School of Health, Care and Social Welfare
SEDENTARY BEHAVIOR AND
PHYSICAL ACTIVITY DURING A
6-MONTHS MULTIMODAL LIFESTYLE
INTERVENTION IN PERSONS WITH
MILD STAGE OF ALZHEIMER´S
DISEASE
Secondary analyses of existing data
EDIT VAS
Main Area: Physiotherapy Level: Second cycle
Credits: 30 hp
Programme: Master´s Programme In Physiotherapy with Specialization In Behavioral Medicine
Course Name: Master´s thesis in Physiotherapy with a Specialization in
Supervisor: Maria Sandborgh Co-supervisor: Charlotta Thunborg Seminar date: 2021-May-27
CONTENTS
1 BACKGROUND ...4
1.1 Dementia and Alzheimer’s disease ... 4
1.2 Physical activity and sedentary behaviour in dementia ... 5
1.3 The role of physiotherapy in dementia ... 7
1.4 Theoretical support for behaviour change in dementia ... 7
1.5 Physical-activity and sedentary behaviour in mild Alzheimer´s disease ... 9
1.6 The model of multimodal lifestyle interventions ...10
2 PROBLEM FORMULATION ... 10
3 AIM ... 11
3.1 Study questions: ...11
4 METHODS ... 11
4.1 Study design ...11
4.2 Sample and setting ...12
4.2.1 Inclusion and exclusion criteria ...13
4.2.2 Sample ...14
4.3 Measurements ...15
4.3.1 Physical activity and sedentary behaviour assessment ...15
4.3.2 Outcome expectancies and exercise self-efficacy ...16
4.4 Procedure ...17
4.5 Analysis and data management ...17
4.6 Ethical considerations ...18
5 RESULTS ... 19
5.1 Physical activity and sedentary behaviour measured by Actigraph ...19
5.2 Physical activity and sedentary behaviour measured by questionnaire ...19
6 DISCUSSION... 23 6.1 Summary of Results ...23 6.2 Results discussion ...23 6.3 Method discussion ...24 6.4 Ethical discussion ...27 7 CONCLUSIONS ... 28
8 CLINICAL IMPLICATIONS AND FURTHER RESEARCH... 28
9 REFERENCES ... 30
Intervention ...39
Multimodal Lifestyle intervention ...39
Multimodal lifestyle intervention + medical food ...39
Control group ...40
APPENDIX A: MIND-AD TRIAL
APPENDIX B: INCLUSION AND EXCLUSION CRITERIA TO THE MIND-AD TRIAL APPENDIX C: THE INDICATOR QUESTIONS OF THE NATIONAL BOARD OF HEALTH AND WELFARE QUESTIONNAIRE
APPENDIX D: OUTCOME EXPECTATIONS FOR EXERCISE SCALE APPENDIX E: SELF-EFFICACY FOR EXERCISE SCALE
ABSTRACT
Purpose: The objective was to study how sedentary behaviour (SB) and physical activity (PA)
change during a 6-months multimodal lifestyle intervention including a Multimodal Lifestyle Intervention, a Multimodal Lifestyle and Medical food intervention and a control subgroup, for people with prodromal Alzheimer's disease (AD). Further, the aim was to explore the predictive value of outcome expectancies and self-efficacy beliefs on level of PA in the two intervention subgroups.
Methods: A secondary analysis of existing data (n=66) from MIND-AD trial with a
descriptive evaluation design was conducted. Descriptive and non-parametric statistical analysis were used for between- and within groups analysis. To explore the predictive value of a model with self-efficacy and outcome expectancies at baseline on PA at 6 months, regression analysis was conducted. Effect size was calculated for between-group and within-group differences.
Results: Objectively measured PA increased in the Multimodal Intervention subgroup. SB
did not change during the intervention. Outcome expectancies for that impact of exercise is beneficial for health in the long run increased during the intervention. Participants higher initial outcome expectancies for the impact of exercise would lead to less AD-related difficulties predicted higher PA level at 6 months. Self-efficacy for exercise or outcome expectancies for the impact of exercise on AD-related difficulties did not change during the intervention.
Conclusions: PA increased in the Multimodal Intervention subgroup. This difference could
not be shown with PA measured by questionnaire which indicates that objective measurements are better suited to measuring PA in people with prodromal AD than subjective measurements. Increased outcome expectancies for that impact of exercise are beneficial for health in the long run demonstrate the participants strengthened intentions to improve their long-term health. Increasing outcome expectancies to manage AD-related difficulties can be an important part of interventions targeting PA in people with prodromal AD.
Keywords: Alzheimer´s disease, physical activity, sedentary behaviour, self-efficacy,
1
BACKGROUND
1.1 Dementia and Alzheimer’s disease
Dementia is a pathological disorder of intellectual functions with deterioration of memory and logical thinking as well as personality changes and emotional disturbances (Larsson & Lundgren, 2010). Dementia affects the behaviour and the ability to perform everyday activities. There are three main ethology-based categories of dementia: primary degenerative, vascular and secondary dementias. The different types of vascular dementia are called stroke-related, post-stroke, single and multi-infarct, subcortical and mixed (vascular and Alzheimer´s disease) dementia. Secondary dementia develops secondarily as a result of a physical disease or injury. Alzheimer´s disease (AD), a primary degenerative dementia, is the most common disorder among the different types of dementia (Larsson & Lundgren, 2010). AD is most often classified into mild/early, moderate and severe stages. During the mild stage of Alzheimer's disease, a slow decline of memory and reduced spontaneity are often seen. The person sometimes forgets words, has difficulties to orient themselves and begin to show motivational difficulties and lack of initiative ability. An increased tiredness, which can be interpreted as depression, can also be seen among the first signs of mild Alzheimer’s (Larsson & Lundgren, 2010).
The predementia stage of AD, the so-called prodromal AD, refers to a stage where memory loss is present but not so severe that it would affect the person´s everyday activities. In prodromal AD, there are biomarkers from cerebrospinal fluid and imaging that can show pathological changes (Dubois, et al., 2010).
Due to the lack of curative treatments, dementia is a burdensome diagnosis for the individual and society. The prevalence of dementia in Europe between 1993 and 2018 shows a prevalence rate of 7.1% (Bacigalupo et al., 2018) while the prevalence of AD in Europe was estimated at 5.05% for both men and women between 1995 and 2015 (Niu et al., 2017). Both Finland (1.71%) and Sweden (1.82%) had a higher percentage of dementia in the population than the average in EU (1.55%) (https://www.alzheimer-europe.org/Policy/Country-comparisons/2013-The-prevalence-of-dementia-in-Europe).
