ContentslistsavailableatScienceDirect
Japanese
Dental
Science
Review
j o u r n a l ho me p a g e : w w w . e l s e v i e r . c o m / l o c a t e / j d s r
Review
Article
Effects
of
the
local
administration
of
antibiotics
on
bone
formation
on
implant
surface
in
animal
models:
A
systematic
review
and
meta-analysis
Ali
Alenezi
a,∗,
Bruno
Chrcanovic
baDepartmentofProsthodontics,CollegeofDentistry,QassimUniversity,SaudiArabia bDepartmentofProsthodontics,FacultyofOdontology,Malm¨oUniversity,Malm¨o,Sweden
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:Received11June2020
Receivedinrevisedform25July2020 Accepted19September2020 Keywords: Dentalimplants Boneformation Animalmodels Drugdelivery Antibiotics Systematicreview
a
b
s
t
r
a
c
t
Purpose:Thisreviewaimedtoevaluatetheeffectsofthelocaldeliveryofantibioticsincorporatedin
implantsurfacesonsomequantitativeparametersofboneformation.
Materialsandmethods:Anelectronicsearchwasundertakeninthreedatabases(PubMed,Scopus,Embase)
inadditiontohandsearching.Thesearchwaslimitedtoanimalexperimentsusingendosseousimplants
combinedwithlocalizedantibioticsrelease.Meta-analyseswereperformedforthepercentagesofbone
volume(BV)andbone-to-implantcontact(BIC).
Results:Ninestudiesmettheinclusioncriteria.Severalmethodswereidentifiedforlocaldeliveryof
antibioticsatthebone-implantinterface,butthemostcommonlyusedmethodwasbycoating
(incor-poratingtheimplantsurfacewiththeantibioticagents).Differentantibioticagentswereused,namely
bacitracin,doxycycline,enoxacin,gentamicin,minocycline,tobramycin,andvancomycin.Therewasno
statisticallysignificantdifferenceinthepercentageofBICbetweenimplantswithorwithoutlocalized
antibioticrelease(P=0.59).Themeta-analysisrevealedhigherBVaroundimplantscoatedwithantibiotics
comparedtocontrolgroups(withoutantibiotics)(P<0.01).
Conclusion:Itissuggestedthatthelocaladministrationofantibioticsaroundimplantsdidnotadversely
affectthepercentageofdirectbonecontactaroundimplants,withatendencyforaslightlybetterbone
formationaroundimplantswhencombinedwithlocaladministrationofantibiotics.Itisamatterof
debatewhethertheseinvivoresultswillhavethesameeffectintheclinicalsetting.However,therisk
ofbiasofthesestudiesmay,tosomeextent,questionthevalidityoftheseresults.
©2020TheAuthor.PublishedbyElsevierLtdonbehalfofTheJapaneseAssociationforDental
Science.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/
licenses/by-nc-nd/4.0/).
1. Introduction
Implantrestorationstoreplacemissingteethbecametodayone ofthemaintreatmentmodalitiesindentalpracticewithmillions ofimplantsplacedeveryyeararoundtheworld[1,2].Manyofthe long-termstudiesonimplantrestorationsreportedsurvivalrates exceeding90%after10yearsoffollow-up[3,4].
However,implantassociatedinfectionsremainagreatthreat thatmayleadtoseveralcomplicationssuchasmarginalboneloss, complexrevisionprocedures,andeventuallyimplantfailure. Bio-materialassociatedinfectionsareseenasabigchallengesincethey aredifficulttotreat[5,6].Asaresultoftheseofinfections,aseries
∗ Correspondingauthorat:DepartmentofProsthodontics,CollegeofDentistry, QassimUniversity,P.O.Box6700,Burydah,51452,SaudiArabia.
E-mailaddresses:dr.ali.alenezi@qudent.org,4110@qu.edu.sa(A.Alenezi).
ofinflammatoryresponsesaregenerated,whichcouldcomplicate theintegrationofimplantsandbonehealing[7].
Thebacterialinvasiontotheimplantsite isbelievedto hap-penfollowingthetraumatothehardandsofttissueafterimplant surgery [8]. Following that, different bacterial strains, mainly Staphylococcusepidermidis,attachtoimplantsurfacetostimulate thesynthesisofextracellularmatrix[9,10].Thepresenceofthis matrixwillfacilitatethebiofilmformationand,ifnoactionsare taken,couldleadtoinfection[11,12].Suchcasesrequireearly treat-menttoavoidtheneedofadvancedprocedurestokeeptheimplant. In dentistry, antibiotics are occasionally prescribed prior to implantsurgerytodecreasetheriskofinfections [13].The sys-temicadministrationofantibioticsisstilltheconventionalmethod used,althoughconventionalantibioticadministrationcouldhave limitationsrelatedtotheantibioticconcentration[14].Improper antibioticconcentrationinthebloodcouldbringsomeunwanted
https://doi.org/10.1016/j.jdsr.2020.09.003
1882-7616/©2020TheAuthor.PublishedbyElsevierLtdonbehalfofTheJapaneseAssociationforDentalScience.ThisisanopenaccessarticleundertheCCBY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
sideeffects,beingtoxicataveryhighlevel,andineffectiveatavery lowlevel[15].
