Microbial Pathogenesis xxx (xxxx) xxx
Available online 23 November 2020
0882-4010/© 2020 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Porphyromonas gingivalis initiates coagulation and secretes polyphosphates
—
A mechanism for sustaining chronic inflammation?
Jessica Neilands, Bertil Kinnby
*Dept of Oral Biology and Pathology, Faculty of Odontology, Malm¨o University, Malm¨o, Sweden
A R T I C L E I N F O Keywords: Fibrin Porphyromonas gingivalis Polyphosphates Inflammation Periodontitis A B S T R A C T
Background: Periodontitis is a chronic inflammation resulting in destruction of tooth-supporting bone. Chronic inflammation is characterized by extravascular fibrin deposition. Fibrin is central to destruction of bone; monocytes bind to fibrin and form osteoclasts, thus providing a link between coagulation and the tissue destructive processes in periodontitis.
The oral microbiome is essential to oral health. However, local ecological changes, such as increased biofilm formation, result in a dysbiotic microbiome characterized by an increase of protease-producing species e.g. Porphyromonas gingivalis. Proteases initiate inflammation and may cleave coagulation factors. Polyphosphates (polyP) may also provide bacteria with procoagulant properties similar to platelet-released polyP. P. gingivalis has also been found in remote locations related to vascular pathology and Alzheimer’s disease.
Objectives: The aim of this study was to investigate procoagulant activity of ten different species of oral bacteria present in oral health and disease as well as presence of polyP and fibrin formation in planktonic and biofilm bacteria.
Methods: Oral bacteria were studied for protease production and procoagulant activity. The presence of polyP and formation of fibrin was observed using confocal microscopy.
Results: P. gingivalis showed strong protease activity and was the only species exerting procoagulant activity. Confocal microscopy showed polyP intracellularly in planktonic bacteria and extracellularly after biofilm for-mation. Fibrin formation emanated from planktonic bacteria and from both bacteria and polyP in biofilm cultures.
Conclusions: The procoagulant activity of P. gingivalis could explain its role in chronic inflammation, locally in oral tissues as well as in remote locations.
1. Introduction
Periodontal inflammation, periodontitis, is a chronic inflammatory condition characterized by destruction of tooth-supporting bone with loss of teeth as the ultimate consequence. A general feature of inflam-matory lesions is extravascular fibrin deposition [1], that in the course of a normal healing process is degraded through plasmin-mediated fibri-nolysis. Coagulation is a hallmark of inflammation; connections and interactions between inflammation and coagulation are well docu-mented and have seen a recent surge in interest [2–6]. Without proper resolution, persistent fibrin deposits in the tissue have a strong proin-flammatory effect supporting sustained inflammation [1,2,7–14]. This is reflected in several studies showing the importance of plasminogen/-plasmin for successful healing [15–17], including the restitution of lost
tooth-supporting bone in experimental periodontitis in mice [18]. Fibrin has been demonstrated to be central to the destruction of bone in the pathogenesis of osteoporosis [10] as well as periodontitis. In experimental periodontal inflammation, tissue deposits of fibrin have been observed located close to bone resorption [18], a phenomenon that is also evident in human plasminogen deficiency [19], characterized by ligneous periodontitis [20]. Monocytes bind to fibrin and form osteo-clasts, thus providing a link between coagulation and the tissue destructive processes in periodontitis [10].
The oral cavity is the host to billions of bacteria belonging to several hundreds of species. This microbiome is essential to oral health and under normal circumstances beneficial to the host [21]. The subgingival microbiome has been characterized in individuals with periodontitis as well as periodontally healthy individuals; some bacterial species were * Corresponding author. Section for Oral Biology and Pathology, Faculty of Odontology, Malm¨o university, S-20506, Malm¨o, Sweden.
E-mail address: Bertil.Kinnby@mau.se (B. Kinnby).
Contents lists available at ScienceDirect
Microbial Pathogenesis
journal homepage: www.elsevier.com/locate/micpathhttps://doi.org/10.1016/j.micpath.2020.104648
found to be more prevalent in periodontal health while others were more common in periodontitis [22]. However, a perturbed microbiome following local ecological changes, such as increased biofilm formation on subgingival tooth areas, i.e. plaque accumulation below the gum line, initiates periodontal inflammation [23,24]. This dysbiotic state is characterized by an increase of protease-producing species and pheno-types. A notable consequence is an increased presence of Porphyromonas gingivalis [25], a well-studied organism known for its production of potent proteases; mainly the cysteine proteases arginine gingipain (Rgp) and lysine gingipain (Kgp) [26]. These proteases are able to elicit innate immune reactions through the action on toll-like receptors and protease-activated receptors, thereby initiating a pro-inflammatory response [27–30]. Furthermore, gingipains have been shown to cleave and activate coagulation factors in vitro [31–33]. Thus, in addition to being involved in the triggering and perpetuation of inflammation through their action on protease-activated and toll-like receptors [29, 30], bacterial proteases may also initiate coagulation leading to extra-vascular fibrin deposition; resulting in further contribution to develop-ment and sustaindevelop-ment of chronic inflammation, and in the case of periodontitis, resorption of tooth-supporting bone.
Inflammation-related coagulation can be triggered through activa-tion via the contact system, Factors XI and XII [34]. These factors can be activated by kallikrein, kininogen and, as recently demonstrated, also by polyphosphates (polyP) [35], large polymers of inorganic phosphate that in mammals are stored in and released from platelets. Bacterial polyP are generally tenfold longer than in mammals [36] and the levels of polyP vary 1000-fold between different bacterial species and different metabolic states [37]. Thus, polyP may also provide bacteria with strong procoagulant properties [38]. The initiation of procoagulant activity resulting in fibrin formation could be an important phenotypic trait of oral bacteria in sustaining chronic inflammation of the periodontium. The purpose of this study was therefore to investigate the procoagulant activity of a range of different oral microbial species prevalent in health and in periodontitis.
2. Materials and Methods 2.1. Bacterial strains
Eight different strains of P. gingivalis were used in this study. The clinical isolates SUB1, 33F, 1A, 50A, 16A, were freshly isolated from subgingival pocket samples of patients with documented periodontal disease. After recovery of the samples on Brucella agar, the clinical strains were identified as P. gingivalis through morphology (glossy black- pigmented colonies containing small Gram-negative coccoid rods) and physiological testing (positive for trypsin as well as indole production but negative for fluorescence and β-galactosidase activity). In addition, the reference strain W50 (ATCC 53978) and its corresponding arginine gingipain (Rgp) knock-out strain E8 and lysine gingipain (Kgp) knock- out K1A were used [39]. Prevotella intermedia, Fusobacterium nuclea-tum, Actinomyces næslundii, Parvimonas micra, were also isolated from patients with established periodontitis as described above, each strain from a different patient, whereas Streptococcus mitis and Veillonella par-vula/dispar 10BB had been isolated from healthy male donors. The identities of all these strains were confirmed by 16S rRNA gene sequencing. In addition, reference strains Rothia dentocariosa ATCC 17931, Streptococcus sanguinis ATCC 10556, Streptococcus constellatus NCTC 10714 were used in this study.
