environment through the discharge
to contaminate soil and groundwater without previous treatment when liquid manure is utilized as top soil cover.
WWTPs, which may contain pharmaceuticals, may be used agricultural land leading to a high risk for soil
risk of run et al. 2010).
Fig. 1.
(adapted from Ternes, 1998)
LWR
2
groups of pharmaceuticals that have been found in the water such as antibiotics, antidepressants, analgesics, anti-inflammatories, anticoagulants, stimulants and antihypertensives among others (
et al., 2013). These drugs are designed in order to be effective at low levels in our bodies and to be resistant against e.g. stomach acid and microbial degradation. The fact that these substances are found in trace concentrations requires the development of more sophisticated and advanced analytical tools in order to accurately determine its concentration (e.g liquid chromatography coupled to tandem spectrometry [LC-MS/MS]).
The most relevant characteristics of these pharmaceuticals
and chemically complex structures with high diversity in shape, structure and molecular weight; ii) some of them are lipophilic and
certain degree of hydrophilicity. This depends on their octanol/water partition coefficient (Log KOW) since those pharmaceuticals with Log
higher than 3 (e.g. diclofenac) tend to be attached to fat matrices and those with Log KOW lower than 3 (e.g. metoprolol) have tendency to
be attached to sludge and suspended particles in water (Ejhed et al. 2012) pharmaceuticals are polar molecules whose ionization level depends on the pH of the medium; iv) their persistence in the environment differs depending on the pharmaceutical. Some substances paracetamol) are degraded through the wastewater treatment
meanwhile others (e.g. naproxen) can persist for more than one year -Utrilla et al., 2013). Actually, those pharmaceuticals with Log K higher than 3 have tendency to bioaccumulate (Ejhed et al. 2012 According to previous studies about the fate and transport of pharmaceuticals in the environment (Fig.1), pharmaceuticals
the influent of WWTPs through the sewage system that excreted faeces and urine from households. Therefore,
pharmaceuticals come into the aquatic environment through the discharges from WWTPs. Veterinary drugs pose also a considerably risk to contaminate soil and groundwater without previous treatment when liquid manure is utilized as top soil cover. Furthermore the sludge from WWTPs, which may contain pharmaceuticals, may be used
agricultural land leading to a high risk for soil contamination as well as risk of run-off polluting both surface and groundwater resources (Santos et al. 2010).
Fate and transport of pharmaceuticals in the environment (adapted from Ternes, 1998).
LWR – EX – 2015:16
groups of pharmaceuticals that have been found in the water such as inflammatories, anticoagulants, stimulants and antihypertensives among others
(Rivera-n order to be effective at against e.g. stomach acid and The fact that these substances are found in trace requires the development of more sophisticated and ical tools in order to accurately determine its coupled to tandem mass pharmaceuticals are: i) large diversity in shape, structure are lipophilic and others have . This depends on their octanol/water ) since those pharmaceuticals with Log tend to be attached to fat matrices have tendency to (Ejhed et al. 2012) tion level depends their persistence in the environment Some substances (e.g. are degraded through the wastewater treatment process, sist for more than one year hose pharmaceuticals with Log Kow
(Ejhed et al. 2012). he fate and transport of harmaceuticals end up into through the sewage system that transport Therefore, most of the pharmaceuticals come into the aquatic environment through the a considerably risk to contaminate soil and groundwater without previous treatment when Furthermore the sludge from WWTPs, which may contain pharmaceuticals, may be used as fertilizer in contamination as well as off polluting both surface and groundwater resources (Santos
Oxidation of pharmaceuticals by chlorine dioxide in wastewater effluent
3
Luo et al. (2014) presents in his study a review of average influent and effluent concentrations of pharmaceuticals from different WWTPs around the world where the influent concentration of most of the pharmaceuticals is between 0.1 µg/L and 10 µg/L. The highest concentrations, above 10 µg/L, belong to nonsteroidal anti-inflammatory drugs (NSAIDs) such as diclofenac, ibuprofen and naproxen. For instance, concentration levels up to 603 µg/L of ibuprofen were detected in the influent of four WWTPs in Spain (Santos et al. 2010). These NSAIDs can be classified as one of the most relevant group of pharmaceuticals since daily load of these pharmaceuticals to the WWTPs is in the order of grams (Coelho et al. 2010). This is due to the fact that to their application level worldwide is very high, probably because they can be bought without medical prescription.
In Sweden, a large study performed by Stockholm Water, regarding pharmaceuticals in Stockholm´s water environment (Wahlberg et al., 2010) states that NSAIDs such as paracetamol, ibuprofen, naxopren and ketoprofen have the highest concentration in the incoming water of Henriksdal and Bromma wastewater treatment facilities. The highest concentration in incoming wastewater was reached by paracetamol with a value of 85 µg/L.
