Development of Peptide Nucleic Acid
Based Artificial Ribonucleases
PNAzymes
Sara Andersson
Master Degree Thesis
2010-06-03
Supervisors
Prof. Roger Strömberg Dr Merita Murtola Karolinska Institutet Department of Biosciences and Nutrition Huddinge, Sweden
Examiner
Assoc. Prof. Simon Dunne Mälardalen University, Eskilstuna/Västerås
Abstract
Since mRNA plays such a vital role in the transfer of genetic code from DNA to proteins, approaches involving interference with mRNA are of great interest for use in therapeutics. Amongst these, peptide nucleic acid based nuclease systems (PNAzymes) are being developed for in vivo gene silencing in future. The PNAzymes hybridize to the RNA, bringing a catalytic group to the phosphodiester bond and inducing the cleavage. A study on PNAzymes has shown high site- and sequence-specificity in the cleavage of target RNA sequences and this also occurs at a faster rate than previously reported oligonucleotide-based artificial ribonucleases. However, an increase in rate is still needed for gene silencing applications to be useful therapeutically. This previous study used 2,9-dimethyl-5-aminophenanthroline conjugated to a PNA strand in order to cleave target RNA.
Illustration 1. A phenanthroline derivative attached to a PNA strand designed to work as a
PNAzyme and a schematic picture of the RNA cleavage by an artificial ribonuclease.
The aim of this project was to synthesize 2,5,9-triaminophenanthroline with tert-butyloxycarbonyl groups (Boc-groups) in the 2- and 9-positions, activate the amino group in the 5-position with phenyl chloroformate and conjugate it to PNA and then compare the cleavage efficiency of the resulting PNAzyme with those carrying the 5-aminophenanthroline and 2,9-dimethyl-5-aminophenanthroline derivatives. In the suggested reaction pathway to produce the Boc-protected 2,5,9-triaminophenanthroline, 5 steps out of 7 were successfully achieved. Cleavage studies on all three phenanthroline derivatives will be performed when the final derivatives are synthesized.
Table of Contents
Abstract ...1
Table of Contents ...2
Abbreviatons and definitions ...3
Introduction ...4
Project aim ...4
Background...4
Gene expression ...5
Ribozymes and DNAzymes ...7
siRNA ...7
Antisense oligonucleotides ...8
Artificial ribonucleases ...9
Results and discussion ... 12
Experimental ... 17 Materials ... 17 Instruments ... 17 Synthesis... 17 Acknowledgements ... 20 References ... 21
Abbreviatons and definitions
2´,3´-cAMP 2´,3´- cyclic adenosine monophosphate
A Adenosine
Boc tert-Butyloxycarbonyl
C Cytidine
Dapa Diaminopropionic acid
DCM Dichloromethane
DNA Deoxyribonucleic acid
G Guanosine
HPLC High performance liquid chromatography
LNA Locked nucleic acid
mRNA messenger RNA
MS Mass spectroscopy
Mtt 4-methyltrityl
NMP N-methylpyrrolidone
PNA Peptide nucleic acid
PNAzyme PNA based enzyme
RISC RNA induced silencing complex
RNA Ribonucleic acid
RNase Ribonuclease
siRNA small interfering RNA
T Thymidine
TIS Triisopropylsilane
TLC Thin layer chromatography
tRNA transfer RNA
Introduction
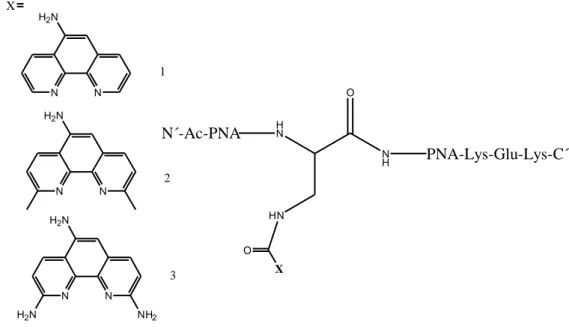
Project aimThe aim of this study was to develop artificial ribonucleases that can be used as artificial RNA restriction enzymes in vitro which can in the future be used for in vivo gene silencing. The project involved synthesis of different phenanthroline derivatives that then will be converted to PNA-attached Cu2+ chelates and used to cleave RNA. The phenanthroline derivatives were planned to be activated at the 5-position with phenylchloroformate and conjugated to a diaminopropionic acid linker on a peptide nucleic acid (PNA) strand. Two of the phenanthroline derivatives, 5-aminophenanthroline 1 and 2,9-dimethyl-5-aminophenanthroline 2, were available for conjugation. 1 was commercially available and 2 was previously synthesized by Hans Åström1. Synthesis of the third derivative, 2, 5, 9-triaminophenanthroline was the major part of this thesis work.
H N HN O N H X O N N H2N N N H2N N N H2N NH2 H2N X N´-Ac-PNA PNA-Lys-Glu-Lys-C´ 1 2 3
Figure 1 Phenanthroline derivatives conjugated to PNA.
PNAzymes carrying the 2,9-dimethyl-5-aminophenanthroline-Cu2+ complex were shown to be site-specific and to cleave target RNA faster than any previously reported oligonucleotide-based artificial nuclease system.2 An interesting question to be answered is whether amino groups in the 2- and 9-position will increase the cleavage rate additionally.