In Finland, dementia, including AD, has been one of the main reasons for mortality. In 2018, 19% of all deaths was because of dementia, including AD. (Official Statistics of Finland, 2018). During a 10 years period the number of deaths caused by dementia/AD has been doubled in Finland. Between 2016 and 2017 there was an increase with 200 persons who died in dementia, including AD (OSF, 2017). Approximately 25 thousand Swedes fall ill in a dementia disease in Sweden every year. The number of people with dementia is probably going to increase after 2020 when the large number of people born in the 1940´s reaches an advanced age (Socialstyrelsen, 2018). According to Skoog et al. (2017), the prevalence of AD in 85-year-olds between 2008 and 2010 was 11.6% in Sweden.
A common way of evaluating the cognitive aspects of mental functions is the Mini-Mental State Examination (MMSE) questionnaire. It is called “mini” because it does not assess mood or abnormal mental experiences. The first section of MMSE includes assessment of orientation, memory and attention. The second section tests the ability to name, follow verbal and written commands, and the ability to write a meaning and copy a polygon (Folstein et al., 1975).
1.2 Physical activity and sedentary behaviour in dementia
Any bodily movement that leads to increased energy consumption is defined as physical activity (Caspersen et al., 1985). Physical activity that is planned and has a structure with the purpose of maintaining or improving physical fitness and strength, is defined as physical exercise. Physical fitness refers to the ability to complete tasks without getting fatigued. Health-related components in physical fitness are cardiorespiratory endurance (aerobic fitness), muscular endurance, muscular strength, body composition and flexibility (Caspersen et al., 1985). Cardiorespiratory endurance is for example walking, jogging, or swimming. Physical inactivity defined as an inadequate physical activity level that does not meet the physical activity recommendations of 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity aerobic activity per week in combination with resistance exercise (World Health Organization, 2010). Sedentary behaviour (SB) is defined as any waking behaviour with less energy expenditure than 1.5 metabolic equivalents in sitting, reclining (position between sitting and lying) or in lying position. Sedentary bout is a period of uninterrupted sedentary time. SB pattern is a term used to describe SB accumulated throughout the waking time during a day or a week by specifying the timing, the duration and frequency of sedentary bouts and breaks (Tremblay et al., 2017).
Wang and Tsai (2016) compared physically active with physically inactive elderly adults (mean age 67 years in both groups). The results showed higher behaviour accuracy in physically active elderly compared to physically inactive elderly. Behaviour accuracy was defined as the percentage of correct responses measured in reactions time to a cognitive task (Wang & Tsai, 2016). Maasakkers, et al. (2020) did not find any association between SB and cognitive function in elderly people between 66 and 75 years without dementia. According to the authors` hypothesis, this result can be explained by the different level of mental activity that different SB require and suggest that SB should be studied in more domain-specific behaviour categories (Maasakkers, 2020). To the author´s knowledge, there are no studies about the effects of SB in early Alzheimer’s or dementia.
Physical activity can be assessed either by self-report tools or wearable monitors (Ainsworth et al., 2015). Self-report tools can be questionnaires, logs, or diaries in order to register the frequency, duration, intensity and type of physical activity. The different types of physical activity monitors are pedometers (measuring steps at walking speed), accelerometers (recording accelerations in one or more planes), heart rate monitors and multi-sensor systems. Accelerometers can be used to enable categorization of PA into sedentary, light, moderate and vigorous according to the duration and frequency of PA (Ainsworth et al., 2015).
People with prodromal AD have some cognitive limitations that can eventually hinder them to answer a questionnaire. Trigg et al. (2007) has therefore studied how elderly people over the age of 65 years with dementia (82% with AD, mean MMSE score 18) could answer a self-reporting questionnaire about quality of life. Participants were able to complete the questionnaire that included questions about feelings and evaluations of quality of life (Trigg et al., 2007).
According to Farina et al. (2014) physical exercise has a positive influence on global cognitive function in AD. Exercise seems to have a nutritive impact on the neuronal function by increasing synaptic plasticity, neurogenesis and vascular function at the same time as the brain´s cerebral vascular perfusion increases (Farina et al., 2014). Physical activity and
physical exercise have positive effects on the brain anatomically, cellularly and molecularly through different processes that include increasing brain plasticity (De la Rosa et al., 2020). A high-intensity functional weight-bearing exercise program was carried out in people with dementia diagnosis, of which 36% had Alzheimer´s disease (Toots, et. al., 2016). The exercise program consisted of lower-limb strength exercises and static and dynamic balance exercises in walking and standing (Toots et al., 2016; Littbrand et. al., 2006). Independence in ADL was increased at 4 months in the exercise group but declined at 7 months for both participants with AD and other dementia diagnosis. Those participants who did not have Alzheimer´s disease, but had another kind of dementia diagnosis, had an increased balance ability and a postponed decline in ADL ability at 4 and 7 months according to the interaction analysis. This postponed decline in ADL ability could not be shown for participants with AD which can depend on the fact that participants with other dementia diagnosis had better cognitive function than participants with AD (Toots et al., 2016).
It seems to be effective to combine physical and cognitive exercise to increase both physical and cognitive functions in people with mild cognitive impairment or dementia (Karssemeijer et al., 2017). There was a positive small-to-medium effect of the combined exercise intervention on the global cognitive function according to the metanalysis. The effect on activities of daily living was moderate-to-large. The physical exercise component included both resistance and aerobic exercise interventions (Karssemiijer et al., 2017). Furthermore, resistance training increased balance, isometric upper- and lower limb muscle strength, gait ability and decreased time needed to complete time-up-and-go test in a group of elderly people with dementia (Cadore et al., 2014).
Physical activity level, in the form of walking activity, was studied in people with diagnosed mild-to-moderate AD between baseline and a one-year follow-up in a longitudinal study (Winchester et al., 2013). The participants were categorized either as sedentary (not engaging in any activity) or active (engaged in walking activity one or more hours per week). The active participants had a significantly higher MMSE score compared to the sedentary participants. The follow-up analysis showed dose-dependent effect of walking on cognition. Sedentary participants declined significantly in their MMSE score between baseline and the one-year follow-up. Active participants who walked for one or more hours per week had a stabilized cognitive function while active participants who walked for two or more hours per week had a significant increase in MMSE-score (Winchester et al., 2013).