1.2. Pharmaceuticals and legislation
Discharge of pharmaceuticals is not yet regulated and thus they do not have maximum discharge guidelines and standards. Even though increasing concentrations of pharmaceuticals are entering the environment every day, there is a gap in legislation regarding the environmental contamination produced by them (Luo et al. 2014; Bel et al., 2011; Oller et al. 2011; Santos et al.,2010). This gap in legislation may be due to the absence of consensus and good understanding of which substances should be regulated and to which level. Currently, the World Health Organization says that it is very unlikely that trace concentrations of pharmaceuticals found in drinking water are a risk for human health. This is because the margin of safety between detected concentrations in drinking water and those that can lead to toxic effects is quite substantial (WHO, 2012). However, this current gap in legislation could change if more data concerning long-term exposure is collected and if more knowledge about how a continuous exposure during several generations may affect a whole population is acquired. Besides, information related to the combined or simultaneous exposure to pharmaceuticals is not known yet (Santos et al., 2010).
Recently, the European Parliament has decided for the first time, to include three pharmaceuticals in a ‘watch list’ of priority substances (Directives 2000/60/EC and 2008/105/EC) in the field of EU Water Framework Directive (European Parliament, 2013). One of these three pharmaceuticals is diclofenac, a commonly-used generic painkiller that belongs to NSAIDs group and that has shown alterations of the kidney and gills of fishes. In some countries such as India, Nepal and Pakistan the manufacture and veterinary use of diclofenac is banned due to imminent extinction of local vultures. The other two substances are sex hormones 17 alpha-ethinylestradiol (EE2) and 17 beta-estradiol (E2), which are suspected to disrupt the endocrine system in humans and harm fish reproduction. By including these substances in the “watch list”, monitoring data will be gathered to establish appropriate measures to determine the risk posed by those substances (European Parliament, 2013). Nowadays, the Swedish government together with Swedish Association of Local Authorities has developed a National
Raquel Alcala Borao
Pharmaceutical Strategy that presents seven different action plans of which one of them focus on reducing the impact o
environment (Lä
As there are no specific guidelines for maximum discharge of pharmaceuticals in the environment, the efficiency of WWTPs is usually given as percentage removal between inlet and outlet. However, taking into account possible
be more interesting to focus on final concentrations at the effluent of WWTPs. One approach would consist on comparing Predicted No Effect Concentration (PNEC) with the environmental concentrations (EC) at the recipie
compare PNEC with EC and if the ratio EC/PNEC is below 1 then long-term negative effects would not be expected.
1.3. Removal of
Current municipal
as P and N as well as reducing
secondary and optional tertiary treatment process meant yet to eliminate micropollutants
the fate of micropollutants in the conventional systems removal efficiency.
1.3.1.
The main goal of primary treatment is to remove suspended solids from the water through aerated grit chamber followed by sedimentation tank. A study performed by Behera et al. (2011) shows that the maximum removal efficiency
estriol) meanwhile other pharmaceuticals such as ibuprofen and naproxen
due to the fact that
dissociated in water and not bound
study performed by Hörsing et al. (2011) indicates that studied pharmaceuticals
water phase.
In secondary treatment, where substances are transformed by biological degradation through activated sludge and secondary sedimentation, NSAIDs
(ibuprofen, ketoprofen 75% (Salgado et al., 2012 The study
for 44 different pharmaceuticals performed at Henriksdal how they we
Fig. 2.
(adapted from Wahlberg et al.
LWR
4
Pharmaceutical Strategy that presents seven different action plans of which one of them focus on reducing the impact of medicines on the environment (Läkemedelsverket, 2014).
As there are no specific guidelines for maximum discharge of pharmaceuticals in the environment, the efficiency of WWTPs is usually given as percentage removal between inlet and outlet. However, taking into account possible-ecotoxicological effects in the ecosystem, it would be more interesting to focus on final concentrations at the effluent of WWTPs. One approach would consist on comparing Predicted No Effect Concentration (PNEC) with the environmental concentrations (EC) at the recipient. A dilution factor needs to be considered
compare PNEC with EC and if the ratio EC/PNEC is below 1 then term negative effects would not be expected.
Removal of pharmaceuticals in wastewater treatment plants
Current municipal WWTPs can control and eliminate par
as P and N as well as reducing the organic load through primary, secondary and optional tertiary treatment process. However they are not meant yet to eliminate micropollutants. Therefore is important to follow
fate of micropollutants in the conventional systems removal efficiency.
Conventional treatments
The main goal of primary treatment is to remove suspended solids from the water through aerated grit chamber followed by sedimentation tank. study performed by Behera et al. (2011) shows that the maximum removal efficiency was generally quite low (~ 28% for diclofenac and estriol) meanwhile other pharmaceuticals such as ibuprofen and naproxen were not removed at all in this primary treatment
due to the fact that most of the substances have more dissociated in water and not bounded to the sludge particles study performed by Hörsing et al. (2011) indicates that studied pharmaceuticals presented a high affinity to be water phase.
In secondary treatment, where substances are transformed by biological degradation through activated sludge and secondary sedimentation, NSAIDs diclofenac presented low removal (<25%) meanwhile other (ibuprofen, ketoprofen, acetaminphen) presented higher removal above 75% (Salgado et al., 2012; Falås et al., 2012).
