Grafting materials for alveolar
cleft reconstruction
- a systematic review
Pegah Mirdamadian
Raha Salahshour Nargi
Supervisors: Manjula Herath, Aron Naimi-Akbar, Jonas Becktor
Department of Oral and Maxillofacial Surgery and Oral Medicine
Master Thesis in Odontology (30 ECTS)
Malmo University
Dentistry Program
Faculty of Odontology
2
Abstract
Aim: The aim of this literature study was to systematically review the scientific evidence on
the most effective donor sites and/or bone substitute material for secondary alveolar cleft grafting in alveolar cleft patients.
Material and method: In order to acquire a systematic and transparent reporting this literature review was conducted according to the PRISMA statement. The literature search was
performed in the following four databases; PubMed, CENTRAL, Web of Science and Scopus. The quality of the included studies was assessed using the revised Cochrane Risk of Bias 2 tool (RoB 2 tool).
Result: The search identified 4754 studies. Five RCT studies was included in this systematic review and assessed different donor site or bone substitute materials. Two studies showed low risk of bias and three moderate risk of bias. Only one study showed a statistically significant difference when comparing iliac bone to substitute material however all studies presented substitute materials with satisfactory results.
Conclusion: According to the data from this systematic review no clear conclusion can be drawn regarding what the most effective bone donor site and/or tissue engineered bone substitute material to use in secondary bone grafts. Based on the available evidence iliac bone could still be regarded as a benchmark, but more research and RCT’s of high quality are required, especially for artificial bone substitute materials.
Sammanfattning
Syfte: Syftet med denna litteraturstudie var att systematiskt granska den vetenskapliga
evidensen gällande det mest effektiva bentagningsstället och/eller bensubstitutmaterialet vid sekundär bentransplantation hos patienter med käkspalt.
Material och metod: För att uppnå en systematisk och transparent rapportering av denna litteraturstudie följdes PRISMA statement. Litteratursökningen gjordes i följande fyra databaser; PubMed, CENTRAL, Web of Science och Scopus. Kvaliteten av inkluderade studier granskades med hjälp av Cochrane Risk of Bias 2 tool (Rob 2 tool).
Resultat: Sökningen identifierade 4754 studier. Fem RCT studier inkluderades i denna systematiska översikt vilka värderade olika bentagningsställen eller bensubstitut. Två studier bedömdes ha låg risk för bias och tre artiklar måttlig risk för bias. Endast en studie visade på en statistiskt signifikant skillnad vid jämförelse av höftben med bensubstitut däremot
presenterade samtliga studier substitutmaterial med tillfredsställande resultat.
Konklusion: Denna systematiska översikt visade att ingen klar slutsats kan dras gällande vilken det mest effektiva bentagningsstället eller bensubstitutsmaterialet är för sekundär bentransplantation hos patienter med käkspalt. Baserat på tillgänglig evidens kan transplantat från höftbenet fortfarande anses vara bäst lämpat men mer forskning samt RCT studier av hög kvalité erfordras, särskilt för artificiella bensubstitutmaterial.
3
Table of Contents
1. Introduction ... 4
1.1Cleft lip and/or palate ... 4
1.2 Embryology and classification ... 5
1.3 Treatment ... 6
1.4 Aim and issue ... 8
1.5 Clinical, societal and ethical importance ... 8
2. Material and method ... 8
2.1 Protocol and registration ... 8
2.2 Research specification ... 8
2.3 Eligibility criteria ... 8
2.4 Information sources ... 9
2.5 Search strategies ... 9
2.6 Study selection ... 10
2.7 Quality assessment and data extraction ... 10
3. Results ... 11
3.1 Literature search ... 11
3.2 Quality assessment and study characteristics ... 11
4.1 Methodological discussion ... 18 4.2 Result discussion ... 19 4.3 Conclusion ... 19 Acknowledgements ... 20 Funding ... 20 5. Reference ... 21 Appendix 1 ... 25 Appendix 2 ... 28 Appendix 3 ... 34 Appendix 4 ... 44
4
1. Introduction
1.1 Cleft lip and/or palate
Cleft lip and/or palate (CL/P) is a collective term used to describe an opening or division that is not anatomically normal, which arises early in pregnancy as a result of the improper fusion of soft tissue and/or bone which are required for normal facial development (1). The cleft can occur in novo (non-syndromic), be hereditary or part of a syndrome (syndromic). Available data worldwide indicates that the incidence approximately is 1 in 700 however with ethnic and geographical discrepancy (2).The incidence in Sweden is approximately 2.0/1000 live births corresponding to 200 children born per year with this congenital deformity (3). Cleft lip isolated or in combination with cleft palate is predominant in boys, while isolated cleft palate is more common in girls (4).
The condition has a multifactorial etiology, no individual causal factor is known to trigger the development of the clefts although genetic and environmental factors (alcohol, smoking, folic acid deficiency…) can be causative. The etiology of non-syndromic forms on CL/P is still not well understood (5)
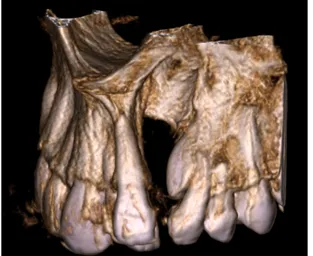
Alveolar cleft is a bony discontinuation of the alveolar bone that occur in approximately 75% of cleft lip and palate patients (6). This type of cleft is usually located between the lateral incisor and canine (Fig. 1, 2). Since the alveolar bone functions as a supportive structure for the facial development and the teeth a number of consequences have been reported. Because of the abnormal development of the alveolar process dental anomalies like agenesis,
supernumerary teeth, peg-shaped lateral incisor as well as rotated teeth adjacent to the cleft occur. There is also evidence of delayed eruption in cleft patients (7). Oronasal fistulae, the communication between the oral and nasal cavity is also reported and can cause speech disturbances (8). Due to the fact that CL/P affects the appearance children may be stigmatized and several studies has reported a psychosocial association with the condition (9).
An additional type is the submucosal palatal cleft type which is a cleft in muscle tissue with an overlying intact mucosa. The mucosal coverage often results in this cleft being unnoticed during the neonatal period and discovered when the child starts talking as a nasal emission occurs due to velopharyngeal insufficiency becomes evident and the speech becomes nasal (10).
Figure 1. 3D-model of alveolar cleft between the lateral incisor and canine.
By: Manjula Herath
Figure 2. Axial CBCT x-ray of alveolar cleft between lateral incisor and canine.
5
1.2 Embryology and classification
During the development of the face there are two important structures involved – the pharyngeal arches and the neural crest cells. The pharyngeal arches arise during the fourth week of embryonic development and are precursors for many structures. The neural crest cells derive from the neuroectoderm and are specialized cells that contribute to the mesenchyme of the head (ectomesenchyme) (11).
By the end of embryonic week 4, five facial prominences will develop around the primitive mouth, the stomatodeum, which occurs as a result of the proliferation of mesenchymal cells of neural crest origin. The unpaired frontnasal prominence forms the forehead, nose and
midsection of the upper lip. The bilateral maxillary prominences will form the maxilla, lateral aspects of the upper lip and the palate and the bilateral mandibular prominences give rise to the mandible and the lower lip. Normal facial development occurs as the result of the fusion between the five prominences between week 5 and 12 of pregnancy, clefts are the result of the absence of fusion (12).
Development of clefts
The palate begins to develop during week 5 and is not completed until week 12, the most critical time is between weeks 6 and 9 (12). From the frontnasal prominence originates the median palatine process which gives rise to the primary palate from where the lip, alveolar bone and anterior hard palate will develop. Disruptions during this period can result in clefting of the primary palate and causes lip and/or alveolar clefts.
From the maxillary prominences the lateral palatal processes will grow and form the
secondary palate, which consists of the hard palate posterior to the nasopalatine foramen and the soft palate. Disruptions in fusion during this time of merging results in palatal clefts (13).