2.2. Bacterial growth
All bacteria were stored in skimmed milk at − 80 ◦C and prior to each
experiment grown on Brucella agar at 37 ◦C under anaerobic conditions
(10% H2, 5% CO2 in N2). Single colonies from the Brucella agar were
inoculated into pre-reduced Brain Heart Infusion Broth (BHI) (Acume-dia, Neogen, Lansing, MI, USA) and incubated over-night anaerobically
at 37 ◦C. Bacteria were also allowed to form biofilms. Single colonies of
P. gingivalis were suspended in 10% prereduced equine serum (Håtuna lab AB, Bro, Sweden) to a final optical density, at 600 nm, of 0.1. Two hundred μL suspension was added to the wells of an Ibidi® 8-well μ-slides (Ibidi® μ-Slide, Ibidi GmbH, Martinsried, Germany) and incu-bated over night or for 7 days anaerobically at 37 ◦C before being
analyzed with confocal microscopy for detection of polyP and visuali-zation of fibrin formation as described below.
2.3. Reagents
Pooled platelet-poor normal human plasma was obtained from George King Bio-Medical Inc (GK-0010-1). Before each experiment the plasma was thawed rapidly in a 37 ◦C water bath to avoid
cry-oprecipitation. Thromboplastin from rabbit brain (Fluka 44 213) was used as a positive control in the coagulation assays. Purified arginine gingipain (Rgp), (GingisREX B0-GRX-005) was obtained from Genovis AB, Lund, Sweden. Alexa546-labeled fibrinogen (Invitrogen F13192) was
reconstituted to a concentration of 5 mg/mL in 0.1 M NaHCO3 pH 8.2
and sterile-filtered. FITC-labeled casein (Anaspec Inc, Fremont, CA, USA) was used for the evaluation of proteolytic activity. For the pro-duction of non-glycosylated PAI–2 in E. coli the vector pLex (Invitrogen) was used. Primers for the amplification of human PAI–2 cDNA were constructed and the DNA was amplified through PCR. The PAI–2 DNA was then ligated into the vector containing the TCS-His sequence (Rapid DNA ligation kit, Boehringer Mannheim 1 635 379). The vector with the PAI–2 and TCS-His inserts was then transformed into competent E. coli. The cells were plated on RMG-Amp plates and incubated at 30 ̊C over night. Resulting colonies were inoculated into RM-Amp medium and incubated at 37 ̊C over night. Production of PAI–2 was induced by inoculating induction medium (Invitrogen) with cells to an OD550 of 0.1.
This culture was grown at 30 ̊C until an OD550 of 0.5 was reached. At this
point tryptophan was added to a concentration of 100 μg/mL and the cells were incubated at 37 ̊C for 34 h. The cells were then centrifuged and disrupted by repeated freeze/thaw cycles. Recombinant PAI–2 was isolated on Ni-NTA-agarose columns.
2.4. Protease activity assay
Bacteria were grown over night as described above and proteolytic activity was assayed using FITC-labeled casein. Two hundred μL of bacterial suspension was added to two μL FITC-labeled casein (0.1 mg/ mL final concentration) and end-point measurements of the resulting fluorescence was measured after 60 min in a Clariostar® fluorometer (BMG Labtech, Germany; using excitation and emission wavelengths of 488 and 538 nm respectively) and expressed as relative fluorescent units (RFU). Three independent experiments were performed and results were expressed as mean ± 95%CI.
2.5. Coagulation assay
Since the turbidity of the bacterial suspensions interfered with the absorbance readings, coagulation assays were performed on culture supernatants. The bacterial suspensions prepared as described above were centrifuged (3000g, 15 min, 4 ◦C) and the supernatants were then
assayed. Procoagulant activity was studied with a functional assay [40]; 70 μL plasma was mixed with 70 μL bacterial supernatant sample or gingipain solution and coagulation was initiated by adding 70 μL 25 mM CaCl2. The reaction was carried out in 96-well microtiter plates (Nunc
Maxisorp) and the process was followed spectrophotometrically at 405 nm, the absorbance increasing as a fibrin clot is formed. The clotting time was used as a measure of the procoagulant activity. The time from addition of CaCl2 to the inflection point of the absorbance curve was
registered. Rabbit thromboplastin (Fluka #44213) was used as a positive control. The culture medium brain-heart infusion (BHI) was used as negative control.
2.6. Detection of polyphosphates
Planktonic suspensions and biofilm cells of P. gingivalis were pre-pared as described above. The planktonic suspensions were transferred to an Ibidi® μ-slide and incubated anaerobically for 2 h to allow the cells to adhere to the surface. The Ibidi μ-slide was then centrifuged at 1200g for 10 min. DAPI (4’,6-Diamidino-2-Phenylindole) was added to a final concentration of 18 μM (5 μg/mL) after which the reaction mixture was incubated in the dark for 60min to allow for DAPI staining to occur. Fluorescence was excited in a spinning disk confocal microscope (Nikon Eclipse TE2000 with a CFI Plan Apokromat 60x oil lens, Nikon Corp., Tokyo, Japan). For visualization of DNA the excitation wavelength was 378 nm and the emission wavelength was 456 nm. Excitation at 415 nm and emission at 550 nm showed polyP [41]. Images were acquired with a Photometrics Prime 95B camera using Nikon NIS-Elements software. 2.7. Visualization of fibrin formation
Fifty μL plasma (George King Biomedical) was diluted with 50 μL McIlvaine’s citrate-phosphate buffer (pH = 7.0) and 1 μL of Alexa564- labeled fibrinogen and 1 μL DAPI (as above) were added. Seventy μL of this mixture was added to an Ibidi® 8-well cell followed by addition of 70 μL of bacterial suspension. Following incubation, the Ibidi® cell was transferred to the microscope and coagulation was initiated through the addition of 70 μL CaCl2. Formation of fibrin was followed in real time by
spinning disk confocal microscopy; excitation wavelength 546 nm and emission wavelength 570 nm [42].
2.8. Control experiments
In order to verify the influence of protease activity on the procoa-gulant activity, supernatants of over-night grown P. gingivalis 50A were preincubated for 30 min at 37 ◦C with plasminogen activator inhibitor
type 2 (PAI-2) (10 nM final concentration), previously shown to inhibit gingipain activity [43]. Coagulation assays were then performed as described above. Three independent experiments were performed based on separate cultures.
The P. gingivalis strain E8 with the gene for arginine gingipain knocked out was studied with respect to procoagulant activity and release of polyP. Bacteria were seeded to Ibidi cells and allowed to form biofilms for one week. Then biofilm supernatants were carefully removed and assayed for procoagulant activity as described above. Presence of polyP and fibrin formation in the biofilms was studied with confocal microscopy as described above.