Background
Traditional drugs are developed to inhibit or activate one or several functions of biomolecules such as proteins. Drug design has been a matter of finding small organic molecules to be use
as medicine and the development has been based on the knowledge of a biological target. Now, scientists are trying to cure diseases in an even earlier stage. This new type of therapy involves interference with gene expression and means that biomolecules like proteins are produced in decreased amounts or not at all. There are many possible ways to achieve the desired effect and for that reason the general sequence of events in gene expression is described first.
Gene expression
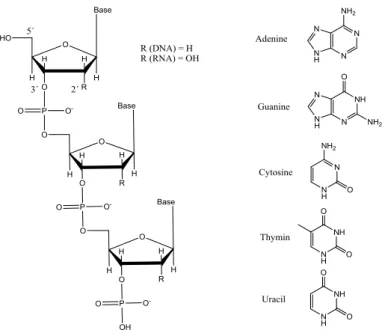
DNA and RNA, our natural carriers for genetic information, consist of nucleic acids that provide directions for building proteins in the body. DNA serves as the storage unit of genetic information, while RNA expresses the information and transfers it to the ribosomes where it is translated into proteins. They are similar in structure but differ in their chemistry and therefore their roles within in the cell. Both DNA and RNA molecules are built up from strands of nucleotides each containing three different components; a pentose sugar, a phosphate group and one of four different types of nitrogenous bases, figure 2. The sugar molecule in DNA is deoxyribose and for RNA it is ribose. The bases attached to the sugar molecule are built up from purine or a pyrimidine with different substituents. DNA contains the purines adenine (A) and guanine (G) and the pyrimidines thymine (T) and cytosine (C). In RNA, thymine is replaced by uracil (U).3
The nucleotides are connected to each other with 3´-5´-phosphodiester linkages to form long strands. A DNA molecule is double-stranded and built up of two anti-parallel strands with the nucleotides running in the opposite direction from one another. One strand running from 3´ to 5´ and the other strand running in the opposite sense from 5´ to 3´. In this double helix, the strands are linked together by base-pairing. Each base in the strand forms specific hydrogen bonds to the base in the other strand directly across from it. In a DNA double helix, adenine and thymine bind together by two hydrogen bonds while guanine and cytosine bind together by three hydrogen bonds. In RNA, uracil binds to adenine in a similar way as thymine does in DNA.3 The hydrogen bonding between the bases are called Watson-Crick from the men who proposed the double helix structure in 1953. Watson and Crick also suggested the mechanism for replication of this structure.4
Expression of a gene, a certain sequence of nucleotide bases, is a two step process involving
transcription and translation. The DNA is not able to produce proteins itself, it has to be
transcribed to RNA and then translated from the RNA base sequence to the amino acid sequence of a polypeptide chain. RNA polymerase binds to the promoter on the DNA strand with help of different transcription factors to uncoil it temporarily from the helix structure in order to start transcription. When uncoiled, ribonucleotides are based-paired with the 3´ to 5´ template DNA strand with the help of polymerase and other transcription factors to produce 5´ to 3´ mRNA. The strand elongation continues until it reaches the terminator, where the RNA polymerase and the completed pre-messenger RNA (pre-mRNA) are released.4
When pre-mRNA is processed and ready, it travels to the ribosomal ribonucleic acid (rRNA), which is the central component of the ribosome. The function of rRNA is to provide a mechanism for decoding mRNA into amino acids and to interact with the transfer RNA (tRNA). tRNAs have a site for amino acid attachment, and a site called an anticodon. The anticodon is an RNA triplet complementary to the mRNA triplet that codes for their cargo amino acid. The first tRNA sequence, complementary to the start codon of mRNA, is attached in a process called initiation in the ribosome. The next tRNA is then attached and another amino acid is added to the first one. This process, called translation, is repeated and amino acids are added to form a polypeptide. When the ribosome discovers a stop codon in the mRNA sequence, the translation and polypeptide formation ends. The polypeptide is then released into the cytoplasm.3,4
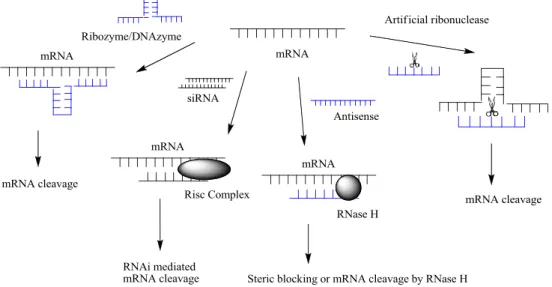
Since mRNA plays such a vital role in the transfer from DNA to proteins, approaches of interfering with mRNA are of great interest for use in therapeutics. The major advantage with inhibition at the gene-level is the specificity of blocking the production of certain proteins by synthesizing a complementary sequence to the target mRNA. This method of interfering with mRNA is called oligonucleotide-based technology and is performed to prevent the above mentioned processes from taking place with the assistance of short strands of DNA or RNA sequences, called oligonucleotides. Different approaches of oligonucleotide-based therapy are shown in figure 3, and involve amongst others ribozymes, DNAzymes, siRNAs, antisense oligonucleotides and artificial ribonucleases to cleave target mRNA.5
Figure 3. Different approaches of oligonucleotide-based therapy to cleave target mRNA.
