Photophysical and Chemical Approaches
to Cellular Biophysics
Victor Akpe
Licentiate Thesis in Biological Physics
ISRN KTH/FYS/--08:54--SE ISBN 978-99-7415-200-5
KTH AlbaNova University Center Cell Physics
SE-106 91 Stockholm SWEDEN
Akademisk avhandling som med tillstånd av Kungl Tekniska högskolan framlägges till offentlig granskning för avläggande av teknologie licentiatexamen i biologisk fysik torsdagen den 18 december 2008 klockan 11.00 i FA32, AlbaNova Universitetscentrum, Roslagstullsbacken 21, Stockholm.
Abstract
The central theme in this thesis is reversibility. Two main attempts has been made to approach reversibility in cellular systems from both chemical and physical points of view.
Reversibility of immunolabeling of proteins on the cell surface has been adressed by development of new fluorescent substances optimized for CALI (Chromophore-Assisted Laser Inactivation of protein). Aluminum phthalocyanine (AlPc) is here identified to be a good candidate for a new generation of fluorophores for efficient hydroxyl radical generation. It is shown that cells can be reversibly labeled with antibody-AlPc conjugates. In experiments on living cells the AlPcs were not only active as classic fluorophores but also as photocatalytic substances with destaining properties.
Reversibility of cell immobilization is also reported, where cells cultured in microstructures were immobilized and 3D supported using hydrogels. Hydrogel formulation and application was optimized to achieve a system where both viability and ease of use was satisfied. Gel reversibility was actualized with pH and enzyme treatment. The developped method offers the possibility of stop flow culturing cells in controlled and reusable 3D environments.
1 Introduction 1 2 Materials 2 2.1 Fluorescent Dyes ... 2 2.2 Gels ... 4 2.3 Gelatin ... 4 2.4 Hydrogels ... 5 2.5 Cell cultures ... 6 3 Experimental Setups 7 3.1 Setup for Chromophore- Assisted Laser Inactivation (CALI) Technique ... 7
3.2 Confocal microscopy ... 8
3.3 Setup for immobilized dyes in gelatin using a FRAP based approach ... 9
3.4 Setup for Time Resolved Laser Flash Photolysis ... 10
3.5 Setup for singlet oxygen and photodegradation quantum yields ... 11
3.6 Setup for the chemical synthesis ... 12
4 Results and Discussion 13 4.1 Summary of Paper 1... 13
4.2 Summary of paper 2 ... 15
5 Conclusions 21
6 Acknowledgements 22
This thesis is based on the following studies:
I. Victor Akpe, Tebello Nyokong, Hjalmar Brismar. Photophysics and Photochemistry of Zinc, Aluminum and Zinc octakis (benzylthio) phthalocyanine. TRITA-FYS 2008 :44, KTH, (2008).
II. Victor Akpe, Erik Vernet, Torbjörn Gräslund, Hjalmar Brismar. Characterization studies of Aluminum Phthalocyanine Binding to antibodies from SKBR 3 Cell line. TRITA-FYS 2008:45, KTH, (2008).
III. Thomas Liebmann, Susanna Rydholm, Victor Akpe, Hjalmar Brismar. Self- assembling Fmoc dipeptide hydrogel for in situ 3D cell culturing. BMC Biotechnology. 7:88 (2007).
Contributions by the author
The contributions of Victor Akpe to the publications listed above include:
I. Experimental design, characterization, physicochemical measurements, interpretation of the data and writing.
II. Experimental design, major part of the contribution for the characterization of the antibody-dye conjugates, interpretation of the data and writing. III. Design and supervision of the chemical synthesis.
1 Introduction
1 Introduction
Dyes are used in many biological applications, especially in their applications as fluorescent probes in bioassays [1, 2], cell biology [3], in vivo imaging [4], flow cytometry [5, 6], and as reactive dyes [7, 8]. Reactive dyes in recent years have also been seen to advance in the photodynamic therapy treatment (PDT) of cancer [9-13], in the CALI (Chromophore-Assisted Laser Inactivation) technique [14-16], which can be used to remove protein at the cell surface. This method offers specific and non-invasive approach for protein inactivation at both cellular and molecular level.
Cellular biophysics involves the application of biophysical approaches to address the mechanisms of biological processes. This thesis therefore borrows backgrounds in the Biological, Chemical, Physical, and Mathematical Sciences. Thus, the studies in this thesis include:
• Synthesis and Characterization of novel Zinc, Aluminum and Tin Octakis (Benzylthio) phthalocyanines (paper 1-technical report 1).
• Photophysics and photochemistry of Zinc, Aluminum and Tin Octakis (Benzylthio) phthalocyanines (paper 1-technical report 1).
• The use of CALI protocol to study reversible antibody staining, where the system investigated consists of HER 2 antibody coupled to AlPc dye and Alexa fluorophore (paper2-technical report 2).