Besidesthesystemicwayofadministration,thereisalsothe release of antibiotics using local drug delivery systems, which isrecognizedasapromisingmethodtosuppresslocalinfection, alsominimizingthesideeffectsassociatedwiththeconventional administrationofantibiotics.AstudybyMoojenetal.showed bet-terformationofbonearoundimplantscoatedwithantibioticsin comparisontocontrolimplants[16].Theauthorssuggestedthat thiseffectwasaconsequenceofthereducedinfectionratesinthe antibioticgroup,andclaimedthatinfection-freebonemayallow betterboneformationcomparedtoinfectedbone[16].
Mostoftheusedmethodswerebasedonincorporatingor coat-ing theimplantsurface withtheantibioticagents,and someof thesetechniquessucceededtoprovidesustainedantibioticrelease [17,18].Thereleaseofantibioticsdirectlyattheimplantsitecould providelowbuteffectivetherapeuticdosesofdrugcomparedtothe conventionalmethods.Apossiblenegativeeffectofthismethod would be therisk of interferingwith thebone healing process around theimplant. It hasbeenreported in someexperiments that antibiotics could have negative influenceon thefunctions of osteoblasts and osteoclasts [19,20]. Moreover, immobilizing antibacterialagentsontothesurfacesofimplantscouldresultina rapidburstreleaseofantibioticsandlowantibacterialeffects[21]. Theaimofthepresentstudywastoreviewandevaluatethe effectsofthelocaldeliveryofantibioticsincorporatedinimplant surfacesonsomequantitativeparametersofboneformation.
2. Materialsandmethods
2.1. Searchstrategies
Anelectronicsearchwithouttimerestrictionswasundertaken in December2019inPubMed,Scopus,andEmbase.The follow-ingterms,relatedtothreemaincomponents(bone,implant,and antibiotics),wereusedinthesearchstrategiesineachdatabase:
(“boneremodeling”OR“boneformation”OR“bone regenera-tion” OR“bonedevelopment”OR“bonegrowth”OR “osseointe-gration”)AND(“biocompatiblecoatedmaterials”OR“endosseous dental implantation” OR “dental implants” OR “implants” OR “bone-implantinterface”)AND(“antibiotics”OR“antibacterial”OR “antimicrobial”OR“infection”OR“drugdelivery”OR“drugdelivery device”OR“drugdeliverysystem”OR“drugrelease”OR“localdrug delivery”)AND(“animalexperimentation”OR“animalmodels”OR “animals”OR“invivo”).
In addition,thereferencelistofthestudiesandtherelevant reviewsonthesubjectwerealsochecked,besideshandsearching ofimplant-relatedjournals.
2.2. Inclusionandexclusioncriteria
Eligibilitycriteriaincludedpublicationsevaluatingtheuseof localizedantibioticsdeliverywithendosseousimplantsinanimal studies.Theimplantinsertionneededtobecombinedwitha antibi-otics agentthat wasadministratedlocallyor releasedfromthe implant surface. Theantibioticagent shouldhave beenapplied locallybeforeoratimplantinsertion.Onlypublicationswrittenin Englishwereconsidered.
2.3. Studyselection
ThestudywasdesignedbasedonthePRISMAguidelinesto per-formsystematicreviewsandmeta-analysis[22].Potentialstudies identifiedintheinitialsearchwererequiredtomeettheinclusion criteria.Theabstractsofthestudiesidentifiedwereread indepen-dentlybythetwoauthorsofthisstudy.Fulltextswerereadfor
thestudiesappearingtomeettheinclusioncriteria,orforwhich therewereinsufficientdatainthetitleandabstracttomakeaclear decision.Disagreementswereresolvedbydiscussionbetweenthe authors.
2.4. Dataextraction
Thefollowing datawerethenextracted onastandard form, whenavailable:typeofantibioticagent,deliverysystem/method, typeofanimal,numberofanimals,numberofimplants,timeperiod betweentheimplantationsurgeryandtheeuthanasiaofthe ani-mals,meanvalues andstandarddeviationofthepercentagesof bonevolume(BV)andbone-to-implantcontact(BIC)aroundthe implants.
2.5. Riskofbiasinindividualstudies
Theanalysisof theriskof biasfor theincludedstudieswas performedaccordingtotheSystematicReviewCentrefor Labora-toryAnimalExperimentation’s(SYRCLE)riskofbiastoolforanimal studies[23].
2.6. Dataanalysis
PercentagesofBVandBICwerethecontinuousoutcomes eval-uated.Weightedmeandifferenceswereusedtoconstructforest plots.Thestatisticalunitwasthenumberofimplantsusedinthe experimentsineachgroup.
Wheneveroutcomesofinterestwerenotclearlystated,thedata werenotusedforanalysis.TheI2statisticwasusedtoexpressthe
percentageofthetotalvariationacrossstudiesdueto heterogene-ity.Theinversevariancemethodwasusedforrandom-effectswhen therewasstatisticallysignificant(P<0.05) heterogeneity,and a fixed-effectsmodelwasusedwhenheterogeneitywasnot statisti-callysignificant.Theestimatesofaninterventionwereexpressedin meandifference(MD)inpercentage,witha95%confidenceinterval (95%CI).ThesoftwareReviewManager(version5.3.3,TheNordic CochraneCentre,TheCochraneCollaboration)wasusedtoperform themeta-analysis.
