D O C T O R A L D IS S E R TA T IO N I N O D O N T O LO g y V IC T O R IA F R ö jD m A L m ö U N IV E R S IT y mALmö högSkOLA
VICTORIA FRöjD
ON CA
2+
INCORPORATION
AND NANOPOROSITy OF
TITANIUm SURFACES AND
ThE EFFECT ON ImPLANT
PERFORmANCE
isbn 91-7104-315-2 O N C A 2 + IN C O R PO R A TIO N A N D N A N O PO R O SI Ty O F T IT A N IU m S U R FA C ES A N D T h E E FF EC T O N Im PL A N T P ER FO R m A N C EO N C A2 + I N C O R P O R A T I O N A N D N A N O P O R O S I T Y O F T I T A N I U M S U R F A C E S A N D T H E E F F E C T O N I M P L A N T P E R F O R M A N C E
Malmo University
Faculty of Odontology Doctoral Dissertations 2010
© Victoria Fröjd 2010 ISBN-91-7104-315-2 Holmbergs, Malmö 2010
VICTORIA FRÖJD
ON CA
2+
INCORPORATION
AND NANOPOROSITY OF
TITANIUM SURFACES AND
THE EFFECT ON IMPLANT
PERFORMANCE
Malmo University, 2010
Faculty of Odontology
University of Gothenburg
Department of Biomaterials
Publikationen finns även elektroniskt, se www.mah.se/muep
Dedicated to my brilliant brother, my always supporting father, my loving mother,
my darling grandmother, my admirable cousin Camilla, and my dear friend Malin Olsson, who I value deeply.
This thesis represents number 40 in a series of investigations on implants, hard tissue and the locomotor apparatus originating from the department of Biomaterials/Handicap Research, University of Gothenburg and the department of Prosthodontics, Malmö University, Sweden.
1. Anders R Eriksson DDS, 1984. Heat-induced Bone Tissue Injury. An in vivo investigation of heat tolerance of bone tissue and temperature rise in the drilling of cortical bone. Thesis defended 21.2.1984. External examiner: Docent K-G. Thorngren.
2. Magnus Jacobsson MD, 1985. On Bone Behaviour after Irradiation. Thesis defended 29.4.1985. External examiner: Docent A. Nathanson.
3. Fredrik Buch MD, 1985. On Electrical Stimulation of Bone Tissue. Thesis defended 28.5.1985. External examiner: Docent T. Ejsing-Jörgensen.
4. Peter Kälebo MD, 1987. On Experimental Bone Regeneration in Titanium Implants. A quantitative microradiographic and histologic investigation using the Bone Harvest Chamber. Thesis defended 1.10.1987. External examiner: Docent N. Egund.
5. Lars Carlsson MD, 1989. On the Development of a new Concept for Orthopaedic Implant Fixation. Thesis defended 2.12.1989. External examiner: Docent L-Å Broström. 6. Tord Röstlund MD, 1990. On the Development of a New Arthroplasty. Thesis defended 19.1.1990. External examiner: Docent Å. Carlsson.
7. Carina Johansson Techn Res, 1991. On Tissue Reactions to Metal Implants. Thesis defended 12.4.1991. External examiner: Professor K. Nilner.
8. Lars Sennerby DDS, 1991. On the Bone Tissue Response to Titanium Implants. Thesis defended 24.9.1991. External examiner: Dr J.E. Davis.
9. Per Morberg MD, 1991. On Bone Tissue Reactions to Acrylic Cement. Thesis defended 19.12.1991. External examiner: Docent K. Obrant.
10. Ulla Myhr PT, 1994. On Factors of Importance for Sitting in Children with Cerebral Palsy. Thesis defended 15.4.1994. External examiner: Docent K. Harms-Ringdahl. 11. Magnus Gottlander MD, 1994. On Hard Tissue Reactions to Hydroxyapatite-Coated Titanium Implants. Thesis defended 25.11.1994. External examiner: Docent P. Aspenberg. 12. Edward Ebramzadeh MScEng, 1995. On Factors Affecting Long-Term Outcome of Total Hip Replacements. Thesis defended 6.2.1995. External examiner: Docent L. Linder. 13. Patricia Campbell BA, 1995. On Aseptic Loosening in Total Hip Replacement: the Role of UHMWPE Wear Particles. Thesis defended 7.2.1995. External examiner: Professor D. Howie.
14. Ann Wennerberg DDS, 1996. On Surface Roughness and Implant Incorporation. Thesis defended 19.4.1996. External examiner: Professor P.-O. Glantz.
Stability and Osseointegration. Thesis defended 3.6.1997. External examiner: Professor J. Brunski.
16. Lars Rasmusson DDS, 1998. On Implant Integration in Membrane-Induced and Grafter Bone. Thesis defended 4.12.1998. External examiner: Professor R. Haanaes. 17. Thay Q Lee MSc, 1999. On the Biomechanics of the Patellofemoral Joint and Patellar Resurfacing in Total Knee Arthroplasty. Thesis defended 19.4.1999. External examiner: Docent G. Nemeth.
18. Anna Karin Lundgren DDS, 1999. On Factors Influencing Guided Regeneration and Augmentation of Intramembraneous Bone. Thesis defended 7.5.1999. External examiner: Professor B. Klinge.
19. Carl-Johan Ivanoff DDS, 1999. On Surgical and Implant Related Factors Influencing Integration and Function of Titanium Implants. Experimental and Clinical Aspects. Thesis defended 12.5.1999. External examiner: Professor B. Rosenquist.
20. Bertil Friberg DDS MDS, 1999. On Bone Quality and Implant Stability Measurements. Thesis defended 12.11.1999. External examiner: Docent P. Åstrand.
21. Åse Allansdotter Johnsson MD, 1999. On Implant Integration in Irradiated Bone. An Experimental Study of the Effects of Hyberbaric Oxygenation and Delayed Implant Placement. Thesis defended 8.12.1999. External examiner: Docent K. Arvidsson-Fyrberg. 22. Börje Svensson DDS, 2000. On Costochondral Grafts Replacing Mandibular Condyles in Juvenile Chronic Arthritis. A Clinical, Histologic and Experimental Study. Thesis defended 22.5.2000. External examiner: Professor Ch. Lindqvist.
23. Warren Macdonald BEng, MPhil, 2000. On Component Integration in Total Hip Arthroplasty: Pre-Clinical Evaluations. Thesis defended 1.9.2000. External examiner: Dr A.J.C. Lee.
24. Magne Røkkum MD, 2001. On Late Complications with HA Coated Hip Asthroplasties. Thesis defended 12.10.2001. External examiner: Professor P. Benum. 25. Carin Hallgren Höstner DDS, 2001. On the Bone Response to Different Implant Textures. A 3D analysis of roughness, wavelength and surface pattern of experimental implants. Thesis defended 9.11.2001. External examiner: Professor S. Lundgren. 26. Young-Taeg Sul DDS, 2002. On the Bone Response to Oxidised Titanium Implants: The role of microporous structure and chemical composition of the surface oxide in enhanced osseointegration. Thesis defended 7.6.2002. External examiner: Professor J.-E. Ellingsen.
27. Victoria Franke Stenport DDS, 2002. On Growth Factors and Titanium Implant Integration in Bone. Thesis defended 11.6.2002. External examiner: Associate Professor E. Solheim.
28. Mikael Sundfeldt MD, 2002. On the Aetiology of Aseptic Loosening in Joint Arthroplasties and Routes to Improved cemented Fixation. Thesis defended 14.6.2002. External examiner: Professor N Dahlén.