• Evaluation of the mechanism that is involved in the laser inactivation of proteins (paper 2-technical report 2).
• Immobilization of the free dyes (MPc, Alexa, and Rh6G) in commercial gel to study the: 1) phtotostability of the dyes; 2) the triplet state dynamics of the dyes (paper 2-technical report).
• Lastly, the synthesis of self- assembling Fmoc dipeptide hydrogel and application in 3D cell culturing. (Paper 3).
2 Materials
All the chemicals used were either purchased from Merck, Sigma-Aldrich or Fluka. The metallopthalocyanine dyes were either synthesized or modified while alexa 488 fluorophore, rhodamine 6G and alexa antibody were purchased from Invitrogen (molecular probes). Two gel types were used in this thesis; commercially obtained edible gel and prepared synthetic gel from the laboratory. The detailed materials used are contained in each separate publication. Also the two cell types, SKBR 3 and MDCK cell cultures are contained in the different papers. All other materials not mentioned have been explained in the different papers.
2.1
Fluorescent Dyes
Fluorescent dyes are used routinely in the Life sciences to trace the presence of biomolecules in cells and other biological systems. They are also used in many areas of research in the applied sciences, such as reactive dyes mentioned earlier, fluorescence recovery after photobleaching (FRAP) [17], Fluorescence Resonance Energy Transfer (FRET)[18], Fluorescence activated cell sorting (FACS) [19, 20], Time Resolved Fluorescence (TRF)[21], Fluorescence Polarization (FP)[22], Fluorescence Correlation Spectroscopy (FCS)[23] and many more.
Aluminum phthalocyanine (AlPc) has been used in paper 2, particularly for its photodriven properties. Like in most phthalocyanines, a sulphonyl chloride (SO2Cl) can easily be introduced into the ringstructure of the molecule, thereby increasing the possibility for coupling to antibodies. Previously reported is the drug delivery application of a stable sulphonamide bond [24-26]. It is for this reason that metallophthalocyanine dyes are commonly used as reactive dyes rather than dyes with carboxylic ends which are mostly used for cell staining. Other phthalocyanine (Pc) dyes synthesized are also reported in paper 1 where the characterization, photophysical and photochemical properties have been discussed. Figure 2-1 shows the chemical structures of some of the Fluorescent dyes used.
2 Materials
2.2
Gels
Gel is an apparent solid-like material formed from a colloidal solution. Different types of gel exist depending on the application it is intended for. In this thesis, two types of gels have been used, commercially obtained gelatin which is used as food ingredients and the synthesized hydrogel used for the 3D cell culturing. The interesting thing about gels in general is that they are superabsorbant and have the propensity for taking in water.
2.3
Gelatin
The decision to use edible gelatin in this study is based on its ready availability and convenience in preparation. The different dyes (AlPc, Alexa and Rhodamine) were stirred in the gelatin prepared and stored in the fridge at 4oC for 10 minutes. For a longer time and a lower temperature could impede the jelly-like nature of the gelatin. Gelatin exists in most cases as an amphoteric protein with isoionic point between 5 and 9 depending on the process method-acid or alkaline pretreatment. The protein is made up of peptide triplets of glycine-x-y, where proline has the preference for the x position and hydroxyproline the y position. Gelatin has several uses, particularly as animal glue, gelling binder in gummy products and are also known to have thermally reversible gelling properties with water. In order to study the photobleaching property of the different dyes, the free dyes were immobilized in gelatin. This enabled the study of the recovery profiles observed for the different dyes. For more reading on gelatin, the reader is directed to Dr Coles (Univ. Pretoria) extensive website on the subject [27].
N N N N N H CH CH3 C O N H CH H C O C O N H CH O CH2 CH2 CH2 NH C+ NH2 NH2 C H CH C O N H H CH CH2 CH2 C O O -C O OH C O N H CH H C O C O
Figure 2-2: Typical gelatin structure that consists of polypeptide chains of (-Ala-Gly-Pro-Arg-Gly-Glu-4Hyp-Gly-Pro-) units.
2 Materials
2.4
Hydrogels
Hydrogels are water-swollen, cross- linked polymeric structures obtained by reactions of monomers or by hydrogen bonding. They tend to mostly absorb a great volume of water as compared to dry weight. Like gelatin, a hydrogel has a three dimensional insoluble polymeric networks. Hydrogels are used in numerous biomedical applications including, transdermal drug delivery [28, 29], wound healing [30, 31], bioadhesive carriers [32, 33], capsules in the encapsulated form [34, 35], implantation coatings [36] . In this thesis, the procedure employed by Xu et al [37] was similarly used to produce 9-fluorenyl methyl carbamate (Fmoc). The application enabled the compartmentalization of the immobilized cells in the microfluidic chamber [38].
Figure 2-3: Firm Fmoc hydrogel formed in a conical shape to demonstrate morphological stability. Hydrogel layering is illustrated by colored dyes in dispersion media.