3. Results
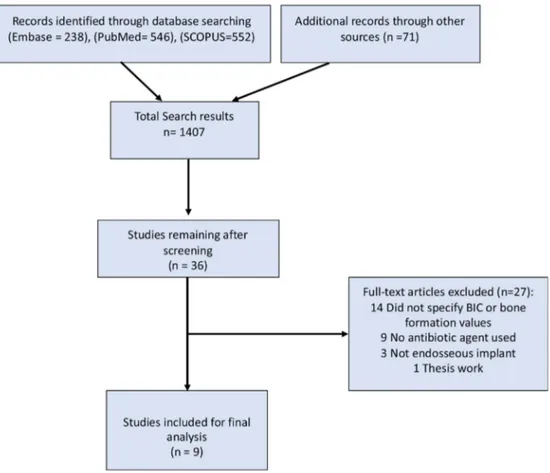
ThesummaryofthestudyselectionprocessisshowninFig.1. Thesearchprocessusingthe3selecteddatabasesandthehand searchingresultedin1407papersthatwereinitiallyscreened.The secondscreeningphaseforpapersthatappearedtomeetthe inclu-sioncriteriaresultedin38articlesthatweresubjectedtofulltext reading,forwhichtwowerecitedinmorethanonedatabase (dupli-cates),and27wereexcludedfornotmeetingtheinclusioncriteria (TableS1–Supplementalmaterial).Thus,atotalof9publications wereincludedinthereview.Detailsoftheincludedstudiesare showninTable1.
The main method for delivering the antibiotic agents was byloadingantibioticsintocoatedlayersontheimplantsurface. Theselayerscanbeformedashydroxyapatite(HA)ormadefrom polymeric materials. The animal group mainly used to exam-ine local antibiotics release from implant surface wasrodents, eitherratsorrabbits.Differentantibioticsagentswereinvestigated, namelybacitracin,doxycycline,enoxacin,gentamicin,minocycline, tobramycin,andvancomycin(Table1).
Theincludedstudiesshowedaconsiderableriskofbias,dueto lackofinformationregardingmanyoftheresearchsteps(TableS2 –Supplementalmaterial).
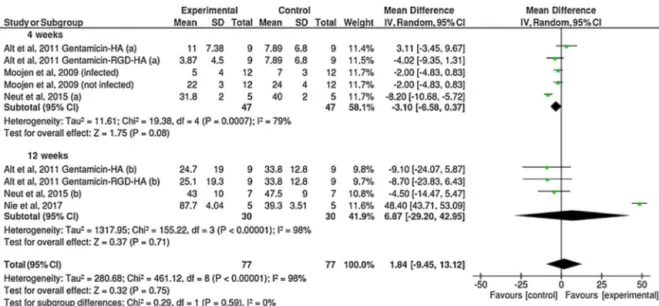
FiveoutofthenineincludedstudiesreportedBICmeanvalues. Ingeneral,resultsofthepercentageofBICrevealedsimilarvalues betweenimplantswithorwithoutantibioticscoating(Fig.2).The
Fig.1.Studyscreeningprocess.
Table1
Detailsoftheincludedstudies.
Study Antibiotic Animalmodel Implantsite No.ofanimals
used
MethodsforBIC andbonevolume measurements
Drugdeliverysystem
Adamsetal.[24] Vancomycin Rat Femur 11 CTanalysis Vancomycin-containingsol-gelfilmon
titaniumalloyrods Moojenetal.[16] Tobramycin Rabbit Tibia 72 Histological
evaluation
Tobramycin-loadedperiapatite-coated titaniumfoamimplants
Altetal.[25] Gentamicin Rabbit Tibia 45 Histological
evaluation
Gentamicin–hydroxyapatite(gentamicin– hydroxyapatite)andgentamicin–RGD (arginine–glycineaspartate)–hydroxyapatite coatings
Fassbenderetal.[26] Gentamicin Rat Tibia 72 CTanalysis Gentamicinlocallyappliedfromapolymeric coatingofintramedullarynails
Walteretal.[27] Doxycycline Rabbit Tibia 10 CTanalysis Bindingofdoxycyclineontoatitanium zirconiumalloysurface
Neutetal.[28] Gentamicin Beagle Femur 12 Histological
evaluation
Poly(lactic-co-glycolicacid)
gentamicin-loadedhydroxyapatite-coated surface
Nieetal.[29] Bacitracin Rat Femur 15 Histological
evaluationandCT analysis
Bacitracinimmobilizationonthetitanium surface
Lietal.[30] Enoxacin Rat Femur 12 CTanalysis Enoxacinloadedintotitanium-nanotubesand immobilizedtypeIcollagen/hyaluronic multilayercoatingonthesurfaceoftheTi-NT Shapiroetal.[31] Minocycline Rat Femur 22 CTanalysis Minocyclinefemoralintramedullaryinjection followedbyimplantationoftitaniumalloyrods
BICmeasurementsweredividedintotwosubgroupsaccordingthe reportedhealingtime,4weeksand12weeks.Nosignificant differ-enceswereobservedbetweenthegroups(P=0.59),withamean differenceofonly1.84%.SixstudiesreportedthepercentageofBV aroundtheimplants(Fig.3).Theresultssuggestedahigher per-centageofBVaroundimplantscoatedwithantibioticscomparedto thecontrolgroup(withoutantibiotics)(P=0.0002),althoughwith smallmeandifference(4.14%).