Ribozymes and DNAzymes
Ribozymes and DNAzymes are both molecules with catalytic action. Natural ribozymes are able to catalyze the hydrolysis of their own phosphodiester bonds and also the bonds of another RNA strand. Ribozymes are enzymes by themselves, which means that they do not require proteins in order to function. DNAzymes, close relatives to ribozymes, are also capable of cleaving an RNA sequence specifically.6
siRNA
Small interfering RNAs (siRNAs) are double-stranded fragments about 20 nucleotides in length, produced from enzymatic cleavage of double-stranded RNA. They are used by the cell in a process called RNA interference to prevent mRNA from being used as a translation template. siRNA is separated and integrated into an RNA-induced silencing complex (RISC). The RISC complex base-pairs to the target mRNA and induces cleavage of the mRNA. Many
studies of siRNA have been performed since its discovery in order to develop its use as therapeutic method.6,7
Antisense oligonucleotides
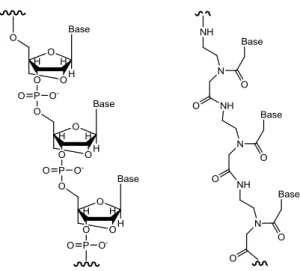
Natural oligonucleotides exhibit antisense properties in vitro and are therefore also candidates for therapeutics. Unfortunately, in vivo, DNA and RNA become degraded by nucleases and are not biologically stable. For that reason, analogue oligonucleotides have been developed.8 In antisense oligonucleotide therapy, the synthetic oligonucleotide hybridizes with target mRNA which can lead to destruction of RNA by the enzyme Ribonuclease H (RNase H). Furthermore, antisense oligonucleotides are also capable of binding to the start site region in translation by hybridization with target mRNA, resulting in a steric blockade of the target. The first modifications made on the oligonucleotides that gave resistance to exo- and endonucleases in the blood cells, but still activated RNase H, was the change of the phosphate backbone to a phosphorothioate backbone. Substitution of one oxygen atom with sulfur stabilized the backbone somewhat, but also gave some toxicity through nonspecific binding to proteins. A second generation of modified oligonucleotides involved O-methyl and 2´-methoxy ethyl modifications and resulted in improved binding to mRNA, increased stability to enzymatic degradation, but weak activation of RNase H. Some of the third generation antisense oligonucleotides have involved larger or total changes of the backbone. Two promising candidates are locked nucleic acid (LNA) and peptide nucleic acid (PNA), figure
4. LNA, a RNA nucleotide modified with an extra bridge connecting the 2' oxygen and 4'
carbon, gives high affinity, specificity to a target RNA strands and stability in vivo.5,6,9 Peptide nucleic acid (PNA) is built up of an achiral pseudopeptide, N-(2-aminoethyl) glycine units with the nucleobases attached.10 It was proposed in year 1991 by Peter E. Nielsen et al., a Danish scientist, with computer-assisted model building. PNA-DNA duplexes in anti-parallel conformation have shown to be more stable than the corresponding DNA-DNA duplex. The melting point (Tm) is higher for PNA-DNA than DNA-DNA. The stability of
PNA-RNA is also higher than for DNA-DNA. PNA is of great interest in the development of antisense oligonucleotide therapy but the duplex form with RNA is not recognized by RNase H, which means that the turnover and reuse of the PNA will not be realized when targeting mRNA.8 This could potentially be solved if the PNA itself would act as an RNA cleaving enzyme, that is if the PNA could be modified to become an artificial ribonuclease.
Figure 4. Backbone of locked nucleic acid (LNA) and peptide nucleic acid (PNA).
Artificial ribonucleases
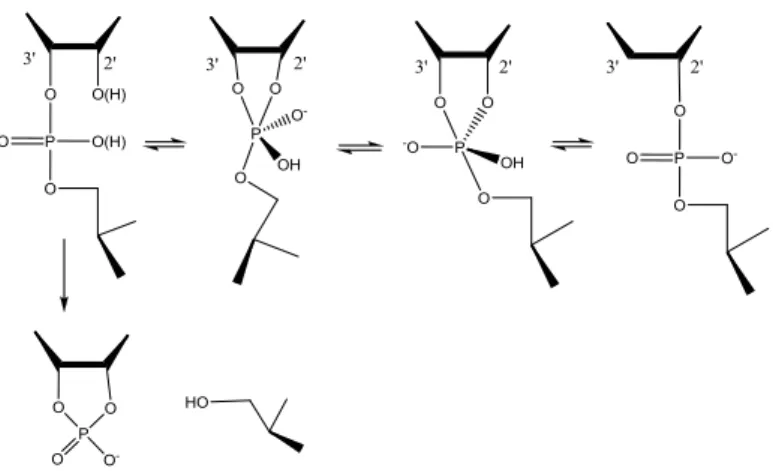
Artificial ribonucleases are mimics of natural ribonucleases and are thereby able to cleave phosphodiester bonds in RNA. In the body, RNA is often (for example by RNase H) cleaved by an attack on the phosphorous atom by the 2´-hydroxyl or 2´-oxyanion to form a phosphorane intermediate, figure 5. This is also typical for nonenzymatic cleavage of RNA, where degradation of phosphorane, departure of the 5´-linked nucleoside, in many cases is the rate-limiting step in this process when it leaves as an oxy anion. This process can be accelerated by reducing electron density at the departing 5´-oxygen (for example by protonation). The overall reaction can also be enhanced by 2´-OH deprotonation and reduction of the electron density at the phosphorous to facilitate the formation of the phosphorane intermediate, which would breakdown faster if it is not protonated. The secondary structure affects the stability of phosphodiester, due to geometric reasons. In a double helix, base-pairing hinders cleaving from occuring as the departing 5´-linked nucleoside is prevented from adopting an axial position. Oxygen as the nucleophile can only enter and leave through an axial position.11 Another possible product of this transesterification reaction might be a 2´,5´-isomer. Both cleavage and isomerization of RNA is shown in figure