2.5
Cell cultures
Two cell culture types were used in this thesis. SKBR 3 and MDCK. The SKBR 3 cell line was obtained from American Type Culture Collection (Manassas, VA). All cell culture reagents were from Invitrogen (Carlsbad, CA). The MDCK cell line (renal epithelial cell line, originally obtained from African Green Monkey embryos) was prepared in the laboratory according to standard protocols. The SKBR 3 cells were cultivated in RPMI 1640 medium supplemented with 15 % PBS and 1% Antibiotic-Antimycotic in 5% CO2 at 37°C.
3 Experimental Setups
3 Experimental Setups
IR spectra (KBr pellets) were recorded on a Perkin-Elmer spectrum 2000 FTIR spectrometer. H-nuclear magnetic resonance (NMR, 400 MHz) spectra were obtained using the Bruker EMX 400 NMR spectrometer. UV-visible spectra were recorded on a Varian 500 UV/visible/NIR spectrophotometer. Fluorescence excitation and emission spectra were recorded on a Varian Eclipse spectrofluorimeter. Photo-irradiations were done using a General electric Quartz line lamp (300W). A 600 nm glass cut off filter (Schott) and a water filter were used to filter off ultraviolet and infrared radiations respectively. An interference filter 700 nm with a band width of 40 nm was additionally placed in the light path before the sample. Light intensities were measured with a POWER MAX 5100 (Molelectron Detector Inc.) power meter. Triplet absorption and decay kinetics were recorded on a laser flash photolysis system, the excitation pulses were produced by a Nd: YAG laser (Quanta-Ray, 1.5 J / 90 ns) pumping a tunable dye laser (Lambda Physic FL 3002, Pyridine 1 dye in methanol). The analyzing beam source was from a Thermo Oriel xenon arc lamp, and a photomultiplier tube was used as a detector. Signals were recorded with a two-channel digital real-time oscilloscope (Tektronix TDS 360); the kinetic curves were averaged over 256 laser pulses. The mass spectra were recorded on MALDI (Matrix Assisted Laser Desorption Ionization) where the mass-to-charge ratio was recorded on a time of flight device. The Confocal images were recorded using a LSM 510 META (Carl Zeiss, Jena) with visible and UV laser modules. ND-1000 (Nanodrop, Wilmington, DE) using Nanodrop 2.5.1 software was used to measure the absorbance of the free dye and the antibody labeled dye. Spectral recordings in paper 2 were performed using a Cary 50 Bio UV-Vis Spectrometer (Varian, Palo Alto, CA) using Cary Win UV Simple Reads Application 2.0 software and a LS50B Luminescence Spectrometer (Perkin Elmer, Waltham, MA) using FL Win Lab 3.0 software.
3.1
Setup for Chromophore- Assisted Laser Inactivation (CALI) Technique
Chromophore- assisted laser inactivation (CALI) is a technique that selectively inactivates protein of interest to elucidate their in vivo functions [39]. This technique has a wide
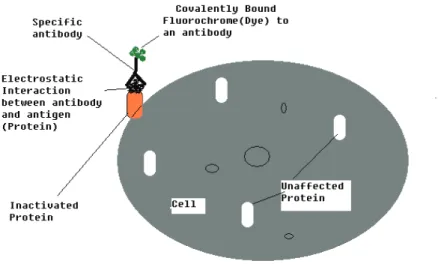
Here, AlPc was modified and labeled with antibody from Mouse c-erbB-2 Ab-2. Essentially, the principle relies on high level of monochromatic light source, regulated level of output power and changeable irradiated area, labeled dye molecule at the physiological pH. Upon irradiation of the labeled dye region with pulsed laser light, the protein is inactivated. The hydroxyl radicals migrate to the antibody-antigen extracellular matrix of the cell where protein is inactivated within effective damage distance between 15Å-34Å radius. Figure 3-1 shows a schematic diagram for the CALI principle. The experimental setup is further explained in paper 2.
Figure 3-1: Illustration of the basic principle for CALI protocol. The protein of interest is presented as orange that has been inactivated. The choice of the excitation light source is dependent on the excitation wavelength of the fluorochrome (dye).
3.2
Confocal microscopy
Confocal laser scanning microscopy (CLSM) is a powerful tool used in biological sciences, medicine, materials science, etc. A microscope objective is used to focus a laser beam onto the specimen where it excites fluorescence. Fluorescent radiation is collected from the objective lens and efficiently directed to the detector via a dichroic beam splitter. The emission filter selects the choice of the wavelength and it also blocks the
3 Experimental Setups
achieved. Light coming from planes above or below the focal plane is out of focus. It is therefore important to arrange the pinhole in front of the detector on a plane conjugate to the focal plane of the objective. For more readings on the principles and applications, the reader is directed to [40-42].
Figure 3-2: Principles of a confocal microscope (from [43]).