3. Results
3.1. Proteolytic activity in oral bacteria
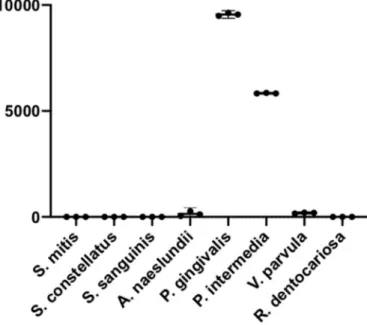
To assess the proteolytic activity of the bacteria included in this study, FITC-labeled casein was used. Of the 10 species of oral bacteria tested, only two species, P. gingivalis and P. intermedia, displayed pro-teolytic activity as seen by an increase in RFU. The propro-teolytic activity was slightly higher in P. gingivalis with a mean of 10 × 103 (95% CI
9.4–9.7 × 103) RFU compared to 5.8 × 103 (95%CI 5.8–5.9 × 103) RFU for P. intermedia. None of the other species showed any increase in RFU (Fig. 1).
3.2. Procoagulant activity in oral bacteria
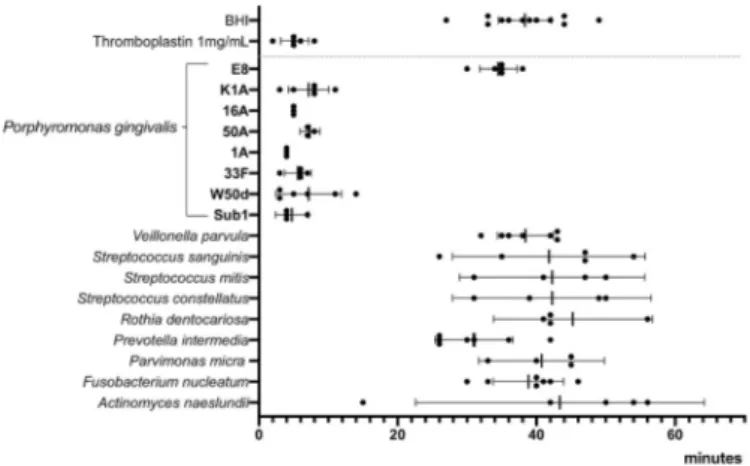
Ten species of oral bacteria that had been tested for proteolytic ac-tivity were also tested for procoagulant acac-tivity and compared to the positive control, thromboplastin. Of these species only P. gingivalis exhibited procoagulant activity. The rate at which supernatants of P. gingivalis initiated coagulation was similar to that of 1 mg/mL throm-boplastin (Fig. 2). Six different strains of P. gingivalis were then tested to
evaluate strain differences in the ability to initiate coagulation. All six strains of P. gingivalis showed strong procoagulant activity which sug-gests that this is a species-related trait (Fig. 3). P. intermedia that showed proteolytic activity in the FITC-casein assay did not induce coagulation which indicates that proteolytic activity as such does not induce coagulation.
3.3. Arginine gingipain initiates coagulation
To evaluate the involvement of gingipains in procoagulant activity of P. gingivalis, the knock-out strains of P. gingivalis W50, K1A and E8 were used. Supernatants from overnight cultures of K1A that has the gene for Kgp knocked out, but possesses Rgp, showed strong procoagulant ac-tivity. However, the strain E8 that has the gene for Rgp knocked out did not initiate coagulation (Fig. 3). Thus, these results suggest that Rgp and not Kgp is involved in the initiation of coagulation by P. gingivalis.
Fig. 1. Protease activity in suspensions of eight species of oral bacteria
measured using FITC-labeled casein and expressed as RFU after 1 h incubation. The graph shows individual values (and mean ± 95%CI) from three biological replicates of each species.
Fig. 2. Representative results of a coagulation assay. Shorter time (x-axis)
equals higher procoagulant activity. Y-axis displays absorbance as a measure of produced fibrin clot. Supernatants from overnight cultures of ten species of oral bacteria; Actinomyces naeslundii (An), Fusobacterium nucleatum (Fn), Parvimonas micra (Pm), Prevotella intermedia (Pi), Rothia dentocariosa (Rd), Streptococcus constellatus (Sc), Streptococcus mitis (Sm), Streptococcus sanguinis (Ss), Veillonella parvula (Vp) and Porphyromonas gingivalis (Pg). Positive control thromboplastin 1 mg/mL (green graph), negative control culture medium BHI (orange), Por-phyromonas gingivalis (red). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4. Polyphosphates were detected in bacterial suspensions and biofilms DAPI detects polyP in addition to DNA with the appropriate excita-tion and emission wavelengths [40] and was therefore used for detection of polyP in planktonic and biofilm cells of P. gingivalis. In overnight planktonic cultures of P. gingivalis, polyP were primarily detected intracellularly (Fig. 4). However, after culturing bacteria as biofilms, increased amounts of polyP appeared and they seemed to be more present extracellularly. This was evident already in overnight cultures and more pronounced after one week of incubation (Fig. 5). Taken together, these results suggest that P. gingivalis can store polyP and release them extracellularly and that this appears to be dependent on the bacterial mode of growth.
3.5. Visualization of fibrin formation with P. gingivalis by confocal microscopy
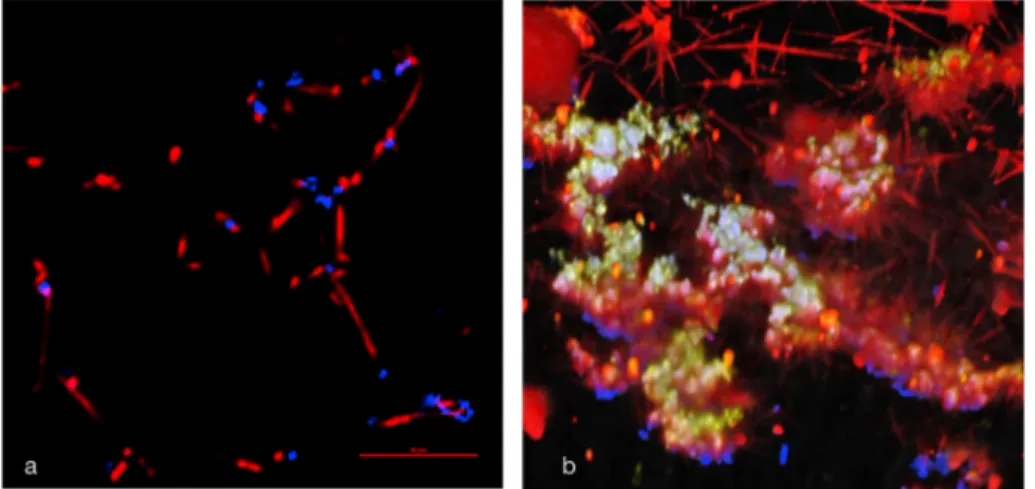
Fibrin formation was followed in real time by spinning disk confocal microscopy using Alexa564-labeled fibrinogen. In planktonic over-night cultures it could be observed that fibrin seemed to originate from the surface of P. gingivalis (Fig. 6a). In biofilm cultures, fibrin formation also originated from the bacterial surfaces but could in addition be seen starting near extracellular polyP and fibrin strands were observed extending and forming meshworks (Fig. 6b).