29. Christer Slotte DDS, 2003. On Surgical Techniques to Increase Bone Density and Volume. Studies in the Rat and the Rabbit. Thesis defended 13.6.2003. External examiner: Professor C.H.F. Hämmerle.
30. Anna Arvidsson MSc, 2003. On Surface Mediated Interactions Related to Chemo-mechanical Caries Removal. Effects on surrounding tissues and materials. Thesis defended 28.11.2003. External examiner: Professor P. Tengvall.
31. Pia Bolind DDS, 2004. On 606 retrieved oral and cranio-facial implants. An analysis of consecutively received human specimens. Thesis defended 17.12. 2004. External examiner: Professor A. Piattelli.
32. Patricia Miranda Burgos DDS, 2006. On the influence of micro-and macroscopic surface modifications on bone integration of titanium implants. Thesis defended 1.9. 2006. External examiner: Professor A. Piattelli.
33. Jonas P Becktor DDS, 2006. On factors influencing the outcome of various techniques using endosseous implants for reconstruction of the atrophic edentulous and partially dentate maxilla. Thesis defended 17.11.2006. External examiner: Professor K. F. Moos 34. Anna Göransson DDS, 2006. On Possibly Bioactive CP Titanium Surfaces. Thesis defended 8.12.2006 External examiner: B. Melsen
35. Andreas Thor DDS, 2006. On platelet-rich plasma in reconstructive dental implant surgery. Thesis defended 8.12.2006. External examiner: Prof E.M. Pinholt.
36. Luiz Meirelles DDS MSc, 2007. On Nano Size Structures For Enhanced Early Bone Formation. Thesis defended 13.6.2007. External examiner: Professor Lyndon F. Cooper. 37. Pär-Olov Östman DDS, 2007. On various protocols for direct loading of implant-supported fixed prostheses. Thesis defended 21.12.2007. External examiner: Prof B Klinge
38. Kerstin Fischer DDS, 2008. On immediate/early loading of implant supported prostheses in the maxilla. Thesis defended 8.2.2008. External examiner: Professor Kristina Arvidson Fyrberg
39. Alf Eliasson 2008. On the role of number of fixtures, surgical technique and timing of loading. Thesis defended 23.5.2008. External examiner: Kristina Arvidson-Fyrberg. 40. Victoria Fröjd DDS, 2010. On Ca2+ incorporation and nanoporosity of titanium surfaces and the effect on implant performance. Thesis to be defended 26.11.2010. External examiner: Professor J. E. Ellingsen
List of papers Bone tissue (in rabbit): Importance of surface topography as well as anodization and Ca2+ incorporation for osseointegration
Study I Fröjd V, Franke-Stenport V, Meirelles
L, Wennerberg A. Increased bone
contact to a Ca2+ incorporated oxidized c.p. titanium implant: an
in vivo study in rabbit. Int J Oral Maxillofac Surg 2008 37(6): 561-6
Study II Fröjd V, Wennerberg A,
Franke-Stenport V. Importance of Ca2+
modifications for osseointegration of smooth and moderately rough anodized titanium implants – a removal torque and histological evaluation in rabbit. Accepted for
publication in Clin Impl Dent Relat Res 2010.
Oral mucosa:
Impact of nanoporosity for the sealing of oral mucosa
Study III Wennerberg A, Fröjd V, Olsson M,
Nannmark U, Emanuelsson L, Johansson P, Yvonne J, Kangasniemi I, Peltola T, Tirri T, Pänkäläinen T,
Thomsen P. Nanoporous TiO2 thin
film on titanium oral implants for enhanced human soft tissue adhesion - a histological evaluation in three different levels of resolution. Clin
Impl Dent Relat Res E published ahead of print 2009. Biofilm accumulation (in vitro): Influence of surface topography, anodization and Ca2+ incorporation, and nanoporosity on multi-species bacterial adhesion and biofilm formation
Study IV Fröjd V, Chávez de Paz L, Andersson
M, Wennerberg A, Davies J,
Svensäter G. In situ analysis of
biofilm formation on titanium surfaces. Submitted.
Study V Fröjd V, Linderbäck P, Wennerberg
A, Chávez de Paz L, Svensäter G, Davies J. Microbial biofilm
formation on smooth nanoporous TiO2 coated and anodized Ca2+ modified and titanium surfaces.
TAbLE OF CONTENTS
ABstrAct ... 17
IntrODUctIOn ... 21
Indications for biomedical titanium implants and research within the field ...21
Principles for integration of biomaterials ...22
Integration into bone tissue...22
Integration into oral mucosa and soft-tissues ...26
titanium as a biomaterial ...27
Bioactivity ...29
Methods for titanium surface processing ...30
surface properties of titanium implants ...34
aimed for bone integration ...34
Macro design ...34
Micro topography ...34
nano topography ...35
chemistry ...35
surface properties of titanium implants aimed for soft tissue integration...44
Macro design ...44
Micro topography ...44
nano topography ...45
chemistry ...45
complications with titanium implant treatments ...48
Nature of the complications ...48
Extent ...48
AIMs ... 55
MAtErIAls AnD MEthODs ... 57
surface processing ...57
Anodic oxidation ...57
sol-gel derived nanoporous tiO2 coating ...58
Blasting process ...58
surface characteristics measurements ...59
Optical interferometry ...59
scanning electron microscopy ...61
transmission electron microscopy ...62
X-ray photoelectron spectroscopy ...62
Ellipsometry ...62
In vivo evaluations – rabbit model ...62
Animals and surgical technique ...62
Biomechanical evaluation ...63
Preparation of histological specimen ...63
light microscopy evaluations ...63
In vivo evaluations – human model ...64
Investigation design and patient selection ...64
X-ray imaging ...64
sample retrieval ...65
Preparation of histological specimen ...65
light microscopy evaluations ...65
transmission electron microscopy analysis ...66
In vitro models ...66
Bacterial strains and culture ...66
16s rrnA fluorescence in situ hybridization ...67
confocal laser scanning microscopy ...68
Biofilm biovolume quantifications ...69
statistics ...69
rEsUlts ... 71
surface properties ...71
topography ...71
chemistry ...74
Osseointegration - study I and II ...75
Interaction with oral mucosa – study III ...78
histological investigation ...78
DIscUssIOn ... 83
surface processing ...83
surface characteristics measurements ...84
In vivo evaluations – rabbit models ...84
In vivo evaluations – human model ...86
In vitro evaluations ...86
surface characteristics ...87
Osseointegration - study I and II ...88
sealing of oral mucosa – study III ...89
Bacterial adhesion and biofilm formation - study IV and V ...89
sUMMAry, FUtUrE PrOsPEctIVEs, AnD DErIVED hyPOthEsIs ... 93
cOnclUsIOns ... 95
POPUlärVEtEnskAPlIG sAMMAnFAttnInG ... 97
AcknOwlEDGEMEnts ... 99
AbSTRACT
Introduction: Titanium implants are commonly used as replacements
for missing teeth with successful long-term performance. The aim of the research performed in the field is to enable successful osseointegrated implant treatments for compromised as well as healthy bone beds, and to establish a rapid osseointegration to shorten the treatment period for the patients. In some cases bone resorption occurs around oral implants and the surrounding conditions may alter when surfaces aimed at being integrated in the bone are exposed to the extensive oral microbiota. Biofilms are most probably constantly present on exposed intraoral surfaces but may during certain conditions be associated with pathological conditions in the surrounding tissues. Implant treatments depend on a stability through the osseointegration, as well as a sealing of oral mucosa for the defence against extensive biofilm accumulation.