3.3
Setup for immobilized dyes in gelatin using a FRAP based approach
The experimental setup for the immobilized dyes in gelatin is essentially the same as in FRAP (fluorescence recovery after photobleaching) method; only here the cells are not alive. The dyes are immobilized according to the protocol reported in paper 2. The essence of immobilization is to restrict the movement of the dye molecules in order to capture the triplet state dynamics and photostability of the dyes. The setup also help in clarifying what could be referred to as irreversible photobleached dye [44] and reversible photobleached dye [45].
(1) (2) (Recovery of the green area )
(Full recovery) (4)
Figure 3-3: Immobilized AlClPc (SO2Cl)4 dye. Picture captured during FRAP experiment of the bleached region using 633nm full laser power.
Figure 3-4: Immobilized Alexa-488 dye. Picture captured during FRAP experiment of the bleached region using 488nm full laser power.
3.4
Setup for Time Resolved Laser Flash Photolysis
Flash photolysis a technique generally used for monitoring photochemical reactions. For reactions with moderate rate decay, flash lamps (typically Xenon lamps) are used since they provide moderate time response. For faster reactions, lasers are usually used to make up for the slow decay time of light emission from a flash lamp. Several lasers are available for this purpose. Here, we used neodymium-yttrium aluminum garnet (Nd-YAG) solid state laser. It is a synthetic mineral that produces light in the IR region of the spectrum at 1064nm upon excitation by flash lamp. To convert IR light into Visible and UV region, a special optic is used.
3 Experimental Setups
limit and upper limit of the excitation wavelength of the dye. From the experiment, the triplet lifetimes, triplet quantum yields were calculated.
Nd: YAG
LASER Dye LASER
Xenon lamp Sample cell holder Monochromator (w ith PMT) computer Focusing mirror Collimating lens UV -Vis spe c. Oscilloscope Figure 3-5: Diagram of a time resolved laser flash photolysis setup.
3.5
Setup for singlet oxygen and photodegradation quantum yields
The setup for singlet oxygen and photodegradation is similar as in photolysis experiment, but here the laser is excluded. A simple setup is depicted in the schematic diagram below (Figure 3-6). For more details on the experimental setup, the reader is directed to read this article [46].
Figure 3-6: Schematic diagram of the setup. 1= Light source (General electric quartz line lamp); 2= Lens (convex); 3= Water filter; 4= Schott glass cut-off filter ; 5= Interference filter; 6= Quartz cell.
3.6
Setup for the chemical synthesis
All the MPc dyes used were either synthesized or modified. The preparation and characterization are contained in the different papers. The MPc dyes synthesized in paper 1 are all novel MPcs and have been characterized by known standard techniques using IR, UV-Vis spectrophotometer, NMR, Mass spectrometer. Most MPcs are mostly synthesized using high temperature and usually the finished raw products are very difficult to purify. A lithium pentanoloate route is employed in the synthesis of paper 1. It requires low heat (< 80oC) in comparison to the traditional method that goes up to 180oC. The traditional method was employed initially during the synthesis but the method did not give a stable phthalocyanine. The alternative method proved to be a better choice. Once the LiPc (Lithium phthalocyanine) has formed, the metal of interest is introduced into the ring of the phthalocyanine, thereby displacing the Lithium metal. The LiPc is the intermediate stage in the phthalocyanine formation with known characteristic features of phthalocyanines. The first confirmation of a newly formed MPc is usually a significant shift in the UV-Vis spectrum position. The photophysical and photochemical properties of these dyes reveal their potential applications as photocatalytic and photoactivatable compounds. Attempt to convert the benzene end positions to thiol groups proved abortive. The thiol end groups in the phthalocyanine could for instance be used as immobilization support on goal plates, coupling extension to antibodies. In paper 2, commercial aluminum phthalocyanine was first modified by dissolving in oleum (which is a mixture of conc. H2SO4 and SO3), followed by several other steps in the modification. For more on the preparation and subsequent modification, the reader is referred to paper 2.
4 Results and Discussion
4 Results and Discussion
In this thesis, two approaches were conducted to study reversibility in cellular systems from both chemical and physical points of view. The first study (paper 1) reports on the photophysics and photochemistry of octakis (benzylthio) phthalocyanines, and suggests their potential application as photoactivatable dyes and as photocatalysts. The second study (paper 2) reports on the combination of FRAP technique and the CALI protocol to study and evaluate Aluminum phthalocyanine as a photocleavable agent. Below, a brief summary of the two studies is provided.
4.1
Summary of Paper 1.