5.12
Due to difficulties in cleaving double-stranded RNA, an optimal target is to cleave RNA at a place where it is single stranded, e.g. RNA loop or bulge. These regions contain nucleotides with no complementary bases in the opposite RNA strand and are potential targets for site-selective cleavage.13 It is also known that metals are involved in the cleavage of RNA by acting as catalysts. The mechanism of metal ion promoted cleavage of RNA is typically not
known in detail for artificial ribonuclease systems, but several suggestions involve coordination of the metal ion with the oxygen atoms in the phosphodiester. Coordination might catalyze hydrolysis of the phosphodiester bonds by nucleophile activation, leaving group activation or Lewis acid activation. More than one type of activation may also occur to give an even faster cleavage.14
Figure 5. The two different products formed in transesterification of RNA, the isomerized
and the cleaved product.
Artificial ribonucleases consist of two parts, a moiety for sequence recognition that hybridizes it with the target RNA and a catalytic group to cleave the phosphodiester bond. Some different modifications of the sequence-recognizing moiety in oligonucleotide-based artificial nucleases have been tested, but most variation is found with regard to the catalytic group. These are often divided into categories depending on what type of active conjugate group is present, e.g. lanthanide ion chelates, Cu2+ and Zn2+ chelates, metal ion independent groups. It has been found that, a loop or bulge is preferred in order to cleave RNA efficiently.11 Another important feature in the design of artificial nucleases is the capability of reuse of the artificial enzyme to cleave more than one target RNA molecule, that is, to get turnover of the substrate. A goal of this study is to develop artificial nucleases that can be used for in vivo gene silencing. Even though the cleavage occurs quite quickly, the current system does not give sufficient cleavage rates to be clinically useful; an improvement of about one order of magnitude is needed for gene silencing applications in therapeutics.
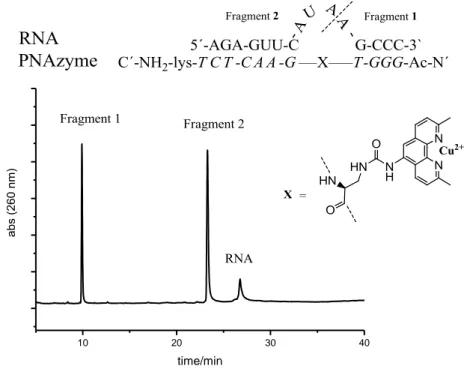
PNA-based nuclease systems (PNAzymes) developed by Merita Murtola12 have shown promising results in the cleavage of target RNA sequences. PNAzymes are individually modified artificial enzymes of PNA capable of sequence-selective cleavage of target RNA. Artificial PNAzymes selectively hybridize with the target RNA, which brings the catalytic
group close to a phosphodiester linkage and induces cleavage of the RNA. This study of PNAzymes showed considerably faster cleavage rates than any other reported oligonucleotide-based artificial nuclease system and has been shown to give high site- and sequence-specificity, see figure 6.11,12
10 20 30 40
time/min
Figure 6. Selective cleavage of target RNA by PNAzyme with IE-HPLC chromatogram after
1h in 37 °C. PNAzyme:RNA, 1:1 (4 mikroM each), in Buffer: 10, mikroM Cu2+, 10 mM HEPES, 0,1 M NaCl.
This project involves the synthesis and evaluation of different phenanthroline derivatives to be used as PNA-attached Cu2+ chelates that cleave target RNA. 5-Aminophenanthroline is commercially available and 5-amino-2,9-dimethylphenanthroline was synthesized by Hans Åström1. 2,5,9-Triaminophenanthroline with tert-butyloxycarbonyl groups (Boc-groups) in the 2- and 9-positions is a synthetic target in this study. The phenanthroline derivatives will be activated with phenylchloroformate and conjugated to a diaminopropionic acid (dapa) linker moiety that is inserted into a PNA strand.
The previous study used 2,9-dimethylphenanthroline-Cu2+ as the scissor-acting part to cleave RNA with good results.10 Since 2,9-diaminophenanthroline-Cu2+ seems to cleave 2´,3´-cAMP about 1000 times faster than 2,9-dimethylphenanthroline- Cu2+ 15, this molecule is of great interest and will be synthesized and evaluated.
PNA-synthesis will be carried out on an automated peptide synthesizer and conjugation to the phenanthroline derivatives will be performed on solid supports by making use of the activated carbamates in reaction with a free amine on the PNA. The kinetics of RNA cleavage for all three PNAzymes will be followed by HPLC analysis and compared.