Classification
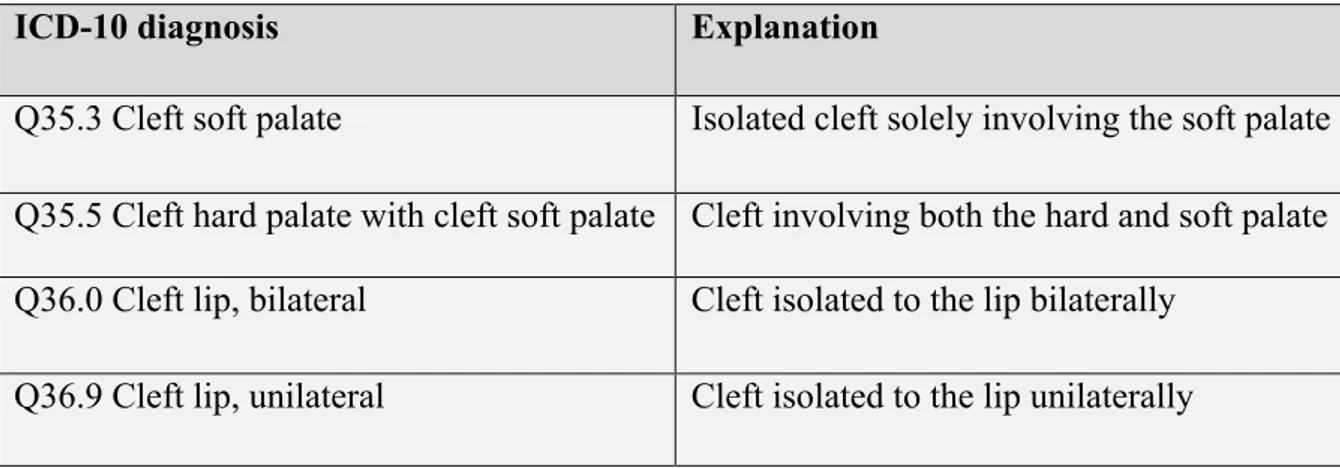
Cleft types are classified depending on where the cleft is located. Through time there has been numerous classification systems for recording cleft lip and palate malformations (14). In table 1 we present a classification according to ICD-10 (International Statistical Classification of Disease and Related Health Problems – Tenth Revision) published by the World Health Organization (WHO), following cleft-diagnoses are registered in the Swedish cleft lip and palate registry (15,16).
Table 1. Classification of the subclasses of CL/P with explanation of location according to ICD-10.
ICD-10 diagnosis Explanation
Q35.3 Cleft soft palate Isolated cleft solely involving the soft palate Q35.5 Cleft hard palate with cleft soft palate Cleft involving both the hard and soft palate Q36.0 Cleft lip, bilateral Cleft isolated to the lip bilaterally
6
1.3 Treatment
Management of cleft lip and palate patients require a multispecialty approach with qualified personnel (17). In Sweden the team typically involves a plastic surgeon, an orthodontist, a speech therapist, a maxillofacial surgeon and ENT-specialists. Other professions as psychologists may also be included (18).
Cleft lip and palate can be diagnosed prenatally with ultrasound. In these cases, parents can meet the treatment team before the delivery of the child and receive information. After birth, feeding the infant can be difficult due to poor intraoral suction. Special bottles are used in these cases. Early interventions include treatment with retrusion tape, stent and palatal molding to optimize the tissue positions presurgical (17,18).
There are multiple approaches for surgical treatment of cleft lip and palate patients, and it differs between practices (18). The euro cleft project which compared surgical approaches of different practices between 1996-2000, registered 201 centers and 194 different approaches (19) Alveolar cleft surgery can be separated into early intervention called boneless bone grafting (3 months) (20), early secondary (2-5 years, primary dentition), and secondary repair which is the widely accepted treatment (approximately 8-12 years, after the eruption of centrals but before canine eruption) (13).
Secondary surgical procedure is performed on patients with mixed dentition (17,21). The ideal time is when the canine root is approximately 1/3 developed, at the age of 9-12
according to El Deeb et al (22). Surgery at earlier stages might affect the development of the maxilla and is therefore not recommended. During secondary alveolar bone grafting, the cleft is surgically exposed and bone grafting is performed (21). The goal is to provide a bony support for the development of a normal occlusion, a stable palate, bony base for the nose, and improved esthetics (17,21). Fig. 3-8 demonstrates the steps of secondary alveolar bone grafting with iliac bone.
The most common and preferred donor site for bone grafting is iliac cancellous bone, because it is easy to harvest and has a high osteoinductive potential. In more recent studies iliac cancellous bone is incorporated with platelet rich plasma, with the goal to enhance
osteogenesis during the grafting procedure. PRP is a good source for growth factors and can be extracted from the autologous whole blood during surgery (23). Other possible donor sites are the cranium, tibia, mandibular ramus and symphysis (20). However, it is important to know that harvesting autogenous bone blocks is followed by risks such as post-operative donor site morbidity, post-operative pain and increased blood loss as well as infections (24,25)
Q37.2 Cleft soft palate with bilateral cleft lip
Bilateral cleft lip with incomplete palate Q37.3 Cleft soft palate with unilateral cleft
lip Unilateral cleft lip with incomplete palate
Q37.4 Cleft hard and soft palate with bilateral cleft lip
A bilateral cleft engaging lip, alveolar bone and palate
Q37.5 Cleft hard and soft palate with
7
Other sources of bone graft material are allogeneic bone-graft substitutes such as deproteinized bovine bone matrix (DBBM) as well as alloplastic materials such as
hydroxyapatite (HA) and tricalcium phosphate (TCP), which are used as autologous grafts for reconstruction of alveolar clefts (20). Recombinant human bone morphogenetic protein-2 (rhBMP-2), an osteogenic agent, in combination with demineralized bone matrix has been used as an alternative for cleft repair (26).
Figure 3. Alveolar cleft located in quadrant one with displaced teeth
Photo: Manjula Herath
Figure 4. Marking of alveolar cleft area for surgery
Photo: Manjula Herath
Figure 5. Harvesting bone from the iliac bone for grafting of alveolar cleft
Photo: Manjula Herath
Figure 6. Blocks of harvested iliac bone
Photo: Manjula Herath
Figure 7. Bone grafting with harvested iliac bone in the alveolar cleft quadrant 1
Photo: Manjula Herath
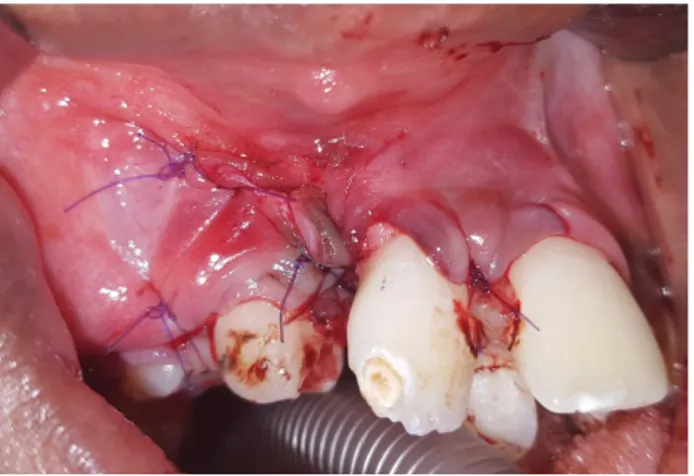
Figure 8. Periostal flap repositioned and sutured after grafting
8
1.4 Aim and issue
The aim of this literature study was to systematically review the scientific evidence on donor sites and bone substitute for secondary alveolar cleft grafting in alveolar cleft patients.
Research question
What is the most effective bone donor site and/or tissue engineered bone substitute material to use in secondary bone grafts in alveolar cleft patient?
1.5 Clinical, societal and ethical importance
Secondary alveolar bone is a widely accepted treatment by professionals but there is no universal consensus regarding type of bone, donor site and need for bone substitute. The latest Cochrane report on the topic was published in 2011 and included two trials, the authors concluded that due to high risk of bias the evidence is insufficient to conclude that one intervention is superior another (27).
Systematic reviews are important to identify, evaluate the current evidence and summarize available scientific studies in order to e.g. assess the best treatment of choice and make it accessible for health care workers. A systematic review of high quality follows a strict design based on a pre-specified and reproducible method and if done well provide reliable data on effects of different interventions. Besides this, systematic reviews can also highlight potential knowledge gaps which can be used to plan further research (28). This particular systematic review also captures the societal perspectives of alveolar bone grafts as it could help to lower costs in the health sector.
2. Material and method
2.1 Protocol and registration
A priori protocol for this systematic review was registered in PROSPERO (registration number CRD42020197451).
2.2 Research specification
In order to formulate a structured and focused research question in terms of patient, intervention, control group and clinical outcome PICO was used (29).
What is the most effective bone donor site and/or tissue engineered bone substitute material to use in secondary alveolar bone grafts in alveolar cleft patients?