Aims: The present thesis has aimed at investigating the impact of Ca2+ incorporation to anodized titanium surfaces for osseointegration, and whether Ca2+ incorporation would compensate for potential shortcomings of a minimal surface roughness. We have further aimed at investigating the adhesion of oral mucosa to nanoporous TiO2 surfaces clinically as well as histologically, and at evaluating the bacterial adhesion and biofilm formation on the test surfaces in
vitro.
Methods: The osseointegration of smooth (average height deviation
<0.5 µm) and moderately rough (average height deviation 1-2 µm) Ca2+ incorporated anodically oxidized surfaces, minimally (average
height deviation 0.5-1 µm) and moderately rough anodically oxidized surfaces, and minimally and moderately rough Al2O3 blasted surfaces, was investigated with a rabbit model in two studies: one histological and one combined biomechanical and histological study. Oral mucosa adhesion to sol-gel derived, smooth nanoporous TiO2 coated and turned surfaces with similar microtopography was investigated in an experimental study in humans, where the samples were evaluated clinically and histologically at three different levels of resolution. All histological sections were evaluated both quantitatively and qualitatively. To study bacterial adhesion and biofilm formation on the surfaces as well as the possibility to mechanically remove adhered bacteria with a smooth toothbrush without dentifrice, multi-species bacterial models (with or without the presence of saliva) combining 16S rRNA fluorescence in situ hybridization and confocal laser scanning microscopy were used. Surface topography and chemistry was characterized using optical interferometry, scanning electron microscopy, X-ray photoelectron spectroscopy, and atomic force microscopy.
Results: Smooth Ca2+ incorporated anodically oxidized implants had significantly more bone in contact compared to minimally rough anodically oxidized and blasted implants when placed in rabbit tibia. Moderately rough Ca2+ incorporated anodically oxidized implants had significantly higher removal torque compared to moderately rough anodically oxidized and smooth Ca2+ incorporated anodically oxidized implants, and, at the same time, the removal torque of smooth Ca2+ incorporated anodically oxidized implants did not significantly differ from that of moderately rough blasted or anodically oxidized surfaces when placed in rabbit tibia.
Nanoporous TiO2 coated abutments had significantly more oral mucosa in contact with the surface as well as significantly less marginal bone resorption when only stable implants were evaluated compared to turned control surfaces. The clinical appearance was, furthermore, assumed to be advantageous for the nanoporous surfaces.
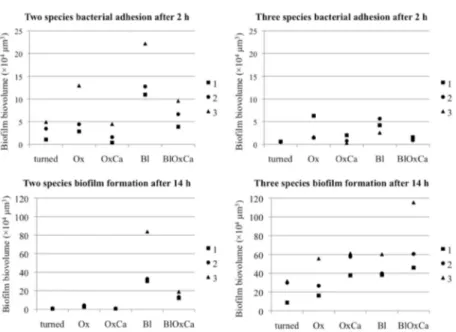
Increasing the surface roughness led to larger biofilm biovolumes
in vitro. At the same time, Ca2+ incorporation tended to decrease biofilm formation when compared to control surfaces. Nanoporosity
or Ca2+ incorporation did not seem to effect biofilm formation when compared to turned surfaces. Moderately rough blasted surfaces generally adhered largest biofilm biovolumes and presented the greatest amount of remaining bacteria after mechanical cleaning.
Conclusions: Within the limits of the studies in the present thesis,
Ca2+ incorporation may enhance osseointegration and compensate for minimal surface roughness in rabbit tibia. Nanoporosity may hold advantages for oral mucosa adhesion; however, no clear conclusions can be drawn. Increased surface roughness may increase bacterial adhesion and biofilm biovolume in vitro, and moderately rough blasted surfaces were most difficult to clean from adhered bacteria.
INTRODUCTION
Indications for biomedical titanium implants and research
within the field
Since the discovery of osseointegration of titanium by Brånemark and, almost contemporary Schroeder and Schulte, installation of titanium implants have become an established treatment for replacements of teeth. Today, more than two million oral implants are placed in the United States annually. If implants per capita are considered, South Korea is the leading country closely followed by Italy, Sweden, and Switzerland. Moreover, titanium implants are used for, for example, amputation prostheses and, if rarely, total hip replacements.
Treatment with oral titanium implants may improve the quality of life for edentulous patients11. In general, titanium dental implants have high survival rates of 90-98 percent over twenty years12, 13. However, when approaching compromised patients or patients with unfavourable bone quality there may be a need for specific implant surface designs and/or particular surgical techniques to improve chances for a successful treatment. The complications that do occur in relation to titanium implants, for example bone resorption and biofilm infections, exemplify an area where further research is needed to hopefully control such events. Another aspect is the aesthetics in relation to implants that still could be improved in many cases, and which are of importance for a large group of patients receiving implant treatment.
The field of oral implants is one example of close links between research and industry and findings in the laboratory often becomes clinically applied. Most new designs and surfaces are considered not
to deviate from existing implants, and the process to reach the clinic is, therefore, rather short. However, a risk with the commercial approach is that the desire to launch “news” may get in conflict with the need for control of possible side-effects with the products, which could result in unnecessary suffering for the patient.
In conclusion, implants need to perform in three biological arenas: in relation to bone tissue, soft tissue, and microbial biofilms, and all these aspects should be considered when developing new implant surfaces. This thesis has aimed at initiating investigations of surfaces in relation to more than one of these “arenas”.
Principles for integration of biomaterials
Surface characteristics of implants seem to effect the inflammatory response in the surrounding tissues 14. An inflammatory response to installed titanium implants, added to the one caused by the surgical trauma have been noted15, 16. One aspect of the modulation of the body response to biomaterials is complement activation (mainly C3 derivates and C5a mediates inflammation)17, where there are differences between various surfaces18-20. However, numerous studies and clinical experience have proven titanium to be a most proper material to use for replacements of lost teeth. Furthermore, the inflammation process may be of importance for the tissue healing around implants14 and, therefore, a controlled activation of a transient inflammatory response may even be a positive reaction. A literature review reveals that implant surface characteristics may influence the biological response.
Integration into bone tissue
Brånemark et al. coined the term osseointegration in 1977 and defined it as: “re- and new-formed bone tissues enclose the implant with perfect congruency to the implant form and surface irregularities thus establishing a true osseointegration of the implant without any interpositioned connective tissue”21. Studies report an unmineralized zone of some hundred nanometres between titanium implants and
bone22, 23. This layer is suggested to mainly consist of proteoglycans 23, 24.
However, studies using transmission electron microscopy of the interface between bone and titanium show an intimate contact and presence of hydroxyapatite at the immediate implant surface 25, 26.
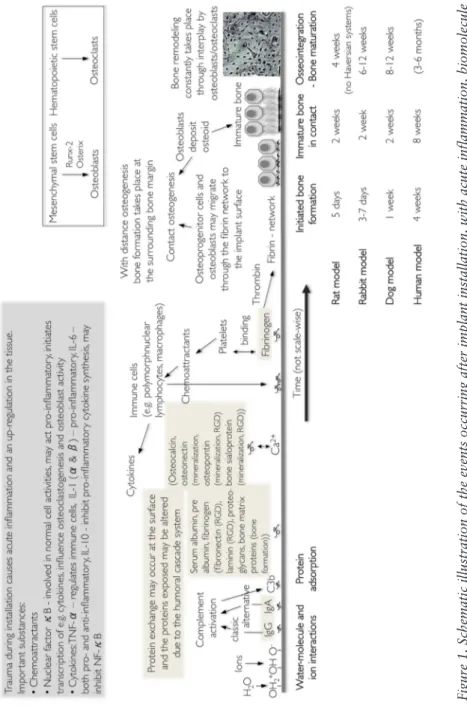
The integration of biomaterials into bone, or any tissue, relies on healing mechanisms involving the stages of haemostasis, inflammation, regeneration, and remodelling. Chemotaxis and recruitment of cells are of crucial importance. However, with new techniques and knowledge, trials to modify the mechanisms after implant installation, to speed up the process as well as having an enhanced healing and integration, are performed with many implant surfaces. The terms distance and contact osteogenesis was first coined by Osborn and Newsely in 198027, reflecting on whether bone formation is initiated at the border of the old bone or at the implant surface. Suggestively, contact osteogenesis or a combination of contact and distance osteogenesis may occur with certain surface characteristics of bioactive nature2. Possible ways to affect the osseointegration is either via the living compartments of the bone, i.e. the cells, or by physico-chemistry with mechanical interlocking28 or attraction forces. In order to modulate the response, an understanding of the naturally occurring events is of great importance.