Results and discussion
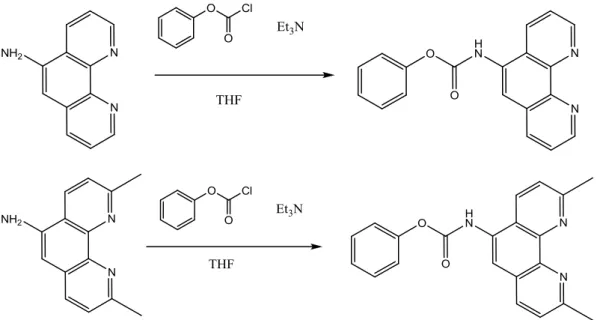
For synthesis of the PNAzymes, the phenanthroline derivatives first need to be activated with phenylchloroformate and then conjugated to a diaminopropionic acid (dapa) linker on a PNA strand. The activation reactions for 5-aminophenanthroline and then 5-amino-2,9-dimethylphenanthroline were performed in quite good yield, 44 % and 59 % respectively (after chromatography). The reaction with 5-aminophenanthroline was also repeated. According to 1H NMR the products seemed quite pure after chromatography, only some peaks with small integrals were found to be side-products. Perhaps the chromatography could have been optimized to remove these side-products, but it was considered not to be necessary since the products were not likely to interfere with the next reaction, where these side-products would anyway be removed in the HPLC-purification.
Figure 1. Activation of 5-aminophenanthroline and 5-amino-2,9-dimethylphenanthroline with
phenylchloroformate.
To be able to conjugate the diaminophenanthroline to a PNA strand, an amino group in the 5-position on the phenanthroline was needed. To avoid formation of side-products when reacting the triaminophenanthroline with phenylchloroformate in the activation step,
butyloxycarbonyl (Boc) groups in the 2- and 9- positions should also be introduced. The Boc-groups will be removed after conjugation with PNA.
2,5,9-triaminophenanthroline, is a new compound that has never been synthesized before and as such more challenging to synthesize. A reaction scheme was devised to obtain compound
7, see scheme 1. The three first intermediates (2, 3 and 4) were synthesized according to a
literature reference16.
Scheme 1. Suggested reaction pathway to synthesize the 5-nitrophenanthroline with
Boc-protected amino groups in the 2- and 9-positions and a numbering scheme for phenanthroline derivatives.
The first step involved an alkylation of the 1,10-phenanthroline (1) nitrogens with 1,3-dibromopropane The propylene chain bridged the two nitrogens. Since bromide acts as a good leaving group, this reaction was reasonably straightforward. This was confirmed by 1H NMR which showed a very pure product (2) in a good yield of 70%.
In the second reaction, potassium ferrocyanide was used to oxidize (1,10-propano)-[1,10]-phenanthrolindium dibromide (2) in the 2- and 9-positions. This reaction was performed twice, the first time with column chromatography as the purification method and the second time without since the product did not contain impurities or side-products after work-up. One possible explanation for the impurities in the first run might be that the temperature of the solution was not in the range of 2-5 °C during the entire addition of 2 to the oxidant mixture. Compared to the literature14, the 37% yield of (1,10-propano)-[1,10]-phenanthroline-2,9-dione (3) was satisfactory for this reaction.
Chlorination of 3 gave a relatively pure product according to NMR. The propylene chain was cleaved from the phenanthroline molecule as expected in the reaction. Due to the risk of losing product in chromatographic purification, the synthesis was continued without further purification. The remains from the released propylene chain should not interfere with the next reaction.
2,9-Dichlorophenanthroline (4) was reacted with a mixture of nitric and sulfuric acids in order to nitrate the 5-position. Synthesis was performed in a similar matter as for the nitration of 1,10-phenanthroline by S. N. Shukla et al17 but with some modifications. A yield of 54% was achieved for this nitration in comparison to 85 % for the parent 1,10-phenanthroline17.
The nitration reaction was repeated several times during the project. In the first reaction, orange yellow crystals were formed, collected and confirmed to be pure product by both MS and NMR spectroscopy. In the other nitration reactions, an orange yellow precipitate was visible directly after the mixture was diluted with water. The second nitration resulted in orange red crystals after base addition. A possible side-product could have been 2,9-dichlorophenanthroline-5,6-dione. No 5-nitro-2,9-dichlorophenanthroline (5) could be isolated from the second reaction mixture. A third reaction was performed, this time the crystals were collected directly after the dilution with water. The remaining solution was made neutral and a second crop of crystals were collected. Crystals were dissolved in dichloromethane and concentrated, which was a modification of the original reaction in the reference17. MS and NMR confirmed pure product in a reasonable good yield of 54%.
TLC was difficult to interpret as the starting material and the product possessed very similar Rf-values. A way to confirm that the 2,9-dichlorophenanthroline (4) had reacted into 5-nitro-2,9-dichlorophenanthroline (5) was to change the wavelength of the UV-lamp from 254 to 365 nm. Both compounds were visible under 254 nm irradiation but under 365 nm irradiation the 5-nitro-2,9-dichlorophenanthroline (5) had a more purple coloration.
As 5-nitro-2,9-dichlorophenanthroline (5) has not been previously reported, additional NMR experiments were performed (see Appendix I).