A multimodal approach is to incorporate physical exercise, cognitive- and support group interventions in persons with dementia. Findings from a randomized control trial (RCT) show that a multimodal intervention can improve cognitive and physical functioning and behavioural outcomes, such as self-esteem, for persons in the early and middle stages of dementia (Burgener et al., 2008).
Physical activity and exercise have a delaying effect on the cognitive decline on people in predementia stage (prodromal AD) according to De la Rosa et al. 2020. An active lifestyle as a part of a multimodal intervention is recommended to older people. The type of exercise and its components such as duration, frequency, intensity is not yet known and need to be studied for people with prodromal AD (De La Rosa et al., 2020).
1.3 The role of physiotherapy in dementia
Physiotherapy with specialization in behavioural medicine incorporates psychosocial, behavioural, and biomedical aspects relevant to health and disease (International Society of Behavioural Medicine, 2014). Physiotherapists participate in rehabilitative interventions in the care of people with dementia by maintaining mobility and function as well as balance ability (Chavan, 2018). Fall incidents are associated with cognitive impairment. Rehabilitation of trauma injuries, such as fractures, are therefore more common in elderly people with dementia than in elderly with intact cognitive ability (Kempenaar, 2005). Mobility problems are due to musculoskeletal problems, for example weakness in thigh muscles, that affect and decrease gait ability (Kempenaar, 2005). People with dementia often have physical impairment due to rigidity or reduced balance. Physiotherapists can target the specific problems with specific exercises and can prescribe a walking aid. The most important issue for the physiotherapist is the awareness that certain mobility problems can be associated with the person´s cognitive impairment because of dementia. The lack of motivation or fear of falling are two examples of how mobility can be affected in dementia (Kempenaar, 2005). In the early stage of dementia, it is important to increase the quality of life (QOL) by setting goals for the rehabilitation that are connected to the activities in everyday life. To increase QOL in this early stage, it is important to focus on social participation as a goal for rehabilitation (Maki et al., 2018). Rehabilitation needs to incorporate participant involvement, caregiver education, enjoyable exercises and physical activities. Problem solving, to overcome obstacles to start and maintain the physical activity and physical exercise, requires a structured behaviour problem solving approach (Teri et al., 2008). The most common behaviour problem in dementia, including AD, is the lack of intrinsic (inner) motivation due to increased apathy (Steinberg et al., 2008). Intrinsic motivation, which is the individual´s ability to motivate him or herself, is rooted in cognitive functioning. Cognitive resources are needed to be able to anticipate future states that translate into motivators for the present behaviour (Bandura, 1997).
1.4 Theoretical support for behaviour change in dementia
In persons with AD, PA and SB are affected by cognitive and emotional behavioural problems and the most frequent behavioural problems are aggression and activity disturbances. Since persons with dementia commonly show behavioural disturbance, the biopsychosocial approach can help in understanding these problematic behaviours (Van der Mussele et. al., 2013). The biopsychosocial approach targets the individual´s social context and psychological well-being as well as the person´s thoughts, beliefs and emotions while attending medical comorbidities due to increased vascular risk factors and neuropsychiatric complications of the disease in order to maintain the functionality level (Farinde, 2012). Non-pharmacological treatments in dementia for behavioural and psychological symptoms are recommended before considering pharmacological treatments. Some of the non-pharmacological treatments are cognitive rehabilitation, social activity/interaction, physical activity and exercise and environmental interventions (Cankurtaran, 2014). A self-management approach can be an appropriate way to deal with behavioural problems due to impaired cognition in people with dementia and mild cognitive impairment (Quinn et al., 2016). Self-management of long-term conditions, such as dementia, can be used to increase knowledge about the disease, the person´s physical and psychological well-being and self-efficacy (Quinn et al., 2016).
Self-efficacy theory is one of the most common theories within Social-Cognitive Theory. According to Social-Cognitive Theory, the model of reciprocal determinism is based on the mutual interaction between the person´s behaviour, the individual and environmental factors (Bandura, 1997). Outcome expectancies and self-efficacy are two central constructs within the theory. Self-efficacy, a person´s belief in his/her ability to be able to execute certain behaviours to reach a desired outcome, has proven to be crucial for lifestyle choices and health behaviours. Outcome expectancies are the individual's expectation that certain behaviour may lead to desired outcomes (Bandura, 1997). It is important to make the person with dementia feel confident in his or her abilities to perform a specific behaviour with specific techniques that can be incorporated in an intervention (Quinn et al., 2016). Efficacy beliefs can activate positive biological processes in the body, especially when coping with the early stages of a disease like AD. In health-related behaviour, efficacy beliefs, such as self-efficacy and outcome expectations, are crucial (Bandura, 1997). Both efficacy beliefs and outcome expectancies affect how the person adopts and incorporates a new behaviour pattern by generalizing it to different situations and how the new behaviour is maintained over time. Self-efficacy is needed in every step on the way in a behaviour change process: in the phase when the person considers making a behaviour change as well as in the phase of gathering efforts and motivation to do the change. With a stronger self-efficacy, the individual can make efforts to make positive behaviour health changes and reduce hindering factors. To adopt a new behaviour, the person needs to form an intention before trying to carry out the action. In the forming of these intentions, positive and high outcome expectancies are needed to make the decision to initiate a behaviour change (Bandura, 1997). It can probably be difficult for a person with dementia or cognitive impairment to make a realistic judgement of their own ability because it requires cognitive ability.
There are two approaches to the theoretical relationship between expected outcomes and self-efficacy (Williams, 2010). The first approach is that self-self-efficacy rating is not causally influenced by outcome expectations. To make a distinction between self-efficacy and outcome expectations, the researcher could use the phrase “if I wanted to” added to the question about self-efficacy. This could be for example: “I could exercise two times per week for 60 minutes if the weather was bothering me, if I wanted to”. The second approach is that self-efficacy is influenced by outcome expectations. As an example, it can be difficult to determine if the person wants to exercise due to the expected negative outcomes, such as pain or tiredness, or because of the lack of perceived capability to perform exercise. To handle this problem, the researcher needs to inform the participants that they should think about the expected outcomes as well when they are rating self-efficacy (Williams, 2010).