In summary, these results illustrate that biofilm cells of P. gingivalis could have two ways to induce fibrin formation, via a mechanism associated with the cell surface, most likely surface-associated or released gingipains and via polyP, closely surrounding the bacteria. 3.6. Control experiments
The influence of protease activity on the procoagulant activity was further demonstrated by incubating supernatants from over-night cul-tures of P. gingivalis 50A with plasminogen activator inhibitor type 2 (PAI-2) (10 nM final concentration) for 30 min at 37 ◦C. These
experi-ments showed that inhibition with a protease inhibitor reduced the procoagulant activity (Fig. 7).
Since extracellular polyP were abundant in biofilms formed by P. gingivalis after one week incubation we decided to study the contri-bution of polyP to the procoagulant actvity by allowing the strain E8, with the gene for arginine gingipain knocked out, to form biofilms. Coagulation assay of these supernatants showed a slightly accelerated clotting compared to negative PBS control (Fig. 8), however slower than all the Rgp-producing strains. Confocal microscopy showed a high amount of extracellular polyP and fibrin formation occurring around extracellular polyphosphates (Fig. 9).
4. Discussion
Coagulation results in the formation of fibrin and extravascular fibrin accumulation leads to sustained chronic inflammation and thereby activation of bone-degrading mechanisms that are pathogenetic in periodontitis. They aim of this study was therefore to investigate different oral bacteria for their ability to exhibit procoagulant activity resulting in the formation of fibrin which then possibly could be important for the initiation and progression of periodontitis.
Proteases are involved in all steps of the coagulation process, therefore proteolytic activity of the bacterial species chosen for this study was investigated. Not unexpectedly, two species, P. gingivalis and P. intermedia, that previously have been shown to possess proteolytic activity also did so in this study. P. gingivalis produces large amounts of gingipains while P. intermedia produces a range of different proteases,
Fig. 3. Result of procoagulant assays with supernatants from overnight cultures
of 17 strains of oral bacteria (10 species; Actinomyces naeslundii, Fusobacterium nucleatum, Parvimonas micra, Prevotella intermedia, Rothia dentocariosa, Strepto-coccus constellatus, StreptoStrepto-coccus mitis, StreptoStrepto-coccus sanguinis, Veillonella parvula and Porphyromonas gingivalis). All tested P. gingivalis strains except for E8 (arginine gingipain knockout) showed rapid procoagulant activity. All other species were negative. These experiments were performed on 3–7 biological replicates. Mean and 95%CI.
Fig. 4. In overnight planktonic cultures of P. gingivalis, polyP were primarily
detected intracellularly. DAPI was used for staining, excitation wavelength 378 nm and emission wavelength 456 nm showed DNA (blue), excitation wave-length 415 nm and emission wavewave-length 550 nm showed polyP (yellow); colocalization appears white. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 5. P. gingivalis incubated as biofilms exhibited extracellular polyP. DAPI
was used for staining, excitation wavelength 378 nm and emission wavelength 456 nm showed DNA (blue), excitation wavelength 415 nm and emission wavelength 550 nm showed polyP (yellow); colocalization appears white. Over night culture b) One week culture. Movies showing all the slices and a 3D rendition of z-stacks corresponding to Fig. 5b are available as supporting in-formation in Data in Brief. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
among them a cysteine protease, interpain. Both these groups of en-zymes are known to interfere with various immune functions e.g. degradation of IgG and complement factors [44–46].
Somewhat surprisingly F. nucleatum did not display any proteolytic activity although it has been shown to produce a proteolytic enzyme called fusolisin, a serine protease important for supplying peptides and amino acids for its metabolism; fusolisin has been shown to be produced in bacteria grown in BHI as was used in this study. However, fusobac-teria have been shown to vary in their ability to hydrolyze casein [47] and it cannot be excluded that proteolytic activity might have been detected using another substrate. None of the other bacterial species tested displayed any proteolytic activity.
The procoagulant activity of the different bacterial species associated with both health and periodontitis [22] was then investigated by adding bacterial culture supernatants to plasma and CaCl2 and following the
change in absorbance spectrophotometrically. Out of the ten species tested only P. gingivalis showed procoagulant activity. Several different strains of P. gingivalis showed procoagulant activity which suggests that it is a species-dependent rather than strain-dependent trait. P. intermedia, which also displayed proteolytic activity in our study, did however not initiate fibrin formation which suggests that the proteolytic enzymes produced by P. intermedia do not affect the coagulation
Fig. 6. Fibrin formation in P. gingivalis cultures was
observed by spinning disk confocal microscopy. Alexa564-labeled fibrinogen (red fluorescent;
excita-tion wavelength 488 nm, emission wavelength 570 nm) was added to plasma which then was added to a bacterial biofilm formed in an Ibidi® culture cell and coagulation was initiated by adding CaCl2. DAPI
staining, excitation wavelength 378 nm and emission wavelength 456 nm, showed DNA (blue) and exci-tation wavelength 415 nm and emission wavelength 550 nm showed polyP (yellow); colocalization of DNA and polyP appears white. a). In over-night planktonic cultures it could be observed that fibrin seemed to originate from the surface of P. gingivalis. b).In biofilm cultures of P. gingivalis incubated for one week extracellular polyP were seen and fibrin strand formation could be seen originating from the surface of the bacteria as well as from polyP. 3D image constructed from Z-stacks comprising 33 0.6 μm slices. Movies showing all the slices and a 3D rendition of the z-stack are available as supporting information. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 7. Supernatants from P. gingivalis strain 50A subjected to procoagulant
activity assay. Preincubation of supernatants for 30 min with PAI-2 (10 nM) inhibited the procoagulant activity. The graphs show mean and sd of three separate over-night cultures. Y-axis displays absorbance as a measure of pro-duced fibrin clot, x-axis shows time.
Fig. 8. Coagulation assay of P.gingivalis strain E8 with the gene for arginine
gingipain knocked out. The bacteria were cultured as biofilms for one week and the supernatants were assayed for procoagulant activity. The results showed accelerated coagulation compared to negative control but slower than all the Rgp-producing P. gingivalis strains (cf Fig. 3). Results from triplicate assays of two biological replicates. The graphs show mean and SEM. Positive control thromboplastin (1 mg/mL; TF), negative control PBS.
