Clinical Assessment of Disturbed Central Pain
Modulation in Orofacial Pain
Erik Öjstedt
Simon Pankalla
Supervisor
Per Alstergren ProfessorOrofacial Pain Unit, Faculty of Odontology
Master thesis in Odontology (30 ECTS)
Malmö University
Program in Odontology
Faculty of Odontology
Abstract
Objective. To retrospectively investigate clinical variables that can predict the presence of disturbed central pain modulation (DCPM).
Material and methods Medical records of 86 patients examined at the Orofacial Pain Unit at Malmö University from September 2012 to December 2013 were examined regarding pain intensity, pain distribution, pain-related disability, psychosocial variables, referred pain as well as somatosensory changes. Based on these variables, the patients were divided into a disturbed central pain modulation (DCPM) group and a non-DCPM group. Allodynia, hyperalgesia, dysesthesia, increased wind-up, regional/general pain distribution and aftersensation were considered as markers for DCPM. Non-parametric statistics were used and a probability level of P<0.05 was considered as significant.
Results. The degree of unspecific physical symptoms and the number of sites eliciting pain referral were significantly higher in the DCPM group. In the multivariate regression model, the independent variables physical symptoms, stress, pain duration, characteristic pain
intensity, pain-related disability, number of sites with referred pain, maximum mouth opening with and without pain, anxiety, and number of pain eliciting jaw movements significantly predicted DCPM (LR Chi2 = 26.89, p = 0.003, Pseudo R2 = 0.29).
Conclusion. This study indicates that stress, anxiety, orofacial pain and its consequences, unspecific physical symptoms and jaw dysfunction are clinical signs of DCPM in patients with orofacial pain. Also, high number of palpations sites with referred pain over the masseter and temporal muscles and the TMJ indicate presence of DCPM.
Sammanfattning
Syfte. Studiens syfte var att retrospektivt undersöka vilka kliniska variabler, bedömda under specialistundersökning av orofacial smärta, som kan förutsäga närvaro av en störd central smärtmodulering (DCPM).
Material och metod. DC/TMD-data hämtades ur patientjournaler från 86 patienter som undersökts på Orofaciala smärtenheten på Malmö Universitet under perioden september 2012 till och med december 2013. Undersökta variabler omfattade smärtintensitet, smärtutbredning, smärtrelaterad nedsatthet, psykosociala variabler, refererad smärta samt kliniska fynd under somatosensoriska undersökningar. Baserat på denna data delades patienterna upp i en DCPM-grupp och en DCPM-grupp utan DCPM. Allodyni, hyperalgesi, dysestesi, wind-up, regional/generell smärtutbredning samt eftersensation ansågs vara markörer för DCPM. Icke-parametriska statistiska analyser användes och en sannolikhetsnivå på P<0,05 ansågs vara signifikant. Resultat. Graden av ospecifika fysiska symptom och antalet refererande smärtor var signifikant högre i DCPM-gruppen. Den multivariata logistiska regressionen visade att ospecifika fysiska symptom, stress, smärtduration, smärtintensitet, smärtrelaterad nedsatthet, antalet refererande smärtpunkter, maximal gapning med och utan smärta, ångest samt antalet smärtinducerande käkrörelser var signifikanta marörer för DCPM (LR Chi2 = 26.89, p =
0.003, Pseudo R2 = 0.29).
Slutsats. Denna studie indikerar att stress, ångest, smärtduration, smärtintensitet,
smärtrelaterad nedsatthet, antalet refererande smärtpunkter, maximal gapning med och utan smärta samt antalet smärtinducerande käkrörelser är associerat med DCPM hos patienter med orofacial smärta.
TABLE OF CONTENTS
INTRODUCTION ... 5
PAIN ... 5
PAIN RECEPTORS ... 6
BASIC NERVOUS SYSTEM PHYSIOLOGY ... 6
FUNCTIONAL LEVELS OF THE CENTRAL NERVOUS SYSTEM ... 7
SPINAL SOMATOSENSORY PATHWAY ... 7
OROFACIAL SOMATOSENSORY PATHWAY ... 8
PAIN MODULATING SYSTEMS IN THE CENTRAL NERVOUS SYSTEM ... 10
RISK FACTORS IN DEVELOPING CHRONIC PAIN ... 12
PSYCHOSOCIAL FACTORS IN CHRONIC PAIN ... 13
LOCAL AND WIDESPREAD PAIN ... 13
SENSITIZATION ... 14
REFERRED PAIN IN THE OROFACIAL REGION ... 14
DISTURBED CENTRAL PAIN MODULATION ... 15
CLINICAL SIGNS OF DISTURBED CENTRAL PAIN MODULATION ... 17
OROFACIAL PAIN, TEMPOROMANDIBULAR DYSFUNCTION AND DISTURBED CENTRAL PAIN MODULATION ... 17
DIAGNOSTIC CRITERIA FOR TEMPOROMANDIBULAR DISORDERS ... 18
ASSESSMENT OF DISTURBED CENTRAL PAIN MODULATION ... 18
SIGNIFICANCE ... 19
HYPOTHESIS ... 19
AIM ... 19
MATERIALS AND METHODS ... 20
SUBJECTS ... 20
RECRUITMENT OF PATIENTS/HEALTHY INDIVIDUALS ... 20
CLINICAL EXAMINATIONS ... 20
SOMATOSENSORY EXAMINATION ... 21
DATA EXTRACTION ... 21
DISTURBED CENTRAL PAIN MODULATION ... 21
BLINDING AND DATA HANDLING ... 22
STATISTICS... 22 ETHICAL CONSIDERATIONS ... 22 RESULTS ... 23 LOGISTIC REGRESSION ... 28 DISCUSSION ... 29 METHODOLOGICAL CONSIDERATIONS ... 30 CONCLUSION ... 32 REFERENCES ... 33 SUPPLEMENTS ... 39
INTRODUCTION
Patients often seek consultation with their general dentist to assess pain-related complaints. In some cases, there are no signs of odontogenic causes but a neuromuscular background might be suspected. Temporomandibular disorders (TMD) constitute a group of diseases affecting the masticatory muscles, the temporomandibular joint (TMJ) and/or adjacent tissues. TMD is the second most common musculoskeletal condition that may result in pain and disability, with a prevalence of 5-12% in the general population, making it a major public health
problem(1,2). Furthermore, insufficient treatment brings an economic burden on society(2). The standard treatment approach in pain-related TMD, aims to reduce symptoms. If the outcome of treatment is unsuccessful, the general practitioner might consider referring the patient to a specialist who can further examine the patient. One important factor is then to assess if there may be an underlying disturbed central pain modulation (DCPM). This assessment may be a challenge but is important since DCPM requires a multidisciplinary treatment plan to improve the possibilities of successful treatment effect(3,4).
Pain
The pain experience is multidimensional. Its nature depends on factors like somatosensory inputs from peripheral tissues in combination with activity in the higher brain’s
somatosensory, affective and cognitive centers. In between these there is important pain modulation in the spinal cord and the lower brain(5). Another fact that makes the experience of pain such a complicated issue are the underlying mechanism: nociceptive pain, neuropathic pain and nociplastic pain, which describes the pain that falls outside of the two previously described definitions, e.g. pain elicited by activation of peripheral nociceptors without any signs of tissue- or neural damage, and is argued to be a consequence of plastic transformation of neural circuits(6–8). Others have also included psychogenic pain, idiopathic pain and inflammatory pain. Psychogenic pain has long been a subject of controversy but is now recognized as a true pain state by many experts. It is thought to be initiated by activities in the central nervous system, more precisely cognitive and affective centers, but the understanding of this is far from complete(9). Idiopathic pain is simply a pain state for which the origin or cause is unknown.