The study carried out by Wahlberg et al. (2010) presents a mass balance for 44 different pharmaceuticals performed at Henriksdal
how they were reduced during the different treatment stages
2. Mass flow for pharmaceuticals at Henriksdal´s WWT (adapted from Wahlberg et al., 2010).
LWR – EX – 2015:16
Pharmaceutical Strategy that presents seven different action plans of medicines on the As there are no specific guidelines for maximum discharge of pharmaceuticals in the environment, the efficiency of WWTPs is usually given as percentage removal between inlet and outlet. However, taking cal effects in the ecosystem, it would be more interesting to focus on final concentrations at the effluent of WWTPs. One approach would consist on comparing Predicted No Effect Concentration (PNEC) with the environmental concentrations considered in order to compare PNEC with EC and if the ratio EC/PNEC is below 1 then
in wastewater treatment plants
eliminate particles, nutrients through primary, . However they are not . Therefore is important to follow fate of micropollutants in the conventional systems to evaluate the
The main goal of primary treatment is to remove suspended solids from the water through aerated grit chamber followed by sedimentation tank. study performed by Behera et al. (2011) shows that the maximum 28% for diclofenac and estriol) meanwhile other pharmaceuticals such as ibuprofen and in this primary treatment. This can be have more affinity to be particles. Actually a study performed by Hörsing et al. (2011) indicates that 61 of the 75 be dissociated in In secondary treatment, where substances are transformed by biological degradation through activated sludge and secondary sedimentation, the diclofenac presented low removal (<25%) meanwhile others ) presented higher removal above al. (2010) presents a mass balance for 44 different pharmaceuticals performed at Henriksdal WWTP and
treatment stages (Fig 2).
Oxidation of pharmaceuticals by chlorine dioxide in waste
The average removal for the 44 pharmaceuticals was around 50% the highest removal took place in the biological degradation step. al. (2012) studied
activated sludge plants with nitrogen removal such as ibuprofen and paracetamol
3). On the other hand, some pharmaceuticals such as metropolol or hydrochlorothiazide
concentration was
3.). The reason of this effe explanation is that during biological
transformed back to parent compounds. In addition to this, some pharmaceuticals may be enclosed in faeces and thus released during biological
compared to the influent (Luo et al., 2014). Consequently
completely remove complete barrier for Utrilla et al.
be totally removed after second
need additional methods to be removed removal during the process
Some
effectively removed by biodegradation alone are: i) the low concentration of these substances compared to other pollutants in wastewater
be enough to active enzymes that are able to ii) some
that can remain they can inhibit growth
quite improbable that they can
microorganisms; iii) the degree of removal will depend on the chemical nature (structure and molecular weight)
on the operational conditions of the WWTP 2008).
needed to increase the removal of pharmaceuticals and decrease the potential impact of the WWTPs effluents in the environment
Fig. 3.
plants with nitrogen removal. First number in parentheses indicates the number of facilities where removal was calculated and the second number indicates the
were studied. (
Oxidation of pharmaceuticals by chlorine dioxide in wastewater effluent
5
average removal for the 44 pharmaceuticals was around 50% the highest removal took place in the biological degradation step.
al. (2012) studied the average removal of some pharmaceuticals in activated sludge plants with nitrogen removal where some substances such as ibuprofen and paracetamol where removed by almost
On the other hand, some pharmaceuticals such as metropolol or ydrochlorothiazide had a negative removal what means tha concentration was higher at the effluent compared to the
The reason of this effect is not totally clear but one possible explanation is that during biological treatment, these substances can be transformed back to parent compounds. In addition to this, some pharmaceuticals may be enclosed in faeces and thus released during biological treatment increasing the effluent concentration levels compared to the influent (Luo et al., 2014).
Consequently, it can be stated that conventional WWTPs are not able to completely remove pharmaceuticals and therefore they
complete barrier for them (Vona et al., 2015; Luo et al. 2014;
et al., 2013). Actually only 25% of these persistent substances can be totally removed after secondary treatment, 50% of the substances need additional methods to be removed and 25% shows a negative removal during the process (Hörsing et al., 2014).
of the reasons that can explain why pharmaceuticals cannot be effectively removed by biodegradation alone are: i) the low concentration of these substances compared to other pollutants in wastewater
be enough to active enzymes that are able to eliminate pharmaceuticals; some pharmaceuticals have stable and complex chemical structure that can remain for long time. Besides, many of them are bioactive so they can inhibit growth or metabolism of microorganism and therefore is quite improbable that they can be used as energy or carbon source for microorganisms; iii) the degree of removal will depend on the chemical
(structure and molecular weight) of the pharmaceutical operational conditions of the WWTP (Klavarioti et al., 2009 Thus more advanced and specific treatment techniques are needed to increase the removal of pharmaceuticals and decrease the potential impact of the WWTPs effluents in the environment
. Average removal and standard deviation in activated sludge plants with nitrogen removal. First number in parentheses indicates the number of facilities where removal was calculated and the second number indicates the total number of facilities that
studied. (Falås et al., 2012).