A health-related assessment explores the determinants of behavioural and environmental risk factors to a health problem. Determinants can be biological, psychological, or social factors that are associated with the behaviour that caused the health problem (Bartholomew, 2016). While certain individual factors (genetics, personal characteristics, social factors, demographic) may not be changeable or are difficult to change, the psychological factors, such as self-efficacy or outcome-expectations can be influenced by interventions (Bartholomew, 2016).
Behaviour change techniques (BCT) are observable components that can alone or in combination with other BCTs be used to support behaviour change (Kok et al., 2016). In older adults (≥60 years), three BCTs have been found to be associated with higher physical activity behaviour effects sizes: barrier identification/problem solving, provision of rewards contingent on successful behaviour and modelling/demonstrating the behaviour (French et al.,
2014). In people with more severe forms of dementia, BCTs should not rely on cognitive ability but should be grounded on non-conscious approaches to health promotion (Nyman, 2019). In the early stage of AD, the individual has enough cognitive capacity to engage with behaviour change techniques to increase their physical activity level. As AD is a progressive disease, BCTs that do not rely on the cognitive processes of the person diagnosed with AD are recommended. BCTs that rely on the cognitive capacity of the caregiver are often used in the clinical context. To promote PA, caregivers should use BCTs such as goal setting, social support, social reward, coping planning and reminders of distal rewards with older people with dementia (Nyman, 2019).
BCTs need to be chosen carefully so that they can lead to the desired behaviour change (Kok et al., 2016). BCTs can be theory-based with evidence coming from different theories explaining the working mechanism of the different BCTS. Theories can also explain a causal link between BCT implementation and behaviour change. BCTs need to clarify the specific determinants for behavioural change and the theory-based methods they incorporate. BCTs that can increase self-efficacy are mastery experience, verbal persuasion, interpretation of physical emotional states and modelling. To increase outcome expectations, the following theory based BCTs can be used: direct experience, repeated exposure, and self-re-evaluation (Kok et al., 2016). A previous study has shown that self-efficacy for adhering to resistance training can mediate the effect of intervention targeting self-efficacy and outcome expectations on exercise training frequency in people participating in cardiac rehabilitation (Millen & Bray, 2009). Therefore, it would be interesting to study if self-efficacy and outcome expectancies for exercise have any predicative value on physical activity in people with prodromal AD.
1.5 Physical-activity and sedentary behaviour in mild Alzheimer´s
disease
Physical activity plays an important role in both preventing dementia and AD and in slowing down the effects of AD in the early stage of the disease (Farina et al., 2014). Sedentary behaviour is associated with increased cognitive impairment and needs to be targeted in AD (Falck, et al., 2017). Impaired cognitive function is associated with SB, measured by questionnaires and accelerometer, according to a systematic review of Falck et al. (2017). The association between SB and impaired cognitive function was also explored by Hartman et al. (2018). People diagnosed with dementia were compared with cognitively healthy older people (< 60 years) by measuring physical activity and SB with an accelerometer. The results confirmed the previous findings of Falck et al. (2017). Participants diagnosed with dementia had significantly lower activity counts and more hours spent in lower-intensity activities than the control group. Also, the relative sedentary time was significantly higher in participants with dementia compared to the control group. Waking time spent in light-to-moderate or moderate-to-vigorous intensity physical activity was significantly lower in the dementia group compared to the control group (Hartman et al., 2018).
Daily average and diurnal physical activity- and sedentary pattern has been studied in a group of participants with mild AD (mean age 73.5 years) by measuring physical activity during a seven days period with an accelerometery (Varma & Watts, 2017). The mild AD group was compared to older adults without cognitive impairment. The diurnal activity pattern showed that physical activity varied over the day in peak activity, maximum activity, minutes during
waking hours and the time at which the peak activity minute occurred. The mild AD group had less moderate-intensity physical activity, less deviation from a non-varying physical activity pattern and lower peak activity than the control group. The greatest differences in the daily pattern were found in the morning between 7am and 12pm when the mild AD groups had a lower moderate-intensity physical activity and lower peak activity compared to the control group. On the other hand, mild AD was not associated with more SB or less low-intensity physical activity across the day (Varma & Watts, 2017).
1.6 The model of multimodal lifestyle interventions
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) study was carried out in Finland as a multidomain RCT (Ngandu et al., 2015). The inclusion criteria were CAIDE risk score and cognitive capacity slightly lower than the mean expected for their age (Kivipelto et al., 2006). The multidomain components of the intervention included physical activity, nutrition counselling, cognitive- and social stimulation, monitoring of the vascular and metabolic risk factors and computerized cognitive training, showed positive effects on cognition (Ngandu et al., 2015). The Multimodal preventive trials for Alzheimer´s Disease: towards multinational strategies (MIND-ADMINI) study was an adapted FINGER-model and has targeted people with prodromal AD by combining lifestyle/vascular multimodal intervention with medical food with the multimodal components from the FINGER study. Prodromal stage of AD is where the early symptoms that signal the onset of AD occur and precede the diagnosable symptoms that develop along with AD. The MIND-ADMINI is a multinational trial with the cooperation of four countries: Sweden, Finland, Germany and France.
2
PROBLEM FORMULATION
Prevention is now highlighted as a key element in managing the dementia epidemic since no drug treatment is available. Decreased physical activity and increased sedentary behaviour are two out of several modifiable risk factors for developing dementia and AD. The behavioural and psychological symptoms of dementia affect the individual even in the early stages of AD and requires a biopsychosocial approach from the caregiver. Furthermore, the moderate-intensity physical activity levels are lower, and time spent in sedentary behaviour is higher in the early stages of AD compared with cognitively healthy older adults. This could negatively affect the progression of AD. A multimodal healthy lifestyle intervention can be introduced in the early stages of AD and it should incorporate physical, social, vascular/metabolic control and cognitive components to address the multifaceted needs of persons with AD. Previous studies have mostly focused on intervening one lifestyle component at the time ( i.e., physical activity, treatment for vascular risks) or the psychological factors such as depression or self-esteem outcomes in AD but has not applied the theoretical frame of self-efficacy and outcome expectancies. The health implications of the theoretical concepts of exercise self-efficacy and outcome expectations for exercise in persons with early signs of AD participating in a multimodal healthy lifestyle intervention is unknown. Furthermore, it is unknown how these
(i.e., self-efficacy and outcome expectations) can change during an intervention period and how these changes relate to changes in physical activity.