Depending on the time lag between stimulus being produced and the actual sensation of pain there is slow pain and fast pain. Fast pain is mediated through A-delta fibers whilst slow pain is mediated through the unmyelinated C fibers(8). Slow pain is felt about one second after a certain stimulus is produced. The pain intensity usually increases slowly over time. Fast pain, on the other hand, is felt instantly, in less than 0.1 seconds. Fast pain is sometimes referred to as sharp or acute. It is the sort of pain you would feel from a needle sting or when the skin is burned. Most deep tissues of the body do not mediate this sharp, fast pain, as the A-delta fiber count is low. Slow pain is sometimes referred to as aching or chronic pain. It is associated with tissue destruction and in contrast to fast pain, slow pain can be mediated from both superficial skin and deep tissues(7). Slow, aching pain is an important phenomenon in the process of healing since tenderness in a tissue prevents movement of that body part. Normally
the immune system gives rise to the slow pain in response to tissue damage. It is therefore also referred to as inflammatory pain(10).
Pain may further be divided into acute or chronic pain. Chronic pain is defined as a pain lasting for more than three months (8) and seldom shows evidence of tissue damage or any signs of peripheral tissue inflammation. Hence, its definition is somewhat like the one
describing nociplastic pain. This sort of pain is usually the result of dysfunction in the central nervous system’s (CNS) different pain barriers. Chronic pain, lacking signs of tissue damage, is related to the rise of multiple syndromes, including fibromyalgia, temporomandibular joint disorders (TMD) and tension-type headache of chronic sort(10).
Pain receptors
Pain receptors, are free nerve endings more commonly referred to as nociceptors. They are found both in superficial skin and in some deep tissues such as arterial walls, joint surfaces, periosteum of bone, etc. Even though most other deep tissues have few pain-inducing nerve endings, these areas can produce slow, aching pain from tissue damage. Nociceptors are excited through three types of stimuli: thermal, mechanical and chemical stimuli. In a simplified way of looking at it, fast pains, i.e. receptors of A-delta fibers, are usually elicited by mechanical or thermal stimuli, while the slower propagating C-fibers are polymodaly active and can be evoked by any source of pain stimulant, but most commonly by chemical stimuli(7,8).
Chemical nociception arises through chemical compounds like bradykinin, serotonin,
histamine, potassium ions, acid, acetylcholine and proteolytic enzymes that react with the free nerve endings and their receptors. How easily these receptors are activated is partly dependent on different sensitizing and habituating molecules in the vicinity. Substance P and
prostaglandins are examples of sensitizing molecules(7).
Basic nervous system physiology
The nervous system's various activities are initiated almost always by an excitation of sensory receptors. These sensory transmissions can directly give rise to conscious reactions in the higher brain regions, or the memories of the experience are stored subconsciously in the brain to later influence a bodily reaction. Peripheral neural receptors are excited and transmit signals through an action potential to neurons in sensory areas of the spinal cord via synapses. Sensory areas in the reticular substance of the medulla, pons, mesencephalon (midbrain), the thalamus, and cerebral cortex are also conducted through(11).
Different synapses transmit signals at different levels of ease. This entails synapses’ ability to determine in what direction the signal is to be spread, depending on the magnitude of the transmitted incoming action potential. In addition, other areas of the nervous system may transmit signals that facilitate or inhibit signal transmission through different synapses. The nervous system processes incoming information in order to cause an appropriate level of
bodily response. More than 99 per cent of the incoming sensory information is normally seen as irrelevant by higher levels of the nervous system and therefore is not acted upon. However, when important sensory information excites the sensory areas of the CNS the signal is
channeled into the brain’s motor regions and pain matrix to give rise to a desired bodily response(11).
The typical neuron in the brain's motor cortex receives incoming signals through synapses on the dendrites or cell body. A single central neuron may have anywhere between 200 to 200000 such receptors. Outgoing signals from these neurons mediate through a single axon that can branch out to other connected neurons in the CNS, PNS, or other peripheral tissues (muscle cells, glandular cells, etc.)(11).
The motor part of the nervous system consists of neurons that excite and provoke
movement/activity of skeletal and smooth muscles, as well as glandular cells. These tissues, affected by the motor neurons, are referred to as effectors of the nervous system. The activities of the effector tissues are regulated by the nervous system from multiple different levels. The spinal cord, reticular substance of the medulla, pons and mesencephalon, the basal ganglia, the cerebellum, and the motor cortex all contribute in some way. The higher regions of the CNS are mostly concerned with more complex reactions to stimuli of sensory areas, partly controlled by thoughts and memories of experiences being processed by the brain. The lower regions e.g. spinal cord level, are responsible for instantaneous responses in the effector tissues(11).
Functional levels of the central nervous system
There are three major controlling levels of the CNS: spinal cord level, subcortical level (lower brain) and cortical level (higher brain).
Neural circuits in the spinal cord can function even without a connection to the brain. It can cause muscle reflexes, withdrawal reflexes from painful objects, etc.
The lower levels of the brain (medulla, pons, mesencephalon, hypothalamus, thalamus, cerebellum and basal ganglia) control many of the bodily activities commonly known as the subconscious. Additionally, these areas of the lower brain contribute to emotional patterns such as reaction to pleasure and pain. The cortical level of the brain, or the cerebral cortex, houses a complex functionality. The cortex is a huge storehouse of memories that never functions alone, but in association with the lower centers of the nervous system. In this way, the brain’s cortex helps the lower levels of the CNS to function with better precision(5,7).
Spinal somatosensory pathway
The somatosensory nervous system includes nociceptive, mechanosensory, propriosensory and thermosensory pathways from the periphery to CNS. The spinal relay of mechanosensory receptor’s path to cortical centers consists of three individual neurons. The primary
mechanosensory neurons ascend as thickly myelinated A-beta fibers, and their somas are located in spinal root ganglia at different levels, just outside the spinal cord. As their axons
enter the spinal cord they form two ascending columns, the gracile fasciculus and cuneate fasciculus, which connect with second order neruons, whose somas are located in the gracile and cuneate nucleus, respectively, both located in the medulla. Mechanosensory and
thermosensory information from the upper trunk to the neck and back of the head will travel through the cuneate fasciculus, while the lower body regions will send projections ending up in the gracile fasciculus. The secondary neurons transmitting mechanosensory and
thermosensory input from the body, excluding the orofacial region, project to connect with third order neurons in the lateral subnucleus of the ventral posterior thalamic nucleus. From here, third order neurons will project axons to mainly the primary sensory cortical
region(5,12). The mechanosensory pathway consists of predominantly monosynaptic
projections, i.e. one first order neuron innervates one second order neuron, and the third order neuron is interconnected with a single second order neuron, with only minimal convergence between adjacent axon terminals. Through this, the mechanosensory system maintains place specificity. This also entails that a single action potential could elicit a second order neuron. Therefore, modulation of mechanosensory projections at the medullary interface is thought to be maintained through interneuron activities, a mechanism referred to as lateral inhibition and facilitation. For example, as second order mechanosensory and nociceptive axons ascend, they give off branches to different pain modulating centers that connect with interneurons. These interneurons extend to synapses in the spinal dorsal horns as well as the thalamus, where inhibition or facilitation is conducted through the activity of different
neurotransmitters(12).
The spinal nociceptive neural relay consists of three relay neurons as well, though they interconnect at different levels of the CNS than the mechanosensories do, and their fibers are thinner, i.e. A-delta and C-fibers. The first order neuron somas are located in dorsal root ganglia, but they connect with second order neurons, whose somas are located already in the spinal dorsal horns. Before the connection is made with the second order neurons, the axons of first order neurons branch and send projections a few segments caudally and a few
segments rostrally. This implies that first order nociceptors synapse with several second order neurons. Simultaneously, each second order neuron receives input from a large number of first order neurons, corresponding to a broad peripheral region. The primary afferent nociceptors of A-delta and C fibers mainly synapse with second order neurons of different nuclei. Second order neurons that receive direct inputs from C fibers will also receive indirect A-delta and C fiber innervation through excitatory interneurons. Hence, these second order neurons are referred to as nociceptive specific. Most nociceptors mediating through A-delta fibers synapse with second order neurons that also receive inputs from branching
mechanosensory A-beta fibers. Therefore, they are referred to as wide dynamic range cells. The axons of the second order neurons form the spinothalamic tract, which lead to third order neurons in the ventral posterior thalamic nuclei. Wide dynamic range cells and nociceptive specific neurons terminate in mainly different subnuclei, but do converge in a few
regions(5,12).