Fluorescence emission spectra of the MPc dyes are mirror images of the absorption spectra, suggesting that the absorbing species is also the fluorescing species. The photophysical and photochemical parameters of the MPcs are listed in Table 1. The ΦF values in Table 1 demonstrate the influence of the central metal on the fluorescence efficiency. The value of ΦF may be influenced by several factors including aggregation and the heavy atom effect. Fluorescence quantum yield values are expected to be larger for MPcs containing lighter atoms (e.g. Al), and smaller for those MPcs containing heavier atoms (e.g. Sn and Zn). Heavy atom effect results in intersystem crossing of the heavier atoms, hence less fluorescence. The observed trend for the fluorescence quantum yields is as expected, in this regard with compound 5 having a value almost double that of the other compounds. Triplet quantum yields (ΦT, Table 1) are correspondingly larger for compounds 4 and 6 compared to compound 5, due to the heavy atom effect.
Table 1: The influence of the central metal (compound 4,5 and 6) on the fluoresce efficiency (ΦF)
Compounds ) (nm Q λ ) (nm F λ ) ( s T μ τ 02 . 0 ±F φ 01 . 0 ± T φ 5 10 d
φ
02 . 0 ± Δ φ T S φ φΔ = Δ K 1 − s d Zn (4) 710 719 630 0.13 0.53 0.03 0.43 0.81 4.76 ZnPcSmixi 675 682 530 0.14 0.86 13.65 0.72 0.84 2.58 x 103 Al (5 ) 699 707 810 0.31 0.40 0.04 0.36 0.90 4.94 AlPSmixi 685 690 800 0.39 0.52 5.79 0.48 0.92 0.72 x 103 Sn (6) 703 717 610 0.15 0.47 0.33 0.35 0.74 54.1The triplet lifetimes are lower for compounds 4 and 6 compared to compound 5, again due to the heavy atom effect( Figure 4-1). The triplet quantum yield values (0.40-0.53) are within the typical range for phthalocyanines in DMSO. Also the long lived triplet states (610 µs – 810 µs) values may find potential applications as photocatalysts and also for Photodynamic therapy (PDT). Table 1 shows that ΦIC values are higher for the compound 4, compared to the other two compounds, suggesting that more electronic energy of the excited singlet state is lost through internal conversion to the ground singlet state for compound 4 compared to compounds 5 and 6.
The efficiency of energy transfer from the triplet state of a photosensitizer to ground state molecular oxygen is quantified by a quantity SΔ (Table 1). SΔis the fraction of the
triplet state quenched by ground state molecular oxygen.The efficiency of quenching was fairly high for all the molecules (0.74-0.90), confirming the suitability of these molecules for application as photocatalysts. Compound 5 showed better quenching ability followed by compound 4, with compound 6 showing the least quenching ability. The degradation of the molecules under irradiation is used to study the photostability of the molecules. This is especially important for those molecules intended for use as photocatalysts. Table 1 gives the photodegration quantum yields (Φd) for compounds 4 to 6. These values are generally lower than reported for MPcs, showing that the thiophenyl substituent imparts a degree of stability onto the MPc compounds.
∆
A
time (s)
Figure 4-1: Singlet depletion curve for compound 4 in DMSO. λexc =699nm
4 Results and Discussion
the photodegradation of the compounds in air, oxygen, DABCO (a radical oxygen scavenger), deuterated DMSO (singlet oxygen is longer lived in DMSO) and nitrogen. A faster rate was observed in oxygen than in nitrogen, air or deuterated DMSO, suggesting that oxygen is involved in the photodegradation. Since the compounds were found to be stable in deuterated DMSO, molecular oxygen is likely not involved in the photodegadation progress but rather oxygen radicals and/or singlet oxygen.
A b so rb an ce 0 ≤ t (s) ≤ 800. where t is at 100s interval
Figure 4-2: Kinetic curves for the photodegradation of 5 in DMSO in the presence of (i) oxygen, (ii) nitrogen, (iii) DABCO and (iv) Deuterated DMSO. Excitation wavelength ~ 700nm.
4.2
Summary of paper 2
According to the CALI approach, hydroxyl radical and other oxygen species produced are products of photochemical processes that occur at the triplet state during charge transfer of electrons of the organic dye to a nearby target, in this case antigen. Figure 4-3 shows the photochemical pathways that MPc dyes can take during photoactivation with 633nm laser light. Both pathways result in the generation of hydroxyl radical and hydroxyl ions, where the hydroxyl ions are probably associated in the aqueous
environment. Hydroxyl radical in particular have short-lifetimes in aqueous
environments, giving an effective action radius of approximately15 Å. Within this action radius, it is argued that proteins may be denatured and decoupled.
Figure 4-3: Reaction pathways common to Metallophthalocyanines (MPcs)
Two mechanisms are therefore proposed for the reversible process; one is the case where the MPc dye is detached from the antibody but the antibody is still attached to the protein at the surface. This possibility may arise if the hydroxyl radical is short-lived in the aqueous environment before it gets to the antibody-antigen attachment. When this happens, the dye area being destained will appear dark indicating that the dye is no longer in that region but probably been washed away in the aqueous environment (see Figure 4-4). The other possibility is the migration of the hydroxyl radical to the site of the antibody-antigen interaction site, where the hydroxyl radical causes the denaturing of the protein at the cell surface (Figure 4-5).