Some attempts of synthesizing 5-nitro-2,9-diaminophenanthroline were made without success. The first attempt was to aminate the 2- and 9-positions by bubbling ammonia gas through a flask with 5 together with acetamide in phenol at 160°C for 8 hours. A mixture of different compounds could be seen on TLC, but unfortunately none of them were possible to identify as the 5-nitro-2,9-diaminophenanthroline. One way to improve the reaction could be to perform it in a pressure-vessel.
Another attempt to synthesize 5-nitro-2,9-diaminophenanthroline 7 by adding amino groups with Boc-protection in the 2- and 9-positions in one step was performed. 5-Nitro-2,9-dichlorophenanthroline was reacted with tert-butyl carbamate, sodium phenolate and a palladium catalyst as a phosphonium tetrafluoroborate salt in a mixture. The mixture was left to react overnight at 100°C to obtain the desired product, but without success. Several products formed, but no product that could be isolated and identified as the desired product. A problem that could explain this result, is that unintentionally only one equivalent of tert-butyl carbamate was added, and the project time ran out before I could repeat the reaction.
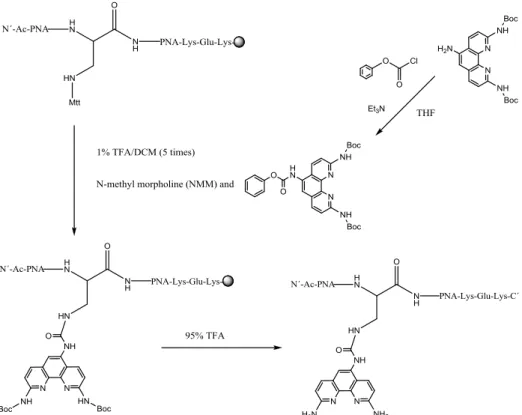
If the target molecule would have been synthesized, it would have been activated in the same manner as the other phenanthroline derivatives and later attached to the PNA strand, figure 7. In the activation step, the Boc-protection groups in the 2-and 9-positions are needed to maintain regiospecificity.
Figure 7. Activation of 2,5,9-triaminophenanthroline with phenyl chloroformate and
conjugation to the dapa-linker in PNA. Mtt deprotection by treatment of 1% TFA/DCM. Phenanthroline conjugation in the presence of NMM. 95% TFA cleaves off all protection -groups and releases the product from the solid support.
The PNA is made on solid support with a diaminopropionic acid linker (dapa-linker) in the middle. Amino acids, glutamic acid and lysine, are attached to increase the solubility of the PNA. A methyltrityl (Mtt) group on the dapa-linker will be cleaved off by treating it with 1% TFA in DCM 5 times. The activated phenanthroline molecule is added with a little base present. After the conjugation the Boc groups and the solid support are cleaved off with 95% TFA containing 2.5% TIS and 2.5% H2O. After HPLC purification and freeze drying
(repeated three times), the PNAzyme will be ready for use in cleavage studies.
No cleaving studies were performed since synthesis of the most interesting phenanthroline, 2,5,9-triaminophenanthroline, was not completed. The aim was not totally achieved since the target molecule was not produced, 5 out of 7 steps were performed and resulted in the 5-nitro-2,9-dichlorophenanthroline. This molecule has not previously been reported and makes me a little bit satisfied, even though I really wanted to get to the final product.
During my 20 weeks of work at Karolinska Institutet I have learnt a lot of things in the area of organic synthesis and drug development. I have learnt to work with a project for a limited
period of time and I have realized that things often take longer time than expected when working in the laboratory. It is not possible to skip a step in a reaction because the synthesis did not work out in the desired way. You always have to work to solve the problems in order to continue on in your work. There will always be ups and downs when working with chemistry; you never really know how the reaction will turn out. Is it not even sure that the same reaction will turn out as the previous one, but that is the fascination with chemistry.
Experimental
MaterialsThe materials used are reported in Appendix II. Instruments
NMR
400 MHz Ultrashield Bruker Avance DRX-400
1
H NMR spectra were recorded at 400 MHz
13
C NMR spectra were recorded at 100.6 MHz
Mass-spectroscopy
Micromass LCT, scanning between m/z 100-2000 Kd scientific pump
Scales
Sartorius BP310S, 310 g ± 0.001 g
Mettler Toledo AND HR-60, 60 g ± 0.1 mg
UV
Vilber Lourmal UV-lamp, 254 and 365 nm
Synthesis
Synthesis of phenylcarbamoyl-1,10-phenanthroline
5-Aminophenanthroline (400 mg, 2.05 mmol) was suspended in dry THF (40 ml) and Et3N
(428 µl, 3.07 mmol) and phenylchloroformate (385 µl, 3.07 mmol) was added under Ar. The THF was evaporated and the solid was dissolved in CHCl3. The mixture was washed with
saturated NaHCO3-aq and dried over MgSO4. The mixture was filtered, evaporated and dried
under vacuum overnight. Chromatography (15% MeOH in CHCl3) was performed on silica.
Yield after purification was 44 %. 1H NMR (400 MHz, CHCl3, 25°C) δ= 9.15 (d, 3J = 4.4 Hz,
= 8.1, 4.5 Hz, 1H), 7.61 (t, 3J = 8.4, 4.3 Hz, 1H), 7.40 (t, 3J = 7.7, 7.9 Hz, 2H), 7.28-7.21 (m,
4H), 5.78 (s, 1H). MS+ peak at 316.37 (molecular weight 315.33).