Orofacial somatosensory pathway
In contrast to the sub-orofacial regions that send somatosensory information through spinal nerves, the orofacial regions conduct through cranial nerves. The trigeminal nerve is the main conducting cranial nerve of the orofacial somatosensation. First order neurons of
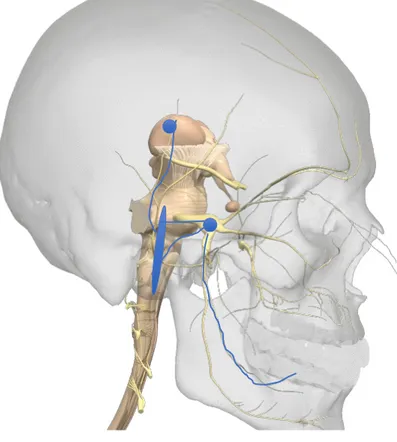
mechanoception and nociception have their somas located in the trigeminal ganglia, just next to the pons. However, these two sensory types have their somas located separately in different regions in the trigeminal ganglia. These neurons project axons to second order neurons, located in a widespread manner, stretching from the upper most cervical spinal segments to the central regions of the pons. Mechanoceptors transcend mainly to the second order neurons in the pons, and the nociceptive terminations are more abundant in the lower medulla-upper spinal cord. However, this is somewhat simplified. The intermixing of the mechanoceptor and nociceptor axon’s synaptic pattern with second order neurons is far greater in the trigeminal system than it is in the spinal somatosensory system. Second order neurons project their axons through the trigeminothalamic path, which terminates in the thalamic ventral posterior nuclei (Figure 1)(5,12).
Figure 1. Orofacial nociceptive
pathway. The peripheral axon leads to the soma of the first order neuron located in the trigeminal ganglion (lower right blue dot). The blue sphere demonstrates the wide distribution of second order neuron somas in the trigeminal nucleus, stretching from central parts of pons to the medulla-spinal interface. Somas of third order neurons are located in the thalamic nuclei (higher left blue dot). Modified from (13).
As for nociceptive transmission through both the spinal somatosensory pathway and the trigeminal one, several cortical and subcortical structures are activated. Included are the primary somatosensory cortex, secondary somatosensory cortex, and insular cortex. These centers are thought to incorporate the pain quality, intensity, duration and location. Primary motor cortex and cerebellum are both part of the motoric response to somatosensory
sensations. Lastly, affective and cognitive regions of prefrontal cortical centers and the limbic system, such as the anterior cingulate cortex, basal nuclei, hippocampus, and amygdala are
also involved in processing of pain(14). The prefrontal cortex, more specifically the anterior cingulate, is of great importance for the expectation and psychological management of the painful sensation. It is tightly interconnected with most of the structures of the limbic system and is sometimes argued to be the cortical part of that system. Activities in amygdala are associated with fear and anxiety, often the result of unfamiliar situations, such as great pain without an obvious explanation. Hippocampal activity seems to be important for memory storage, including memories of painful situations(15).
The limbic system could in a sense be viewed as a set of different anatomical structures, with partly opposing physiological functions, that all aim to influence the hypothalamus. Through hypothalamic influence, control over the endocrine system as well as autonomic nervous system is achieved and hence, the bodily reaction changes thereafter. For example,
sympathetic activation leading to an increased level of stress hormones could be the result of an overwhelming amygdala activity on hypothalamic neurons(15,16).
Pain modulating systems in the central nervous system
The central nervous system has a system of structures that modulate incoming nociceptive signaling. The central system involves mainly the following components; a nociceptive signaling inhibitory complex of nuclei in the rostral ventral medulla (RVM); locus coeruleus; and the periaqueductal gray (PAG)(5,17). The exact mechanisms controlling these different central nuclei are not well understood. However, compelling evidence demonstrates that neural nuclei in these regions are responsible for the up-regulated excretion of
neurotransmitters like norepinephrine and serotonin, which modulate pain signaling through binding adrenergic receptors and serotonin receptors, respectively, on spinal dorsal horn neurons(14,17). The PAG is a region located in the mid brain, surrounding the cerebral aqueduct. Ramifications of the ascending nociceptive signaling relay provide this region with somatosensory information. This lateral innervation seems to be important for the resulting quality and further propagation of the main somatosensory signal. PAG also communicates with several other central structures, such as the thalamus, primary cortical somatosensory center, cerebellum, and several limbic structures. Upon activation of its opioid receptors, neurons of PAG stimulate neurons in the RVM primarily, but also parts of the locus
coeruleus(5). Endogenous opioids such as endorphins decrease the subjective pain sensation through the binding of mu-opioid receptors on PAG neurons. This is mimicked through the use of morphine, for example(18).
The raphe magnus nucleus is a serotonergic nucleus that lies within the RVM(19). Just like PAG, RVM receives and projects signals from and to several central regions involved in pain modulation. Apart from PAG, it communicates with the thalamus and the noradrenergic locus coeruleus. RVM could be considered the final transmission station for pain modulation. It houses descending neurons to the trigeminal nuclei as well as the spinal dorsal horns. The projecting neurons have two basic functions that have been identified; pain -facilitating and – inhibiting neurons. Hence, they are commonly referred to as on-cells and off-cells,
respectively. Studies have found the pain facilitating neurons to be upregulated by noxious stimuli, simultaneously the inhibitory cells’ activity decreases. The opposite reaction occurs when the opioid receptors of RVM cells are activated, i.e. the pain inhibiting cells are
activated and the stimulatory cells are downregulated(14,19,20). Upon activation of RVM cells the spinal dorsal horns and trigeminal nuclei are exposed to an increased level of serotonin. Whether this neurotransmitter is released by ascending RVM axon terminals or interneurons is not fully elucidated(14,21,22). Additionally, the role of serotonin in modulation of ascending nociceptive signaling seems complex and could be bidirectional. Studies on rodents have demonstrated that activation of the serotonin receptors 5-HT2 and
5-HT3 results in a pro-nociceptive action, whilst activation of different sub groups of 5-HT1 and
5-HT7 results in anti-nociceptive actions(23,24).
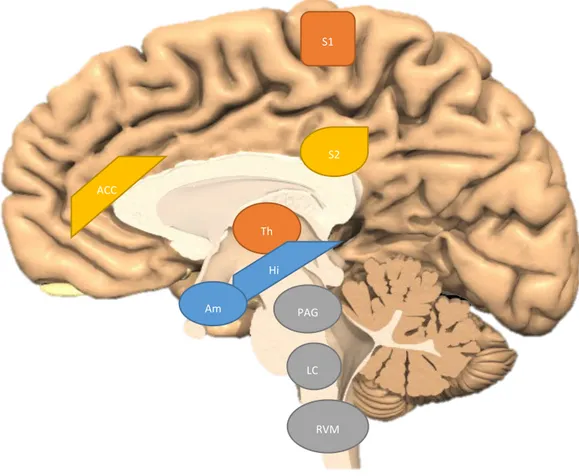
Figure 2. Important central nervous system structures in pain processing. S1 – primary somatosensory cortex,
S2 – secondary somatosensory cortex, ACC – anterior cingulate cortex, Th – thalamus, Hi – hippocampus, Am – amygdala, PAG – periaqueductal gray, LC – locus coeruleus, RVM – rostral ventral medulla. Modified from (25).
Since the bidirectional nature of serotonin does not offer a complete picture of the brainstem’s analgesic system, some studies have investigated the role of other neurotransmitters(21,26).