2.3 Eligibility criteria
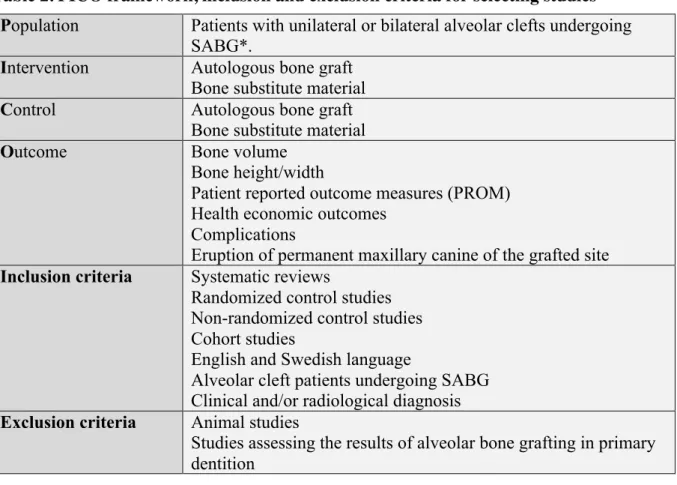
Studies included in this systematic review were randomized controlled trials (RCT), non-randomized controlled trials (N-RCT), cohort studies, systematic reviews and meta-analysis Eligible studies were selected according to PICO and inclusion/exclusion criteria as presented in Table 2.
9
Table 2. PICO framework, inclusion and exclusion criteria for selecting studies
*SABG will be defined as receiving alveolar bone grafting at stages later than the primary dentition, including those undergoing surgery in the mixed dentition and mature dentition stages. Studies assessing the results of alveolar bone grafting in the primary dentition will be excluded.
2.4 Information sources
An electronic search was performed in the following separate databases; PubMed, CENTRAL (Cochrane Central Register of Controlled Trials), Scopus and Web of Science until the 12th of
August. No filters were used in the search. In order to acquire a systematic and transparent reporting this literature review was conducted according to the PRISMA statement (30) (Appendix 1).
2.5 Search strategies
A main search strategy was developed for PubMed in collaboration with a university librarian this strategy was then adapted to the remaining databases. (Table 3) For PubMed and
CENTRAL, a combination of MeSH-terms and free text was used and for Scopus and Web of Science exclusively the free text was adopted. Titles and abstracts retrieved were imported to EndNote (Reference Management software) for removal of duplicates. Reference
management was carried out with the aid of Rayyan, a web and mobile app for systematic reviews (31).
Population Patients with unilateral or bilateral alveolar clefts undergoing
SABG*.
Intervention Autologous bone graft
Bone substitute material
Control Autologous bone graft
Bone substitute material
Outcome Bone volume
Bone height/width
Patient reported outcome measures (PROM) Health economic outcomes
Complications
Eruption of permanent maxillary canine of the grafted site
Inclusion criteria Systematic reviews
Randomized control studies Non-randomized control studies Cohort studies
English and Swedish language
Alveolar cleft patients undergoing SABG Clinical and/or radiological diagnosis
Exclusion criteria Animal studies
Studies assessing the results of alveolar bone grafting in primary dentition
10
2.6 Study selection
The list of publications after duplicate removal was divided between two independent groups, group 1 (ANA, PM, RS) and group 2 (MH, JB). Each reviewer in the groups excluded
irrelevant articles based on title and abstract, uncertainties regarding including a publication based on title and abstract were retained until the following step: full-text screening. Studies for full-text screening was divided between group 1 and 2, all potentially eligible studies were read in full-text by each review respectively, any disagreement was discussed in the separate groups until consensus decision was reached. Studies were included if fulfilled PICO and inclusion and exclusion criteria. Excluded articles read in full-text were recorded and will be referenced in this review. If enough applicable studies of sufficient quality a meta-analysis was planned be performed.
Table 3. Search strategy in PubMed, CENTRAL, Web of Science and Scopus
Due to time constraints, it was agreed at this stage to only proceed with RCT’s for this master thesis. This systematic review will be further developed for publication and will include RCT, non-RCTs, cohort studies and systematic reviews as planned.
2.7 Quality assessment and data extraction
The Cochrane risk of Bias 2 tool was developed 2008 for assessing the quality of RCT studies. The tool was revised 2019 and the revised Risk of Bias 2 tool (RoB2 tool) was developed (32). The tool consists of domains of bias where each focus on different aspects of the studies; randomization process, intended intervention, outcome data and reported result. Each domain present questions aiming at different features of the studies relevant for judging the risk of bias of that particular domain which is expressed low, high or some concern. (Appendix 2) Lastly the overall risk of bias for the study is decided by adding the risk of
Database Indexing terms Abstract (n)
PubMed (((”Cleft Palate” [Mesh]) OR (”Cleft Lip” [Mesh]))) OR ((“cleft lip”)) OR (“cleft palate”)) OR (cleft)))
AND
(((“Alveolar Ridge Augmentation”[Mesh]) OR
(“Alveoloplasty”[Mesh]) OR (“Surgery, Oral”[Mesh]) OR (“Alveolar Bone Grafting”[Mesh])) OR ((Alveoloplasty) OR (“oral surgery”) OR (“alveolar bone grafting”) OR (“bone graft”))) 1 664 CENTRAL 111 Web of Science 753 Scopus 2226 Total 4754
11
biases of all domains (33). For this systematic review quality assessment was carried out in concordance by four reviewers (ANA, MH, PM, RS).
3. Results
3.1 Literature search
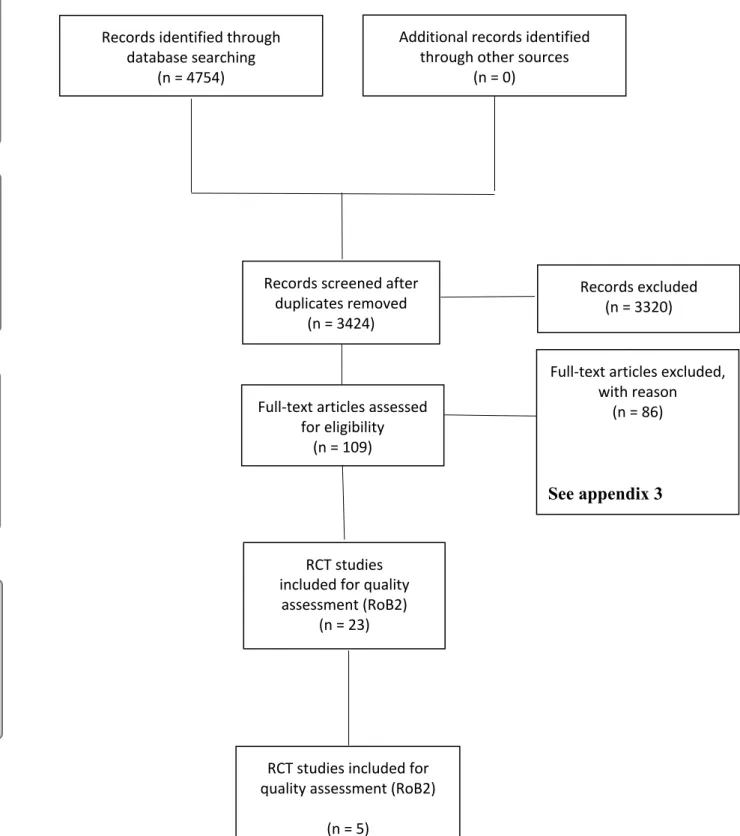
The literature identification yielded a total of 4754, the number of publications retrieved from each data base is presented in table 3. A flow chart of the screening process for systematic reviews according to PRISMA is presented in Fig. 9. After duplicate removal 3424 articles remained and after title-abstract screening a total of 109 publications were assessed for full-text screening. A total of 86 studies were excluded during the full-full-text assessment and are presented in Appendix 3. The most common reason for excluding a study was wrong study type.
In February 2021 an update on the PubMed (table 3) search was made, which resulted in 77 new title and abstracts, none of which met the inclusion criteria.