4. Discussion
Implantsuccessfultreatmentisbelievedtobeamatterofgood integrationwithbone.Thisintegrationisachievedafteraseries ofhealingphasesfollowingimplantsurgery,anddifferentfactors areknowntoaffecttheprocess[32,33].Itisimportanttoensure properboneformationlevelstoobtaingoodimplantintegration withbone.Withthatinmind,theamountofBVaroundimplants
Fig.2.Forestplotforthecomparisonofthepercentagesofbone-to-implantcontactbetweenimplantscoatedornotwithantibioticsagents,accordingtohealingtime.
Fig.3. Forestplotforthecomparisonofthepercentagesofbonevolumeformationbetweenimplantscoatedornotwithantibioticsagents.
waschosenasthemainoutcomeforthisreview,inordertohelp evaluatewhetherantibioticscoatingwouldinterferewithimplant osseointegration.ThevaluesofBVandBICwereusedconsistently intheliteratureasadescriptionofosseointegration.
Forlocalantibioticdeliveryapplications,thesubjectofstudyin thepresentreview,itisnecessaryfortheselectedcarriermaterialto exhibitgreatbiocompatibilitywithlittleantigenicproperties[34]. Inaddition,thesematerialsshouldensurereleaseofthe therapeu-ticagentatthetargetsiteinacontrolledrateandduration[35].In theirexperiment,Altetal.coatedthegentamicin–hydroxyapatite (gentamicin–HA)andgentamicin–RGDonsteelk-wires[25].The drug release analysisshowedan initialburstrelease of around 65% ofthegentamicinduringthefirsthour followedbyslower releasekineticsinthelater24h.Moreimportantly,theinfection ratedecreaseddramaticallyforgentamicin-coatedk-wires com-paredtothek-wireswithoutantibiotics.
According to the present results, it is suggested that the release of antibiotics locally around implants has no signifi-cant influence on the percentage of BIC. One of the possible explanationsforthisfindingistherelativelylowamountof antibi-otics that can be released by these drug delivery techniques. The results from the literature do not show a consensus on this matter.Altetal.investigated theeffectsof gentamicin–HA andgentamicin–(arginine–glycine–aspartate)–HAcoatingsonnew bone formation [25]. In their experiment, 250g/cm of gen-tamicin was coated on steel k-wires inserted in rabbits’ tibia for the observation periods of 4 and 12 weeks. The
quantita-tive and qualitative histological evaluation revealed better BV andbetterdirectimplantcontactinthecontrolgroupcompared withgentamicin coatinggroup but withnostatistically signifi-cantdifferences.Meanwhile,otherreportswithvariousantibiotics concentrationswereassociatedwithdifferentBVlevelswhen com-paredwithcontrolimplants.Forinstance,Neutetal.showedthat theboneingrowtharoundpoly (lactide-co-glycolide)-gentamicin-HA-coatedpinsinfemoralcondylesofdogswasnotimpairedby thepresenceofthegentamicin-loadedcoating[28].Althoughin theiranimal experimentthebone growwasslightlyless inthe pinscoated with10g/mlgentamicin compared tothecontrol group.
Ontheother hand,theresultsoftheanalysisof BVsuggest thatthelocalreleaseofantibioticshasaslightlypositiveinfluence onthepercentageofBVintheregionaroundtheimplants.Some experimentsshowedthatdifferentconcentrationsof antibiotics canbeassociatedwithdifferenteffectsonbonecell.Forinstance, Adamsetal.[24]. investigatedthetotal bonevolumeformation aroundimplantrodsimplantedintoinfectedfemurofrats,andthe implantscoatedwithsol–gelfilmscontainingvancomycinshowed slightly highertotal bone volume in comparisonto thecontrol group.Walteretal. foundthat 141g/cm2 doseof doxycycline
revealedanosteoinductiveeffects byenhancingthe differentia-tionofosteoprecursorcells atanearlystage[27]. Ontheother hand,Edinetal.[36].reportedthathighconcentrationsof van-comycin(morethan10,000gml−1)couldcausecelldeath,while concentrationslowerthan1000gml−1hadnegligibleeffectson
thereplicationofosteoblasts.Miclauetal.reportedthatosteoblasts deathcanoccuratconcentrationhigherthan400gml−1[20].
Thereweretwomainkindsofanimalsforthistypeof experi-ments,dogsandrodents.Thesefindingsaresimilartowhatwas reported ina previousreview [37].Small animalslikeratsand rabbitsarecommonlyusedinimplantresearchevenwiththe bio-logical dissimilaritiesthattheirboneshavecomparedtohuman bones[38,39]. Thiscanbeunderstoodsincetheseanimalshave shorterhealingtime,whichenableevaluationofboneformation aroundimplantsatdifferenthealingphases[39,40].
Antibioticsagentswithvariousspectrawereexaminedamong theincludedstudies,andgentamicinwasthemostcommonused agent.Gentamicinbelongstoaminoglycosidegroupofantibiotics andiscommonlyprescribedtopreventimplantassociated infec-tionsandotherperiodontalinfections[41,42].Gentamicinhasa relativebroadantibacterialspectrumbutmainlyforGram-negative bacteria.Theantibacterialmechanismofgentamicinisbasedon interruptingproteinsynthesisbybindingthe30Ssubunitofthe bacterialribosome[43].