Y-aminobutyric acid (GABA) and glutamate/glutamatic acid are two unidirectional
RVM LC PAG S1 S2 ACC Th Hi Am
neurotransmitters that inhibit and activate receptors respectively. Studies have found GABAergic as well as non-GABAergic (exhibiting neither GABA nor serotonin) neural axons to be abundant in both the tract from PAG to RVM and from RVM to the spinal dorsal horns. GABAergic axon projections from PAG terminate on both on- and off-cells in RVM. This is true for the non-GABAergic axons as well. Furthermore, the cell populations of both on- and off-cells seem to have some GABAergic and some non-GABAergic axons(21). This information indicates a highly complex neural interaction from PAG to the spinal dorsal horn nociceptive afferents, which presumably involves interneuron activity as well.
The central nervous system’s descending pain modulation system also includes a
noradrenergic pathway. The locus coeruleus, situated in the pons, is one of the regions that descend axons, thus releasing norepinephrine in the spinal dorsal horns and the trigeminal nuclei. Studies have shown that stimulation of the locus coeruleus, as well as its connected higher centers of PAG, RVM and the thalamus, could result in an increased level of norepinephrine in the cerebrospinal fluid, i.e. also in the vicinity of spinal dorsal horn
neurons(27). The adrenergic effect from stimulation of PAG, RVM and the thalamus is most likely indirect, as they lack noradrenergic neurons, the norepinephrine release is the result of their interaction with neurons in locus coeruleus(28). Just as in the case of serotonin, there seems to be a bidirectional receptor effect in the biochemistry of norepinephrine acting on dorsal horn neurons. Activation of α2-adrenergic receptors is associated with pain inhibition,
whilst α1-adrenergic receptors create pain facilitation.
Overall, several types of descending pathways co-exist as pain modulators in the analgesic system of the CNS. It is not fully understood how these neurotransmitter systems interact and function in response to different pain conditions(29).
Risk factors in developing chronic pain
Even though the processes leading to chronic pain cannot yet be explained in detail, a few important risk factors have been found. Factors like: female gender; a prolonged pain duration; trauma in early childhood; genetics leading to a predisposition of lower pain thresholds; and there is also evidence indicating that people who have been overly protected from traumatic situations during their childhood tend to have a greater risk of developing chronic pain later in life due to the increased catastrophizing of pain sensations(6,30,31). Depression, stress, anxiety and prolonged periods of pain also seem to be important risk factors and might also be initiating factors for the development of chronic pain. These last-mentioned factors have generally been seen as consequences/symptoms of chronic pain disorders. However, studies show that prolonged periods of pain and psychological factors are associated with an alteration of central pain thresholds as well as the descending pain
Psychosocial factors in chronic pain
The psychosocial components of pain are usually divided into affective factors, such as anxiety, depression, and stress; and cognitive factors, including the individual’s thoughts and expectations of the pain experience. Pain catastrophizing is an example of a cognitive factor.
Not long ago, chronic pain was seen as only a somatic symptom of a disease. Today it is recognized to be an illness on its own. It affects the psychosocial status, likewise, the psychosocial status contributes to an altered pain experience(6). For instance, some studies have shown that in certain populations, chronic pain increases the risk of depression by 2.4 – 4.0 times. It has also been shown that having a deeply manifest depression increases the risk of both neuropathic and non-neuropathic pain by six and three times respectively(36–38). The presence of stress, as a consequence of chronic pain, could be explained by the fear that many affected individuals do experience. Fear is a common response to pain, which the patient does not know the origin of and pains that does not seem to ease over time. As mentioned earlier, fear and anxiety are consequences of activity in the amygdala, which in itself is associated with an increase of sympathetic stress response. Stress hormones such as epinephrine and glucocorticoids, further enhance the amygdala activity(16).
Hence, psychosocial factors such as depression, stress and anxiety have a close but complex relationship to chronic pain. Patients exhibiting depression have altered serotonin levels peripherally, but especially in the CNS. This is also true for patients experiencing chronic/dysfunctional pain, which is an example of how they share some patterns of neurobiological markers. Serotonin, accompanied by GABA (gamma-aminobutyric acid), norepinephrine and glutamate regulate the nociceptive transmission centrally. Through adjustment of the amount of these substances, the brain’s cognitive and affective centers may influence the pain experience, especially that of chronic pain(11,35).
The importance of this correlation between chronic pain and psychosocial status is the ability to direct the correct treatment. Very few therapies have proven to help individuals with chronic pain, an exception is cognitive behavioral therapy (CBT). It is believed that through CBT, the activity of inhibitory neural tracts descending from PAG down to spinal level neurons in the nociceptive path may increase and hence reduce the afferent pain signaling. (39,40)
Local and widespread pain
Local pain implies that the pain stays within a limited area, tenderness of one masticatory muscle for example. Pain experiences in a broader region, e.g. the whole orofacial area or soreness in many structures innervated by the same cranial nerve indicates regional pain distribution. An individual having a pain depiction involving a greater part of the body would imply a general pain distribution. The presence of several widely distributed pain conditions simultaneously is strongly associated with a centrally disturbed pain modulation(41). It is also a predictor of developing pain-associated TMD as well as worsening the treatment prognosis of different pain-associated disorders(42,43). Just as the presence of a greater bodily pain
experience seems to elevate the risk of developing pain disorders in the orofacial area, the presence of painful TMD disorders seem to increase the risk of pain arising in other bodily locations(41,42,44). Hence, you could readily argue that there could be a reciprocal
relationship. The link between widespread pain and DCPM is thought to be the hallmarks of central sensitization. Decreasing activity in the analgesic system of CNS, as well as increased sensitivity in the second-order neurons in the spinal cord, would partly explain the elevated risk of pain-development in new bodily regions(7,45–47).
Sensitization
Most sensory receptors in the nervous system adapt to their stimuli through a phenomenon called neural plasticity. The longer the stimulus is active, the harder it is to excite the receptor. Pain receptors, on the other hand, do not adapt in the same way. In contrast, the excitation of nociceptive fibers progressively amplifies if the stimulus remains. Thus a weaker, normally not so painful stimulus is sufficient to produce a higher level of painful sensation once the receptors are sensitized(7,45). This phenomenon is called hyperalgesia and it is an important function to protect from further injuries. During an inflammation and/or trauma to a specific bodily site, neurons are typically suffering great chemical stimuli as well as direct trauma, which might lead to cell death. During healing, damaged neurons will try to restore the innervation of the healing tissue. This process involves different levels of disorganized sprouting of nerve fibers that can accumulate to create a traumatic neuroma, i.e. a more dense neural aggregation, with a greater density of neural receptors per tissue volume. Since the likelihood of a peripheral signal being transmitted centrally depends on the quantity and intensity of signals reaching the second order neurons in the spinal cord, stimuli to a neuroma could with a greater ease result in a painful sensation(48).
Even though the broad literature today considers central mechanisms to constitute the main role of chronic pain, peripheral mechanisms are believed to account for at least in part the initiation and perhaps the upholding of the pain sensation that gradually lead to central neural plasticity(6,35). For example, A-beta fibers normally conduct non-noxious sensations and decrease the stimulatory effect of painful signaling through A-delta and/or C fibers to
secondary spinal neurons. In individuals with a central sensitization A-beta as well as A-delta and C fibers stimulates the secondary neurons equally and thus producing a higher inflow of stimuli to the somatosensory cortex(49). Regardless whether the increased input through central neurons is caused by sensitized nociceptors or ectopic changes in central or peripheral neurons, it is a significant drive in central sensitization and its clinical expressions(50).
Referred pain in the orofacial region
The phenomenon when pain is perceived from a different bodily location than the actual tissue damage is commonly known as referred pain(7,51). For instance, during some tissue injuring illnesses in visceral organs, the pain may appear in more superficial tissue, which is a normal neural function. The degree of pain intensity and spatial spread varies between
individuals and may be abnormally high. Among patients suffering from orofacial pain or temporomandibular dysfunction, referred pain is a common finding with a prevalence of up to
80% according to some authors(51). The explanation for this phenomenon is that the
nociceptor innervating the area with referred pain converges with the nociceptor innervating the damaged tissue to the same second-order neuron in the spinal cord nucleus (see figure 3). As mentioned in the previous section, prolonged nociceptive pain may lead to central
sensitization of pain. Presence of or increased referred pain may thus be a clinical sign of DCPM(52).