3.2 Quality assessment and study characteristics
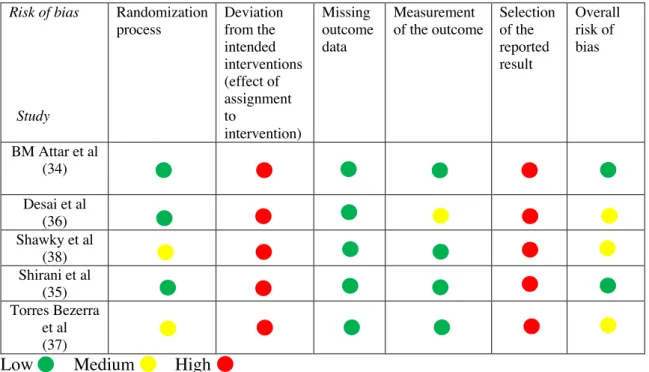
Quality assessment using RoB-2 tool was performed on 23 RCT’s, which identified 2 studies of low risk of bias (B.M Attar et al. 2017; Shirani et al. 2017) (34,35) and 3 studies were considered having some concern (Desai et al. 2019; Torres Bezerra et al. 2019; Shawky et al. 2016) (36–38). In the remaining 18 studies a high risk of bias was found. The most common reason for high risk of bias was because poorly described or unclear randomization process. Data extraction with the key characteristics for each of the 5 included studies are presented in Table 5 (reference number here). Studies excluded based on high risk of bias is presented in Appendix 4. Quality assessment of some concern and low risk of bias studies are presented in Table 4.
As the results from the included studies were not comparable no meta-analysis was undertaken.
Table 4 Risk of bias within each study according to the revised RoB-2 tool
Low Medium High Risk of bias Study Randomization process Deviation from the intended interventions (effect of assignment to intervention) Missing outcome data Measurement of the outcome Selection of the reported result Overall risk of bias BM Attar et al (34) Desai et al (36) Shawky et al (38) Shirani et al (35) Torres Bezerra et al (37)
12
PRISMA 2009 Flow Diagram
Figure 9. PRISMA flow diagram presentering the search steps with reasons for exclusion
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal. pmed1000097
For more information, visitwww.prisma-statement.org
Records identified through database searching
(n = 4754)
Additional records identified through other sources
(n = 0)
Records screened after duplicates removed
(n = 3424)
Records excluded (n = 3320)
Full-text articles assessed for eligibility
(n = 109)
Full-text articles excluded, with reason
(n = 86)
See appendix 3
RCT studies included for quality
assessment (RoB2) (n = 23)
RCT studies included for quality assessment (RoB2)
(n = 5) In cl u d ed E li gi b il it y Sc re eni ng Id en ti fi cat ion
13 First author, year, country, reference Population Study group material Control group material
Outcome Follow up Main findings Adverse effects Conclusion
BM Attar et al, 2016, Iran (34) n: 10 study group 10 control group Age: 8-14 years Mandibular symphysis (2/3 autologous, 1/3 allogeneic) and L-PRF
Iliac bone - Volume of residual alveolar defects (cm3) -Volume of regenerated bone (cm3) -Percentage bone reconstruction (%) 1 week, 1 mo., 3 mo., 6 mo., 12 mo. Study group Preop volume: 0.89 +0.29cm3 After 12mo: 0.28+0.15cm3 Volume of new bone
formed/%bone reconstruction: 0.61+0.19cm3 / 69.57%+10.13%
Control group
Preop volume: 0.95+0.27cm3 After 12mo: 0.26+0.13cm3 Volume of new bone
formed/%bone reconstruction: 0.69+0.19cm3 / 73.86%+6.93%
Temporary sensory
complications in chin and lower lip in study group with resolution within 3 mo. Study could walk normally the day after surgery. Control group mean time needed before walking normally 5.9 + 2.1 days Satisfactory results, fistula repaired. No statistically significant difference between the groups.
Desai et al, 2019, India (36) n: 20 study group /20 control group Age: 9-18 years Anterior iliac bone + PRF Anterior iliac bone Bone resorption (postop vertical bone height in relation to interdental bone height) 4-point scale: Immediate postoperative, 3 mo., 6 mo., 9 mo. Study group:
3 mo. 14 patients grade 1
After 6mo and 9mo – 19 patients grade 1 resorption, 1 patient grade 2.
Control group:
3 mo. – 16 patients grade1,
1 patient (5%) in study group and control group 6 patients (30%) showed serious discharge. No significant difference between groups at 3 and 6mo, but significant after 9 mo.
PRF favors reduced bone resorption and
14 Grade 1: 0–25 % bone resorption Grade 2: 25–50% Grade 3: 50–75% Grade 4: 75– 100% 1 patient grade 2
6 mo. 14 patients grade 1. 6 patients grade 2
9 mo. 12 patients grade 1, 8 patients grade 2. Dehiscence 4 patients study group (20%), 8 patients (40%) in control group. improved wound healing. Shirani et al, 2016, Iran (35) n: 8 study group 8 control group Age: study group mean age 10.6 years, control group mean age 10.4 years Iliac + PRGF FDBA + PRGF Volume of regenerated bone (cm3)
6 mo. Study group:
- baseline volume cleft before surgery (v1) – 1.3 cm3
- mean regenerated bone volume 6 months (v2) – 1.0 cm3
-v2/v1 (ratio of bone formation) = 0.7 (70%)
Control group:
- baseline volume cleft before surgery (v1) – 1.2 cm2
- mean regenerated bone volume 6 months (v2) – 0.6 cm3
-v2/v1 (ratio of bone formation) = 0.5 (50%) 1 patient in study group excluded due to dehiscence and infection. Iliac + PRGF resulted in scar at surgical site Iliac bone in combination with PRGF was more successful.
15 Shawky et al, 2000, Egypt (38) n: 12 study group 12 control group Age: 9-14 years Anterior iliac bone + PRF Anterior iliac bone - Volume of residual alveolar defects(cm3) -Volume of regenerated bone (cm3) -Percentage bone formation (%) -Density (Hus) of newly formed bone
6 mo. Study group:
- baseline volume of defect: mean 0.96 + 0.259 cm3
- final volume of defect: mean 0.18 + 0.08 cm3
-Mean amount of newly formed bone volume 0.78 cm3.
-82.6% new bone.
-Bone density – mean 360.82 HU
Control group:
- baseline volume of defect: mean 0.9 + 0.28 cm3
- final volume of defect: mean 0.275 + 0.05 cm3
-Mean amount of newly formed bone volume 0.62cm3.
-68.38 new bone %
-Bone density – mean 384.03 HU
No adverse effects
Percentage of newly formed bone in study group was statistically significant increase in comparison to the control group. Difference in bone density not statistically significant. Torres Bezerra et al, 2019, Brazil (37) n: 20 Control group: 10 Study group: 10 patients Autogenous bone graft from mandibular symphysis Bio-Oss associated with PRP - Mean volume of residual alveolar defects (mm) -Mean area of residual alveolar defect (mm)
1-year postop Study group:
Baseline area: 345.81mm2 Baseline volume: 374.88mm3 1year post op:
Area: 265.08mm2 Volume: 254.12mm3
No adverse effects
Significant reduction regarding mean area and mean volume within groups. No statistically significant difference between study and control group
16
Abbreviations: n, number om patients; L-PRF, leukocyte-rich-plateletal-rich-fibrin; mo., months pre-op, preoperative; PRF, platelet rich fibrin; FDBA, freeze-dried bone allograft; PRGF, plasma rich in growth factors; HU, Hounsfield scale; Bio-Oss, Deproteinized Bovine bone graft; PRP, platelet rich plasm
Mean age: Study group: 15.6 years Control group: 14.5 years Control group: Baseline area: 274.39mm2 Baseline volume: 277.87 mm3 1year post op:
Area: 132.72mm2 Volume: 96.19mm3
regarding mean area and mean volume.
17
This systematic review included results from five RCT’s, two articles of low and three articles of moderate risk of bias. Common to these articles was that they compared autogenous bone graft such as iliac bone or mandibular symphysis to a combination of autogenous bone with supplementary material or a bone substitute material.
Majority of the studies used iliac bone as bone grafting material for either the study or control group. Torres Bezerra et al (37)was the only exception, although they also used autogenous bone graft but from the mandibular symphysis. Shirani et al (35)used a combination of iliac bone with PRGF for their study.
Anterior iliac bone with or without PRF/PRP
Shawky et al (38) and Desai et al (36) investigated the effect of adding PRF to iliac bone and compared it to solely iliac bone as a grafting material. Shawky et al only included patients with unilateral cleft in both study and control group, but Desai et al also included some patients with bilateral cleft in both groups. The biggest difference between the two studies was that use of different outcome measures. Shawky et al investigated volume of residual alveolar defect, volume of regenerated bone, percentage bone formation and density, while Desai et al measured bone resorption and wound healing.