Fig. 9. Confocal imaging of Porphyromonas gingivalis cultured anaerobically
for one week in Ibidi cells. Staining with DAPI for visualization of DNA (exc 378 nm, em 456 nm; blue) and polyphosphates (exc 415 nm, em 500 nm; yellow). Fibrin visualized with Alexa564-labeled fibrinogen (red). Colocalization
of polyP and fibrin appears orange. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
cascade. Interpain, the main protease produced by P. intermedia, is a cysteine protease involved in a range of reactions such as complement degradation, heme acquisition and breakdown of albumin [48]. Gingi-pains are also cysteine proteases, can degrade complement and are involved in acquisition of heme and although gingipains and interpain share some common properties, interpain did not seem to have the property of initiating coagulation, thus it is clear that a specific pro-teolytic activity is required to induce procoagulant activity. Of all the oral bacteria tested for procoagulant activity P. gingivalis strains pro-ducing arginine gingipain were the only ones able to initiate coagula-tion. The initiation was rapid corresponding to a high concentration of thromboplastin. Thus, it could be concluded that the initiation of coagulation occurs through an enzymatic mechanism dependent on arginine gingipain, which is in agreement with the findings of Imamura et al. [31–33] who showed that arginine gingipain could cleave and activate coagulation factors IX and X as well as prothrombin in vitro. The conclusion is corroborated by the finding that supernatants from over-night cultures of the P. gingivalis strain E8, that has the gene for Rgp knocked out, did not display any procoagulant activity, while the strain K1A that has the gene for lysine gingipain knocked out displayed the same activity as the wild type strains. The fibrin formation generated by P. gingivalis in this study adds a new dimension to previous data on the cleavage of coagulation factors mentioned above by showing a relevant biological consequence, fibrin formation, that could have a significant influence on the pathogenesis of periodontitis.
The results from this study thus indicate that the procoagulant ac-tivity of P. gingivalis primarily was dependent on arginine gingipain activity. However, we also showed that bacteria cultured as biofilms released polyphosphates to the extracellular environment. PolyP were detected by fluorescent confocal microscopy in both planktonic and biofilm cells. In overnight planktonic suspensions they were predomi-nantly found intracellularly and only occasionally extracellularly. In biofilm cells however, extracellular polyP were observed both in 24-h and one-week old biofilms. In bacteria, polyphosphates are important for the ability to adapt to nutritional deficiencies and other environ-mental stresses, but they have also been shown to be important in bio-film formation and virulence expression [49]. The synthesis of polyphosphates is catalyzed by the enzyme polyphosphate kinase (PPK) and was first discovered in Escherichia coli [50]. PPK and thereby the levels of polyP in various bacteria have been shown to be affected by mesalamine, an anti-inflammatory drug used in ulcerative colitis [51]. The reduction of polyP made the bacteria less tolerant to inflammatory oxidants and also changed their biofilm properties. It was suggested that this could alter the ability of the bacteria to colonize and survive in a chronically inflamed environment. In P. gingivalis, PPK activity has been shown to be important for biofilm maturation and in stationary phase long term survival, a metabolic state resembling that of biofilm cells [52]. It is therefore conceivable that polyP also may be important for the ability of P. gingivalis to persist in inflammatory environments.
Confocal microscopy of over-night planktonic cultures showed the formation of fibrin strands originating from the surface of the bacteria consistent with coagulation being initiated by released or surface- associated proteases. However, in the one-week biofilm cultures it was evident that fibrin formation in addition started from polyP. Thus, the microscopy findings further indicate that P. gingivalis in addition to Rgp also may initiate coagulation through the release of polyP. In order to demonstrate the relative influence of Rgp and polyP, we therefore also cultured the Rgp knockout strain E8 as one-week biofilms. Microscopy of these showed ample presence of polyP and fibrin formation starting around extracellular polyP. No evidence of fibrin formation at the bac-terial surface was seen. The fibrin formed in these cultures looked denser and strand formation was less evident than in wild-type cultures. This may have a similar background as the knotted appearance of fibrin formed in the presence of polyP observed by Whyte et al. [53]. Pro-coagulant activity assays of supernatants from the Rgp knock-out (E8) biofilm cultures showed a slightly accelerated clotting, albeit slower
than that of the Rgp-producing strains. We therefore conclude that polyP contribute to the procoagulant activity of P. gingivalis biofilm cells, although the main effect appears to be due to Rgp activity. However, if stored polyP were to be released in tissues, they would have the po-tential to contribute to deposition of fibrin that is more stable and highly resistant to fibrinolysis [54]. In addition, polyP per se have also been shown to have direct proinflammatory effects [55]. Intracellular polyP may thus represent a significant procoagulant potential that could be released under certain circumstances, such as during biofilm growth.
Locally in the subgingival area the procoagulant activity of P. gingivalis would be beneficial for the microorganisms since sustained inflammation caused by the presence of fibrin leads to an increased flow of protein-rich gingival fluid, a nutrient source for these bacteria with a proteolytic metabolism. The ability of P. gingivalis to induce coagulation is likely to contribute to its role in chronic inflammation, in oral tissues as well as in other distant organs such as aorta and brain. Fibrin deposits [56] as well as P. gingivalis and its gingipains have been seen associated with amyloid formation in brain tissue from Alzheimer’s patients [57]. Persistent extravascular fibrin deposits are strong inducers of chronic inflammation and have been seen in osteoporosis [10] as well as peri-odontitis [18], conditions in which the activation of osteoclast forma-tion through the binding of monocyte receptors to fibrin is of crucial importance, but also in other chronic inflammatory conditions [2,3,8]. The formation of extravascular fibrin and its contribution to sustained chronic inflammation with activation of osteoclasts is thus a plausible pathogenic mechanism in periodontitis. P. gingivalis that is found in increased amounts due to the dysbiosis initiating this condition is likely to contribute through its ability to induce increased extravascular fibrin deposition. In addition, it is noteworthy that bacteria originating from the oral microbiota also can be found in other parts of the body. In particular P. gingivalis has been studied and found to be associated with systemic pathology, including cardiovascular disease, Alzheimer’s, and Parkinson’s disease [58–63].
The procoagulant activity of P. gingivalis could explain its role in chronic inflammation, locally in oral tissues as well as in remote loca-tions. Further studies aim at studying possible interactions with other bacterial species associated with periodontal inflammation and their influence on the protease production and procoagulant activity of P. gingivalis in order to gain an increased insight in the pathogenesis of periodontitis and possibly also other inflammatory conditions where P. gingivalis has been found.
Author contributions
Both authors conceptualized, designed and performed experiments (J. Neilands microbiology, B. Kinnby coagulation) and contributed equally to analyzing data and writing the manuscript.
Conflict of interest
The authors declare no conflict of interest. Acknowledgments
The study was supported by The Foundation for Dental Research in Malm¨o founded by Bertil Rohlin for Rohlin-Dentalen Malm¨o, Odonto-logical Research Region Skåne and the Knowledge Foundation. References
[1] J.L. Degen, T.H. Bugge, J.D. Goguen, Fibrin and fibrinolysis in infection and host defense, J. Thromb. Haemostasis: JTH 5 (Suppl 1) (2007) 24–31, https://doi.org/ 10.1111/j.1538-7836.2007.02519.x.