3
AIM
The objective of this study was to study how sedentary behaviour and physical activity change during a 6-months multimodal lifestyle intervention including a Multimodal Lifestyle Intervention subgroup, a Multimodal Lifestyle and Medical food intervention subgroup and a control subgroup, for people with prodromal Alzheimer's disease. Further, the aim was to explore the predictive value of outcome expectancies and self-efficacy beliefs on level of physical activity in the two intervention groups.
3.1 Study questions:
1. What is the difference between the three subgroups at 6 months for physical activity and sedentary time?
2. How does physical activity change for each subgroup over time from baseline to 6 months, measured subjectively and objectively?
3. How does sedentary behaviour change for each subgroup over time from baseline to 6 months, measured subjectively and objectively?
4. How do self-efficacy and outcome expectancies for physical exercise change during the intervention, measured at baseline and at 6 months in the two intervention subgroups together?
5. What is the predictive value of self-efficacy and outcome expectancies at baseline on the level of physical activity at 6 months for the two intervention subgroups together?
4
METHODS
4.1 Study design
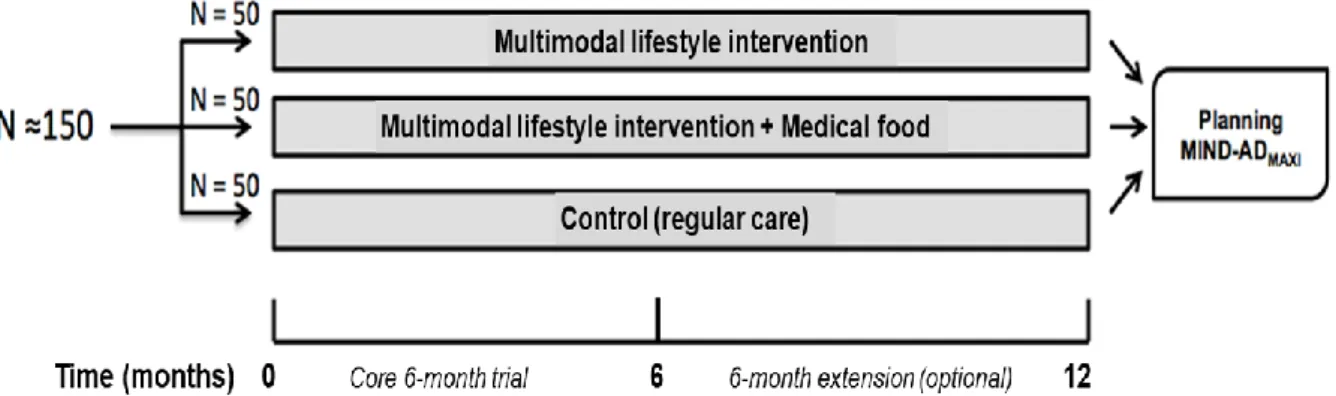
The main study (MIND-AD) was a RCT with three arms, see fig 1. This master´s degree study is a sub-study with a descriptive evaluation design and is a secondary analysis of existing data from the MIND-AD trial (figure 1), (appendix A), (Trial registration: ClinicalTrials.gov
study concerns individuals with prodromal Alzheimer’s disease.
Figure 1: Design of the MIND-AD trial. Copied with permission from the authors (Trial registration: ClinicalTrials.gov NCT03249688)
4.2 Sample and setting
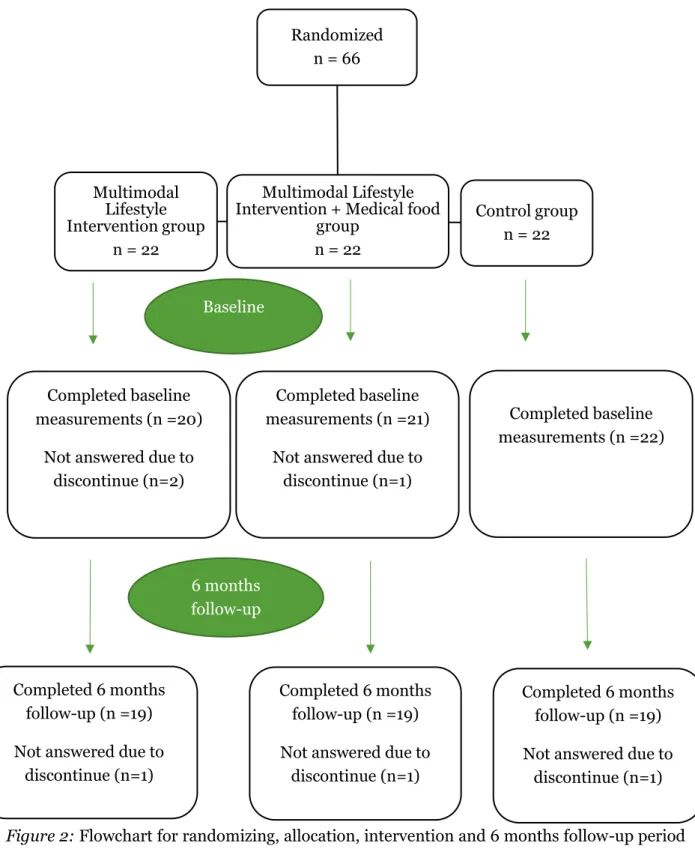
Sample size in the main MIND-AD trial was not calculated because the primary outcome measures concerned feasibility and safety of the multimodal intervention. The number of participants was therefore based on experiences from previous studies, such as the FINGER study (Ngandu et al., 2015). The number of participants that were considered adequate for the original study aim was 150 participants with 50 participants in each arm. Every participant had a study partner, who was either a relative or a close friend (Trial registration: ClinicalTrials.gov NCT03249688). In this sub-study, 66 participants from Sweden and Finland were included with 22 participants in each trial-arm at baseline. 20 participants completed the baseline measurements in the Multimodal Lifestyle intervention subgroup and 21 participants in the Multimodal Lifestyle intervention + Medical food subgroup. All the participants in the control subgroup completed the baseline measurements. 19 participants in each subgroup continued to the 6 months follow up (Figure 2), (Trial registration: ClinicalTrials.gov NCT03249688). The 6-month MIND-AD trial was extended with an additional 6-month period but because of a high drop-out rate at the 12 month follow-up, data from this follow-up was not included in this sub-study.