Shawky et al reported positive results when PRF was added to iliac bone, when investigating the quantity of new bone formed. There was a higher percentage of new bone formation but when measuring bone density, the density was a bit lower in the study group, even though the difference was not statistically significant.
Desai et al also reported satisfactory results when using iliac bone + PRF. There was a significant difference when measuring bone resorption at 9 months, indicating that bone resorption was reduced when PRF was used. Additionally, less adverse effects were documented for the study group suggesting that wound healing was also improved.
Iliac bone versus other alternatives
Two of the studies included in this review compared iliac bone to other alternatives for bone grafting on patients with unilateral cleft. Attar et al used a combination of mandibular symphysis, allograft and L-PRF as intervention in the study group, and iliac bone in the control group. When measuring bone volume and percentage bone reconstruction both groups showed successful results, although the difference between the groups was very small and not significant. Patients where bone graft was harvested from iliac bone required approximately 5.9 days before they could walk normally. When grafting from the chin, 3 patients
experienced temporary sensory complications in chin and lower lip that was resolved within 3 months.
Shirani et al (35,37) presented results for both secondary and tertiary grafting but presented them separately, therefore results could be assessed for this review. The study group consisted of autogenous iliac bone with PRGF and the control group consisted of an allograft (FDBA) with PRGF. In both groups mean regenerated bone volume was close to the baseline volume cleft before surgery, but a higher ratio of bone formation was evident in the study group, which made iliac bone in combination with PRGF the more successful choice.
Mandibular symphysis as grafting material
Torres Bezerra et al (37) and Attar et al (34) both included bone grafting from mandibular symphysis in their study. As mentioned, Attar et al used a combination of grafting materials
18
in their study group, bone from mandibular symphysis included and compared to iliac bone. Torres et al study aim was to compare the outcome of autologous bone grafting from
mandibular symphysis to an artificial material called Bio-Oss in combination with PRP. At first, they included 28 patients in their study but then decided only 20 patients were eligible. No further explanation was given. They operated on patients with unilateral cleft, except for one patient in the study group that had a bilateral cleft which they recorded as 2 separate clefts and therefore registered the number of patients to study group as 11. Both alternatives demonstrated satisfactory results with significant reduction in mean area and volume within each group separately. When looking at the 1 year post operational volume and area
reduction, the change was bigger for the control group. Even though the difference was not statistically significant, Torres Bezerra et al indicated that Bio-Oss in combination with PRP was a good option for alveolar bone grafting material.
4. Discussion
4.1 Methodological discussion
In order to avoid missing important studies when doing a systematic review Cochrane is recommending using at least three different data bases for the search (39). The three databases considered most important are; CENTRAL Medline and Embase. The authors of this
systematic review did not have access to Embase therefore databases Web of Science and Scopus was also searched.
Of the total 23 RCT’s identified after full-text screening, 18 were excluded (Appendix 4), due to high risk of bias regarding the randomization process. Randomization means that the selection of patients for different interventions takes place in a random way and not based on patient characteristics, e.g. by coin tossing or sealed envelopes. The majority of the studies mentioned that the trial was randomized but no more than that. There was no detailed description of the sequence generation process.
Domain 2 of the RoB-2 tool assesses the risk of bias regarding effect of assignment to
intervention (Appendix 2). None of the included RCT’s achieved to fulfill the criteria to blind the patients and operators (Fig. 8). Since the five articles either included the iliac bone or mandibular symphysis both the study participants and the surgeons were aware of the intervention as bone is harvested, therefore for this systematic review this domain was regarded less relevant. There was also an overall high risk of bias on domain 5 assessing the risk of bias in selection of the reported result as none of the included studies mentioned any protocol or pre-specified analysis plan, but in relation to the aim of this systematic review and the general trend of absence of mentioned protocol it was regarded irrelevant. Based on this, articles with high risk of bias on one or both of these domains could still be evaluated as having some concern or low on the overall risk of bias. Domain 1 (randomization) was regarded the most important and in order for a study to be judged as low risk of bias this domain had to be estimated low.
The systematic review by Cochrane from 2011 on this topic included two RCT’s, “Dickinson et al, 2008 (40) and Segura-Castillo, 2005” (41) although considered as high risk of bias. None of these were included in this systematic review. The study by Dickinson et al was excluded due to high risk of bias because of unclear description of randomization. The publication by Seguro-Castillo was excluded during the title-abstract screening and it was decided to investigate why there was a discrepancy between the assessment done by Cochrane and this systematic review. The title that the authors based the exclusion on was “Technical
19
Strategies – Reduction of Bone Resorption by the Application of Fibrin Glue in the
Reconstruction of the Alveolar Cleft” and exclusion was motivated because the article was considered focusing on different techniques rather than different grafting materials. The study was assessed in full-text and met the criteria for quality assessment but was in the later step excluded due to inadequate description of randomization sequence.
4.2 Result discussion
Only one included study, Shirani et al (35) examined the use of allograft. In this particular study the allograft FDBA in combination with PRGF did not perform better than iliac bone and PRGF, the ratio bone formation in the iliac bone group was 70% and in the allograft group 50%. But because the advantages of using allografts such as avoid second operation site for harvesting bone, decreased surgical time, decreased risk of post-operational pain and recovery more studies on allografts need to be carried and possibly with combination other supplementary material other than PRGF.
Shawky et al (38) reported a statistically significant increase when combining PRF with iliac bone and Desai et al described that PRF reduced bone resorption and reduced healing. Desai et al did not report a statistically significant difference as Shawky et al did but the results point towards that combining iliac bone with either PRF and PRP is satisfactory and could be an alternative to solely using iliac bone. There is a need for more RCT studies of high quality on this topic in order to draw a conclusion but there is a low certainty that there is an effect. Complications to the intervention was documented in three of the studies. Attar et al (34) reported sensory complications when grafting was made from the chin symphysis as well as patients having problems with walking after iliac bone surgery. Shirani et al and Desai et al both reported complications such as dehiscence when iliac bone was used as a bone grafting material. Torres Bezerra et al (37) compared Bio-Oss in combination with PRP to autogenous material and reported reduction of mean area and mean volume of the cleft in both groups although the difference between the groups was not significant. Bio-Oss in combination with PRP showed favorable results and could be utilized as an alternative to autologous bone but more studies are needed and with larger sample groups.
Of the excluded articles in the full-text stage (Appendix 3), six where found to be on going trials which are only registered in Clinical Trials database (42). The authors of these will prior to publication be contacted in order to acquire possible preliminary results.
The main limitation when it came to assemble the results and constructing a meta-analysis was that the studies showed a substantial heterogenicity in regarding the selection of patients, interventions and outcome. Therefore, meta-analysis was precluded.
Because of language restriction this systematic review might have missed RCT’s that might have affected the outcomes and conclusions of this review.
4.3 Conclusion
According to the data from this systematic review no clear conclusion can be drawn regarding which is the most effective bone donor site and/or tissue engineered bone substitute material to use in secondary bone grafts. Based on the available evidence, iliac bone could still be regarded as a benchmark.
20
As mentioned previously the latest systematic review on the topic was published 2011 by Cochrane, although a decade has passed since no new evidence or considerable progress has been made in the field of secondary alveolar bone grafting. Of the 23 RCT studies that this systematic review yielded in, solely 5 studies passed the criteria of adequate quality for inclusion. With this said, RCT’s of high quality are required with respect to a clear
presentation of the randomization process as well as measurement of outcome data. Further, future studies should aim to limit age to 8-12 years old (mixed dentition) in order to avoid including patients with permanent dentition, as it could affect the results. Lastly, studies examining artificial bone substitute material is of interest to examine further with the prospect to replace autologous bone in the future.
Acknowledgements
We would like to thank Martina Vall at the library of Malmo University for excellent assistance with the search strategy.
Funding
21
5. Reference
1. LKG Registret. Vad är läpp-käk och gomspalt (LKG)? 21 September 2020. 2020. [cited 23 February 2021] Available from https://lkg-registret.se/patientinformation/vad-ar-lapp-kak-och-gomspalt-lkg
2. Mossey PA, Modell B. Epidemiology of Oral Clefts 2012: An International Perspective. Frontiers of Oral Biology. 2012;16.