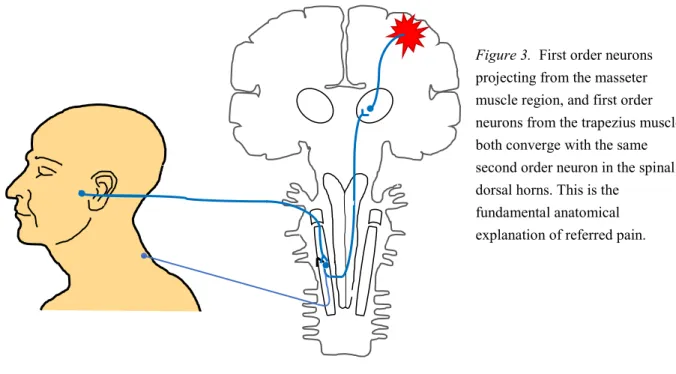
To treat the patient’s symptoms, it is crucial to find and treat the source of actual tissue damage. Luckily, there seems to be a concordance in the way these specific trigger points refer the pain to other body sites in most individuals. Making a parallel to the field of dentistry, where the source of temporal headache in some cases arise from trigger points in the trapezius muscle(51,53). This is due to nociceptive afferents innervating muscles, skin, and joints of the head, but also the area around the shoulders, all converging with the same group of second-order neurons in the dorsal horns of the spinal cord(52).
Figure 3. First order neurons
projecting from the masseter muscle region, and first order neurons from the trapezius muscle, both converge with the same second order neuron in the spinal dorsal horns. This is the
fundamental anatomical explanation of referred pain.
Disturbed central pain modulation
In most cases with chronic pain, hyperalgesia or allodynia originates from neural plasticity in the spinal cord or thalamus, creating a sensitization of central neurons(45). Central
sensitization is one consequence of a disturbed central pain modulation (DCPM) and is caused by neural functional changes in the somatosensory system of the spinal cord, brainstem and the brain’s pain matrix(46). These central changes include altered activity in descending inhibitory and facilitating neurons from PAG (periaqueductal gray), RVM and locus coeruleus, toward spinal neurons in the dorsal horn and trigeminal nuclei, facilitated excitation of second-order neurons in the spinal cord and neurons in the thalamus due to lowered thresholds.
As mentioned in a previous section, the descending pain modulation system of the brain stem is composed of inhibitory and facilitating neural activity, acting on spinal and thalamic levels of conduction. These are thought to normally maintain a baseline state of sensory processing. Dysfunction in this system, through altered transmitter and receptor availability, is thought to be one underlying cause of DCPM(14). As a consequence, the pain sensation may be
amplified, increased in duration and spatial extension. Hence, a stimulus that does not seem to produce or originate from any tissue damage could still elicit a painful sensation(45,47). The components of the descending pain inhibitory tracts have synaptic connections to several cortical and limbic regions. These higher centers are known to be active during pain
experiences, and the activity seems to differ depending on whether the pain is acute or chronic. These regions are therefore thought to play a role in the chronification of pain and development of the disturbed central pain modulation(17,54).
Central changes in patients with a chronic pain disorder do not only include altered release of neurotransmitters and expression of synaptic receptors. Recent studies have demonstrated changes in the volume and density of cortical and limbic gray matter, i.e. changes in density of neural somas and dendrites, in patients demonstrating chronic pain from different
origins(17,55,56). One study could even demonstrate a reverse gray matter plasticity in patients who were treated successfully for their chronic back pain(17,57). Other studies have put their interest to white matter changes in these individuals, elucidating the fact that changes in density occur there as well(17,58). The increase of neural somas and axons in some
regions, and a decrease in other, point to the conclusion that chronification of pain is in part due to increased connectivity between some central regions and a decrease between other. Hippocampus is one limbic structure where an association between increased neural density and development of chronic pain has been proven to exist. A study performed on rodents found that by blocking neurogenesis of hippocampal cells, the magnitude of developed neuropathic pain after nerve injury decreased(59). This is of interest, since hippocampal neurogenesis is also associated with memory formation and learning abilities(60). Earlier findings suggest that individuals experiencing chronic pain show impaired memory skills(61). These findings could be perceived as contradictory, however, none of these studies could explain the causal relationship between hippocampal neurogenesis and the aforementioned clinical effects. Hence, memory and learning processes could be involved in chronification of pain.
As mentioned, several psychosocial factors have been associated with pain perception. Emotional stimuli, such as a traumatic experience, will enhance the attachment of memories from that situation. It is therefore argued that strong emotions in conjunction with painful events could store the memories of the painful event and evoke pain in future similar physical activities and/or emotions(62,63).
From recent studies, the neural plasticity leading to chronification of pain has been attributed to a heightened level of pro-inflammatory mediators centrally, i.e. neuroinflammation. It is being argued that peripheral pain, resulting from tissue- and/or neural damage is associated with an increase of central pro-inflammatory mediators. Increased levels of sympathetic stress
hormones (epinephrine and norepinephrine) seem to result in the same state of
neuroninflammation. Through this inflammatory state, stress and peripheral tissue damage produce sensitizing plastic changes throughout the somatosensory pathway, i.e. hyperalgesic priming(64). This theory has been reinforced by pre-clinical studies on rodents, where
antagonists of specific important pro-inflammatory cytokines, such as IL-1β, proved to reduce symptoms of chronic pain, only when aimed at receptors in the CNS(64,65).
Clinical signs of disturbed central pain modulation
There is no consensus about the clinical presentation or definition of DCPM. However, there are some clinical signs that are considered highly likely to be present due to DCPM and therefore may be used to assess its presence. The lowered thresholds and decreased central inhibitory activity lead to more incoming pain signaling, which causes an unproportionally high pain sensation for which the umbrella term is hyperesthesia. The phenomenon of exaggerated pain perception to a normally painful stimulus is called hyperalgesia. The same mechanism may also lead to pain sensation from a normally not painful stimulus. In that case, it is referred to as allodynia. In neuropathic pain, allodynia is associated with an ectopic activity in A-beta fibers that changes its conductive pattern from a non-painful transmission to a painful one(8,45,47,50).
Clinically, an individual with DCPM often also presents dysesthesia, which is the perception of an unpleasant sensation that arises from evocation or spontaneously and which usually is painful as well. It should not be confused with paresthesia which also presents as an abnormal sensation, though not an unpleasant one(8,45,47,50,52). Furthermore, clinical signs like after-sensation (when pain persists even after the stimulus has been removed) and hefty temporal summation/wind-up (gradually increased pain due to repeated provocation with the same stimulation intensity) are also argued to be symptoms of DCPM(45,47,66).
Every healthy individual will to some extent present a temporal summation effect when a certain area is painfully provoked repeatedly. This is explained by the cumulative afferent pain signaling by first-order peripheral neurons, which eventually triggers the second-order neuron in the spinal dorsal horn beyond its excitation threshold. In individuals who suffer from DCPM, the second-order neurons in the dorsal horn may be pre-sensitized with a lower threshold. Thus the wind-up effect will be much greater(1,45,52).
Orofacial pain, temporomandibular dysfunction and disturbed central pain
modulation
The concept of temporomandibular disorders (TMD) includes multiple different disorders in the temporomandibular joint, jaw muscles and adjoining tissues. Underlying causes for the onset of chronic pain in these conditions is not well understood, though common symptoms are orofacial pain and reduced jaw function(46). There are no strong connections between chronic orofacial pain and peripheral tissue damage. Peripheral and central sensitization, on the other hand, seem to enhance the pain symptoms in these kinds of disorders(46,67). Patients experiencing chronic orofacial pain often seem to have DCPM. Since different
sources of pain arise from different processes, it is important to target these specific mechanisms. The fact that clinical signs of disorder are often lacking makes it difficult to direct the correct method of treatment(10,46).