Today, research starts to focus on the gene-regulation and molecular mechanisms of cells involved in osteogenesis around implant29. In a thesis by Omar (2010), the molecular mechanisms of osseointegration, the importance of the mesenchymal progenies and the hematopoietic derived cells (for example platelets, neutrophils, endothelial cells, monocytes, lymphocytes, and osteoclasts), and growth factors that initiate other cellular events have been discussed. Polymorphonuclear leukocytes, macrophages, and osteoclasts will phagocytise injured tissues to allow for regeneration of newly formed tissue. The effect of cytokines or other transcription factors varies with the cell type and the specific cell surface receptor they bind to; consequently, one substance may activate various intracellular cascades30. The multi-function of several molecules involved in the healing cascades reveal the complexity of the biological system as well as the fact that we do not have complete knowledge of the mechanisms. There is, furthermore, a close relation between all cells involved, from the mesenchymal stem cells to the osteoclasts; for example osteoblasts release factors that activates osteoclasts31.
Cells may interact with surfaces via receptors/adhesion molecules, such as immunoglobulin’s, cadherins, and integrins. Integrins
may form complexes with RGD (Arginine-Glycine-Aspartic acid) sequences and may, thereby, be attached to certain RGD containing proteins (e.g. fibronectin) or other with cell receptors coating a surface 32.
When bone formation occurs, osteoblasts first lay down a seam of osteoid, an unorganized non-mineralized matrix of collagen. Mineral/hydroxyapatite crystals are, thereafter, being deposited in gaps within the arranged collagen α-triple helix33. Examples of key factors in bone healing that enable osseointegration are presented in Figure 1.
Summary, discussion, and specific relevance to the present thesis: Osseointegration of titanium implants have been extensively investigated, however, we do not possess complete understanding of the mechanisms behind osteogenesis and bone healing around implants. Studies on biomolecular and cellular effects of titanium surface characteristics are being more common and will probably improve the possibility to more accurately modify an “optimal” surface. Whether this “optimal surface” would possess properties that have desired effects not only regarding bone-tissue, but in addition with respect to soft-tissue and microbial biofilms, is to be discussed in this thesis.
Figure 1. Schematic illustration of the events occurring after implant installation, with acute inflammation, biomolecule
adsorption, bone tissue healing, and remodelling
1, 2 . Rat model 3, 4 , rabbit model 5-8 , dog model 9, human model 10
Integration into oral mucosa and soft-tissues
The oral mucosa surrounding implants has been suggested to have both similarities and differences to the gingiva around teeth. From the marginal bone level to the marginal gingiva about 1-1.5 mm of connective tissue are suggestively in close contact with the implant, followed by about 2 mm of junctional epithelium, and a keratinized oral epithelium34. Hemidesmosomes as well as a basal lamina have been found at the interface between titanium and epithelial cells35. This relation does seem to be re-established if disrupted followed by healing36. If only disrupted by clinical probing, the epithelial attachment is suggested to heal in five days37. One difference between the peri-implant mucosa and the gingiva surrounding a tooth is the arrangement of the subepithelial connective tissue. Collagen fibres project from the root cement into the connective tissue proper, while a commercially pure titanium implant is surrounded by dense, connective tissue with collagen fibres and fibroblasts extended mainly in parallel with the implant surface in man38-40. There are, however, findings of random 41, circular42 , or even perpendicular fibre orientations43, around specific surfaces. A tight sealing between the oral mucosa and the implants is believed to be of importance for the defence against extensive biofilms44 and the protective mechanisms seem to correspond to the gingiva surrounding a tooth42. However, an in vivo study in dogs suggested that peri-implant tissues may have decreased defence capacity when it is poorly vascularised45.
In an in vitro study, multiple integrin subunits in human gingival fibroblasts were found grown in contact with titanium implant surfaces and, furthermore, titanium surface roughness altered cellular morphology but appeared to have limited effects on the integrin expression46. However, connective tissue cells are greatly influenced by an extra-cellular matrix, and in vitro results may only vaguely reflect the physiological situation47.
Summary, discussion, and specific relevance to the present thesis: For oral implants, the relation to soft-tissue has not been explored in as great extent as regarding bone-tissue. However, soft-tissue attachment is of importance for the infection resistance and the aesthetics of implant treatments. There are surface modifications suggested to improve soft-tissue adhesion and one such has been investigated in this thesis.
Titanium as a biomaterial
A major reason behind titanium being a gold standard for osseointegration is the native titanium oxide layer (about 2-7 nm thick) established instantly when in contact with oxygen. The oxide layer offers a stable and corrosion resistant outer layer of the bulk material, giving the material outward characteristics more of a ceramic and makes the metal biocompatible. The native oxide of a titanium surface is depending on the bulk material but mainly consists of titanium oxide and has an amorphous structure. If titanium is oxidized further (thermally or electrochemically), TiO2 may assume three crystalline phases: anatase, rutile, and, if not as common, brookite48.
Titanium may exist in two crystallographic forms: the hexagonal close-packed crystal structure, named the alpha structure, and a body-centred cubic structure, named the beta structure. In few words, the two phases, or a combination of them, gives the metal somewhat different properties. The beta phase of titanium appears when titanium is heated above 883°C and solidified rapidly and stabilized using stabilizing elements. 49Commercially pure titanium (ASTM F67, grade 1 to 4) is composed of 98.9-99.5 weight percent pure titanium; all has alpha crystallinity, and the differences between the grades are the content of impurities (carbon, nitrogen, iron, hydrogen, and oxygen). To further increase the mechanical properties, for example, when used as total hip replacements or amputation prosthesis carrying substantial load, alloys of titanium are used for biomedical applications and have been shown reasonably biocompatible. Titanium alloys are considerably harder materials compared to commercially pure titanium. Ti-6Al-4V is the most commonly used titanium alloy in the biomedical field and also called titanium grade 5; it has a mixture of alpha and beta phased crystals, as have many of the other titanium alloys.49
Some studies indicate similar response to titanium alloys as to commercially pure titanium50, whereas others have indicated somewhat weaker bone responses to the alloy compared to commercially pure titanium51. Originally, the Brånemark concept strictly advocated titanium grade 1 for osseointegrated implants, but today most commercial titanium oral implants are made from commercially pure grade 4, and some from titanium alloy (grade
5). What needs to be kept in mind is that the resultant surface characteristics of a certain surface process may differ between different grades of titanium when these possess different mechanical as well as chemical properties. Focus will be on commercially pure titanium in the following parts of the thesis, where all studies were performed with titanium grade 4.