[2] D. Davalos, K. Akassoglou, Fibrinogen as a key regulator of inflammation in disease, Semin. Immunopathol. 34 (1) (2011) 43–62, https://doi.org/10.1007/ s00281-011-0290-8.
[3] M. Del Rosso, F. Margheri, S. Serratì, A. Chilla, A. Laurenzana, G. Fibbi, The urokinase receptor system, a key regulator at the intersection between inflammation, immunity, and coagulation, Curr. Pharmaceut. Des. 17 (19) (2011) 1924–1943, https://doi.org/10.2174/138161211796718189.
[4] M. O’Brien, The reciprocal relationship between inflammation and coagulation, Top. Companion Anim. Med. 27 (2) (2012) 46–52, https://doi.org/10.1053/j. tcam.2012.06.003.
[5] S. Danckwardt, M.W. Hentze, A.E. Kulozik, Pathologies at the nexus of blood coagulation and inflammation: thrombin in hemostasis, cancer, and beyond, J. Mol. Med. 91 (11) (2013) 1257–1271, https://doi.org/10.1007/s00109-013- 1074-5.
[6] A.T. Long, E. Kenne, R. Jung, T.A. Fuchs, T. Renn´e, Contact system revisited: an interface between inflammation, coagulation, and innate immunity, J. Thromb. Haemostasis 14 (3) (2016) 427–437, https://doi.org/10.1111/jth.13235. [7] B.M. Connolly, E.Y. Choi, H. Gårdsvoll, A.L. Bey, B.M. Currie, T. Chavakis, S. Liu,
A. Molinolo, M. Ploug, S.H. Leppla, T.H. Bugge, Selective abrogation of the uPA- uPAR interaction in vivo reveals a novel role in suppression of fibrin-associated inflammation, Blood 116 (9) (2010 Sep 2) 1593–1603, https://doi.org/10.1182/ blood-2010-03-276642.
[8] M.J. Flick, C.M. LaJeunesse, K.E. Talmage, D.P. Witte, J.S. Palumbo, M. D. Pinkerton, S. Thornton, J.L. Degen, Fibrin(ogen) exacerbates inflammatory joint disease through a mechanism linked to the integrin αMβ2 binding motif, J. Clin. Invest. 117 (11) (2007) 3224–3235, https://doi.org/10.1172/JCI30134. [9] C. Jennewein, N. Tran, P. Paulus, P. Ellinghaus, J.A. Eble, K. Zacharowski, Novel
aspects of fibrin(ogen) fragments during inflammation, Mol. Med. 17 (5–6) (2011) 568–573, https://doi.org/10.2119/molmed.2010.00146.
[10] H.A. Cole, T. Ohba, J.S. Nyman, H. Hirotaka, J.M.M. Cates, M.J. Flick, J.L. Degen, J.G. Schoenecker, Fibrin accumulation secondary to loss of plasmin-mediated fibrinolysis drives inflammatory osteoporosis in mice, Arthritis & Rheumatology (Hoboken, N.J.) 66 (8) (2014) 2222–2233, https://doi.org/10.1002/art.38639. [11] J.P. Luyendyk, J.G. Schoenecker, M.J. Flick, The multifaceted role of fibrinogen in
tissue injury and inflammation, Blood 133 (6) (2019 Feb 7) 511–520, https://doi. org/10.1182/blood-2018-07-818211.
[12] M.P. Motley, D.H. Madsen, H.J. Jürgensen, D.E. Spencer, R. Szabo, K. Holmbeck, M.J. Flick, D.A. Lawrence, F.J. Castyellino, R. Weigert, T.H. Bugge, A CCR2 macrophage endocytic pathway mediates extravascular fibrin clearance in vivo, Blood 127 (9) (2016) 1085–1096, https://doi.org/10.1182/blood-2015-05- 644260.
[13] M.J. Flick, X. Du, D.P. Witte, M. Jirouˇskov´a, D.A. Soloviev, S.J. Busuttil, E.F. Plow, J.L. Degen, Leukocyte engagement of fibrin(ogen) via the integrin receptor αMβ2/ Mac-1 is critical for host inflammatory response in vivo, J Clin Invest. American Society for Clinical Investigation 113 (11) (2004 Jun 1) 1596–1606, https://doi. org/10.1172/JCI20741.
[14] C. Rubel, G.C. Fern´andez, G. Dran, M.B. Bompadre, M.A. Isturiz, M.S. Palermo, Fibrinogen promotes neutrophil activation and delays apoptosis, J. Immunol. 166 (3) (2001 Feb 1) 2002–2010, https://doi.org/10.4049/jimmunol.166.3.2002. [15] J.D. Vassalli, J.H. Saurat, Cuts and scrapes? Plasmin heals!, Nat. Med. 2 (3) (1996)
284–285.
[16] Y. Shen, Y. Guo, P. Mikus, R. Sulniute, M. Wilczynska, T. Ny, J. Li, Plasminogen is a key proinflammatory regulator that accelerates the healing of acute and diabetic wounds, Blood 119 (24) (2012) 5879–5887, https://doi.org/10.1182/blood-2012- 01-407825.
[17] R. Sulniute, Y. Shen, Y.Z. Guo, M. Fallah, N. Ahlskog, L. Ny, O. Rakhimova, J. Broden, H. Boija, A. Moghaddam, J. Li, M. Wilczynska, T. Ny, Plasminogen is a critical regulator of cutaneous wound healing, Thromb. Haemostasis 115 (5) (2016) 1–9, https://doi.org/10.1160/TH15-08-0653.
[18] R. Sulniute, T. Lindh, M. Wilczynska, J. Li, T. Ny, Plasmin is essential in preventing periodontitis in mice, Am. J. Pathol. 179 (2) (2011) 819–828, https://doi.org/ 10.1016/j.ajpath.2011.05.003.
[19] R. Mehta, A.D. Shapiro, Plasminogen deficiency, Haemophilia 14 (6) (2008) 1261–1268, https://doi.org/10.1111/j.1365-2516.2008.01825.x. [20] ¨O. Günhan, M. Günhan, E. Berker, C.A. Gürgan, H. Yildirim, Destructive
membranous periodontal disease (ligneous periodontitis), J. Periodontol. 70 (8) (1999) 919–925, https://doi.org/10.1902/jop.1999.70.8.919.
[21] P.S. Kumar, M.R. Mason, Mouthguards: does the indigenous microbiome play a role in maintaining oral health? Front Cell Infect Microbiol 5 (2015 May 6) 5721–5729.
[22] L. Abusleme, A.K. Dupuy, N.S. Dutzan, N. Silva, J.A. Burleson, L.D. Strausbaugh, J. Gamonal, P.I. Diaz, The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation, ISME J. 7 (5) (2013 Jan 10) 1016–1025.
[23] P.D. Marsh, Are dental diseases examples of ecological catastrophes? Microbiology 149 (2) (2003) 279–294, https://doi.org/10.1099/mic.0.26082-0.