3. Hagberg C, Larson O, Milerad J. Incidence of Cleft Lip and Palate and Risks of Additional Malformations. The Cleft Palate-Craniofacial Journal. 1998 Jan 15;35(1). 4. Akademiska Sjuhuset. Information om spaltmissbildningar. [cited 23 February 2021]
Available from https://www.akademiska.se/for-patient-och-besokare/ditt-besok/undersokning/lapp--kak--och-gomspalt/
5. Wong F, Hagg U. An update on the aetiology of orofacial clefts. Hong Kong Medical Journal; 2004 Oct 10 p. 331–6.
6. Cho-Lee G-Y, García-Díez E-M, Nunes R-A, Martí-Pagès C, Sieira-Gil R, Rivera- Baró A. Review of secondary alveolar cleft repair. Annals of Maxillofacial Surgery.
2013;3(1).
7. Ranta R. A review of tooth formation in children with cleft lip/palate.
American Journal of Orthodontics and Dentofacial Orthopedics. 1986 Jul;90(1). 8. Bajaj AK, Wongworawat AA, Punjabi A. Management of Alveolar Clefts. Journal of
Craniofacial Surgery. 2003 Nov;14(6).
9. Glener AD, Allori AC, Shammas RL, Carlson AR, Pien IJ, Aylsworth AS, et al. A Population-Based Exploration of the Social Implications Associated with Cleft Lip and/or Palate. Plastic and Reconstructive Surgery - Global Open. 2017 Jun;5(6). 10. Reiter R, Brosch S, Wefel H, Schlömer G, Haase S. The submucous cleft palate:
Diagnosis and therapy. International Journal of Pediatric Otorhinolaryngology. 2011 Jan;75(1).
11. Ulfig N. Embryologi: en kortfattad bok. 1st ed. Wilhelms DB, editor. Studentlitteratur AB; 2012.
12. Bernheim N, Georges M, Malevez C, A DM, Mansbach A. Embryology and epidemiology of cleft lip and palate. 2006;
13. Zreaqat MH, Hassan R, Hanoun A. Cleft Lip and Palate Management from Birth to Adulthood: An Overview. In: Insights into Various Aspects of Oral Health. InTech; 2017.
14. Khan M, Ullah H, Naz S, Iqbal T, Ullah T, Tahir M, et al. A revised classification of the cleft lip and palate. Canadian Journal of Plastic Surgery. 2013 Mar;21(1).
22
15. Becker M, Klinö K. LKG-registret: Nationella kvalitetsregistret för läpp-käk-gomspalt Malmö; 2016 Oct. [cited 24 February 2021] Available from
https://kvalitetsregister.se/hittaregister/registerarkiv/nationelltkvalitetsregisterforlappka kochgomspaltlkg.2314.html
16. World Health Orgnization (WHO). International Statistical Classification of Diseases and Related Health Problems - Tenth Revision. 2011. [cited 24 February 2021] Available from https://www.icd10data.com/ICD10CM/Codes/Q00-Q99/Q35-Q37 17. Nahai FR, Williams JK, Burstein FD, Martin J, Thomas J. The Management of Cleft
Lip and Palate: Pathways for Treatment and Longitudinal Assessment. Seminars in Plastic Surgery. 2005;19(04).
18. LKG Registret. DET NATIONELLA VÅRDPROGRAMMET FÖR LKG. 2020. [cited 25 February 2021] Available from
https://lkg-registret.se/patientinformation/det-nationella-vardprogrammet-for-lkg
19. Shaw WC, Semb G, Nelson P, Brattström V, Mølsted K, Prahl-Andersen B, et al. The Eurocleft Project 1996–2000: overview. Journal of Cranio-Maxillofacial Surgery. 2001 Jun;29(3).
20. Kang NH. Current Methods for the Treatment of Alveolar Cleft. Archives of Plastic Surgery. 2017 May 15;44(3).
21. Hogeman K, Jacobsson S, Sarnäs K. Secondary bone grafting in cleft palate: a follow up of 145 patients. Cleft Palate Journal. 1972 Jan;
22. el Deeb M, Messer L, Lehnert M, Hebda T, Waite D. Canine eruption into grafted bone in maxillary alveolar cleft defects. Cleft Palate Journal. 1982 Jan.
23. Oyama T, Nishimoto S, Tsugawa T, Shimizu F. Efficacy of platelet-rich plasma in alveolar bone grafting. Journal of Oral and Maxillofacial Surgery. 2004 May;62(5). 24. Nissan J, Ghelfan O, Mardinger O, Calderon S, Chaushu G. Efficacy of Cancellous
Block Allograft Augmentation Prior to Implant Placement in the Posterior Atrophic Mandible. Clinical Implant Dentistry and Related Research. 2011 Dec;13(4).
25. Nissan J, Marilena V, Gross O, Mardinger O, Chaushu G. Histomorphometric analysis following augmentation of the posterior mandible using cancellous bone-block
allograft. Journal of Biomedical Materials Research Part A. 2011 Jun 15;97A (4). 26. Francis CS, Mobin SSN, Lypka MA, Rommer E, Yen S, Urata MM, et al. rhBMP-2
with a Demineralized Bone Matrix Scaffold versus Autologous Iliac Crest Bone Graft for Alveolar Cleft Reconstruction. Plastic and Reconstructive Surgery. 2013
May;131(5).
27. Guo J, Li C, Zhang Q, Wu G, Deacon SA, Chen J, et al. Secondary bone grafting for alveolar cleft in children with cleft lip or cleft lip and palate. Cochrane Database of Systematic Reviews. 2011 Jun 15;
23
28. Centre for Reviews and Dissemination U of Y. CRD’s guidance for undertaking reviews in health care. 2009 Jan. Available from
https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf
29. SBU. Ställ tydligare frågor så får du bättre svar. 2019. [cited 25 February 2021] Available from https://www.sbu.se/sv/publikationer/vetenskap-och-praxis/stall-tydligare-fragor-sa-far-du-battre-svar/
30. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. International Journal of Surgery. 2010;8(5).
31. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Systematic Reviews. 2016 Dec 5;5(1).
32. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019 Aug 28; 33. Cochrane. Current version of RoB 2. 2019. [cited 27 February 2021] Available from
https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials
34. Movahedian Attar B, Naghdi N, Etemadi Sh M, Mehdizadeh M. Chin Symphysis Bone, Allograft, and Platelet-Rich Fibrin: Is the Combination Effective in Repair of Alveolar Cleft? Journal of Oral and Maxillofacial Surgery. 2017 May;75(5).
35. Shirani G, Abbasi AJ, Mohebbi SZ, Moharrami M. Comparison between autogenous iliac bone and freeze-dried bone allograft for repair of alveolar clefts in the presence of plasma rich in growth factors: A randomized clinical trial. Journal of
Cranio-Maxillofacial Surgery. 2017 Oct;45(10).
36. Desai AK, Kumar N, Dikhit P, Koikude SB, Bhaduri S. Efficacy of Platelet-Rich Fibrin in Secondary Cleft Alveolar Bone Grafting. Craniomaxillofacial Trauma & Reconstruction Open. 2019 Jan 15;3(1).
37. Bezerra BT, Pinho JNA, Figueiredo FED, Brandão JRMCB, Ayres LCG, da Silva LCF. Autogenous Bone Graft Versus Bovine Bone Graft in Association with Platelet-Rich Plasma for the Reconstruction of Alveolar Clefts: A Pilot Study. The Cleft Palate-Craniofacial Journal. 2019 Jan 12;56(1).
38. Shawky H, Seifeldin SA. Does Platelet-Rich Fibrin Enhance Bone Quality and Quantity of Alveolar Cleft Reconstruction? The Cleft Palate-Craniofacial Journal. 2016 Sep 1;53(5).
39. Carol Lefebvre. Chapter 4: Searching for and selecting studies. Cochrane. [cited 27 February 2021] Available from https://training.cochrane.org/handbook/current/chapter-04
24
40. Dickinson BP, Ashley RK, Wasson KL, OHara C, Gabbay J, Heller JB, et al. Reduced Morbidity and Improved Healing with Bone Morphogenic Protein-2 in Older Patients with Alveolar Cleft Defects. Plastic and Reconstructive Surgery. 2008 Jan;121(1). 41. Segura-Castillo JL, Aguirre-Camacho H, González-Ojeda A, Michel-Perez J.
Reduction of Bone Resorption by the Application of Fibrin Glue in the Reconstruction of the Alveolar Cleft. Journal of Craniofacial Surgery. 2005 Jan;16(1).