Synthesis of 2,9-dimethyl-5-phenylcarbamoyl-1,10-phenanthroline
5-Amino-2,9-dimethylphenanthroline (204 mg, 0.913 mmol) was suspended in dry THF (22 ml) and Et3N (191 µl, 1.369 mmol) and phenylchloroformate (172 µl, 1.369 mmol) were
added under Ar. The THF was evaporated and the solid was dissolved in CHCl3. The mixture
was washed with saturated NaHCO3(-aq) and dried over MgSO4. The mixture was filtered,
evaporated and dried under vacuum overnight. Chromatography (5% MeOH in CHCl3) was
performed on silica. Yield after purification was 59%. 1H NMR (400 MHz, CHCl3, 25°C) δ=
8.81 (s, 1H), 8.18 (d, 3J = 8.3 Hz, 1H), 8.13 (d, 3J = 8.4 Hz, 2H), 7.60 (d, 3J = 8.3 Hz, 1H),
7.52 (d, 3J = 8.5 Hz, 1H), 7.43 (d, 3J = 8.3 Hz, 2H), 7.29 (s, 1H), 7.27-7.25 (m). MS+ peak at
344.47 (molecular weight: 343.38).
Synthesis of (1,10-propano)-[1,10]-phenanthrolidium dibromide (2)
To a solution af 1,10-phenanthroline monohydrate 1 (10.00 g, 50.4 mmol) dissolved in nitrobenzene (80 ml), 1,3-dibromopropane (26 ml) was added dropwise. The mixture was refluxed at 120°C until a crystalline product formed, after 4 h, and cooled overnight. The crystals were collected by filtration and dried under vacuum for 24 h. The crystals were dissolved in water (50 ml) and warmed to 80°C. Ethanol (ca 300 ml) was added slowly with stirring and the solution was left to cool overnight. Crystals were collected in a yield of 70 %.
1
H NMR (400 MHz, D2O, 25°C) δ= 9.64 (d, 3J = 5.8 Hz, 2H, H-2,9), 9.43 (d, 3J = 8.4 Hz, 2H,
H-4,7), 8.56 (S, 2H, H-5,6), 8.52 (dd, 3J = 5.8, 8.4 Hz, 2H, H-3,8), 5.12 (t, 3J = 6.9 Hz, 4H,
H-a), 3.40 (q, 3J = 7.0 Hz, 4H, H-b).
Synthesis of (1,10-propano)-[1,10]-phenanthroline-2,9-dione (3)
NaOH (44.2 g, 1.10 mol) was added slowly with stirring to an aqueous solution of [K3Fe(CN)6] (108 g, 327 mmol in 180 ml water). The flask was placed in an ice/water bath
and cooled to 2-5 °C. Compound 2 (12.503 g, 32.7 mmol) was dissolved in water (25 ml) and added dropwise to the flask maintaining the temperature at 2-5 °C. The reaction mixture was allowed to stir for 2 h, before it was neutralized to pH 7-8 with 4 M HCl. The beige brown solid was partially dissolved in CHCl3 and left to stir overnight, then filtered, concentrated
and dried under vacuum. The resulting solid was subjected to silica gel chromatography (5% MeOH in CHCl3). Yield of reaction was 37 %. 1H NMR (400 MHz, CDCl3, 25°C) δ= 7.73 (d,
3
J = 9.5 Hz, 2H, H-4,7), 7.38 (s, 2H, H-5,6), 6.82 (d, 3J = 9.4 Hz, 2H, H-3,8), 4.33 (t, 3J = 6.5
Hz, 4H, H-a), 2.47 (q, 3J = 6.5 Hz, 2H, H-b).
Synthesis of 2,9-dichloro-1,10-phenanthroline (4)
Compound 3 (3.027 g, 12 mmol) was dissolved in POCl3 (30 ml) and PCl5 (5.0 g, 24 mmol)
was added. The mixture was degassed and refluxed at 110°C under argon overnight. Excess POCl3 was removed by distillation and the solid material was decomposed with ice. The
suspension was neutralized with ammonia solution (30%, aqueous) with cooling. A brown precipitate was collected and dried under vacuum. The mother liquor was extracted with CH2Cl2 (2 x 40 ml) and concentrated. No further purification was performed.1H NMR (400
MHz, CDCl3, 25°C) δ= 8.23 (d, 3J = 8.5 Hz, 2H, H-4,7), 7.85 (s, 2H, H-5,6), 7.67 (d, 3J = 8.4
Hz, 2H, H-3,8), 1.26 (m, 4H, H-a), 0.86 (m, 2H, H-b).