water effluent
average removal for the 44 pharmaceuticals was around 50% and the highest removal took place in the biological degradation step. Falås et the average removal of some pharmaceuticals in some substances emoved by almost 100% (Fig. On the other hand, some pharmaceuticals such as metropolol or had a negative removal what means that the higher at the effluent compared to the influent (Fig ct is not totally clear but one possible these substances can be transformed back to parent compounds. In addition to this, some pharmaceuticals may be enclosed in faeces and thus released during treatment increasing the effluent concentration levels that conventional WWTPs are not able to and therefore they are not a (Vona et al., 2015; Luo et al. 2014; Rivera-Actually only 25% of these persistent substances can
of the substances and 25% shows a negative why pharmaceuticals cannot be effectively removed by biodegradation alone are: i) the low concentration of these substances compared to other pollutants in wastewater may not pharmaceuticals; chemical structures Besides, many of them are bioactive so m of microorganism and therefore is as energy or carbon source for microorganisms; iii) the degree of removal will depend on the chemical of the pharmaceutical as well as Klavarioti et al., 2009; Aga, Thus more advanced and specific treatment techniques are needed to increase the removal of pharmaceuticals and decrease the potential impact of the WWTPs effluents in the environment.
Average removal and standard deviation in activated sludge plants with nitrogen removal. First number in parentheses indicates the number of facilities where removal was calculated total number of facilities that
Raquel Alcala Borao
1.3.2.Advanced
The three main options of additional for pharmaceutical
treatment; ii) oxidation processes.
Improved biological removal increasing the sludge retention time. the perfor
moving bed biofilm
carriers moves freely inside the bioreactor and at the same time they provide sites for adsorption and growth microorganisms
shows that moving bed biofilm carriers can enhance the capacity to remove some compounds such as diclofenac and ketoprofen compared to conventional activated sludge process.
been tested for pharmaceuticals removal is membran
that combines activated sludge biological treatment and membrane filtration.
et al., 2010
treatment with MBR and it gave though
cost it seems that this is not a very efficient technique high level of pharmaceuticals removal.
Physical removal is ab
them. Membrane techniques such as microfiltration (MF), ultrafiltration (UF), nanofiltration (NF) and reverse osmosis
membrane and high differential pressures to remove these substances. However
water, pharmaceuticals are poorly removed because the pores of these membranes are higher than the molecular size of pharmaceuticals. the other hand,
pharmaceuticals. A study performed by Yangali shows that RO membranes are able to achieve and 99%.
membrane technique there are some important drawback related to high el of the reject concentration of Fig. LWR 6
Advanced treatment alternatives for pharmaceutical removal
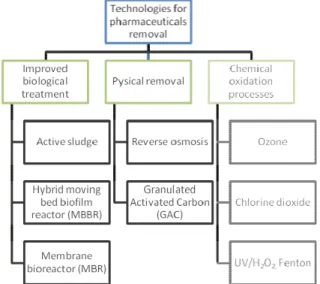
The three main options of additional and advanced treatmen for pharmaceuticals removal are (Fig. 4): i) improved bio treatment; ii) physical removal by sorption and filtration; iii) advanced oxidation processes.
Improved biological removal can be applied by adding carriers or increasing the sludge retention time. Fålas et al. (2012, 2013)
the performance of improved biological removal by usi moving bed biofilm-active sludge process. In this technique,
carriers moves freely inside the bioreactor and at the same time they provide sites for adsorption and growth microorganisms
shows that moving bed biofilm carriers can enhance the capacity to remove some compounds such as diclofenac and ketoprofen compared to conventional activated sludge process. Another technique that has been tested for pharmaceuticals removal is membrane bioreactor (MBR) that combines activated sludge biological treatment and membrane filtration. However a rapport carried out by Stockholm Water
et al., 2010) shows that 32 of 46 substances were still det
treatment with MBR and it gave an average reduction of 80%. Even though these biological treatments has the benefit to have low running cost it seems that this is not a very efficient technique alone
evel of pharmaceuticals removal.
Physical removal is able to remove pharmaceuticals without
them. Membrane techniques such as microfiltration (MF), ultrafiltration (UF), nanofiltration (NF) and reverse osmosis (RO) use a semipermeable membrane and high differential pressures to remove these substances. However even though MF and UF are efficient to remove turbidity from water, pharmaceuticals are poorly removed because the pores of these membranes are higher than the molecular size of pharmaceuticals. the other hand, RO presents greater potential to remove
harmaceuticals. A study performed by Yangali-Quintanilla et al. (2011) shows that RO membranes are able to achieve a removal
%.. Even though high removal levels can be reached with this membrane technique there are some important drawback
related to high electricity consumption and problems with the handling of the rejected flow from the membranes that
oncentration of micropollutants.
Fig. 4. Some of the available technologies for pharma removal.