Figure 2: Flowchart for randomizing, allocation, intervention and 6 months follow-up period for the MIND-AD study
4.2.1 Inclusion and exclusion criteria
The participants were included in the MIND-AD trial according to the following exclusion- and inclusion criteria. In summary, patients were eligible for inclusion in the MIND-AD trial if they were between 60-85 years, had an MMSE score that was less or equal to 24 out of maximum 30 points (Folstein et al., 1975), were diagnosed with prodromal AD and had an available study
Multimodal Lifestyle Intervention + Medical food
group n = 22 Randomized n = 66 Control group n = 22 Multimodal Lifestyle Intervention group n = 22 Completed baseline measurements (n =20)
Not answered due to discontinue (n=2) Baseline 6 months follow-up Completed baseline measurements (n =21)
Not answered due to discontinue (n=1)
Completed baseline measurements (n =22)
Completed 6 months follow-up (n =19) Not answered due to
discontinue (n=1)
Completed 6 months follow-up (n =19) Not answered due to
discontinue (n=1)
Completed 6 months follow-up (n =19) Not answered due to
partner. Patients were excluded if they were diagnosed with dementia or MRI consistent stroke or if they had major depressive disorders. Other exclusion criteria were severe loss of vision or concomitant participation in other trials (appendix A), (Trial registration: ClinicalTrials.gov NCT03249688).
4.2.2 Sample
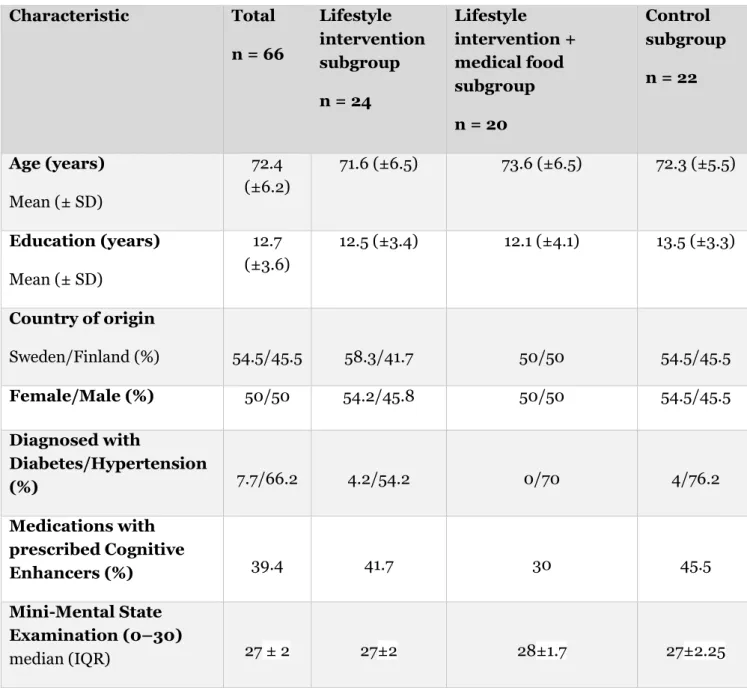
Participants in the total sample (n = 66) were 54.2% female with a median score of 27 on the MMSE test. Participants had an average age of 72.4 years (SD ±6.2). 54.4% was from Sweden and 74% had diabetes or hypertension. 39.4% was medicated with cognitive enhancers (Table 1).
Table 1: Baseline characteristics of the participants
*Cholinesterase inhibitors and glutamate inhibitor
Characteristic Total n = 66 Lifestyle intervention subgroup n = 24 Lifestyle intervention + medical food subgroup n = 20 Control subgroup n = 22 Age (years) Mean (± SD) 72.4 (±6.2) 71.6 (±6.5) 73.6 (±6.5) 72.3 (±5.5) Education (years) Mean (± SD) 12.7 (±3.6) 12.5 (±3.4) 12.1 (±4.1) 13.5 (±3.3) Country of origin Sweden/Finland (%) 54.5/45.5 58.3/41.7 50/50 54.5/45.5 Female/Male (%) 50/50 54.2/45.8 50/50 54.5/45.5 Diagnosed with Diabetes/Hypertension (%) 7.7/66.2 4.2/54.2 0/70 4/76.2 Medications with prescribed Cognitive Enhancers (%) 39.4 41.7 30 45.5 Mini-Mental State Examination (0–30) median (IQR) 27 ± 2 27±2 28±1.7 27±2.25
4.3 Measurements
The following variables and measurements used in the MIND-AD trial (Trial registration: ClinicalTrials.gov NCT03249688) were analysed in the current sub-study.
4.3.1 Physical activity and sedentary behaviour assessment
The level of PA and SB was measured with objective and subjective measurements at baseline and at 6 months in all the three subgroups. Objective measurements of the level of PA and SB was performed with an accelerometer. Participants wore Actigraph (Actigraph of Pensacola, Florida, US) accelerometer on their waist during a seven days period. Participants were instructed to wear the Actigraph during the whole time they were awake, regardless of the type of activity, even during bathing or swimming and during sedentary activities. The Actigraph recorded the intensity of and the energy expenditure associated with the physical activities they were doing. The average of 7 days accelerometer time was calculated in minutes and used as the objective measurement of both physical activity and sedentary behaviour in the sub-study. Actigraph measurement of PA and SB has been used in previous studies in people with dementia (Hartman et al., 2018) and to assess physical activity levels in people with mild Alzheimer´s disease and (Watts et al., 2016). Actigraph measurement can therefore be considered as a valid tool of measurement also for people with prodromal AD.
Subjective measures of PA and SB were self-ratings on the three questions. Two indicator questions of the National Board of Health and Welfare (BHW) and one question from the Swedish School of Sport and Health Sciences (Swedish: Gymnastik- och Idrotsshögskolan, GIH) stationary single-item question (SED-GIH) were used (appendix B). The first two questions assess the frequency and duration of physical activity and physical exercise and is called the BHW-PA questions (Olsson et al., 2016). The answers are calculated by multiplying exercise time by two and adding the number of minutes of every day physical activity time. This generates a continuous outcome, so called “activity minutes” that can be categorized as 1 = 0 minutes, 2= less than 30 minutes, 3 = 30-59 minutes (0.5-1 hour), 4 = 60-89 minutes (1-1.5 hours), 5 = 90-149 minutes ((1-1.5-2,5 hours), 6 = 150-300 minutes (2.5- hours), 7 = more than 300 minutes (>5 hours) for physical activity. The questions can also be answered in numbers of physical activity minutes per week (table mode) by specifying the amount of physical activity for each day of the week (Olsson et al., 2016). In this sub-study, only the indicator question about physical activity was used as a subjective measurement of PA. The BHW PA questionnaire has been tested in a sample including Swedish adults with a median age of 51 years (Olsson et al., 2016). The sensitivity (proportion of the insufficiently active) and specificity (proportion of the sufficiently active) detected by the BHW-PA questions are fair when answering the questions with categorical answer alternatives (0.57-0.76 respectively 0.50-0.71), (Olsson et al., 2016).