Synthesis of 5-nitro-2,9-dichloro-1,10-phenanthroline (5)
2,9-Dichloro-1,10-phenanthroline 4 (1.229 g, 4.93 mmol) was dissolved in concentrated H2SO4 (10 ml) and a mixture of fuming HNO3 (2 ml) and H2SO4 (2 ml) was added. The
mixture was heated to 100 °C for 2 h and then diluted with water. The precipitate was collected and the mother liquor was made more neutral with NaOH (aq) in order to complete precipitation of the yellow product. The precipitated material was combined, extracted with CHCl3 (3*40 ml) and concentrated to yield orange yellow crystals, yield 54 %. 1H NMR (400
MHz, CDCl3, 25°C) 1H NMR (400 MHz, CDCl3, 25°C) δ= 9.04 (d, 3J = 9.0 Hz, 1H, H-4),
8.74 (s, 1H, H-6), 8.40 (d, 3J = 8.4 Hz, 1H, H-7), 7.84 (d, 3J = 9.0 Hz, 1H, H-3), 7.80 (d, 3J =
Acknowledgements
During the work of my Master degree thesis I have had a lot of help and support from other chemists, friends and family.
I would like to thank Professor Roger Strömberg for the opportunity to perform my Masters degree project in his group.
Thank you Dr Merita Murtola for all help and support during my work. You have tried to explain an entirely new area for me in such a short time, thank you for answering my sometimes odd questions.
A great thanks to you Associate Professor Simon Dunne for all help and support during my Masters degree and all these years during my education.
I would also like to thank my friends in the laboratory; Erik, Joanna, Malgorzata, Mikael, Partha, Stefan and all people from JB´s group for good times.
Thank you also Mattias Lindvall for being such a good and supportive friend during this time at Karolinska Institutet and also for the past three years.
Great thanks to all my friends and family for all support and advice during my Masters degree project.
Thank you Mälardalen University for the economic support that made it possible for me to perform my Masters degree project at Karolinska Institutet.
References
1
Aström H, Strömberg R.,(2001) A method for synthesis of an artificial ribonuclease, Nucleosides Nucleotides Nucleic Acids, 20(4-7),1385-8.
2
Murtola M., Wenska M., Strömberg R. (2010) PNAzymes that are artificial RNA restriction enzymes, J.Chem Soc., ID: 2010-008739
3Campbell N. A., Reece J. B., Simon E. J., (2007) Essential Biology with physiology, San
Francisco, Pearson Education Inc, pp 35-53, 171-198.
4
Becker W. M., Kleinsmith L. J., Hardin J., (2006) The world of the cell, San Francisco, Pearson Education Inc. pp 40-73, 649-683.
5
Isaka Y., Imai E., Takahara S., Rakugi H., (2008) Oligonucleotidic therapeutics. Expert opinion, Drug Discovery, 3, pp 991-996.
6
Eckstein F., (2007) The versatility of oligonucleotides as potential therapeutics. Expert opinion, Drug Discovery, 7, pp 1021-1034.
7
Hannon G. J., Rossi J. J., (2004) Unlocking the potential of the human genome with RNA interference. Nature, 431, pp 371-378.
8
Hyrup B., Nielsen P.. (1996) Peptide Nucleic Acids (PNA): Synthesis, Properties and Potential Applications. Bioorganic & Medicinal Chemistry, 4(1), pp. 5-23.
9
Juliano R., Bauman J., Kang H., Ming X., (2009) Biological Barriers to Therapy with Antisense and siRNA Oligonucleotides. Molecular Pharmaceutics, 6(3), pp 686-695.
10
Ratilainen T., Holmén A., Tuite E., Nielsen P. E., Nordén B., (2000) Thermodynamics of Sequence-specific binding of PNA to DNA. Biochemistry, 39, pp 7781-7791.
11
Niittymäki T., Lönnberg H., (2005) Artificial ribonucleases. Organic & Biomolecular Chemistry, 4, pp 15-25.
12
Murtola M., Thesis Karolinska Institutet 2009, ISBN 978-91-7409-725-2.
13
Kuznetsova I. L., Zenkova M. A., Vlassov V. V., (2006) Cleavage of RNA bulge loops by artificial RNases. Russian Chemical Bulletin, International Edition, 55(7), pp 1284-1294.
14
Mikkola S., Stenman E., Nurmi K., Yousefi-Salakdeh E., Strömberg H., Lönnberg H., (1999) The mechanism of the metal ion promoted cleavage of RNA phosphodiester bonds involves a general acid catalysis by the metal aquo ion on the departure of the leaving group. J.Chem Soc, Perkin Trans 2, pp 1619-1625.
15
Wall M., Linkletter B., Williams D., Lebuis A.-M., Hynes R. C., J. Chin., (1999) Rapid Hydrolysis of 2´,3´-cAMP with a Cu(II) Complex: Effect of Intramolecular Hydrogen Bonding on the Basicity and Reactivity of a Metal-Bound Hydroxide. Journal of the American Chemical Society, 121, pp 4710-4711.
16
Frey J., Kraus T., Heitz V., Sauvage J-P., (2007) Synthesis of a Bis-macrocycle Containing Two Back-to-Back Rigidly Connected 1,10-Phenanthroline Units as a Central Core and its Incorporation in a Handcuff-Like Catenane, Chem. Eur. J., 13, pp 7584-7594.
17
Shukla S. N., Gaur P., Mehrotra R., Prasad M., Kaur H., Prasad M., Srivastava R. S., (2008) Tailored synthesis, spectroscopic, catalytic, and antibacterial studies of dinuclear ruthenium (II/III) chloro sulfoxide complexes with 5-nitro-o-phenanthroline as a spacer, Journal of Coordination Chemistry, 62(15), pp 2556-2568.