LWR – EX – 2015:16
pharmaceutical removal
treatment techniques i) improved biological physical removal by sorption and filtration; iii) advanced by adding carriers or , 2013) has studied using a hybrid technique, plastic carriers moves freely inside the bioreactor and at the same time they provide sites for adsorption and growth microorganisms. The result shows that moving bed biofilm carriers can enhance the capacity to remove some compounds such as diclofenac and ketoprofen compared Another technique that has e bioreactor (MBR) that combines activated sludge biological treatment and membrane Water (Wahlberg shows that 32 of 46 substances were still detected after an average reduction of 80%. Even has the benefit to have low running alone to achieve a without degrading them. Membrane techniques such as microfiltration (MF), ultrafiltration RO) use a semipermeable membrane and high differential pressures to remove these substances. even though MF and UF are efficient to remove turbidity from water, pharmaceuticals are poorly removed because the pores of these membranes are higher than the molecular size of pharmaceuticals. On presents greater potential to remove Quintanilla et al. (2011) a removal between 85% Even though high removal levels can be reached with this membrane technique there are some important drawbacks. These are ectricity consumption and problems with the handling contains high
Oxidation of pharmaceuticals by chlorine dioxide in wastewater effluent
7
In order to solve the disadvantages presented by membrane technology, activated carbon adsorption is a technique that can be used to remove persistent/non-biodegradable organic compounds by adsorption processes in the activated carbon pores both with powdered activated carbon (PAC) and granular activated carbon (GAC). In general, efficient removal levels can be achieved with this technology, especially for substances with non-polar characteristics (KOW >2) (Luo et al., 2014).
However its efficacy can be affected by the presence of natural organic matter (NOM) that can compete for binding sites, thus leading to blocked pores. One disadvantage for this technology is that large volumes of activated carbon are needed in order to achieve good removal and that there is no disinfecting effect.
To overcome the problems and limitations presented by physical and biological treatment methods, advance oxidation processes are considered. These technologies are based on redox reactions and on the intermediate action of hydroxyl and other radicals that are able to oxidize non-biodegradable substances to several by-products and in some cases to inert end-products (Klavarioti et al.,2008). Besides, chemical oxidants are advantageous because they have disinfecting effect in water. On the other hand, the main disadvantage for oxidation, regardless which oxidizing agent is used, is the formation of new ecotoxicological active compounds that can have undesirable biological effects. These compounds can be either transformation products and/or by-products. Transformation products are those substances that are partially degraded, although the complete mineralization to carbon dioxide and water is not achieved and they can also be substances that are larger than the parent substance after the treatment. These can be less or more toxic substances in comparison to the parent substance (Hörsing et al., 2014). By-products are new substances that are formed during oxidation for instance organo-halogen by-products such as trihalomethanes (THMs) and chlorophenols that are carcinogenic substances formed after treatment with chlorine (WHO, 2004).
The efficiency of these methods will depend on the characteristics of the wastewater (pH, DOC, alkalinity, inorganic substances) as well as how reactive the oxidant is for the target pollutants. The best treatment efficiencies are reached when these oxidation technologies are combined with other physicochemical and biological processes (Klavarioti et al.,2008). For example, if an effluent with biodegradable substances is treated then biological treatment followed by post-chemical oxidation would be the best option. Thus easily biodegradable compounds are first removed and competition for the chemical oxidant is avoided. On the other hand, it may be interesting to have a biological post-treatment after the chemical oxidation in order to remove by biological degradation those by-products that arise from the incomplete mineralization of pharmaceuticals.
There are several oxidizing agents that can be used for this chemical oxidation. A big emphasis has been set on ozone as a promising chemical oxidation technology to remove pharmaceuticals and other organic micropollutants as well as on chlorine dioxide (ClO2) which is a
weaker oxidizing agent compared to ozone but easier to handle. Other advanced oxidation processes such as the combination of ozone with hydrogen peroxide or UV light, Fenton (Fe2+/H2O2) or combinations
between them have not show results that justify its utilization due to the fact that they are more expensive and complicated to use than ozone or ClO2 alone (Hörsing et al., 2014)
Raquel Alcala Borao LWR – EX – 2015:16
8
2.
AIM OF THE STUDY
Despite the fact that there are several advanced treatment technologies that can be utilized for pharmaceuticals removal, this study has the objective to investigate and evaluate the effectiveness of ClO2 as
chemical oxidation technology in a tertiary step for the removal of pharmaceuticals in biologically treated wastewater effluent. A bench-scale test study was designed to be able to determine the optimal ClO2
dose-reaction time relationship based on the maximum level of selected environmentally relevant pharmaceuticals that is possible to remove with this oxidation treatment. Concentration of the oxidant remaining in solution after removal of the pharmaceuticals wants also be investigated and evaluated. Furthermore the possibility of ClO2 dosage control
strategy for future upscaling wants to be studied by evaluating the relationship between ultraviolet absorption at 254nm and pharmaceuticals removal.
3.
BACKGROUND
As the current investigation is focused on the application of ClO2 as
oxidative agent to remove pharmaceuticals from biologically treated wastewater, some chemical aspects and previous studies with ClO2 are
described.