The primary interaction between an implant and the host is by adsorption of water molecules and ions followed by proteins from the blood plasma. Titanium and titanium alloys have the ability to spontaneously allow calcium phosphate nucleation on the surface in a solution; the calcium phosphate formed on commercially pure titanium most resembles apatite52. Furthermore, ions generally modulate the adhesion of proteins and extracellular Ca2+ has been suggested to link proteins to TiO253, 54. The part of the implants positioned in the bone mainly interacts with plasma proteins, for example, fibrinogen, albumin, immunoglobulin G53, 55. Albumin has been suggested the main salivary protein adsorbed on titanium surfaces56.
Proteins generally adsorb to a surface, possibly unfold, and thereafter bind firmly or are exchanged or covered by other proteins. The protein adsorption may, in addition, be altered by surface characteristics, such as the topography55 and the physico-chemical properties57. Possibly, a protein with an RGD sequence could be sought to express its RGD sequence to the surroundings after having adsorbed to a surface in order to promote cell adhesion. It has also been suggested that surface chemistry increases the biological activity of, e.g. the integrin-binding protein fibronectin, resulting in enhancement of cell adhesion58.
Summary, discussion, and specific relevance to the present thesis: For many years it has been known that titanium as a bulk material possesses qualities appropriate for biomedical implants, nevertheless, it is the native or modified titanium oxide layer that enables the tissue integration. The properties of the surface impact the protein adsorption, and, furthermore, the proteins are of importance for the biological response (by bone cells, soft-tissue cells, as well as bacteria). However, the protein adsorption to the titanium surfaces used has not been investigated within the frame of this thesis.
bioactivity
The term bioactivity indicates that something interacts or stimulates an effect in biological structures. Suggestively, the term “bioactivity” should be addressing a phenomenon and not a specific surface or material. Bioactivity may be defined as involving biochemical bonding, or that a surface attracts certain proteins and/or stimulates bone cells, having a catalytic effect on other processes within the cell. Hench, who has mainly been working with bioglass or bioglass ceramics, defined bioactivity, referring to the biochemical bonding, as: “Bioactivity is the characteristic of an implant material which allows it to form a bond with living tissues” 59. Williams defined bioactivity, aiming at the stimulating effect rather than the bonding, as: “Phenomenon by which a biomaterial elicits or modulates biological activity” 60. One example of bioactive processes is discussed by Davies, with the importance of platelet activation and osteoblasts and pre-osteoblasts migrating through the fibrin network to attach to a surface and deposit osteoid; resulting in de novo bone formation 2, which may also be described as bone induction.
Regardless of the definition, the aim of a surface with bioactive characteristics would be more rapid integration, resulting in shorter healing periods, and, possibly, stronger anchorage of the implant. In the thesis by Göransson (2006) the following surface modifications result in surfaces with properties of possibly bioactive character: fluoride etching, alkali heat treatment, anodization with
e.g. ion incorporation into the oxide layer, hydroxyapatite coating,
and covalent immobilization of proteins61.
Summary, discussion, and specific relevance to the present thesis: Bioactivity is a widely used term. The concept should suggestively be used to describe the nature of surface characteristics, instead of being considered a particular surface character. Suggested bioactive surface characteristics have indicated advantages for osseointegration, and one surface with allegedly bioactive characteristics (i.e. anodically oxidized and calcium ion incorporated) have been investigated in this thesis.
Methods for titanium surface processing
There are a number of processes used to modify titanium implant surfaces. The selected process is depending on the mechanical properties required, as well as which parameters that are aimed to be modified. However, it is difficult to alter the chemistry without altering the topography and vice versa. It is common that methods are combined to achieve the preferred surface characteristics. The resulting surface can be controlled to various degrees with different methods. Some examples will follow.
Physical treatments: Turning process
The turning process of dental implants is often used to gain the macro design of the implant that may, thereafter, be modified. Turned surfaces have mainly been found smooth (average height deviation <0.5 µm) or minimally rough (average height deviation of 0.5-1 µm) and anisotropic due to the turning process. The original Brånemark implant has a turned, minimally rough surface. Today, studies of more than 20 years follow-up times for functional implants are reported with the original Brånemark system implants 13. M ost abutment surfaces have smooth turned or polished surfaces 62.
Grit/abrasive blasting
The processing can produce isotropic surfaces with various roughnesses and chemistries depending on the blasting particle (TiO2 and Al2O3 are commonly used) and its size, as well as the pressure and the distance of the blasting instrument. There are commercial implants with a blasted surface alone (TiOblast™ from Astra Tech AB, Gothenburg, Sweden) or in combination with other surface processes (for example OsseoSpeed™ from Astra Tech AB, and SLA®/SLActive® from Institute Straumann AG, Basel, Switzerland). Most blasted surfaces achieve a minimally to moderately rough (average height deviation of 1-2 µm) surface.
Ultraviolet irradiation of TiO2 crystalline surfaces
By treating a crystalline surface with ultraviolet irradiation, decomposition of organic compounds occur and an extremely clean surface is achieved 63. Furthermore, surface oxygen vacancies appear,
which interact with water molecules and forms hydroxyl-groups with hydrophilic domains on the outermost layer 64. A surface need to be crystalline in order to respond to the treatment in the wanted manner, and cannot have an amorphous outer titania layer. After the surface processing the surface, although being highly hydrophilic, becomes amphiphilic 63 and may, thereby, attract different proteins at different regions. Specific surface chemistry may, in addition, be applied to the surfaces and may, further, influence the photocatalytic effect 65. The surface topography depends on the original surface, but is in general isotropic, and, mostly, minimally 65 and moderately rough surfaces have been investigated in the literature 66, 67.
Electrochemical treatments:
Micro-arch oxidation/anodic oxidation
By using electrochemical oxidation surface topography as well as chemistry may be altered 68. Anodized surfaces acquire an isotropic, porous appearance with pore size and distribution depending on the electrolyte as well as the voltage/current of the oxidizing process. The process can be modified to achieve a relatively high control of the resulting surface, for example, nanotubular structures. The TiUnite® surface of Nobel Biocare™ is anodized and has a moderately rough surface, with porous structures of a diameter 0.5-3 µm in general; it presents phosphor ions in the oxide layer69, which is about 2-8 µm thick and has both anatase crystallinity and an amorphous phase70, 71. The Ospol AB, Malmö, Sweden, surface is a smooth anodized surface with calcium ions within its oxide layer. The Ca (study I) or OxCa (study II, IV, and V) is processed according to the same protocol as the Ospol surface and similar to that presented by Sul et
al. (2002)72.
Chemical treatments: Acid etching
Etching of a surface, mainly via a thermal process using acids that resolves the outermost layer of a material, in general creates an isotropic surface with a negative skewness. Commercial dual acid-etched implants are OSSEOTITE® (BIOMET 3i™), which are etched with HCL/H2SO473 and has a minimally rough surface74. The SLA®/SLActive® surfaces from Institute Straumann AG
(Waldenburg, Switzerland) is firstly blasted, secondly acid etched, resulting in a moderately rough surface. The OsseoSpeed™ surface from Astra Tech AB is the TiOblast™ surface with further etching of hydrofluoric acid, resulting in a chemically modified, moderately rough surface with nanofeatures; the oxide thickness is up to 1 µm and consisting of an inner amorphous layer followed by an outer crystalline layer (anatase and rutile)71.
Alkali heat treatment
By treatment of NaOH followed by heating, an amorphous sodium titanate surface is achieved. The surface have been found to initiate apatite formation in simulated body fluid; the scenario has been suggested to begin with ion exchange between the material (sodium) and the solution (hydronium and, thereafter, calcium)75. When calcium titanate constitutes the outermost surface, adsorption of phosphate and calcium ions occurs. The processing can be performed on various initial surfaces. The surface orientation and roughness depend on the original surface; however, the process tends to create an isotropic surface.