4 Results and Discussion
Figure 4-4: Schematic representation for the detachment of the dye from the antibody but where the antibody is still attached to the protein at the surface.
Figure: 4-5: Schematic representation of the dye bound antibody linked to a protein at the cell surface.
green fluorescent dye (c-erbB-2 Ab-2 Alexa 488) and red fluorescent dye (c-erbB-2 Ab-2 Aluminum phthalocyanine) (Figure 4-6).
A
B
C
4 Results and Discussion
Figure 4-6 : Cell staining and FRAP. (A) antibody-alexa staining (prebleach). Excitation source: 488nm, green light; (B) antibody-AlPc(SO2Cl)4 staining (prebleach). Excitation source: 633nm, red light; (C) antibody-AlPc(SO2Cl)4 staining (prebleach) with excitation source at 633nm and alexa-antibody staining (prebleach) with excitation wavelength at 488nm; (D) postbleach result from (C), where a loss of the red signal and a fading green signal is observed. (D) shows fading compared to (A) but with the signal still present.; (E) Seperation of green signal from (D) using dichroic beam splitter; (F) Seperation of red signal from (D) using the dichroic splitter. (F) shows a gradual loss of red signal when compared with (B) at the pre-bleach state.
During multiple irradiation using different wavelengths (488/633 nm), the antibody-AlPc conjugate was specifically removed from the cell surface while the antibody-Alexa 488 conjugate remained (Figure 4-6). Experiments were further performed with free dyes in gel under short time periods. The AlPc dye shows a fast recovery profile in intensity (Figure 4-7), which clearly indicates a non-permanent photobleaching at the triplet state while the controls show little or no recovery profile (or gain in intensity) indicating a permanent photobleaching.
(2)
800 100 200 300 400 500 600 100 120 140 160 180 200 220 240 time (µs) In te n si ty ( A .u.) Region1 Region2 Region3Figure 4-7 : FRAP profile for AlClPc (SO2Cl) 4 showing full signal recovery.
The absence of the red signal intensity in Fig 4-4 D indicates the dye is cleaved from the extracellular matrix cell. This leaves out two possibilities for the mechanism. One is the case where the MPc dye is detached from the antibody but the antibody is still attached
short-lived in the immediate environment and is usually the case before it gets to the antibody-antigen attachment. When this happens, the dye area being destained will appear dark indicating that the dye is no longer in that region but probably been washed away. The other possibility is the migration of the hydroxyl radical to the site of the antibody-antigen interaction site, where the hydroxyl radical causes the denaturing of the protein at the cell surface.
The results from the experiments suggest there is a multiple interaction of forces taking place, such as covalent or ionic interaction forces, which is dependent on the site of bonding between the antibody and the antigen. In the present study, it may be proposed that hydroxyl can cause the cleavage of the sulphonamide bond such that the dye is detached from the antibody but where the antibody-antigen interaction are held together by ionic forces. In order to validate the two proposed mechanisms, the cell was washed in PBS several times and restained with red dye, MPc-antibody. After 60 minutes of incubation, the area that previously was destain, showed a restaining (Figure 4-8). The restaining of the dye suggests that the MPc-antibody could have been cleaved off and the antibody-protein interaction could also have been separated by the release of hydroxyl radical before being short-lived in the aqueous environment.
Figure 4-8: Recovery of the signal after several washouts followed by incubation of the MPc-antibody.
5 Conclusions
5 Conclusions
In this thesis, the photophysics and photochemistry of octakis (benzylthio) phthalocyanines is reported and the potential of using octakis (benzylthio) phthalocyanines as a photoactivatable dyes is discussed. In addition, the combination of CALI protocol and the FRAP technique was employed to investigate the possible photocleavage mechanism of antibody that is conjugated to phthalocyanine using SKBR3 cell line. We show that using reversible staining it is possible to characterize cellular differentiation without any long term effects on the cells or remaining contamination of the fluorophores. For the CALI approach, the differentiation state of the cell is identified by the unique protein that is expressed in order to allow for sensitive and specific detection of proteins on the cell surface.
6 Acknowledgements
I would like to thank the Swedish Institute for the scholarship support, for providing the opportunity of continuing my studies in Sweden. I am indeed grateful to Professor Hjalmar Brismar; he is truly an exceptional supervisor. He was able to link my chemical background to the reversible staining project and also for sourcing for additional financial support for my studies at KTH. Rick Rogers is also acknowledged for not just his wonderful contributions to the project but also, for introducing me to the CALI principle. A big thank to the Cell physics department - of both former co-workers and the present people: Jacob, Susanna, Gustav, Erland, Padideh, Mårten, Linda, Gerald, Bjorn, Tove, Thomas, and Athanasia. Special thanks to Jacob and Padideh, for their help with the administration of the thesis. A very special thank you is extended to Dr Aman Russom for last minute help with the text. Lastly, I would like to thank my brother, Emeka for the supportive role played throughout my education and my family; Ronke, Shaddai and Jesimiel for their patience and understanding.