Varioustechniqueswereexaminedtoobserveandevaluatethe directboneformationaroundimplants.Itisimportantforanyused techniquetoallowaccuratereadingoftheexperimentaldata.All theincludedstudiesinthisreviewevaluatedtheboneformation usingeitherhistologicalsectionsormicro-CT(CT),orboth. Pre-viously, manyresearchers usedthin histologicalsectionsofthe implantwiththesurroundingbonetoobservedirectbone forma-tionunderlightmicroscopy[44].Thesesectionsneedtobeground downtobefewmicrometersinthicknesstoallowexaminingsingle cellslayer[45].Themaindrawbacksofthistechniquearethatitis atwo-dimensionalevaluationandneedtobepreparedwith sev-eralsawingandgrindingprocedures.Recently,CTisusedmore toobservethetotalamountofbonearoundtheimplantinthree dimensions.TheCTimagespermitobservingtheBVformation andtheentiresurroundingregioninthreedimensions.Forthat, CTimagesarebelievedtobemoredescriptivethanthe histolog-icalsectionswhenassessingboneformationaroundimplants.
Thestudiesincludedinthisreviewusedmethodswerebasedon implantscoatingwhileothersusedcarriermaterialssuchasgelsor polymersforthelocalreleaseofantibiotics.SomestudiesusedHA coatings[16,25].OneofthelimitationsoftheHAcoatingisthatit needshighprocessingtemperaturetobeformed,whichmakeit dif-ficultforanantibioticagenttobeincorporatedinthecoatinglayer [18].Acommonlyusedtechniqueforloadingthetherapeuticagents intoHAcoatinglayerissimplybyimmersionthecoatedimplant indrugsolution.However,severalstudiesreporteduncontrolled release kineticsassociatedwiththis techniquecharacterizedby earlyburstreleaseinthefirsthourformostoftheloadeddrugs [17,28].
Recently, numerous studies examined the use of some biodegradable polymersasimplantcoatingforlocaldrug deliv-ery[46,47].Thesepolymerscandemonstratesustainedandslower releaseratecomparedtotheHAcoating[46].Anothergreat advan-tageofthesepolymercoatingsisthattheyallowtheuseofhigher volumeandseveraltypesofantibacterialagents[47].Forinstance, gentamicinwasloadedintopoly(d,l-lactide)(PDLLA)coatingto treat implantassociated infectioninananimal model[48]. The gentamicin demonstrated sustain release kineticsfrom(PDLLA) coatingthatlastedmorethantwo days.InthestudyofLietal. enoxacinwasloadedintoimmobilizedcollagen/hyaluroniccoated implant[30].Thismethodinvolvedtheuseofacarriermaterialin theformoffoamorpolymericcoating.Inotherwork,Alenezietal. developedathinsurfacecoatingimplantsconsistsofathinpoly (N-isopropylacrylamide)-co-acrylamide(PNIPAAm-AAm)polymer forvancomycinreleaseinvitro[49].Thevancomycindemonstrated sustainreleasefromthesurfaceandwasabletoeradicate Staphy-lococcusepidermidisbacteriainculture.Furthermore,Neutetal.
developedagentamicin-HA-coatingwithaprotectivePLGA[poly (lactic-co-glycolicacid)]-overlayertobeexaminedastreatment option for infectionin cementless total jointreplacement [28]. Thisgentamicincoatedlayershowedresistanceofinfectionand evengoodantibacterialefficacytowardsomegentamicin-resistant staphylococcalstrains.PLGAisanotherpolymermaterialthatis commonlyusedfor encapsulationand release ofwide range of drugsandchemicalagents.Thispolymermaterialisknownforits highbiocompatibilityandfavorablebiodegradablebehaviorthat werefoundtobesuitablefordrugdeliveryapplications[46,50].
Implantsurfacescanalsobemodifiedtoshowspecialnano fea-tures,suchastubesorpores,forantibioticreleasedirectlyfrom implantsurface[51,52].Acommonmethodusedfortheformation ofhighlyporousstructuresonimplantsurfaceisbyanodization [53].Forlocaldrugdeliveryapplications,theseporousstructures canbemodifiedtoexhibithighloadingcapabilities.Somereports revealedthattitaniananotubesloadedwithantibioticscanenhance cellattachment,proliferation,andosteogenicdifferentiation[54]. However,somereleasebehaviortestsshowedthatdrugrelease fromtitaniananotubescanbeassociatedearlyburstrelease,which canleadtotoxicity[55–57].Therefore,acontrolledrelease behav-iorofantibacterialagentsiscrucial.MesoporousTiO2coatingonTi
implantswasexaminedinseveralexperimentsasatoolforlocal drugdeliveryattheboneimplantinterface[58,59].These meso-porouscoatingscanbeformedasuniformedandthinfilmswith highlyporoussurfacethatallowloadingandreleasingofseveral drugsagents.Forinstance,Gallietal.investigatedimplantscoated withmesoporousTiO2filmsincorporatedwithmagnesium[60].In
theirexperiment,thereleaseofmagnesiumfromthecoatedlayer revealedbetterboneformationinrabbitboneafterthreeweeks ofhealingtimeincomparisontonon-loadedmesoporouscoated implants.