3.1. Chemical aspects of chlorine dioxide
ClO2 is an oxidant that has been widely used as disinfectant of high
quality water such as groundwater, treated water and drinking water as well as for wastewater disinfection (Huber et al. 2005). ClO2 is a neutral
compound of chlorine in the +IV oxidation state. When there are not oxidizable substances and in basic medium, it dissolves in water and discomposes into the formation of chlorite and chlorate:
2ClO2 + H2O → ClO2- + ClO3- + 2H+ (Eq.1)
ClO2 is a relatively small, volatile, highly energetic molecule considered
as a free radical. Another important physical property is its high solubility in water, especially in chilled water. Actually ClO2 is 10 times more
soluble than chlorine (above 11 ºC) (EPA, 1999)
Concentrated ClO2 vapor is potentially explosive so it cannot be stored
commercially as gas. Therefore it must be manufactured at the point of use. Dilute solutions of ClO2 must be kept in closed recipient in absence
of light since ClO2 discomposes with sunlight. However an aqueous
solution containing >8 g/l of ClO2 at temperature above 30 ºC is
explosive (Hoigné and Bader, 1994). A common reaction to produce aqueous solution of ClO2 at the point of use is by mixing hydrochloric
acid (HCl) and sodium chlorite (NaClO2):
5NaClO2 + 4HCl → 4ClO2 + 5NaCl + 2H2O (Eq. 2)
ClO2 is usually preferred to chlorine for disinfection of water. The first
reason is because ClO2 does not produce halogen-substituted
disinfection byproducts (DBPs) compounds (e.g. organochlorine and THMs) as chlorine does. This is because ClO2 reacts as an electron
acceptor and H atoms in activated organic C-H or N-H structures are thus not substituted by Cl (Hoigné and Bader, 1994). Besides, chlorine reacts via both oxidation and electrophilic substitution meanwhile ClO2
reacts only by oxidation. Secondly, ClO2 is an efficient disinfectant in a
broad range of pH between 5 and 10 and it is not affected by the presence of ammonia. Actually ClO2 is not consumed by ammonia so it
is considered a virucide when ammonia is presented in water. Thirdly, ClO2 does not oxidize bromide. This means that bromide is not
Oxidation of pharmaceuticals by chlorine dioxide in wastewater effluent
9
transformed into hypobromite and consequently neither bromoform nor bromated are generated in the water solution (Hoigné and Bader, 1994). Fourthly ClO2 is more selective in water compared to chlorine and
therefore lower disinfectant doses are needed (WHO, 2004).
Accordingly to the study of reaction kinetics of ClO2 in water performed
by Hoigné and Bader (1994), ClO2 is a highly selective oxidant with
several functional groups of organic substances such as phenolic compounds, tertiary/secondary amines as well as organosulfur groups. It is known that many pharmaceuticals have phenolic and/or amino functional groups in their structure therefore it is expected that ClO2 will
oxidize rather high number of pharmaceuticals despite its lower oxidation potential compared to ozone (Huber et al., 2005).
3.2. By-products formation with ClO2 as oxidative agent
Formation of DBPs is an issue of major concern due to the fact that these DBPs are potentially hazardous substances for health. When ClO2
is used as disinfectant, the major risk is posed on the formation of inorganic by-products including chlorite (ClO2-) and chlorate (ClO3-)
(WHO, 2004). These are formed due to the reduction of ClO2 to chlorite
when it reacts with organic matter. ClO3- is formed in much lower
concentration than ClO2-; approximately 50%-70% and 30% of
consumed ClO2 is transformed to ClO2- and ClO3- respectively (EPA,
1999). These inorganic by-products can lead to hemolytic anemia at low levels of exposure and higher levels can result in an increase in methemoglobin. Additional studies have shown that chlorite can produce effects on the nervous systems in infants and young children (Veschetti et al., 2004).
There are several post- treatment techniques that can remove chlorite and ClO2 residuals from treated water. However, these post-treatments
are not valid for chlorate since once it is formed, it is stable in water and thus it cannot be removed. These post-treatment techniques are i) addition of reduced-sulfur compounds (e.g. sulfur dioxide and sodium sulfite); ii) Granular activated carbon (GAC) or powdered activated carbon (PAC); iii) adding reduced iron salts (e.g. ferrous chloride and ferrous sulfate) (EPA, 1999). However, the most convenient method is to use reduced iron salts since it has already been proved to be an effective method to remove chlorite with chloride as expected byproduct (Sorlini & Collivignarelli, 2005; Katz & Narkis, 2001). The reduction of iron salts to chloride is governed by the following equation:
4Fe2+ + ClO2- + 10H2O→4 Fe(OH)3(s) + Cl- + 8H+ (Eq. 3)
According to Sorlini & Collivignarelli (2005), complete removal of chlorite can be achieved with a stochometric dose of ferrous ion 3,31 mg Fe2+/mg ClO2- (Katz & Narkis, 2001) at neutral pH (6.5-8.0). This
reaction is kinetically fast with complete ClO2- reduction applying
reaction times of 5 – 15 seconds. It is also possible to remove ClO2 left
in solution by the following ClO2/ Fe(aq)2+ reaction:
4H+ + ClO
2 + 5Fe2+ → Cl- + 5Fe3+ + 2H2O (Eq. 4)
Therefore the theoretical Fe2+ for the complete reduction of 1mg ClO 2 is
4.14 mg of Fe2+.