42. U.S National Library of Medicine. Clinical Trials. Available from https://www.clinicaltrials.gov
25
Appendix 1
Section/topic # Checklist item Reported on page #
TITLE
Title 1 Identify the report as a systematic review, meta-analysis, or both. Main page
ABSTRACT
Structured summary 2 Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and
implications of key findings; systematic review registration number.
2
INTRODUCTION
Rationale 3 Describe the rationale for the review in the context of what is already known. 4-7
Objectives 4 Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons,
outcomes, and study design (PICOS). 8
METHODS
Protocol and registration 5 Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide
registration information including registration number. 8
Eligibility criteria 6 Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered,
language, publication status) used as criteria for eligibility, giving rationale. table 2 9 and Information sources 7 Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify
additional studies) in the search and date last searched. 9
Search 8 Present full electronic search strategy for at least one database, including any limits used, such that it could be
repeated. 10
Study selection 9 State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable,
included in the meta-analysis). 10
Data collection process 10 Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes
26
Data items 11 List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and
simplifications made. Table 2
Risk of bias in individual
studies 12 Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. 10 and 11 Summary measures 13 State the principal summary measures (e.g., risk ratio, difference in means). In results Synthesis of results 14 Describe the methods of handling data and combining results of studies, if done, including measures of consistency
(e.g., I2) for each meta-analysis. 17 and 18
Page 1 of 2
Section/topic # Checklist item Reported on page #
Risk of bias across studies 15 Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective
reporting within studies). -
Additional analyses 16 Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating
which were pre-specified. -
RESULTS
Study selection 17 Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at
each stage, ideally with a flow diagram. Figure 10 and
Appendix 3, 4 Study characteristics 18 For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and
provide the citations. Table 5
Risk of bias within studies 19 Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). Table 4 Results of individual studies 20 For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each
intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. Table 5 Synthesis of results 21 Present results of each meta-analysis done, including confidence intervals and measures of consistency. - Risk of bias across studies 22 Present results of any assessment of risk of bias across studies (see Item 15). - Additional analysis 23 Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). -
27
Summary of evidence 24 Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to
key groups (e.g., healthcare providers, users, and policy makers). 19 and 20
Limitations 25 Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of
identified research, reporting bias). 19
Conclusions 26 Provide a general interpretation of the results in the context of other evidence, and implications for future research. 20
FUNDING
Funding 27 Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the
systematic review. 20
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal. pmed1000097
28
Appendix 2
Risk of bias assessment
Responses underlined in green are potential markers for low risk of bias, and responses in red are potential markers for a risk of bias. Where questions relate only to sign posts to other questions, no formatting is used.
Domain 1: Risk of bias arising from the randomization process
Signalling questions Comments Response options
1.1 Was the allocation sequence random? Y / PY / PN / N / NI
1.2 Was the allocation sequence concealed until participants were enrolled and assigned to interventions?
Y / PY / PN / N / NI
1.3 Did baseline differences between
intervention groups suggest a problem with the randomization process?
Y / PY / PN / N / NI
Risk-of-bias judgement Low / High / Some concerns
Optional: What is the predicted direction of bias
arising from the randomization process? NA / Favours experimental / Favours comparator / Towards null /Away from null / Unpredictable
29
Domain 2: Risk of bias due to deviations from the intended interventions (effect of assignment to intervention)
Signalling questions Comments Response options
2.1. Were participants aware of their assigned
intervention during the trial? Y / PY / PN / N / NI
2.2. Were carers and people delivering the interventions aware of participants' assigned intervention during the trial?
Y / PY/ PN / N / NI
2.3. If Y/PY/NI to 2.1 or 2.2: Were there deviations from the intended intervention that arose because of the trial context?
NA / Y / PY / PN / N / NI
2.4 If Y/PY to 2.3: Were these deviations likely
to have affected the outcome? NA / Y / PY / PN / N / NI
2.5. If Y/PY/NIto 2.4: Were these deviations from intended intervention balanced between groups?
NA / Y / PY / PN / N / NI
2.6 Was an appropriate analysis used to estimate the effect of assignment to intervention?
Y / PY / PN / N / NI
2.7 If N/PN/NI to 2.6: Was there potential for a substantial impact (on the result) of the failure to analyse participants in the group to which they were randomized?
NA / Y / PY / PN / N / NI
Risk-of-bias judgement Low / High / Some concerns
Optional: What is the predicted direction of bias
due to deviations from intended interventions? Favours comparator / Towards NA / Favours experimental /
null /Away from null / Unpredictable
30
Domain 3: Missing outcome data
Signalling questions Comments Response options
3.1 Were data for this outcome available for all, or nearly all, participants randomized?
Y / PY / PN / N / NI
3.2 If N/PN/NI to 3.1: Is there evidence that the result was not biased by missing outcome data?
NA / Y / PY / PN / N
3.3 If N/PN to 3.2: Could missingness in the
outcome depend on its true value? NA / Y / PY/ PN / N / NI
3.4 If Y/PY/NI to 3.3: Is it likely that missingness in the outcome depended on its true value?
NA / Y / PY / PN / N / NI
Risk-of-bias judgement Low / High / Some concerns
Optional: What is the predicted direction of bias
due to missing outcome data? Favours comparator / Towards NA / Favours experimental /
null /Away from null / Unpredictable
31
Domain 4: Risk of bias in measurement of the outcome
Signalling questions Comments Response options
4.1 Was the method of measuring the outcome
inappropriate? Y / PY / PN / N / NI
4.2 Could measurement or ascertainment of the outcome have differed between
intervention groups?
Y / PY / PN / N / NI
4.3 If N/PN/NI to 4.1 and 4.2: Were outcome assessors aware of the intervention received by study participants?
NA / Y / PY / PN / N / NI
4.4 If Y/PY/NI to 4.3: Could assessment of the outcome have been influenced by knowledge of intervention received?
NA / Y / PY / PN / N / NI
4.5 If Y/PY/NI to 4.4: Is it likely that
assessment of the outcome was influenced by knowledge of intervention received?
NA / Y / PY / PN / N / NI
Risk-of-bias judgement Low / High / Some concerns
Optional: What is the predicted direction of bias
in measurement of the outcome? Favours comparator / Towards NA / Favours experimental /
null /Away from null / Unpredictable
32
Domain 5: Risk of bias in selection of the reported result
Signalling questions Comments Response options
5.1 Were the data that produced this result analysed in accordance with a pre-specified analysis plan that was finalized before unblinded outcome data were available for analysis?
Y / PY / PN / N / NI
Is the numerical result being assessed likely to have been selected, on the basis of the results, from...
5.2. ... multiple eligible outcome
measurements (e.g. scales, definitions, time points) within the outcome domain?
Y / PY / PN / N / NI
5.3 ... multiple eligible analyses of the data? Y / PY / PN / N / NI
Risk-of-bias judgement Low / High / Some concerns
Optional: What is the predicted direction of bias
due to selection of the reported result? Favours comparator / Towards NA / Favours experimental /
null /Away from null / Unpredictable
33
Overall risk of bias
This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Risk-of-bias judgement Low / High / Some concerns
Optional: What is the overall predicted direction
of bias for this outcome? NA / Favours experimental / Favours comparator /
Towards null /Away from null / Unpredictable
34
Appendix 3
Table presenting publications excluded on relevance.
Reference Reason for exclusion
Abramowicz S, Katsnelson A, Forbes PW, Padwa BL. Anterior versus posterior
approach to iliac crest for alveolar cleft bone grafting.
Retrospective
Aiyesha Wahaj, Kashif Hafeez, Muhammad Sohail Zafar,
Role of bone graft materials for cleft lip and palate patients: A systematic review,
Systematic Review
Allard RH, Lekkas C, Swart JG. Autologous versus homologous bone grafting in
osteotomies, secondary cleft repairs and ridge augmentations: a clinical study.
Retrospective
Alonso N, Risso GH, Denadai R, Raposo-Amaral CE. Effect of maxillary alveolar reconstruction on nasal symmetry of cleft lip and palate patients: a study comparing iliac crest bone graft and recombinant human bone morphogenetic protein-2
Wrong outcome
Alonso, N. Tanikawa, D. Y. S.; Rocha, D. L; Callan, Rdflw; Ozawa, t.O,; DoAmaral, C. E, R; DeAlmeiuda, A. B. A.; Preliminary Results of Maxillary Alveoalr Cleft Repair using Recombinant Human Bone
Morphogenic Protein-2.
Conference abstract
Amini, P. & Milani, A. & Rahmani, N.. (2016). Radiological assessment of using allograft in combination with platelet-rich fibrin in alveolar cleft grafting. 26. 175-185.