The third question concerned self-rated sedentary time, The Swedish School of Sport and Health Sciences (Swedish: Gymnastik- och Idrottshögskolan, GIH) stationary single-item question (SED-GIH) about SB. There are seven answer categories to the question: “How much time do you sit a normal day, excluding sleep?”. The answer categories are “Virtually all day”, “13–15 hours”, “10–12 hours”, “7–9 hours”, “4–6 hours”, “1–3 hours”, “Never” (Kallings et al., 2019). The SED-GIH question has a moderate concurrent validity with total stationary time and prolonged bouts of stationary time compared to accelerometer. Stationary time is defined as waking behaviour during the time spent lying, reclining, sitting or standing with no
ambulation, regardless of the energy expenditure. The SED-GIH question has shown even good convergent validity as it has a significant correlation with three other questions often used to assess sedentary behaviour. In both the concurrent and convergent validity sample, the participants were recruited from the Swedish population (Kallings et al., 2019).
4.3.2 Outcome expectancies and exercise self-efficacy
The questionnaire used in the MIND-AD trial to assess outcome expectancies include two questions. The first one is about outcome expectations for that exercise if beneficial for health in the long rung and the second one is about the impact of exercise on AD-related difficulties (appendix C). The answers are on a 10-point Likert scale, where every scale step is numbered where 1 stands for “not certain” and 10 for “very certain”. Thus, higher scores indicate higher outcome expectation. The original Outcome Expectations for Exercise (OEE) scale has nine questions with answers on a 5-point Likert-scale (Resnick et al., 20001). The scale is scored by summing the numerical ratings for every answer and by dividing it by the number of responses. The outcome measure is the mean score of all items. OOE scores range between 1 (low outcome expectations) and 5 (strong outcome expectations). Individuals that exercise regularly are considered to have higher outcome expectancies than those who do not. Higher scores on outcome expectations indicate stronger outcome expectancies. The internal consistency (alpha coefficient = 0.89) has been found high. The original OOE scale is tested for reliability and validity in a sample of adults older than 65 years with a MMSE score ≥ 20 living in a continuing care retirement community in a North America city. The results show good internal consistency (alpha =0.87). Furthermore, criterion-related validity is confirmed by the results showing that individuals who exercised regularly had higher outcome expectations for exercise than those who did not exercise. To explore reliability, a structural equation modelling approach was used with R2 estimates. It showed some support for reliability because the majority of R2 estimates were less than 0.5 (Resnick et al., 20001).
The Exercise self-efficacy scale (E-SES) is constructed as a 10-point Likert scale where every scale step is numbered and 1 stands for “not confident” and 10 stands for “very confident” (appendix D). The outcome measure is the mean score of all items (Resnick & Jenkins, 2000). Higher scores indicate higher self-efficacy which stands for the strength of self-efficacy expectations. The questions are the same in the MIND-AD trial as in the original E-SES scale except for the part regarding the frequency and duration of exercise (2 times a week/60 minutes vs. 3 times a week/20 minutes). This difference is needed to adjust the question after the exercise groups in the MIND-AD trial. Validity and reliability have been tested in a sample including older adults (≥65 years) with a MMSE score ≥ 20, living in a continuing care retirement community in a North America city. The internal consistency (alpha coefficient = .92) confirmed the internal consistency of the questionnaire (Resnick & Jenkins, 2000). The construct- and criterion-related validity appears satisfactory as individuals with better physical and cognitive health were more likely to have higher self-efficacy expectations. The lambda estimates were statistically significant (≥0.61, p<0.05) which gives a strong support for that each item in the questionnaire expresses self-efficacy expectations (Resnick & Jenkins, 2000).
4.4 Procedure
In the main study, people with prodromal AD in Finland and Sweden, who appeared to fulfil eligibility criteria, were informed about the study, and were invited to a screening visit. People who met the in- and exclusion criteria were enrolled directly or during a separate baseline visit. Data collection was done by the participants personally visiting the health centre at both baseline and at the 6 months follow up to fill in the questionnaires. Randomization was performed by computer-generated allocation, that was done in blocks of six, two individuals randomly allocated to each subgroup: Multimodal Lifestyle intervention, Multimodal Lifestyle intervention + Medical food and control subgroup, at each site after baseline, by the study nurse. Randomization was balanced across sites. Double blinding was pursued as completely as possible. Participants were not actively told which subgroup they belong to. Investigators conducting outcome measures were blinded for the randomization group and were not involved in the intervention activities. Participants were advised not to discuss the intervention during testing sessions. The research team doctors, research nurses, psychologists and physiotherapists were also blinded.
The master student of this study has received the study protocol of the MIND-AD trial from Charlotta Thunborg, who is the project coordinator in MIND-AD and researcher at Karolinska Institute. After receiving the anonymous and coded dataset with the baseline characteristics, measurements of physical activity, sedentary time, outcome expectations and self-efficacy, the data was analysed during spring 2021 and is presented as a second-year master thesis.
4.5 Analysis and data management
Statistical analyses were performed with IMBM SPSS statistics version 22. The level of significance was set at α ≤ 0.05. for all analyses. For non-normally distributed data the statistical analyses were non-parametric and for normally distributed data, parametric statistical analyses were used. The study questions were analysed as follows.
Between-subject analyses for were conducted with the Kruskall-Wallis test (study question 1, 2, 3). In case of no significant differences between the subgroups, within-subject analyses were performed with the Wilcoxon signed-rank test for non-normally distributed data and paired-Sample T-test for normally distributed data in order to analyse differences in the total sample over time.
Changes in the E-SES and the OEE scales between baseline and the 6-month follow-up were analysed for the two intervention subgroups together by Wilcoxon signed-ranked test (study question 4).