Thelimitationsofthepresentreviewincludethesmallnumber ofincludedstudiesandthevariationsamongeachstudy regard-ingtheanimalspecies,theobservationperiodforboneformation levels,theusedantibioticagentwithdifferentconcentrations,and thetechniquesusedtocalculatethepercentageofbonearoundthe implants.Allthesevariationsandconfoundingfactorsmaylimit thecapacitytodrawfirmconclusions.Theincludedstudieshavea considerableriskofselection,performance,anddetectionbias. Fur-thermore,thefindingsobtainedfromanimalexperimentscannot bedirectlyappliedtohuman.
5. Conclusion
Theresultsofthepresentreviewsuggestthatthelocal adminis-trationofantibioticsaroundimplantsdoesnotadverselyaffectthe directbonecontactwithimplants.Therewasbetterbone forma-tionaroundimplantswhencombinedwithlocalantibioticsrelease incomparisontoimplantswithoutantibiotics,butthemean differ-encewassmall.Itisamatterofdebatewhethertheseinvivoresults willhavethesameeffectinthehumanclinicalsettinginthelong term.However,theriskofbiasofthesestudiesmay,tosomeextent, questionthevalidityoftheseresults.
Funding/grantsupport
Thisstudyreceivednospecificgrantfromanyfundingagency inthepublic,commercial,ornot-for-profitsectors.
Conflictofinterest
AppendixA. Supplementarydata
Supplementary materialrelated tothis article canbefound, intheonlineversion,atdoi:https://doi.org/10.1016/j.jdsr.2020.09. 003.
References
[1]PjeturssonBE,KaroussisI,BurginW,BraggerU,LangNP.Patients’ satisfac-tionfollowingimplanttherapy.A10-yearprospectivecohortstudy.ClinOral ImplantsRes2005;16(2):185–93.
[2]ChenST,BuserD.Estheticoutcomesfollowingimmediateandearlyimplant placementintheanteriormaxilla—asystematicreview.IntJOralMaxillofac Implants2014;29Suppl:186–215.
[3]MoraschiniV,PoubelLA,FerreiraVF,BarbozaEdosS.Evaluationofsurvivaland successratesofdentalimplantsreportedinlongitudinalstudieswitha follow-upperiodofatleast10years:asystematicreview.IntJOralMaxillofacSurg 2015;44(3):377–88.
[4]BerglundhT,PerssonL,KlingeB.Asystematicreviewoftheincidenceof biolog-icalandtechnicalcomplicationsinimplantdentistryreportedinprospective longitudinalstudiesofatleast5years.JClinPeriodontol 2002;29Suppl 3:197–212,discussion232–233.
[5]CostertonJW,MontanaroL,ArciolaCR.Biofilminimplantinfections:its pro-ductionandregulation.IntJArtifOrgans2005;28(11):1062–8.
[6]GulatiK,RamakrishnanS,AwMS,AtkinsGJ,FindlayDM,LosicD. Biocompati-blepolymercoatingoftitaniananotubearraysforimproveddrugelutionand osteoblastadhesion.ActaBiomater2012;8(1):449–56.
[7]Anderson JM. Biological responses to materials. Annu Rev Mater Res 2001;31(1):81–110.
[8]ZhaoL,ChuPK,ZhangY,WuZ.Antibacterialcoatingsontitaniumimplants.J BiomedMaterResBApplBiomater2009;91(1):470–80.
[9]CampocciaD,MontanaroL,ArciolaCR.Thesignificanceofinfectionrelated to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006;27(11):2331–9.
[10]HarrisLG,RichardsRG.Staphylococciandimplantsurfaces:areview.Injury 2006;37Suppl2:S3–14.
[11]HetrickEM, Schoenfisch MH. Reducing implant-related infections: active releasestrategies.ChemSocRev2006;35(9):780–9.
[12]DunneJrWM.Bacterialadhesion:seenanygoodbiofilmslately?ClinMicrobiol Rev2002;15(2):155–66.
[13]ChrcanovicBR,AlbrektssonT,WennerbergA.Prophylacticantibioticregimen anddentalimplantfailure:ameta-analysis.JOralRehabil2014;41(12):941–56. [14]KeenanJR,Veitz-KeenanA.Antibioticprophylaxisfordentalimplant
place-ment?EvidBasedDent2015;16(2):52–3.
[15]ZamanSB,HussainMA,NyeR,MehtaV,MamunKT,HossainN.Areviewon antibioticresistance:alarmbellsareringing.Cureus2017;9(6):e1403. [16]MoojenDJ,VogelyHC,FleerA,NikkelsPG,HighamPA,VerboutAJ,etal.
Prophylaxisofinfectionandeffectsonosseointegrationusinga tobramycin-periapatitecoatingontitaniumimplants—anexperimentalstudyintherabbit. JOrthopRes2009;27(6):710–6.
[17]StigterM,BezemerJ,deGrootK,LayrolleP.Incorporationofdifferent antibi-oticsintocarbonatedhydroxyapatitecoatingsontitaniumimplants,release andantibioticefficacy.JControlRelease2004;99(1):127–37.