Foreign language
Bajestan MN, Rajan A, Edwards SP, Aronovich S, Cevidanes LHS, Polymeri A, Travan S, Kaigler D. Stem cell therapy for reconstruction of alveolar cleft and trauma defects in adults: A randomized controlled, clinical trial.
Wrong population
Balaji SM. Alveolar cleft defect closure with iliac bone graft, rhBMP-2 and rhBMP-2 with zygoma shavings: Comparative study.
35 Bekisz JM, Fryml E, Flores RL. A Review of Randomized Controlled Trials in Cleft and Craniofacial Surgery.
Wrong outcome
Benlidayi ME, Tatli U, Kurkcu M, Uzel A, Oztunc H. Comparison of bovine-derived hydroxyapatite and autogenous bone for secondary alveolar bone grafting in patients with alveolar clefts.
Retrospective
Borba AM, Borges AH, da Silva CS, Brozoski MA, Naclério-Homem Mda G, Miloro M. Predictors of complication for alveolar cleft bone graft.
Retrospective
Borstlap WA, Heidbuchel KL, Freihofer HP, Kuijpers-Jagtman AM. Early secondary bone grafting of alveolar cleft defects. A comparison between chin and rib grafts.
nRCT
Carlini JL, Biron C, Gomes KU, Da Silva RM. Surgical repositioning of the
premaxilla with bone graft in 50 bilateral cleft lip and palate patients.
Retrospective cohort study, wrong study design
Cohen M, Figueroa AA, Haviv Y, Schafer ME, Aduss H. Iliac versus cranial bone for secondary grafting of residual alveolar clefts.
Retrospective
da Rosa WLO, da Silva TM, Galarça AD, Piva E, da Silva AF. Efficacy of rhBMP-2 in Cleft Lip and Palate Defects: Systematic Review and Meta-analysis.
Systematic Review
De Santis, P,; Savoia, A. Allopastic material in cleft palate and lip sequelae
Foreign language Du F, Wu H, Li H, Cai L, Wang Q, Liu X,
Xiao R, Yin N, Cao Y. Bone Marrow Mononuclear Cells Combined with Beta-Tricalcium Phosphate Granules for Alveolar Cleft Repair: A 12-Month Clinical Study.
Wrong publication type
Elfaramawi, T.I. & Faramawey, M.I. & Dahaba, M.M. & Hakam, M.M. (2015). Deproteinized Bovine Bone Graft in Maxillary Alveolar Cleft Reconstruction.
Wrong publication type
Enemark H, Jensen J, Bosch C. Mandibular bone graft material for reconstruction of alveolar cleft defects: long-term results.
36 Franco Bueno, D. Bone Tissue Engineering with Dental Pulp Stem Cells for Alveolar Cleft Repair
NCT, trial not completed/results not published
Freihofer HP, Borstlap WA, Kuijpers-Jagtman AM, Voorsmit RA, van Damme PA, Heidbüchel KL, Borstlap-Engels VM. Timing and transplant materials for closure of alveolar clefts. A clinical comparison of 296 cases.
Retrospective
Freihofer HP, Kuijpers-Jagtman AM. Early secondary osteoplastic closure of the residual alveolar cleft in combination with orthodontic treatment.
Wrong publication type
Gimbel M, Ashley RK, Sisodia M, Gabbay JS, Wasson KL, Heller J, Wilson L,
Kawamoto HK, Bradley JP. Repair of alveolar cleft defects: reduced morbidity with bone marrow stem cells in a resorbable matrix.
nRCT
Giudice G, Cutrignelli DA, Leuzzi S, Robusto F, Sportelli P, Nacchiero E. Autologous bone grafting with platelet-rich plasma for alveolar cleft repair in patient with cleft and palate.
Retrospective
Greived, M. Cost Effectiveness in Alveolar Bone Grafting in Patients with Cleft Lip and Palate.
NCT, trial not completed/results not published
Guo J, Li C, Zhang Q, Wu G, Deacon SA, Chen J, Hu H, Zou S, Ye Q. Secondary bone grafting for alveolar cleft in children with cleft lip or cleft lip and palate.
Systematic Review
Hammoudeh JA, Fahradyan A, Gould DJ, Liang F, Imahiyerobo T, Urbinelli L, Nguyen JT, Magee W 3rd, Yen S, Urata MM. A Comparative Analysis of
Recombinant Human Bone Morphogenetic Protein-2 with a Demineralized Bone Matrix versus Iliac Crest Bone Graft for Secondary Alveolar Bone Grafts in Patients with Cleft Lip and Palate: Review of 501 Cases.
Retrospective
Hebatallah G Samra El-Shamy. Evaluation of Platelet Rich Fibrin/Biphasic Calcium Phosphate Effect Versus Autogenous Bone Graft on Reconstruction of Alveolar Cleft.
NCT, trial not completed/results not published
37 Herford AS, Boyne PJ, Rawson R, Williams RP. Bone morphogenetic protein-induced repair of the premaxillary cleft.
Retrospective
Jahanbin A, Zarch HH, Irani S, Eslami N, Kermani H. Recombinant Human Bone Morphogenic Protein-2 Combined with Autogenous Bone Graft for Reconstruction of Alveolar Cleft.
nRCT
Janssen NG, Weijs WL, Koole R, Rosenberg AJ, Meijer GJ. Tissue engineering strategies for alveolar cleft reconstruction: a systematic review of the literature.
Systematic Review
Kadry, W. Tissue Engineered Constructs for Alveolar Cleft Repair.
NCT, trial not completed/results not published
Kamal M, Ziyab AH, Bartella A, Mitchell D, Al-Asfour A, Hölzle F, Kessler P, Lethaus B. Volumetric comparison of autogenous bone and tissue-engineered bone replacement materials in alveolar cleft repair: a systematic review and meta-analysis.
Systematic Review
Kessler P, Thorwarth M, Bloch-Birkholz A, Nkenke E, Neukam FW. Harvesting of bone from the iliac crest--comparison of the anterior and posterior sites.
Unclear outcome
Khojasteh A, Kheiri L, Motamedian SR, Nadjmi N. Regenerative medicine in the treatment of alveolar cleft defect: A systematic review of the literature.
Systematic Review
Koole R, Bosker H, van der Dussen FN. Late secondary autogenous bone grafting in cleft patients comparing mandibular
(ectomesenchymal) and iliac crest (mesenchymal) grafts.
Retrospective
Kortebein MJ, Nelson CL, Sadove AM. Retrospective analysis of 135 secondary alveolar cleft grafts using iliac or calvarial bone.
38 Kupfer P, Abbott MM, Abramowicz S,
Meara JG, Padwa BL. Cost differences between the anterior and posterior approaches to the iliac crest for alveolar bone grafting in patients with cleft lip/palate.
Retrospective
Lee C, Nishihara K, Okawachi T, Iwashita Y, Majima HJ, Nakamura N. A quantitative radiological assessment of outcomes of autogenous bone graft combined with platelet-rich plasma in the alveolar cleft.
nRCT
Li S, Zhang C, Yuan T. [Osteogenic potential of platelet-rich plasma combined with cells and artificial bone].
Wrong publication type, not primary study
Liang F, Yen SL, Imahiyerobo T, Sanborn L, Yen L, Yen D, Nazarian S, Jedrzejewski B, Urata M, Hammoudeh J.
Three-Dimensional Cone Beam Computed Tomography Volumetric Outcomes of rhBMP-2/Demineralized Bone Matrix versus Iliac Crest Bone Graft for Alveolar Cleft Reconstruction.
nRCT
Luaces-Rey R, Arenaz-Búa J, Lopez-Cedrún-Cembranos JL, Herrero-Patiño S, Sironvalle-Soliva S, Iglesias-Candal E, Pombo-Castro M. Is PRP useful in alveolar cleft reconstruction? Platelet-rich plasma in secondary alveoloplasty.
nRCT
M.E Deeb. Reconstruction of alveolar clefts with mandibular or iliac crest bone grafts: A comparative study.
Discussion paper, not primary study
Ma L, Yali H, Guijun L, Dong F. Effectiveness of corticocancellous bone graft in cleft lip and palate patients: A systematic review.
Systematic Review
MacIsaac ZM, Rottgers SA, Davit AJ 3rd, Ford M, Losee JE, Kumar AR. Alveolar reconstruction in cleft patients: decreased morbidity and improved outcomes with supplemental demineralized bone matrix and cancellous allograft.