7 References:
1. Minard-Basquin, C., et al., A Polyphenylene Dendrimer-Detergent Complex as a Highly
Fluorescent Probe for Bioassays. J. Am. Chem. Soc., 2003. 125(19): p. 5832-5838.
2. Ye, Z., et al., Development of functionalized terbium fluorescent nanoparticles for antibody
labeling and time-resolved fluoroimmunoassay application. Talanta, 2005. 65(1): p. 206-210.
3. De Souza, W., From the cell biology to the development of new chemotherapeutic approaches against trypanosomatids: dreams and reality. Kinetoplastid Biology and Disease, 2002. 1(1):
p. 3.
4. Gray, D.C., et al., In Vivo Imaging of the Fine Structure of Rhodamine-Labeled Macaque
Retinal Ganglion Cells. Invest. Ophthalmol. Vis. Sci., 2008. 49(1): p. 467-473.
5. Dey, P., et al., Combined applications of fine needle aspiration cytology and Flow cytometric
immunphenotyping for diagnosis and classification of non Hodgkin Lymphoma. CytoJournal,
2006. 3(1): p. 24.
6. Berney, M., H.-U. Weilenmann, and T. Egli, Flow-cytometric study of vital cellular functions
in Escherichia coli during solar disinfection (SODIS). Microbiology, 2006. 152(6): p.
1719-1729.
7. Buschle-Diller, G. and M.K. Traore, Influence of Direct and Reactive Dyes on the Enzymatic
Hydrolysis of Cotton. Textile Research Journal, 1998. 68(3): p. 185-192.
8. Ara Ãjo, d.F.F.V., L. Yokoyama, and L.A.C. Teixeira, Influence of Experimental Variables on
Decoloration of Azo Reactive Dyes by Hydrogen Peroxide and UV Radiation. Environmental
Technology, 2007. 28(10): p. 1073 - 1078.
9. Hampton, J., P. Goldblatt, and S. Selman, Photodynamic therapy: a new modality for the
treatment of cancer. Ann Clin Lab Sci, 1994. 24(3): p. 203-210.
10. Cuenca, R.E., et al., Breast Cancer With Chest Wall Progression: Treatment With
Photodynamic Therapy. Ann Surg Oncol, 2004. 11(3): p. 322-327.
11. Tsaytler, P.A., et al., Immediate Protein Targets of Photodynamic Treatment in Carcinoma
Cells. J. Proteome Res., 2008. 7(9): p. 3868-3878.
12. Moghissi, K., et al., Photodynamic therapy (PDT) in early central lung cancer: a treatment
option for patients ineligible for surgical resection. Thorax, 2007. 62(5): p. 391-395.
13. Dahle, J., et al., Cooperative effects of photodynamic treatment of cells in microcolonies. Proceedings of the National Academy of Sciences of the United States of America, 1997.
94(5): p. 1773-1778.
14. Jay, D.G., Selective destruction of protein function by chromophore-assisted laser inactivation. Proceedings of the National Academy of Sciences of the United States of
America, 1988. 85(15): p. 5454-5458.
7 References:
16. Bulina, M.E., et al., Chromophore-assisted light inactivation (CALI) using the phototoxic
fluorescent protein KillerRed. Nat. Protocols, 2006. 1(2): p. 947-953.
17. Sonesson, A.W., et al., Lipase Surface Diffusion Studied by Fluorescence Recovery after
Photobleaching. Langmuir, 2005. 21(25): p. 11949-11956.
18. Sekar, R.B. and A. Periasamy, Fluorescence resonance energy transfer (FRET) microscopy
imaging of live cell protein localizations. J. Cell Biol., 2003. 160(5): p. 629-633.
19. Wernerus, H., P. Samuelson, and S. Stahl, Fluorescence-Activated Cell Sorting of Specific
Affibody-Displaying Staphylococci. Appl. Environ. Microbiol., 2003. 69(9): p. 5328-5335.
20. Cain, C., R. Wilson, and R. Murphy, Isolation by fluorescence-activated cell sorting of
Chinese hamster ovary cell lines with pleiotropic, temperature-conditional defects in receptor recycling. J. Biol. Chem., 1991. 266(18): p. 11746-11752.
21. Sanchez, S.A., et al., Tubulin equilibrium unfolding followed by time-resolved fluorescence
and fluorescence correlation spectroscopy. Protein Sci, 2004. 13(1): p. 81-88.