Post-traumatic trigeminal neuropathy (PTTN) is a condition, which often entails a serious chronic pain situation in the orofacial area. It is caused by injury to branches of the trigeminal nerve, which then causes an altered sensation of touch and pain in the areas innervated by that branch. Hence, PTTN can involve just a few branches or the whole trigeminal nerve complex, which makes the clinical depiction varied to some extent. Common findings in patients with PTTN are the presence of dysesthesia, allodynia, and an increase in temporal summation, which indicates neural plasticity in the damaged nerves different levels of conduction. Often, there are clinical signs of possible trauma to the nerve, e.g. scars from extra-oral surgery, missing teeth after extraction etc. Though, in some cases there are no signs, and the patient might not recall previous trauma. The fact that PTTN produces local and regional symptoms that very much coincide with the symptoms of a more general disturbance in pain modulation, assessing whether the patient is suffering from PTTN or a more general disturbance of pain modulation is difficult(48).
Diagnostic Criteria for Temporomandibular Disorders
The main diagnostic tool for TMDs is the DC/TMD (diagnostic criteria for
temporomandibular disorders). It was put together to facilitate diagnosis, treatment and/or referral to other fields of medicine. DC/TMD consists of a refined version of the axis I and the axis II from the former gold standard tool, RDC/TMD, which was introduced in 1992(68). The final output of the DC/TMD instrument was finalized in 2012 and published in 2014(1). The use of DC/TMD gives the practitioner the potential to assess among the twelve most common pain- and dysfunctional disorders in the orofacial area. Axis I is made out to assess the patients’ physical state and involves a TMD pain screener which is a self-report
instrument to determine the presence of pain-related as well as dysfunctional TMDs(1,2,69). It entails standardized methods to examine, evaluate subjective and objective symptoms as well as clear diagnostic criteria for diagnosis(70).
The axis II is a tool that is used to evaluate the individual’s psychosocial status and pain-related disabilities. It comes in two different levels of thoroughness: a brief assessment and a comprehensive variant with an extended set of tools for assessments. The axis II does not directly contribute to the diagnosis according to DC/TMD. However, the assessment of the individual’s psychosocial status is of great importance to evaluate the possibility of a
successful outcome from standard treatment. Simultaneously, the axis II assessment may help the clinician in the consideration of DCPM involvement. Hence, the decision making in referring the patient to a multidisciplinary care is facilitated(1,70).
Assessment of disturbed central pain modulation
The assessment of DCPM can only be assessed through electroneurophysiological
nor is it reasonable to conduct in the general case of pain assessment. However, as mentioned previously, there are several clinical signs associated with the presence of a disturbed central pain modulation of some sort.
The assessment of these clinical signs is not completely included in the DC/TMD, nor is it in the expanded version(2). Instead, a qualitative somatosensory examination is used, through which the assessment of allodynia, dysesthesia, aftersensation, spatial spread and wind-up is possible.
Significance
DCPM is a prominent phenomenon in chronic pain and involved in several phenotypes linked to chronic pain, like somatosensory disturbances, autonomic changes, as well as
psychological aspects of chronic pain (depression, anxiety, catastrophizing). However, there is no established clinical assessment of the presence of or degree of DCPM. A proper clinical assessment of DCPM has a great potential to improve knowledge of pain mechanisms in chronic pain as well as clinical care.
Hypothesis
The authors of this present study hypothesized that DCPM, as defined in this study, is
associated with the presence and degree of each of the following factors of stress, depression, anxiety, number of stimulated locations with referred pain, and the degree of unspecific physical symptoms, as assessed in the DC/TMD.
The phenomenon of DCPM is multifactorial. Therefore, considering the combination of several clinical and psychosocial factors could present a greater explanatory effect of DCPM, than focusing on single factors. With this in mind, the authors of this study hypothesized that the presence of DCPM could be explained by the factorial combination of depression, stress, anxiety, number of stimulated sites with referred pain.
Aim
The aim of this study was to investigate which clinical variables assessed during a specialist examination of orofacial pain that can predict the presence of DCPM.
MATERIALS AND METHODS
Subjects
Data for this retrospective study were obtained from patients’ medical records from the Orofacial Pain Unit at Malmö University, Faculty of Odontology, Malmö, Sweden, where the patients had undergone a comprehensive examination. From this database, 86 consecutively examined patients referred to the Orofacial Pain Unit were selected. Inclusion criteria for the participating patients were age of 18-75 years and masseter or temporalis muscle myalgia according to Diagnostic Criteria for Temporomandibular Disorders (DC/TMD; Schiffman et al. 2014). Exclusion criteria were psychiatric disease (depression and anxiety were allowed), limitation in the Swedish language that would have prevented the subject to answer the axis II protocol satisfactory. The patients had to be examined later then September 2012 in order to have been examined using the latest DC/TMD version.
Recruitment of patients/healthy individuals
The subjects were informed about the project and signed a consent form. The project has an ethical approval from the regional Ethical Review Board in Lund, Sweden (2015/339).
Clinical examinations
Before the examination all patients and healthy individuals answered the DC/TMD Axis II questionnaires used for assessment of psychosocial status and distress. All participants completed a DC/TMD questionnaire prior to the clinical examination. This questionnaire comprised assessment of degrees of depression (Patient Health Questionnaire-9), anxiety (Generalized Anxiety Disorder-7), number of physical symptoms (Patient Health
Questionnaire-15), stress (Perceived Stress Scale-10), catastrophizing (Pain Catastrophizing Scale), characteristic pain intensity (mean of pain intensity for reported worst, current and average pain; Graded Chronic Pain Scale), pain-related disability (mean of how much facial pain changed the patient’s ability to participate in daily activities, social activities and work; Graded Chronic Pain Scale), and grade (Graded Chronic Pain Scale) as well as pain locations (Pain Drawing).
All participants underwent a clinical DC/TMD examination. This examination comprised standardized assessment of pain locations, headache, mouth opening capacity, pain on mandibular movement, pain on palpation and temporomandibular joint noises. Both examiners that conducted the clinical examinations that made the foundation for this study attended a two-day course and were calibrated in the use of DC/TMD on a specialist level. The department of Orofacial Pain and Jaw Function at Malmö University, Sweden is one of five official DC/TMD Training and Reliability Centers in the world, accredited by the
International Network for Orofacial Pain and Related Disorders Methodology (INfORM, part of IADR: http://www.iadr.org/INfORM/DC-TMD). The examiners were both calibrated to the Reference Standard Examiner level in Swedish and English.
Somatosensory examination
A qualitative somatosensory examination was performed. Extraoral areas innervated by the trigeminal nerve branches and the second cervical nerve (a total of 8 sites per patient) were examined with the use of three different stimuli (touch, cold, pin-prick) to assess
hypoesthesia, hypersthesia, dysesthesia, allodynia, hypoalgesia or hyperalgesia as well as aftersensations and/or spatial spreading. A repeated pin-prick test was performed to assess the degree of wind-up in selected area (nerve branch with most severe somatosensory changes).
Data extraction
From the patients’ records, two previous master students extracted the DC/TMD data. The data extraction was validated by re-exctraction of data from a subsample of five patients, randomly chosen, and checked against the initially extracted data. Data comprised completed questionnaires regarding the patients’ pain, quality of life and health history, as well as clinical findings of the orofacial region. Answers from the questionnaires included demographical factors such as age, gender, profession and civil status as well as general condition.