[18]StigterM,deGrootK,LayrolleP.Incorporationoftobramycinintobiomimetic hydroxyapatitecoatingontitanium.Biomaterials2002;23(20):4143–53. [19]IsefukuS,JoynerCJ,SimpsonAH.Gentamicinmayhaveanadverseeffecton
osteogenesis.JOrthopTrauma2003;17(3):212–6.
[20]MiclauT,EdinML,LesterGE,LindseyRW,DahnersLE.Bonetoxicityoflocally appliedaminoglycosides.JOrthopTrauma1995;9(5):401–6.
[21]ZhaoL,ChuPK,ZhangY,WuZ.Antibacterialcoatingsontitaniumimplants.J BiomedMaterResBApplBiomater2009;91B(1):470–80.
[22]MoherD,LiberatiA,TetzlaffJ,AltmanDG.Preferredreportingitemsfor sys-tematicreviewsandmeta-analyses:thePRISMAstatement.BMJ2009;339, b2535.
[23]HooijmansCR,RoversMM,deVriesRB,LeenaarsM,Ritskes-HoitingaM, Lan-gendamMW.SYRCLE’sriskofbiastoolforanimalstudies.BMCMedRes Methodol2014;14:43.
[24]AdamsCS,AntociJrV,HarrisonG,PatalP,FreemanTA,ShapiroIM,etal. Controlledreleaseofvancomycinfromthinsol-gelfilmsonimplantsurfaces successfullycontrolsosteomyelitis.JOrthopRes2009;27(6):701–9. [25]AltV,BitschnauA,BöhnerF,HeerichKE,MagesinE,SewingA,etal.Effectsof
gentamicinandgentamicin–RGDcoatingsonboneingrowthand biocompat-ibilityofcementlessjointprostheses:anexperimentalstudyinrabbits.Acta Biomater2011;7(3):1274–80.
[26]FassbenderM,MinkwitzS,KronbachZ,StrobelC,Kadow-RomackerA, Schmid-maierG,etal.Localgentamicinapplicationdoesnotinterferewithbonehealing inaratmodel.Bone2013;55(2):298–304.
[27]WalterMS,FrankMJ,SatuéM,MonjoM,RønoldHJ,LyngstadaasSP,etal. Bioac-tiveimplantsurfacewithelectrochemicallybounddoxycyclinepromotesbone formationmarkersinvitroandinvivo.DentMater2014;30(2):200–14. [28]NeutD,DijkstraRJ,ThompsonJI,KavanaghC,vanderMeiHC,BusscherHJ,etal.
Abiodegradablegentamicin-hydroxyapatite-coatingforinfectionprophylaxis incementlesshipprostheses.EurCellMater2015;29:42–55,discussion55-56.
[29]NieB,AoH,LongT,ZhouJ,TangT,YueB.Immobilizingbacitracinontitanium forprophylaxisofinfectionsandforimprovingosteoinductivity:aninvivo study.ColloidsSurfBBiointerfaces2017;150:183–91.
[30]LiH,NieB,ZhangS,LongT,YueB.ImmobilizationoftypeIcollagen/hyaluronic acidmultilayercoatingonenoxacinloadedtitaniananotubesforimproved osteogenesisandosseointegrationinovariectomizedrats.ColloidsSurfB Bioin-terfaces2019;175:409–20.
[31]ShapiroJA,AbuMoussaS,LindsayCP,MasonGB,DahnersLE,WeinholdPS. Locallydeliveredminocyclinemicrospheresdonotimpairosseointegrationof titaniumimplantsinaratfemurmodel.JOrthop2020;20:213–6.
[32]RigoECS,BoschiAO,YoshimotoM,AllegriniS,KonigB,CarbonariMJ.Evaluation invitroandinvivoofbiomimetichydroxyapatitecoatedontitaniumdental implants.MaterSciEngC2004;24(5):647–51.
[33]BerglundhT,AbrahamssonI,LangNP,LindheJ.Denovoalveolarbone for-mationadjacenttoendosseousimplants.ClinOralImplantsRes2003;14(3): 251–62.
[34]ChenFH,GaoQ,NiJZ.Thegraftingandreleasebehaviorofdoxorubincinfrom Fe(3)O(4)@SiO(2)core-shellstructurenanoparticlesviaanacidcleavingamide bond:thepotentialformagnetictargetingdrug delivery.Nanotechnology 2008;19(16):165103.
[35]SlowingII,TrewynBG,GiriS,LinVY.Mesoporoussilicananoparticlesfordrug deliveryandbiosensingapplications.AdvFunctMater2007;17(8):1225–36. [36]EdinML,MiclauT,LesterGE,LindseyRW,DahnersLE.Effectofcefazolin
andvancomycinonosteoblastsinvitro.ClinOrthopRelatRes1996;(333): 245–51.
[37]AleneziA,ChrcanovicB,WennerbergA.Effectsoflocaldrugandchemical compounddeliveryonboneregenerationarounddentalimplantsinanimal models:asystematicreviewandmeta-analysis.IntJOralMaxillofacImplants 2018;33(1):e1–18.
[38]PearceAI,RichardsRG,MilzS,SchneiderE,PearceSG.Animalmodelsfor implantbiomaterialresearchinbone:areview.EurCellMater2007;13:1–10. [39]Wancket LM. Animal models for evaluation of bone implants and devices:comparativebonestructureandcommonmodeluses.VetPathol 2015;52(5):842–50.