22. Inou Ã, S., et al., Fluorescence polarization of green fluorescence protein. Proceedings of the National Academy of Sciences of the United States of America, 2002. 99(7): p. 4272-4277. 23. Al-Soufi, W., et al., Fluorescence Correlation Spectroscopy, a Tool to Investigate
Supramolecular Dynamics: Inclusion Complexes of Pyronines with Cyclodextrin. J. Am.
Chem. Soc., 2005. 127(24): p. 8775-8784.
24. Guianvarc'h, D., et al., Synthesis and Biological Activity of Sulfonamide Derivatives of
Epipodophyllotoxin. J. Med. Chem., 2004. 47(9): p. 2365-2374.
25. von Greyerz, S., et al., Interaction of Sulfonamide Derivatives with the TCR of
Sulfamethoxazole-Specific Human {alpha}{beta}+ T Cell Clones. J Immunol, 1999. 162(1): p.
595-602.
26. Al-Masoudi, N.A. and Y.A. Al-Soud, New Sulphonamide and Carboxamide Derivatives of
Acyclic C-Nucleosides of Triazolo-Thiadiazole and the Thiadiazine Analogues. Synthesis, Anti-HIV, and Antitumor Activities. Part 2. Nucleosides, Nucleotides and Nucleic Acids,
2008. 27(9): p. 1034 - 1044.
27. Link to Dr. Berard Cole and the gelatin site: http://www.gelatin.co.za
28. Brown, M.B., et al., Dermal and Transdermal Drug Delivery Systems: Current and Future
Prospects. Drug Delivery, 2006. 13(3): p. 175 - 187.
29. Scheindlin, S., Transdermal Drug Delivery: Past, Present, Future. Mol. Interv., 2004. 4(6): p.
308-312.
30. Schneider, A., J.A. Garlick, and C. Egles, Self-Assembling Peptide Nanofiber Scaffolds
Accelerate Wound Healing. PLoS ONE, 2008. 3(1): p. e1410.
31. Martin, B.C., et al., Agarose and methylcellulose hydrogel blends for nerve regeneration
applications. Journal of Neural Engineering, 2008. 5(2): p. 221-231.
32. Alsberg, E., et al., Cell-interactive alginate hydrogels for bone tissue engineering. J Dent Res, 2001. 80(11): p. 2025-2029.
33. Peppas, N.A., et al., Physicochemical foundations and structural design of hydrogels in
medicine and biology. Annual Review of Biomedical Engineering, 2000. 2(1): p. 9-29.
35. Kozlovskaya, V. and S.A. Sukhishvili, Amphoteric Hydrogel Capsules: Multiple
Encapsulation and Release Routes. Macromolecules, 2006. 39(18): p. 6191-6199.
36. Huang, H., et al., Protein-Mediated Assembly of Nanodiamond Hydrogels into a
Biocompatible and Biofunctional Multilayer Nanofilm. ACS Nano, 2008. 2(2): p. 203-212.
37. Zhang, Y., et al., Supramolecular Hydrogels Respond to Ligand-Receptor Interaction. J. Am. Chem. Soc., 2003. 125(45): p. 13680-13681.
38. Liebmann, T., et al., Self-assembling Fmoc dipeptide hydrogel for in situ 3D cell culturing. BMC Biotechnology, 2007. 7(1): p. 88.
39. Liao, J.C., J. Roider, and D.G. Jay, Chromophore-Assisted Laser Inactivation of Proteins is
Mediated by the Photogeneration of Free Radicals. PNAS, 1994. 91(7): p. 2659-2663.
40. Fine, A., Confocal Microscopy: Principles and Practice. Cold Spring Harbor Protocols, 2007.
2007(20): p. pdb.top22-.
41. Klaus, A.V. and V. Schawaroch, Novel methodology utilizing confocal laser scanning
microscopy for systematic analysis in arthropods (Insecta). Integr. Comp. Biol., 2006. 46(2):
p. 207-214.
42. Ono, M., et al., Quantitative Comparison of Anti-Fading Mounting Media for Confocal Laser
Scanning Microscopy. J. Histochem. Cytochem., 2001. 49(3): p. 305-312.
43. Kowalewski, J., Mathematical Models in Cellular Biophysics, in Physics. 2007, KTH:
Stockholm.
44. Bagshaw, C.R. and D. Cherny, Blinking fluorophores: what do they tell us about protein
dynamics? Biochem. Soc. Trans., 2006. 34(Pt 5): p. 979-982.
45. Linschitz, H. and J. Rennert, Reversible Photo-Bleaching of Chlorophyll in Rigid Solvents. Nature, 1952. 169(4292): p. 193-194.
46. Durmus, M., V. Ahsen, and T. Nyokong, Photophysical and photochemical studies of long
chain-substituted zinc phthalocyanines. Journal of Photochemistry and Photobiology A:



![Figure 3-2: Principles of a confocal microscope (from [43]).](https://thumb-eu.123doks.com/thumbv2/5dokorg/5481026.142676/15.892.239.674.257.583/figure-principles-confocal-microscope.webp)