Axis I data, i.e. answers to the DC/TMD Symptom Questionnaire and clinical findings, were collected regarding pain and headache localization, mobility of the jaw, masticatory muscle and TMJ palpatory pain, familiar pain, referred pain as well as TMJ sounds. Referred pain is based on eight structures at each side in the orofacial region. Thus, referred pain sites can be obtained from a maximum of 16 structures according to DC/TMD. The investigated structures of referred pain due to palpation consists of I) the masticatory muscles temporalis and
masseter(s), II) temporomandibular joint (lateral pole and around the lateral pole) as well as III) supplemental masticatory muscle areas (posterior mandibular region, submandibular region, lateral pterygoid area and temporalis tendon).
DC/TMD Axis II data (psychosocial factors that may influence or be a consequence of orofacial pain) were assessed. The Swedish version of Axis II of the DC/TMD includes measures to assess dysfunctional chronic pain, depression, anxiety, somatization, stress and catastrophizing. Dysfunctional chronic pain was assessed using the Graded Chronic Pain Scale (GCPS). Depression was assessed with the 9-item Patient Health Questionnaire (PHQ-9). Anxiety was assessed with the 7-item Generalized Anxiety Disorder Assessment (GAD-7). Stress was assessed with the 10-item Perceived Stress Scale (PSS-10). Unspecific physical symptoms were assessed through the Patient Health Questionair-15 (PHQ-15). A full-body pain drawing was used to assess the location and distribution of pain(s).
Disturbed central pain modulation
DCPM in this study was defined as presence of dysesthesia, allodynia, aftersensation, spatial spread, regional/general pain distribution and/or wind-up (increase of 30% or three steps in pain intensity on NRS 0-10). The patients were allocated into two groups according to presence of DCPM or not: the DCPM group and the non-DCPM group.
Blinding and data handling
All data collected from the patients was transferred into an Excel file where a code number replaced all identification for individual patients. The Excel file is stored at MaU internal servers with access limited to the supervisor and the two students working on this project only. The code list is kept on paper in a locked storage at the department.
Statistics
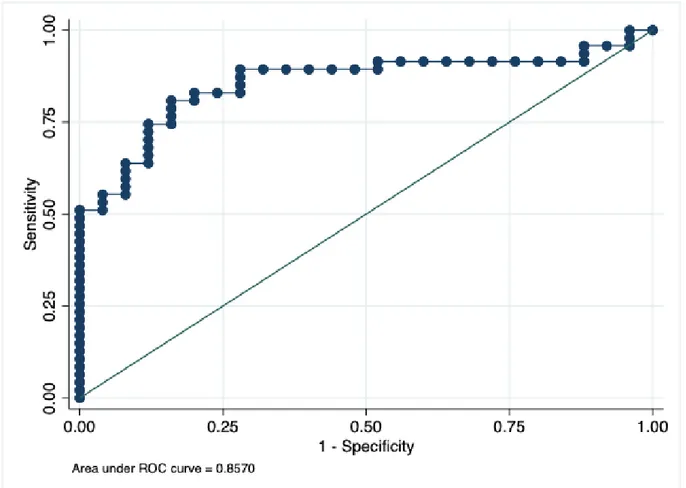
Nonparametric statistics were used for analytical statistical tests. Firstly, the significance of the differences between the DCPM and non-DCPM group regarding the presence and grade of clinical as well as psychosocial variables was analyzed using a Mann Whitney U-test (two-sample Wilcoxon’s test) as a post-hoc analysis (see Table 2). Then the relations between clinical and psychosocial variables were calculated in the whole patient group, as well as in the DCPM group, using Spearman’s rank correlation. Further, a regression analysis was performed using the significant relations from the univariate correlations to assess what combination of variables that best could predict the presence or absence of a disturbed central pain modulation. This was conducted using a multiple logistic regression. An area under the receiver operator characteristic (ROC) curve of >0.70 was considered as a strong explanatory effect of the independent variables.
A probability value of P < 0.05 was considered as significant. Spearman’s Rank Correlation Coefficient (rs) was used to measure strength of relations in the univariate correlations as well as in the multiple logistic regressions. Stata 13.1 was used for the statistical analyses.
ROC-curve
During the past four decades, ROC analysis has become a popular method for evaluating the accuracy of medical diagnostic systems. The most desirable property of ROC analysis is that the accuracy indices derived from this technique are not distorted by fluctuations caused by the use of arbitrarily chosen decision criteria or cut-offs. In other words, the indices of
accuracy are not influenced by the decision criterion (i.e. the tendency of a reader or observer to choose a specific threshold on the separator variable) and/or to consider the prior
probability of the "signal". The derived summary measure of accuracy, such as the area under the curve (AUC) determines the inherent ability of the test to discriminate between the
diseased and healthy populations. Using this as a measure of a diagnostic performance, one can compare individual tests or judge whether the various combination of tests (e.g.
combination of imaging techniques or combination of readers) can improve diagnostic accuracy(71).
Ethical considerations
The data from the patients were collected during the routine clinical examination at the specialist clinic, i.e. no extra assessments or examinations were needed. In patients, the examination procedure may cause brief and minor pain in the masticatory system. However, there was therefore no added risk for the patients to participate in the project compared to the routine examination. The project has an ethical permission, which required consent from each
participating patient/healthy individual. These consents have been obtained for the included patients and data from the individuals that did not consent were excluded.
RESULTS
Table 2 shows the clinical and psychosocial variables in patients with or without DCPM. Table 3 and Table 4 show the significant relations between clinical variables and psychosocial variables in all patients and in the patients with DCPM, respectively. The outcome from the logistic regression model is presented in Figure 4.
Table 1
With DCPM Without DCPM Percentiles Percentiles Median 25th 75th n Median 25th 75th n Age years 38 25 55 54 25 23 61 32 Sex m/w 9/45 11/21Orofacial pain duration years 3.0 2.0 7.8 48 4.0 1.0 7.0 25
Diseases Fibromyalgia # 9 0 Rheumatic disease # 11 3 DC/TMD diagnoses Myalgia # 46 24
Myofascial pain with
referral # 41 16 Arthralgia # 21 11 Headache attributed to TMD # 17 6
TMD = temporomandibular disorders. Myalgia and headache attributed to TMD are reported per patient, arthralgia and degenerative joint disease are reported per joint, i.e. more than one observation per subject, n = number of observations; m/w = number of men (m) and women (w).
Demographic data of 86 patients with chronic orofacial pain, with or without disturbed central pain modulation (DCPM)
n = number of observations, p = probability value, Characteristic pain intensity (0-10) from the Graded Chronic Pain Scale (GCPS), Pain-related Disability score (0-10) from the Graded Chronic Pain Scale (GCPS),
Depression (0-27) from the patient Health Questionnaire-9 (PHQ-9), Anxiety (0-21) from the Generalized Anxiety Disorder Assessment-7 (GAD-7), Stress (0-40) from the Perceived Stress Scale-10 (PSS-10), Unspecific physical symptoms (0-30) from the Patient Health Questionnaire-15 (PHQ15), as well as Number of sites with referred pain (0-12) from the DC/TMD clinical examination.