Depositional treatments: Ion implantation
Ion implantation involves an ion source, an accelerator, and a chamber where the surface is positioned. When ions are sputtered towards a surface using lower energies the process is defined as ”ion beam deposition”. Various ions can be implanted to various depths and in controlled concentrations. Ion implantation can be executed on any initial surface, but with somewhat different outcome (depending on the hardness etc.). The surface topography and orientation is nearly solely depending on the original surface. For a review, see Rautray et al. (2010).76
Sol-gel coatings
Sol-gel technology allows preparation of materials with a wide range of topographical, physical, and chemical properties. Most achieve an isotropic appearance. The sol-gel technique, furthermore, provides a surface coating that is relatively easy to control in great quantities and for larger implant sizes. A solution (“sol”) is prepared with a
specific composition and often matured to retain a solid and a liquid phase. The specimen is, thereafter, dipped at a specific number of times, with drying in between, and finally heat-treated to solidify and sinter the coating. It has been shown that sol-gel matrices can be modified with organic functional groups77, that may incorporate proteins and release them at a controlled rate78. Sol-gel techniques are used to achieve nanotopographical features. An example of sol-gel coating used commercially is the dental implant NanoTite® (BIOMET 3i™), which has a surface treated with discrete crystalline deposition of calcium phosphate that is sol-gel derived.
Summary, discussion, and specific relevance to the present thesis: There are a number of available processes to modify titanium surfaces. Many of them allow for great control of the effect on the surface, which is of importance in the production of commercial implants as well as for research studies. However, it is still difficult to alter only one specific characteristic, since they often affect one another. The surfaces used in the studies of this thesis have frequently been aimed to posses either similar topographic or chemical features. The methods used have been: turning process, blasting with Al2O3, anodic oxidation and incorporation of calcium ions, sol-gel deposition of TiO2, or combinations thereof.
Surface properties of titanium implants
aimed for bone integration
Macro design
The threaded, screw shaped implant is today the dominant design, although, the ideal thread type remains to be described. Screw shaped implants are advantageous to cylindrical implants since they, for example, have improved load distribution and a larger area with close fit to the bone 79. Companies offer both straight walled or tapered implants, and the shape and depth of the threads also varies. Hansson et al.80 suggested enhanced load distribution with microthreads resulting in significantly less bone resorption compared to implants without microthreads81.
Modern implants today presents high survival rates, even if short implants (<10 mm) are assessed82, 83.
Micro topography
Wennerberg proposed in her thesis (1996) an optimal topography for bone integration of titanium implants to be a surface with an average height deviation of 1.5 µm, an average wavelength of about 11.1 µm, and a developed area ratio of 50 percent. The following section will mainly focus on average height deviation or roughness in height, the Sa parameter.
Albrektsson and Wennerberg84 suggest titanium implant surfaces to be divided into smooth (<0.5 µm), minimally rough (0.5-1.0 µm), moderately rough (1.0-1.5 µm), or rough (>2.0 µm) according to their average roughness in height. As can be understood from earlier presented findings, moderately rough surfaces may be considered to stimulate the strongest bone integration. Although the importance of surface parameters has been questioned85, most commonly used commercial implants today have minimally or moderately rough surfaces86. Whereas these modern surfaces have been backed up by published clinical studies with survival rates of about 97 percent for five to ten years follow-up times87-89, possible risks with moderately rough surfaces may be increased risks in cases with peri-implantitis and, to a minor extent, ion leakage. These will further be discussed under the section “Complications with implant treatments”.
nano topography
Nano features of titanium implants have been suggested of importance for bone integration mostly by affecting the wettability, ion and protein adsorption, as well as the cells90. The bone response in a rabbit model was enhanced by a nanofeatured surfaces with hydroxyapatite deposition91, and in another study nanofeatures of titania presented similar, or even a tendency to enhanced, bone response as nanofeatures of hydroxyapatite92. Although, today when discussing nanofeatures it is most often as an additional structural dimension on top of the microtopography; most commercial implants proclaiming a nanofeatured topography (for example OsseoSpeed™ from Astra Tech AB, SLActive® from Straumann AG, and Nanotite from Biomet 3i inc) have a minimally or moderately rough microtopograhy93.
The dimensions of the concept of nanofeatures are similar to that of microfeatures, e.g. both the distribution and the dimensions may be of importance92. With various techniques altered structure of the features can be established, and the effect of orientation and character of nanofeatures are not yet fully evaluated.
chemistry
Ion incorporation of: Phosphorous ions
Phosphorous ions can be found on the TiUnite® surface (Nobel Biocare™) at the rate of approximately two atomic percentage69. Hydrothermal treatment with phosphorous ions have resulted in higher removal torque and bone in contact ratio as compared to turned, acid etched, grit blasted, grit blasted and acid etched, or spark anodized after six weeks in rabbit94.
Fluoride ions
Fluoride ions have high affinity for calcium and phosphate ions, and fluoride-modifications have been found to stimulate mesenchymal bone cells29, 95. Fluoride increases the density of bone96, which may have an impact on the bone-tissue properties adjacent to the implant surface. Furthermore, in vivo models demonstrate advantages with fluoride modifications. Increased bone-tissue contact has been found through studies in dog97, 98 and rat29, together with increased biomechanical strengths in rabbit-studies evaluated for one to three months, as compared to somewhat rougher blasted surfaces99-101.
There is one commercial oral implant surface that is fluoride modified by etching with hydrofluoric acid, OsseoSpeed™ by Astra Tech AB. Yet, Kang et al. (2009) found F on the OsseoSpeed™ surface at very low ratio (0.3 atomic%) when using both X-ray photoelectron spectroscopy and auger electron spectroscopy69.
Magnesium ions
Magnesium implantation has been suggested to increase the hydroxyapatite nucleation and apatite growth on titanium. Magnesium ion modifications increased the adhesion and up-regulated intracellular cascades of human bone derived cells compared to non-modified Al2O3 surfaces102 and Ti6Al4V surfaces103, as well as for mouse osteoblast like cells on smooth and moderately rough surfaces104. Mg2+-implanted surfaces have also shown improved interfacial shear strength105-109, and more rapid osseointegration108, 109 as compared to control implants. In another study, Mg2+ -incorporated micropatterned surfaces increased resonance frequency measurement results as compared to commercially available implants SLA®, Osseotite, TiOblast™, and Mg2+-incorporated TiUnite®; but without the micropatterned threads, the Mg-modifications did not enhance the bone-integration110.
Calcium ions
In the thesis by Sul (2002), possible effects of incorporated Ca2+ were suggested. These are mainly that Ca2+ within the oxide layer moves towards the outer surface and the extracellular body fluids; thereafter, electrostatic interactions between the calcium ions and ions as well as adhesive bone matrix proteins arises, and calcium ions may also stimulate RGD surface receptors and prompt the recruitment of osteoblasts and osteoprogenitor cells111.
Calcium ions may be deposited through, for example, ion implantation, incorporation into the titania during electrochemical oxidation, or through plasma immersion. The dissolution of Ca2+ has been suggested a key factor for hydroxyapatite nucleation on calcium modified surfaces112. Ca2+ implantation accelerates the adsorption of phosphate ions and improves the ability of titanium to induce the formation of calcium phosphate minerals on the surface112-115, possibly, due to a more positively charged
surface and more hydroxyl radicals as compared to unmodified TiO2116. When positioned in simulated body fluid the formation of octacalcium phosphate has been suggested energetically favourable as compared to hydroxyapatite, but since hydroxyapatite is more thermodynamically stable the dominating mineral crystal will shift from octacalcium phosphate to hydroxyapatite over time117. Mainly electrostatic interactions are suggested to be of importance for the mineral nucleation on a surface.