[24] R.P. Darveau, Periodontitis: a polymicrobial disruption of host homeostasis, Nat. Rev. Microbiol. 8 (7) (2010) 481–490, https://doi.org/10.1038/nrmicro2337. [25] G. Hajishengallis, S. Liang, M.A. Payne, A. Hashim, Low-abundance biofilm species
orchestrates inflammatory periodontal disease through the commensal microbiota and complement, Cell Host Microbe 10 (5) (2011) 497–506, https://doi.org/ 10.1016/j.chom.2011.10.006.
[26] R. Pike, W. McGraw, J. Potempa, J. Travis, Lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Isolation, characterization, and evidence for the existence of complexes with hemagglutinins, J. Biol. Chem. 269 (1) (1994) 406–411.
[27] M. Holzhausen, L.C. Spolidorio, R.P. Ellen, M.-C. Jobin, M. Steinhoff, P. Andrade- Gordon, N. Vergnolle, Protease-activated receptor-2 activation: a major role in the
pathogenesis of Porphyromonas gingivalis infection, Am. J. Pathol. 168 (4) (2006) 1189–1199, https://doi.org/10.2353/ajpath.2006.050658.
[28] B.A. Beutler, TLRs and innate immunity, Blood. American Society of Hematology 113 (7) (2009 Feb 12) 1399–1407, https://doi.org/10.1182/blood-2008-07- 019307.
[29] R.A. Giacaman, A.C. Asrani, K.F. Ross, M.C. Herzberg, Cleavage of protease- activated receptors on an immortalized oral epithelial cell line by Porphyromonas gingivalis gingipains, Microbiology 155 (Pt 10) (2009) 3238–3246, https://doi. org/10.1099/mic.0.029132-0.
[30] K. Haruyama, A. Yoshimura, M. Naito, M. Kishimoto, M. Shoji, Y. Abiko, Y. Hasra, K. Nakayama, Identification of a gingipain-sensitive surface ligand of
Porphyromonas gingivalis that induces Toll-like receptor 2- and 4-independent NF- kappaB activation in CHO cells, Infect. Immun. 77 (10) (2009) 4414–4420, https:// doi.org/10.1128/IAI.00140-09.
[31] T. Imamura, J. Potempa, S. Tanase, J. Travis, Activation of blood coagulation factor X by arginine-specific cysteine proteinases (gingipain-Rs) from
Porphyromonas gingivalis, J. Biol. Chem. 272 (25) (1997) 16062–16067, https:// doi.org/10.1074/jbc.272.25.16062.
[32] T. Imamura, S. Tanase, T. Hamamoto, J. Potempa, J. Travis, Activation of blood coagulation factor IX by gingipains R, arginine-specific cysteine proteinases from Porphyromonas gingivalis, Biochem. J. 353 (Pt 2) (2001) 325–331.
[33] T. Imamura, A. Banbula, P. Pereira, J. Travis, J. Potempa, Activation of human prothrombin by arginine-specific cysteine proteinases (gingipains R) from Porphyromonas gingivalis, J. Biol. Chem. 276 (22) (2001) 18984–18991. [34] F. Müller, N.J. Mutch, W.A. Schenk, S.A. Smith, L. Esterl, H.M. Spronk,
S. Schmidbauer, W.A. Gahl, J.H. Morrissey, T. Renne, Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo, Cell 139 (2009) 1143–1156,
https://doi.org/10.1016/j.cell.2009.11.001.
[35] J.H. Morrissey, S.A. Smith, Polyphosphate as modulator of hemostasis, thrombosis, and inflammation, J. Thromb. Haemostasis 13 (Suppl. 1) (2015) S92–S97, https:// doi.org/10.1111/jth.12896.
[36] S.N.J. Moreno, R. Docampo, Polyphosphate and its diverse functions in host cells and pathogens, PLoS Pathog. 9 (5) (2013), e1003230, https://doi.org/10.1371/ journal.ppat.1003230.
[37] A. Kornberg, N.N. Rao, D. Ault-Rich´e, Inorganic polyphosphate: a molecule of many functions, Annu. Rev. Biochem. 68 (1999) 89–125, https://doi.org/10.1146/ annurev.biochem.68.1.89.
[38] S.A. Smith, S.H. Choi, R. Davis-Harrison, J. Huyck, J. Boettcher, C.M. Rienstra, J. H. Morrissey, Polyphosphate exerts differential effects on blood clotting, depending on polymer size, Blood 116 (20) (2010) 4353–4359, https://doi.org/ 10.1182/blood-2010-01-266791.
[39] J. Aduse-Opoku, N.N. Davies, A. Gallagher, A. Hashim, H.E.A. Evans, M. Rangarajan, J.M. Slaney, M.A. Curtis, Generation of Lys-gingipain protease activity in Porphyromonas gingivalis W50 is independent of Arg-gingipain protease activities, Microbiology (Reading, Engl) 146 (8) (2000 Aug 1) 1933–1940, https:// doi.org/10.1099/00221287-146-8-1933.
[40] B.S. Schwartz, Antigen-induced monocyte procoagulant activity. Requirement for antigen presentation and histocompatibility leukocyte antigen-DR molecules, J. Clin. Invest. 76 (3) (1985) 970–977, https://doi.org/10.1172/JCI112097. [41] R. Aschar-Sobbi, A.Y. Abramov, C. Diao, M.E. Kargacin, G.J. Kargacin, R.J. French,
E. Pavlov, High sensitivity, quantitative measurements of polyphosphate using a new DAPI-based approach, J. Fluoresc. 18 (5) (2008) 859–866, https://doi.org/ 10.1007/s10895-008-0315-4.
[42] F.L. Macrae, C. Duval, P. Papareddy, S.R. Baker, N. Yuldasheva, K.J. Kearney, H. R. McPherson, N. Asquith, J. Konings, A. Casini, J.L. Degen, S.D. Connell, H. Philippou, A.S. Wolberg, H. Herwald, R.A.S. Ari¨ens, A fibrin biofilm covers blood clots and protects from microbial invasion, J. Clin. Invest. 128 (8) (2018 Aug 1) 3356–3368, https://doi.org/10.1172/JCI98734.
[43] J. Neilands, F.J. Bikker, B. Kinnby, PAI-2/SerpinB2 inhibits proteolytic activity in a P. gingivalis-dominated multispecies bacterial consortium, Arch. Oral Biol. 70 (2016) 1–8.
[44] J. Potempa, R.N. Pike, Corruption of innate immunity by bacterial proteases, J Innate Immun 1 (2) (2009) 70–87, https://doi.org/10.1159/000181144. Karger Publishers.
[45] M. Potempa, J. Potempa, T. Kantyka, K.-A. Nguyen, K. Wawrzonek, S. P. Manandhar, K. Popadiak, K. Riesbeck, S. Eick, A.M. Blom, Interpain A, a cysteine proteinase from Prevotella intermedia, inhibits complement by degrading complement factor C3, PLoS Pathog. 5 (2) (2009 Feb), e1000316, https://doi.org/ 10.1371/journal.ppat.1000316.