Formation of significant concentrations of organo-halogen and non-halogenated DBPs has not been extensively reported in previous studies. However, a study performed by Richardson et al. (2010) detected organic DPBs after the treatment of drinking water with ClO2. From all the 27
detected organic DPBs only two of them were chlorinated DPBs, meanwhile the others were non-halogenated DPBs (ketones, carboxylic
Raquel Alcala Borao LWR – EX – 2015:16
10
acids and maleic acids). Another study performed by Serrano et al. (2015) states that four brominated HAAs (haloacetic acids) were formed after disinfection of drinking water with ClO2 due mainly to the reaction of
organic matter with bromide present in the water but any THMs were detected.
3.3. Previous studies with ClO2
There are several studies that show promising results using ClO2 to treat
drinking water, surface water and wastewater.
Huber et al. (2005) studied the performance of ClO2 during the oxidation
with pharmaceuticals in water treatment. The oxidation experiments were performed in “natural waters”, surface water and groundwater, where the samples were spiked with pharmaceuticals with concentrations up to 1µg L-1. Results for this study show that diclofenac, one of the
pharmaceuticals that have been recently included in the “watch list” of the Water Framework Directive and that has a low removal level by conventional methods, was completely removed by oxidation with ClO2.
According to this study, diclofenac was oxidized in surface water samples by more than 90% within 1 minute at the lowest tested concentration of 0.5 mg ClO2 L-1.Lee et al. (2010) studied the kinetics of
different oxidants in wastewater samples from effluent of secondary treatment spiked with different pharmaceuticals. The consumption kinetics and decay pattern of ClO2 was similar to chlorine; they showed
an initial phase with a rapid consumption of oxidant within the first 2 minutes and then a slow decrease over 60 minutes of reaction time. On the other hand, ozone was totally depleted in less than 2 minutes. Andersen (2010) studied the oxidation of ClO2 in wastewater effluent
spiked with three steroid estrogens (E1, E2 and EE2) to a concentration of 0.4 µg L-1. This study demonstrates that the three tested steroid
estrogens (E2 and EE2 belongs to the recent “watch list” of emerging pollutants) were removed within the first 30 seconds with 2.5 mg/L and 3.75 mg/L of ClO2 for low COD (~ 15 mg/L) and high COD (~ 40
mg/L) effluents respectively without leaving ClO2 residuals. This may
be due to the fact that steroid substances contain phenolic groups to which ClO2 is a highly selective oxidant. Another study performed by
Hey et al. (2012) examined the removal of 56 different pharmaceuticals spiked in biologically treated wastewater effluents after 18 hours of reaction. In the effluent with low COD (35 mg/L) more than a half of the studied pharmaceuticals were removed by more than 90%, meanwhile one third of the pharmaceuticals resisted degradation even at the higher tested ClO2 concentration (20 mg/L). This group of
pharmaceuticals characterized by being less sensitive to be oxidized by ClO2 included β-blockers such as metropolol and bisoprolol which have
a secondary amine functional group. The most easily oxidized pharmaceuticals (ClO2 concentration between 0.5mg/L and 1.25 mg/L)
were diclofenac, hormones containing phenolic structures (estriol, estrone, ethinyl) and antibiotics like ciprofloxacin.
It can be said that most of the previous studies (Table 1) have mainly tested the disinfection effect of ClO2 in both drinking water and
wastewater. During the last years, more research has been conducted in ClO2 as oxidant for pharmaceuticals but this has been focused on how
(kinetics) and which pharmaceuticals can be removed according to different oxidant concentration as well as the formation of inorganic by products. None of them has however studied how to optimize this technique in order to upscale it and achieve the maximum removal of pharmaceuticals.
Oxidation of pharmaceuticals by chlorine dioxide in wastewater effluent
11
Table 1. Previous studies in ClO2 treatment.
Ap pl i c ati on S i z e Ref erenc e
ClO2 k inet ic s in drink in g wat er B at c h t es t
Hoign é an d B ader, 1994 D rink in g wat e r t reat m ent B at c h t es t K orn, 200 2
I norg anic DB P s in was t e wat er P ilot plant V es c het t i et al. 2004 O xidat i on of ph arm ac eut ic als in nat ural
wat ers B at c h t es t Hube r et al. 200 5
Orga nic DB P s in drink ing wa t e r Full/ pilot s c ale
Ric hards o n et al. 2010 O xidat i on o f m ic ropollut ant s in was t e wat e r
(k inet ic t rans f orm at ions ) B at c h t es t Lee et al. 20 10 O xidat i on in s pik ed (s t er oid es t roge ns )
was t e wat e r B at c h t es t A nders en 201 0 O xidat i o n ph arm ac eut ic als in s pik ed
was t e wat e r B at c h t es t H ey et al. 2 012, 20 13 Orga nic DB P s in drink ing wa t e r Full s c ale S erra no et al. 20 15
3.4. Motivation and possibilities for ClO2 treatment in WWTPs
Even though chlorine and ClO2 approximately react with the same
compounds (Huber et al. 2005), ClO2 is much preferred due to the
toxicity of DBPs generated by the use of chlorine; actually more than 300 DBPs have been addressed due to disinfection with chlorine (Richardson et al., 2000). Besides ClO2 is more selective than chlorine in
water and thus less doses are needed to have the same effect (WHO, 2004).