In vitro studies have generally shown that cells adhere in a
lesser extent to calcium modified surfaces, meanwhile they spread more and seemingly become more activated. Nayab et al. (2005) suggest that early phosphate and calcium precipitation on calcium ion implanted surfaces explain the lesser attachment of bone cells. After a certain time (suggestively 24 hours) these particles undergo conformational changes and an increase in cell adhesion occurs118.
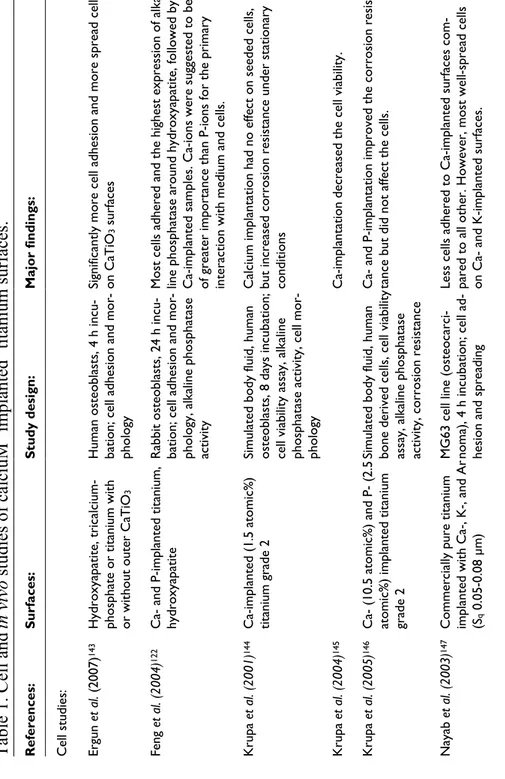
In previously published in vivo studies using anodized commercially pure titanium with incorporated Ca2+, calcium modification enhanced the osseointegration119-121. Possible effects of the calcium ions may be that they attract proteins and growth factors of importance for the bone cells and bone formation118, 122, that they enhance bone cell growth123, 124, and that they function as binding sites for bone mineral crystals125 through ion adsorption as earlier mentioned. Studies in the literature regarding calcium-implanted surfaces are gathered in Table 1.
Calcium ions are incorporated in the commercial Ospol AB surface at approximately two atomic percent, and was also found at somewhat over one atomic percent on the OsseoSpeed™ surface analyzed with X-ray photoelectron spectroscopy69.
Calcium phosphates
There are a number of calcium phosphates that may form biologically as well as synthetically126. Various ions can be incorporated in the apatite crystal, for example CO32- and F-, which results in somewhat different qualities (e.g. solubility)127. Tricalcium phosphate (the β- form mainly used as biomaterial) is a rapidly resorbed crystal but which partly converts into more stable hydroxyapatite with the right composition of the surrounding electrolyte or when surrounded by body fluids. β-tricalcium phosphate is also sintered together with
hydroxyapatite to slow down the degradation rate; the ceramic is then called biphasic calcium phosphate. Octacalcium phosphate is another pre-cursor to hydroxyapatite. The composition of the calcium phosphate is of importance to control when establishing a calcium phosphate coated surface128.
One possible disadvantage with apatites is that they may resolve in a physiologic environment, or fracture from the bulk material. This was primarily a problem when hydroxyapatite was deposited using plasma-spraying methods129, 130. However, by using techniques to deposit thin layers of hydroxyapatite (sol-gel coatings or other refined methods), coat loosening may no longer be a problem. Calcium phosphates or hydroxyapatite coatings have been suggested to create a surface with bioactive characteristics and mineral nucleation occur upon the surface127.
There are a number of methods to coat a surface with calcium phosphates; for example, plasma spraying, electrophoretic deposi-tion, sputter deposideposi-tion, and sol-gel coating. Calcium phosphates are commonly used within the orthopaedic research and the com-mercial dental implant surface NanoTite® (BIOMET 3i™) has a surface treated with sol-gel derived discrete crystalline deposition of calcium phosphate131, 132.
Immobilization of biofunctional molecules/proteins
Proteins can either be adsorbed or covalently immobilized on surfaces. Challenges are to maintain the biofunctionality after immobilization and to control the diffusion rate. For bone-tissue interactions, mainly RGD-peptides133, collagen134, and BMP-2135, 136 have been tested. Furthermore, immobilizing other cell adhesive molecules, e.g. tetra-cell adhesion molecule, may modify and improve the osteogenesis137.
Bisphosphonates
Bisphosphonates are commonly used systematic drugs to treat conditions such as osteoporosis and have an inhibitory effect on osteoclasts. Immobilized bisphosphonates on titanium138, stainless steel139, 140, and hydroxyapatite coated titanium or titanium alloy implants141, 142 have indicated enhanced bone response from various studies in rats and dogs. The application is relatively modestly explored and research within the field is ongoing.
surfaces.
Maj or fi nd in gs : Si gn ifi ca nt ly m or e ce ll ad he si on a nd m or e spr ea d ce lls on C aT iO3 s ur fa ce s Most cell s adhered and the highe st expressi on of al ka-line phosphata se around hydroxyapatite, followed by C a-im pl an te d sa m pl es . C a-io ns w er e su gg es te d to b e of greater imp ortance than P-i on s for the primary interaction with medium and cell s. C al ci um im pl an tat io n ha d no e ffe ct o n se ed ed c el ls , but increased co rrosion resistan ce under station ary conditions Ca-implantation decreased the ce ll viabili ty. Ca- and P-impla ntation improve d the corrosion resis-tance but did no t affect the cell s. Le ss c el ls a dh er ed t o C a-im pl an te d su rf ac es c om -pared to all othe r. However, mo st well -spread ce lls on Ca- and K-i m planted surfaces.M
i
m
pl
an
te
d
tit
an
iu
m
St ud y de si gn : H um an o st eo bl as ts , 4 h in cu -bation; cell adhe sion and mor -phology Rabbit ost eoblasts, 24 h incu-bation; cell adhe sion and mor -ph ol og y, a lk al in e ph os ph at as e activity Simulated body fluid, human osteoblast s, 8 da ys incubat io n; ce ll vi ab ili ty a ss ay , a lk al in e ph os ph at as e ac tiv ity , c el l m or -phology Simulated body fluid, human bo ne d er iv ed c el ls , c el l v ia bi lit y as sa y, a lk al in e ph os ph at as e ac tiv ity , c or ro sio n re si st an ce MG63 cell lin e (osteocarci -no m a) , 4 h in cu bat io n; c el l a d-hesion and sprea dingin
v
iv
o
studies
of
calciu
Su rf ac es : Hydroxyapatite, tricalcium-ph os ph at e or t itan iu m w ith or without out er CaTiO 3 C a nd P -im pl an te d tit an iu m , hydroxyapatite C a-im pl an te d (1 .5 at om ic % ) titanium grade 2 C ( 10 .5 a to m ic % ) an d ( 2. 5 atomic%) implan ted titanium gr ad e 2 C om m er ci al ly p ur e tit an ium implanted with Ca-, K-, and Ar (Sq 0.05 -0.08 µm )Table 1. Cell an
d