[46] T. Maekawa, J.L. Krauss, T. Abe, R. Jotwani, M. Triantafilou, K. Triantafilou, et al., Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host and Microbe, NIH Public Access 15 (6) (2014 Jun 11) 768–778, https://doi.org/ 10.1016/j.chom.2014.05.012.
[47] K.W. Bennett, B.I. Duerden, Identification of fusobacterium in a routine diagnostic laboratory, J. Appl. Bacteriol. 59 (1985) 171–181, https://doi.org/10.1111/ j.1365-2672.1985.tb03318.x.
[48] D.P. Byrne, K. Wawrzonek, A. Jaworska, A.J. Birss, J. Potempa, J.W. Smalley, Role of the cysteine protease interpain A of Prevotella intermedia in breakdown and release of haem from haemoglobin, Biochem J 1 January 425 (1) (2010) 257–264,
https://doi.org/10.1042/BJ20090343.
[49] M.R. Brown, A. Kornberg, The long and short of it - polyphosphate, PPK and bacterial survival, Trends Biochem. Sci. 33 (6) (2008) 284–290, https://doi.org/ 10.1016/j.tibs.2008.04.005.
[50] M. Akiyama, E. Crooke, A. Kornberg, An exopolyphosphatase of Escherichia coli. The enzyme and its ppx gene in a polyphosphate operon, J. Biol. Chem. 5 (1) (1993) 633–639, 268.
[51] J.U. Dahl, M.J. Gray, D. Bazopoulou, F. Beaufay, J. Lempart, M.J. Koenigsknecht, Y. Wang, J.R. Baker, W.L. Hasler, V.B. Young, D. Sun, U. Ursula Jakob, The anti- inflammatory drug mesalamine targets bacterial polyphosphate accumulation, Nat Microbiol 2 (2017) 16267, https://doi.org/10.1038/nmicrobiol.2016.267. [52] W. Chen, R.J. Palmer, H.K. Kuramitsu, Role of polyphosphate kinase in biofilm
formation of Porphyromonas gingivalis, Infect. Immun. 70 (8) (2002) 4708–4715,
https://doi.org/10.1128/IAI.70.8.4708–4715.2002.
[53] C.S. Whyte, I.N. Chernysh, M.M. Domingues, S. Connell, J.W. Weisel, R.A.S. Ari¨ens, et al., Polyphosphate delays fibrin polymerisation and alters the mechanical properties of the fibrin network, Thromb Haemost. Schattauer GmbH 116 (11) (2017 Nov 11) 897–903.
[54] S.A. Smith, J.H. Morrissey, Polyphosphate enhances fibrin clot structure, Blood 112 (7) (2008) 2810–2816, https://doi.org/10.1182/blood-2008-03-145755. [55] S.M. Hassanian, P. Dinarvand, S.A. Smith, A.R. Rezaie, Inorganic polyphosphate
elicits pro-inflammatory responses through activation of the mammalian target of rapamycin complexes 1 and 2 in vascular endothelial cells, Journal of Thrombosis and Haemostasis vol. 13 (5) (2015 May 1) 860–871, https://doi.org/10.1111/ jth.12899. John Wiley & Sons, Ltd.
[56] M.J. Page, G.J.A. Thomson, J.M. Nunes, A.-M. Engelbrecht, T.A. Nell, W.J.S. de Villiers, M.C. de Beer, L. Engelbrecht, D.B. Kell, E. Pretorius, Serum amyloid A binds to fibrin(ogen), promoting fibrin amyloid formation, Sci Rep. Nature Publishing Group 9 (1) (2019 Feb 28) 1–14, https://doi.org/10.1038/s41598-019- 39056-x.
[57] S.S. Dominy, C. Lynch, F. Ermini, M. Benedyk, A. Marczyk, A. Konradi, M. Nguyen, U. Haditsch, D. Raha, C. Griffin, L.J. Holsinger, S. Arastu-Kapur, S. Kaba, A. Lee, M. I. Ryder, B. Potempa, P. Mydel, A. Hellvard, K. Adamowicz, H. Hasturk, G. D. Walker, E.R. Reynolds, R.L.M. Faull, M.A. Curtis, M. Dragunow, J. Potempa, Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease
causation and treatment with small-molecule inhibitors, Science Advances 5 (1) (2019 Jan 1) eaau3333, https://doi.org/10.1126/sciadv.aau3333. American Association for the Advancement of Science.
[58] C. Hayashi, C.V. Gudino, F.C. Gibson, C.A. Genco, Review: pathogen-induced inflammation at sites distant from oral infection: bacterial persistence and induction of cell-specific innate immune inflammatory pathways, Molecular Oral Microbiology 25 (5) (2010) 305–316, https://doi.org/10.1111/j.2041- 1014.2010.00582.x.
[59] J.-L.C. Mougeot, C.B. Stevens, B.J. Paster, M.T. Brennan, P.B. Lockhart, F.K. B. Mougeot, Porphyromonas gingivalis is the most abundant species detected in coronary and femoral arteries, J. Oral Microbiol. 9 (1) (2017) 1281562, https:// doi.org/10.1080/20002297.2017.1281562.
[60] I.M. Velsko, S.S. Chukkapalli, M.F. Rivera, J.-Y. Lee, H. Chen, D. Zheng, I. Bhattacharyya, P.R. Gangula, A.R. Lucas, L. Kesavalu, Active Invasion of Oral and Aortic Tissues by Porphyromonas Gingivalis in Mice Causally Links Periodontitis and Atherosclerosis, in: M. Glogauer (Ed.), PLoS ONE, vol. 9, Public Library of Science, 2014 May 16, e97811, https://doi.org/10.1371/journal.pone.0097811, 5. [61] S. Delbosc, J.-M. Alsac, C. Journe, L. Louedec, Y. Castier, M. Bonnaure-Mallet,
R. Ruimy, P. Rossignol, P. Bouchard, J.B. Michel, O. Meilhac, Porphyromonas gingivalis participates in pathogenesis of human abdominal aortic aneurysm by neutrophil activation. Proof of concept in rats, PloS One 6 (4) (2011), e18679,
https://doi.org/10.1371/journal.pone.0018679.
[62] M. Cortes-Canteli, L. Mattei, A.T. Richards, E.H. Norris, S. Strickland, Fibrin deposited in the Alzheimer’s disease brain promotes neuronal degeneration, in: Neurobiology of Aging [Internet], vol. 36, Elsevier Inc, 2014 Oct 31, pp. 608–617,
https://doi.org/10.1016/j.neurobiolaging.2014.10.030, 2.
[63] B. Adams, J.M. Nunes, M.J. Page, T. Roberts, J. Carr, T.A. Nell, D.B. Kell, E. Pretorius, Parkinson’s disease: a systemic inflammatory disease accompanied by bacterial inflammagens. - PubMed - NCBI, Front. Aging Neurosci. 11 (2019) 555,