When comparing ClO2 and ozone, both chemical oxidants react with
electron-rich groups of organic molecules such as phenolic- and aminogroups. However it has been seen that ozone reacts faster with the same reactive functional group (Huber et al. 2005, Hey, 2013). Regarding the formation of by-products and their toxicity, it is known that inorganic by-products are formed during the treatment with ClO2 (e.g
chlorite). The formation of other significant disinfection byproducts such as organo-halogen and non-halogenated by-products is not very well known yet. In the case of ozonation, there is a risk for the formation of both organohalogen by-products (e.g bromoform with presence of bromide ion) and non-halogenated by-products (e.g aldehydes, ketoacids), as well as inorganic by-products (e.g bromated, iodate) (WHO, 2004).
Regarding the cost of these technologies, treatment with ozone has a large capital cost of 100-300 k€ for a medium size WWTP. This high cost may not be reasonable and profitable for smaller WWTPs although it could be acceptable for larger WWTPs. On the other hand, ClO2
generators are simpler and cheaper (its cost is around 10-30 k€) for both the preparation system and the reaction chamber (Andersen, 2010). However the cost of producing ClO2 can be the same or double
compared to ozone depending on the chemicals that are used to produce the ClO2 solution and the scale of production. Therefore the best
approach could be to use ClO2-treatment for upgrading small scale
WWTPs (<2000 pe) since the capital cost for ClO2 technology is lower
than for ozone and the running costs would not be so high considering treatment for limited period of time and lower scale of consumption.
Raquel Alcala Borao
Previous studies in
optimization of this treatment focused on pharmaceuticals treatment for upscaling
test not only the influence pharmaceuticals removal
for the design of reactor (e.g continuous stirred allows calcula
known.
One additional upscaling
composition and inflow of
control is important in order to avoid high cost and achieve good resource
The hourly pharmaceutical studied by
follows the water flow in a large extent and they reach a minimum value around 7am. This shows that the dosing of the oxidant for pharmaceuticals
full scale process
possible due to their low concentration in water, t Sehlén
oxygen demand was not robust for real absorbance
tested as a parameter oxidant substance.
pharmaceuticals concentration and ultraviolet absorbance since they constitute a very small
absorbing at
dosage of the oxidant
adjusted leading to a more resource efficient process implementation.
Fig. 5
Linköping WWTP (Shelén et al. 2015)
LWR
12
revious studies in ClO2 treatment (Table 1) showed that
optimization of this treatment focused on pharmaceuticals treatment for upscaling has not been deeply studied yet. Therefore, this study aims to test not only the influence of different doses of oxidant pharmaceuticals removal but also the reaction time. This is
the design of reactor (e.g continuous stirred-tank reactor) since it allows calculating its volume once the residence time of the chemical is known.
One additional key issue that needs to be faced when thinking upscaling a new treatment technology to pilot or full
composition and inflow of wastewater varies within time.
control is important in order to avoid high cost and achieve good resource-efficiency within the process.
hourly variation of flow and normalized concentration of pharmaceuticals substances coming into the Linköping WWTP studied by Sehlén et al. (2015) (Fig. 5). The mass flow of pharmaceuticals follows the water flow in a large extent and they reach a minimum value around 7am. This shows that the dosing of the oxidant for pharmaceuticals removal can be adjusted according to the mass fl full scale process. As an online measurement of pharmaceuticals is not possible due to their low concentration in water, this study performed by
et al. 2015 proposes ozone dosage control by measuring oxygen demand (COD) or Spectral Absorption Coefficient (SAC) was not considered a suitable indicator in this case because it is not robust for real-time measurements. Decrease of SAC,
absorbance at 254nm at which organic matter can be quantified as a parameter that can be linked to the removal effect of the oxidant substance. There is not any relationship between pharmaceuticals concentration and ultraviolet absorbance since they constitute a very small proportion of the total amount of substances absorbing at 254nm. This SCA could be monitored on line and thus the dosage of the oxidant for the removal of pharmaceuticals could be adjusted leading to a more resource efficient process implementation.
5. Hourly variation of mass flow of pharmaceuticals in Linköping WWTP (Shelén et al. 2015).
LWR – EX – 2015:16
showed that the optimization of this treatment focused on pharmaceuticals treatment for his study aims to of oxidant in . This is a key factor tank reactor) since it ting its volume once the residence time of the chemical is key issue that needs to be faced when thinking in technology to pilot or full scale is that wastewater varies within time. Thus, dosage control is important in order to avoid high cost and achieve good concentration of substances coming into the Linköping WWTP was mass flow of pharmaceuticals follows the water flow in a large extent and they reach a minimum value around 7am. This shows that the dosing of the oxidant for be adjusted according to the mass flow in online measurement of pharmaceuticals is not his study performed by control by measuring chemical sorption Coefficient (SAC). COD indicator in this case because it is not ecrease of SAC, which is the at which organic matter can be quantified, was that can be linked to the removal effect of the here is not any relationship between pharmaceuticals concentration and ultraviolet absorbance since they proportion of the total amount of substances could be monitored on line and thus the for the removal of pharmaceuticals could be adjusted leading to a more resource efficient process implementation.