R ef er en ce s: Cell studies: Er gu n et al. ( 20 07 ) 143 Feng et al. (2004) 122 K ru pa et al. (2001) 144 K ru pa et al. (2004) 145 K ru pa et al. (2005) 146 Nayab et al. (20 03) 147M aj or fi nd in gs : Ca-implanted su rfaces had le ss ad hered but more spread cells, w ith hig her pr ol ife ra tio n co m pa re d to a ll other surface s. The other io n-i m planted surfaces were similar to controls. . Cells presented a more co mplex morphology (si m ilar to a ct iv e os te ob las ts ) o n al l C a-im pl an te d su rf ac es compared to co ntrols. Surfaces im planted with hi gh dose of Ca had le ss adhered but more spread cell s after 2 h, ho wever, after 24 h more cells co mpared to controls. A fter 24 h there was an up-regulation of a5b1 integrin an d vinculin po sitive adhesion plaqu es with an increas e in cell number, siz e, and gran ula rity on C a-im pl an te d su rf ac es . Ca-implanted su rfaces present ed cells with up -re gu la te d os te opo nt in ( ge ne a ct iv at io n) , b on e morphogenetic protein, and bon e sialop ro te in . N o differences betw een alkaline pho sphatase and os-teonectin. Si gn ifi ca nt ly m or e pr ol ife ra tiv e and m ito tic c el ls , w ith a m or e ra pi d ce ll cy cl e, o n C a-im pl an te d su rf ac es . Ca-implanted su rfaces present ed an increased nu mber of c el ls a fte r 4 day s. C a-im pl an ta tio n of m od er at el y rough surfaces increased the ost eoblastic gene-expression of al kaline phosphata se, ost eopontin, and os te on ec tin . St ud y de si gn : Harvested human bone cells, 4 h or 48/72/96 h incubation; cell adhesion, m orphology, and growth MG63 cells, 4 an d 24 h incuba-tion; cell adhesi on, analysi s of in te gr in a dh es io n m ol ec ul es , vinculin adhesi on plaques MG63 cells, 24 h and 6 days incubation; prot ein expression , os te op on tin g en e ex pr es si on MG63 cells, 24, 48, and 72 h incubation; Ki-6 7 expression, number of mitot ic cell s A pa tit e fo rm at io n, M C T 3T 3-E1 c el ls ; c el l v ia bi lit y (m ito -ch on dr ia l f un ct io n) , g en e ex -pr es si on Su rf ac es : C om m er ci al ly p ur e tit an ium implanted with Ca-, K-, and Ar C om m er ci al ly p ur e tit an ium with low mediu m and high doses of implan te d Ca C om m er ci al ly p ur e tit an iu m , C a-im pl an ta tio n C om m er ci al ly p ur e tit an iu m , C a-im pl an ta tio n Sm oo th t ur ne d co m m er ci al ly pure titanium an d ,moderately rough hydroxyapatite-blasted su rf ac es , C a-im pl an ta tio n R ef er en ce s: Nayab et al. (20 04) 124 Nayab et al. (20 05) 118 Nayab et al. (20 07a) 123 Nayab et al. (20 07b) 148 Pa rk et al. (2008) 149
M aj or fi nd in gs : Si gn ifi ca nt ly m or e bo ne in c on ta ct t o C a-in co rp or at ed su rf ac es w he n pl ac ed in t ib ia . Ca-implanted su rfaces had sign ifi cantly higher mi ner-alization index and osseointegrat ion index. More newly for m ed bone and bo ne in contact w ith C a-im pl an te d su rf ac es . Ca-implanted an d hydroxyapatite surface s prese nted rapid bone formation. Greater bo ne volu mes around C a-im pl an te d su rf ac es t ha n hy dr ox ya pa tite a t al l t im e-po in ts . S ig ni fic an tly le ss d ec re as e in b on e vo lu m e fr om 4 to 8 w ee ks a ro un d C a-im pl an ts c om pa re d to h y-droxyapatite. Th e amount of bon e increased arou nd pu re t ita ni um s ur fa ce s ch ro no lo gi ca lly . T he t hi ck ne ss o f C aT iO3 a ffe ct ed t he a pa tit e fo rm a-tion. There were no differences regarding the soft-tis su e, h ow ev er , C a-im pl an ts s tim ul at ed b on e fo rm a-tion and present ed bone directly on the surface. St ud y de si gn : R ab bi ts , 1 2 w ee ks ; h is to lo gy (bone in conta ct , bone area) Rabbits, 12 wee ks; histolog y Rat, 2, 8, and 18 days; histol -og y Rabbits, 2, 4, an d, 8 weeks; re m ov al t or qu e, b on e vo lu m e Rat, 7 and 28 da ys; so ft tissue and bone respo nse Su rf ac es : Sm oo th a no di ze d C a-incorporated, and minimally ro ug h an od iz ed a nd b la st ed su rf ac es . Turned or laser etched mi -croarc oxidated and Ca-implanted Titanium grade 1 and slightly rougher Ca-impl anted surfaces Titanium grade 2, hydroxyapa-tite, and Ca-imp lanted surafces Titanium grade 2, magnetron sputtered CaTiO 3 R ef er en ce s: In vivo Fr oj d et al. (2008) 119 G uo et al. (2010 ) 150 Hanawa et al. (199 7a ) 151 Ic hi ka w a et al. (2 00 0) 152 O ht su et al. (2007c) 153
M aj or fi nd in gs : In cr ea se d ap at ite fo rm at io n, in cr ea se d ce ll via bil ity after 4 days, and signi fic antly high er removal torq ue an d m or e bo ne in co nt ac t w ith C a-imp la nts . More bone in co ntact with Ca -implanted surfaces. No differences regarding bone area. P-implanted surfaces had the hi ghest remova l tor que values and bone in contact , follo wed by the Ca -im pl an ts . Higher mineral apposition rate after 2 weeks, initi ally enhanced osteo blast adhesion and higher metab olic ac tiv ity , a s w el l a s, m or e ex te ns iv e bo ne in c on ta ct t o anodized Ca- an d P-implanted im plants. Anodi zed Ca- an d P-im pl an te d im pl an ts a cc el er at ed t he p ri m ar y os -te og en ic r es po ns e. Si gn ifi ca nt ly h ig he r re m ov al t orq ue , m or e bo ne in contact, and gre ater bone area ar ound Ca-impla nted su rf ac es . Si gn ifi ca nt ly e nh an ce d os se oi nt egr at io n in t he m ax il-la e. St ud y de si gn : A pa tit e fo rm at io n, M C T 3T -E 1 ce lls (1 a nd 4 d ay s) , r ab bi t (6 w ee ks ); ce ll vi ab ili ty , r em ov al torque, bone in contact R ab bi t, 6 w ee ks ; h is to lo gy (b on e in c on ta ct , b on e ar ea ) Rabbits, 6 weeks ; removal torque, bone in contact Human osteobla sts, rabbits (2 and 4 weeks ); ce ll morphol -og y, a dh es io n, p ro lif er at io n, and metabolic activity, hi sto -morphometry Rabbits, 6 weeks ; removal torque, bone in contact, and bone area within the three first threads Human, 2 mont hs; histolog y Su rf ac es : T i6 A l4 V , C a-im pl an ta tio n w ith di ffe re nt c on ce nt ra tio ns ( al l had smooth surfaces) Blasted and acid-etched, blasted acid-etch ed and Ca-implanted rough surfaces Turned, acid etc hed and P-implanted, grit-b lasted, grit-blasted and acid-etched, ano-dized with Ca-in corporation Anodized with incorporated C a nd P -io ns , a ci d-et ch ed Moderately rough hydroxyapa-tite and Ca-impl anted (40 atomic%) hydroxyapatite Turned (smooth ) and ano-di ze d C a nd P -in co rp or at ed (m in im al ly r ou gh ) su rf ac es R ef er en ce s: Pa rk et al. (2007b) 154 Pa rk et al. (2009b) 155 Pa rk et al. (2009a) 94 R av an et ti et al. (2 01 0) 156 Suh et al. (2007) 157 Sh ib li et a l. (2 00 7) 158