Table 2
WITH DCPM WITHOUT DCPM Percentiles Percentiles Median 25th 75th n Median 25th 75th n P Clinical variablesMouth opening capacity mm 51 46 55 53 48 45 54 30
Number of sites with referred pain 0-12 2 1 4 54 1 0 2 31 0.004
Characteristic pain intensity 0-10 6.0 5.3 8.0 54 5.5 4.7 7.1 32
Unspecific physical symptoms 0-30 11 8 16 54 7 5 12 32 0.001
Psychosocial status Pain-related disability 0-10 3.2 0.8 5.6 54 2.2 0.3 4.4 32 Depression 0-27 8 5 15 54 8 2 12 32 Anxiety 0-21 4 2 9 54 4 2 7 32 Stress 0-40 16 9 22 54 14 8 17 32
Clinical and psychosocial variables in 86 patients with chronic orofacial pain, with or without disturbed central pain modulation (DCPM)
Table 3
Correlation
rs n P
Characteristic pain intensity
Pain-related disability 0.62 86 <0.001
Depression 0.39 86 <0.001
Stress 0.25 86 0,021
Unspecific physical symptoms
Stress 0.69 86 <0.001
Pain-related disability 0.43 86 <0.001
Depression 0.65 86 <0.001
Anxiety 0.50 86 <0.001
Sites with referred pain
Depression 0.29 85 0.008
Pain-related disability 0.24 85 0.024
Maximal mouth opening without pain
Pain-related disability -0.30 83 0.007
Maximal mouth opening
Pain-related disability -0.24 83 0.028
n = number of observations, rs = Spearman’s Rank Correlation Coefficient, p = probability value. Significant correlations between clinical and psychosocial
Table 4
Correlation
rs n P
Characteristic pain intensity
Pain-related disability 0.58 54 <0.001
Depression 0.34 54 0.013
Unspecific physical symptoms
Stress 0.77 54 <0.001
Pain-related disability 0.35 54 0.009
Depression 0.67 54 <0.001
Anxiety 0.51 54 <0.001
Sites with referred pain
Depression 0.30 54 0.030
______________________________________________________________
n = number of observations, rs = Spearman’s Rank Correlation Coefficient, p = probability value. Significant correlations between clinical and psychosocial variables in the
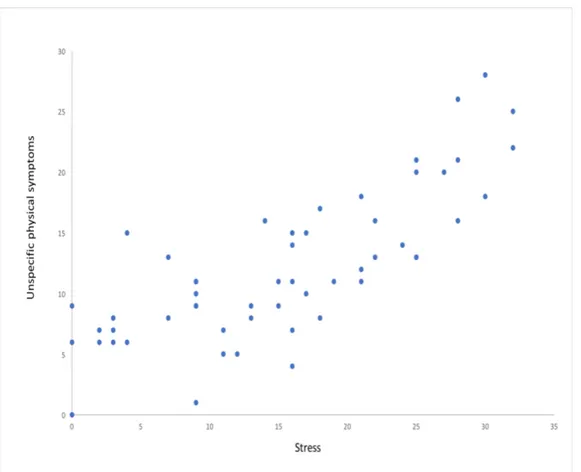
Figure 4. Degree of unspecific physical symptoms was significantly correlated to stress. The diagram describes
Logistic regression
In the multivariate regression model, the combination of the independent variables physical symptoms, stress, pain duration, characteristic pain intensity, pain-related disability, number of sites with referred pain, maximum mouth opening with and without pain, number of jaw movements causing orofacial pain and anxiety significantly predicted DCPM (LR Chi2 =
26.89, p = 0.003 and Pseudo R2 = 0.29; Figure 5.).
Figure 5. Area under ROC curve = 0.8570 indicates that the model based on the combination of the included
DISCUSSION
This study indicates that patients with DCPM show a higher number of unspecific physical symptoms and a higher number of palpatory sites that elicit referred pain. It also indicates that an underlying disturbed central pain modulation is associated with the presence of stress, a prolonged pain duration, characteristic pain intensity, level of pain-related disability, physical symptoms, anxiety, sites with referred pain, maximum mouth opening with and without pain and number of jaw movements causing orofacial pain.
The multivariate logistic regression analysis showed that the following specific factors: stress, prolonged pain duration, characteristic pain intensity, pain-related disability, degree of diffuse physical symptoms, anxiety, number of sites with referred pain, maximum mouth opening with and without pain, and number of jaw movements causing orofacial pain may indicate a disturbed central pain modulation (DCPM) in patients with orofacial pain. Whether these variables are causative factors, maintaining factors or a consequence of DCPM is not possible to tell from our results. These findings suggest that psychosocial factors are associated with DCPM. However, when the psychosocial symptoms were looked at separately, no significant correlation to DCPM could be found in this study. This sets it apart from earlier studies that have shown relations between psychosocial factors and DCPM(34,72).
Hall et al(34) found that depression and stress were common consequences of pain, as well as important markers for development of debilitating consequences of pain, such as
chronification of pain and unspecific physical symptoms. Anxiety, interestingly enough, did not seem to contribute to these disabilities, according to their study. However, depression and stress were demonstrated to play a role in pain experience, where depression was
characterized by loss of self-esteem and decreased incentive, which potentially lowers activity and in turn affects pain related disability. Stress, on the other hand, was seen as persistent high tension, lower threshold for getting upset and irritability(34). In contrast to this study as well as the study performed by Hall et al, associations between the trait of anxiety and DCPM have been found in other studies. Clark et al. showed in their recent study, comprising patients with chronic back pain, that greater symptoms of anxiety and personalities with defensive anxiety traits showed heightened sensitivity in the participants and were highly associated with DCPM(72).
From the entire material used in this study, as well as in the DCPM group alone, the degree of depression was related to the number of sites with referred pain. Anxiety, stress, and
depression were all related to the degree of unspecific physical symptoms. Degree of unspecific physical symptoms and number of sites with referred pain were both associated with the presence of DCPM. Hence, our study still supports an association between DCPM and these psychosocial factors.
According to the regression model, pain duration, i.e. chronification of pain, not in itself but in combination with several other variables, is an indicator of DCPM. Chronic pain is tightly associated with central changes in the somatosensory system. Even though the relationship between chronic pain and disturbance of central pain modulation is not yet understood in detail, nor is it far from always evident on an individual level, the consensus is that the longer
the pain duration, the higher the risk of neural plasticity. The other way around seems to be true as well. More neural plasticity would elevate the risk of unceasing pain(47,56). The finding that patients with DCPM showed more unspecific physical symptoms was not surprising since it is consistent with the current literature covering this field of subject(41,73). This association could be explained by the hyperexcitability of CNS, which is a key factor in DCPM. Neuroplastic changes in other central regions than the somatosensory ones, such as limbic structures, may in part explain the unspecific physical symptoms in various locations and does not only have to include pain, but may also include insomnia, dizziness, heart palpitation, shortness of breath, etc.(74). Limbic structures are tightly connected with several somatosensory regions, and are readily activated during pain sensations. The limbic structures are important for setting emotional states, through hypothalamic homeostatic control, and thus, structural changes in these regions could explain parts of the unspecific physical symptoms in patients with DCPM(15).
This is one of few studies that have analyzed referred pain, as assessed in the DC/TMD, in relation to the presence of DCPM. According to the results in this study, clinical assessment of presence and magnitude of referred pain may be of importance to classify patients more precisely as well as for diagnosis and treatment planning.
The patients in the DCPM-group had more sites with referred pain to palpation than non-DCPM patients. This finding is in line with several previous studies,(47,75) but has not yet been thoroughly looked at before the present study. The number of secondary nociceptive fields, i.e. locations with referred pain has long been argued to be higher in patients with TMD than healthy individuals,(75) especially in women. TMD pain disorders have also been associated with a central disturbance in pain modulation(47). From this, one could draw the conclusion that the phenomenon leading to an increased number of referred pain sites might share a common pathogenesis with disturbance of central pain modulation. Another possible explanation would be that an underlying disturbed pain modulation would enhance the pain sensation from referred sites, just as it has been shown to increase it from trigger points. Hence, the central sensitization would merely facilitate the identification of secondary nociceptive fields, which would be present anyway. As from a more conservative view, a combination of these two mechanisms could also explain our findings.
Methodological considerations
The patient pool used in this study was unevenly distributed gender-wise, with a larger proportion of female participants (three to one). This is especially evident in the group of DCPM, which consisted of five times more women than men. This distribution is however reflected in studies covering the epidemiology of TMD, where the prevalence for women seems to be somewhere around 1,5 to 2 times greater than that for men(76,77). The
epidemiology of disturbed central pain modulation gender-wise, is not clear however, which might be, at least in part, due to shifting diagnostic terms and criteria. Fibromyalgia, which is known to be associated with DCPM, presents a prominent predilection for women(78,79). In a study released 2010 concerning chronic orofacial pain, Aggarwal et al found that women were significantly more likely to develop chronic pain in the oro-facial area than men