[40]JowseyJ. StudiesofHaversiansystemsinmanandsomeanimals.JAnat 1966;100(Pt4):857–64.
[41]ChangWK,SrinivasaS,MacCormickAD,HillAG.Gentamicin-collagenimplants toreducesurgicalsiteinfection:systematicreviewandmeta-analysisof ran-domizedtrials.AnnSurg2013;258(1):59–65.
[42]Norowski Jr PA, Bumgardner JD. Biomaterial and antibiotic strategies for peri-implantitis: a review. J Biomed Mater Res B Appl Biomater 2009;88B(2):530–43.
[43]PopatKC,EltgrothM,LatempaTJ,GrimesCA,DesaiTA.Decreased Staphy-lococcus epidermis adhesion and increased osteoblast functionality on antibiotic-loadedtitaniananotubes.Biomaterials2007;28(32):4880–8. [44]Cano-Sanchez J,Campo-TraperoJ,Gonzalo-LafuenteJC,Moreno-LopezLA,
Bascones-MartinezA.Undecalcifiedbonesamples:adescriptionofthe tech-niqueanditsutilitybasedontheliterature.MedOralPatolOralCirBucal 2005;10Suppl1:E74–87.
[45]DonathK,BreunerG.Amethodforthestudyofundecalcifiedbonesandteeth withattachedsofttissues.TheSage-Schliff(sawingandgrinding)technique.J OralPathol1982;11(4):318–26.
[46]AleneziA,NaitoY,TerukinaT,PrananingrumW,JinnoY,TagamiT,etal. ControlledreleaseofclarithromycinfromPLGAmicrospheresenhancesbone regenerationinrabbitcalvariadefects.JBiomedMaterResBApplBiomater 2018;106(1):201–8.
[47]VirtoMR,ElorzaB,TorradoS,ElorzaMdeL,FrutosG.Improvementof gentam-icinpoly(D,L-lactic-co-glycolicacid)microspheresfortreatmentof osteomyeli-tisinducedbyorthopedicprocedures.Biomaterials2007;28(5):877–85. [48]LuckeM,SchmidmaierG,GollwitzerH,RaschkeM.Entwicklungeiner
biode-gradierbarenundantibiotischwirksamenBeschichtungvonImplantaten.Hefte zuderUnfallchirurg2000;282:362–3.
[49]AleneziA,HulanderM,AtefyektaS,AnderssonM.Developmentofaphoton induceddrug-deliveryimplantcoating.MaterSciEngC2019;98:619–27. [50]RuhePQ,HedbergEL,PadronNT,SpauwenPH,JansenJA,MikosAG.
rhBMP-2releasefrominjectablepoly(DL-lactic-co-glycolicacid)/calcium-phosphate cementcomposites.JBoneJointSurgAm2003;85-ASuppl3:75–81. [51]LinWT,TanHL,DuanZL,YueB,MaR,HeG,etal.Inhibitedbacterialbiofilm
formationand improvedosteogenicactivity ongentamicin-loadedtitania nanotubeswithvariousdiameters.IntJNanomed2014;9:1215–30. [52]Vallet-RegíM,BalasF,ArcosD.Mesoporousmaterialsfordrugdelivery.Angew
ChemIntEd2007;46(40):7548–58.
[53]AleneziA,NaitoY,AnderssonM,ChrcanovicBR,Wennerberg A,JimboR. Characteristicsof2differentcommerciallyavailableimplantswithorwithout nanotopography.IntJDent2013;2013:769768.
[54]LinW-t, TanH-l,DuanZ-l,YueB, MaR, HeG,etal.Inhibitedbacterial biofilmformationandimprovedosteogenicactivityongentamicin-loaded tita-niananotubeswithvariousdiameters.IntJNanomed2014;9:1215–30. [55]LeeK,MazareA,SchmukiP.One-dimensionaltitaniumdioxidenanomaterials:
nanotubes.ChemRev2014;114(19):9385–454.
[56]RathboneCR,CrossJD,BrownKV,MurrayCK,WenkeJC.Effectofvarious con-centrationsofantibioticsonosteogeniccellviabilityandactivity.JOrthopRes 2011;29(7):1070–4.
[57]ZhangM,WeiM,WangD,DuanY.Preparationandcharacterizationofadrug vehicle:polymerbrushimmobilizedAgnanoparticlesontotitanium nano-tubes.MaterLett2014;135:51–4.
[58]HarmankayaN,KarlssonJ,PalmquistA,HalvarssonM,IgawaK,AnderssonM, etal.Raloxifeneandalendronatecontainingthinmesoporoustitaniumoxide filmsimproveimplantfixationtobone.ActaBiomater2013;9(6):7064–73. [59]KarlssonJ,HarmankayaN,AllardS,PalmquistA,HalvarssonM,TengvallP,
etal.Exvivoalendronatelocalizationatthemesoporoustitaniaimplant/bone interface.JMaterSciMaterMed2015;26(1):5337.
[60]GalliS,NaitoY,KarlssonJ,HeW,MiyamotoI,XueY,etal.Localreleaseof mag-nesiumfrommesoporousTiO2coatingsstimulatestheperi-implantexpression ofosteogenicmarkersandimprovesosteoconductivityinvivo.ActaBiomater 2014;10(12):5193–201.