To answer study question 5, a bivariate correlation analysis with Spearman´s rho was carried out to see if there were any significant correlations between efficacy beliefs and outcome expectancies at baseline and PA levels measured by Actigraph at the 6-month follow-up. In case of significant correlations between the variables, the next step was to perform a simple or multiple linear regression analysis, depending on the number of independent variables with significant correlations with the dependent variable, in order to explore the predictive value of a model with self-efficacy and outcome expectancies at baseline on PA at 6 months. In the regression analysis, the standardized coefficients Beta (β) values were presented.
Likert-scale variables were treated as continuous variables in this study (Wu & Leung, 2017). To describe the baseline characteristics, descriptive statistics were used.
The reason why the two intervention subgroups were analysed together was the fact that the Multimodal Lifestyle intervention group and the Multimodal Lifestyle intervention + Medical food group received the same lifestyle intervention consisting of physical and cognitive exercise and social activities. Furthermore, both intervention groups answered questionnaires about self-efficacy and outcome expectancies for exercise. The two intervention groups were therefore considered equivalent from a behavioural medicine aspect.
The two questions about outcome expectations for exercise were designed to capture a general (long term health) and a specific (AD-related difficulties) aspect of AD. For this reason, the two questions were analysed separately. The median of each outcome expectations for exercise questions and the median of the 9 questions in the self-efficacy for exercise questionnaire was used in the analysis. Data was collected in form of mild and moderate/vigorous intensity physical activity by Actigraph. For the study, mild and moderate/vigorous PA was summed for every participant as the objective measurement of PA.
Effect size was calculated for between-group and within-group differences as eta squared (η2) from z-value and the total number of observations, where η2=0.01 is considered a small effect size, η2=0.06 is considered an intermediate effect size and η2=0.18 is considered a large effect size (Lenhard & Lenhard, 2016). Effect size was calculated in those results where the p-value was either ≤0.05 or slightly over 0.05, but not higher than 0.06. The reason for presenting effect size was to give some information about the magnitude of an effect that is not statistically significant but may be of clinical significance (Erceg-Hurn & Mirosevich, 2008). For effect size calculation (η2), the z-value and the total number of observations were reported.
4.6 Ethical considerations
By using secondary data, the value of the investment in data collection is maximized (Morrow et al., 2014). Furthermore, it reduces the burden on participants by not making them participate in a new study. The major ethical consideration in the secondary analysis is that data should be anonymized before it is released to the sub-study author. For this reason, the data set was coded, anonymous and released under safe circumstances through secure technological solution to the author. It was also important to make sure that the previous ethical approval for the MIND-AD trial also applies to the sub-study. The consent of the participants was determined to be valid also for the sub-study. The outcomes of analyses from the sub-study should not allow for the participants to be re-identified. The use of previously gathered data should not result in any damage or distress to the participants.
The MIND-AD trial was conducted according to Good Clinical Practice (Pieterse & Diamant, 2014) and the ethical principles stated in the Declaration of Helsinki (World Medical Association, 2001). According to the Helsinki Declaration, the interests of the patient should outweigh the needs of research. (World Medical Association, 2001). The following ethical principles were applied in the MIND-AD trial. Before enrolment in the study, both the participants and their study partners submitted a written informed consent after receiving information about the aim, method, source of funding of the study and about potential risks or discomfort the study could entail. The participants were informed that consent could be withdrawn at any time without any need of explanation. The subject information was treated
confidentially by collecting made-up subject initials. Identification codes that connected the subjects` names to their identification number were stored in the Investigator Site File. It was ensured that subject information would not be made publicly available. For the main study, both the participants and ethics committee would be informed if any negative event would occur that would imply risk for the participants during the intervention. Any potential burden or risk would lead to suspension if the suspension would not mean risk for the participants health. To prevent any cardiovascular or musculoskeletal injuries related to the exercise in the intervention subgroups, the instructions given to every participant were individually formed to guarantee appropriate performance techniques (Trial registration: ClinicalTrials.gov NCT03249688). The MIND-AD trial has been ethically approved. DNR: 2016/2605-31/1, 2017/1466-32 and 2017/1730-32.
5
RESULTS
5.1 Physical activity and sedentary behaviour measured by Actigraph
There were no significant differences in PA (p=0.19) or SB (p=0.31) between the subgroups at 6 months.
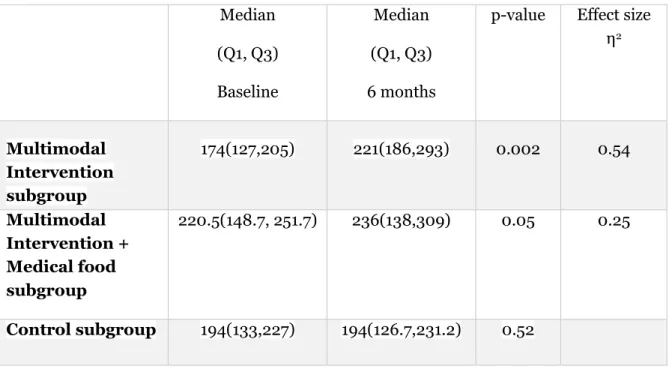
For the Multimodal Intervention subgroup, PA was significantly higher at 6 months compared to baseline (z=-3.114, N = 18, two-tailed). The effect size (η2) = 0.54, indicates a large effect size. For the Multimodal Intervention + Medical food subgroup there was no significant difference in PA between baseline and 6 months follow-up (z=-1.931, N = 15, two-tailed) with a large effect size (η2)= 0.25. For the control subgroup, there was no difference in PA between baseline and 6 months follow-up (z=-0.639, N—ties = 17, two-tailed), (table 3).
There was no difference in SB in the Multimodal Intervention subgroup (z=-1.634, two-tailed), in the Multimodal intervention + Medical food subgroup (z=-1.363, two-tailed) or in the control subgroup (z=-0.544, two-tailed), between baseline and the 6 month follow-up (Table 4).
As no significant differences were found in SB between the subgroups, differences in the total sample were analysed between baseline and the 6 months follow-up. The analyses showed no significant difference (t=-1.590, df=50, mean difference=-28.25, 95%CI:-63.95,7.447, p=0.118, two-tailed), (Table 4).
5.2 Physical activity and sedentary behaviour measured by
questionnaire
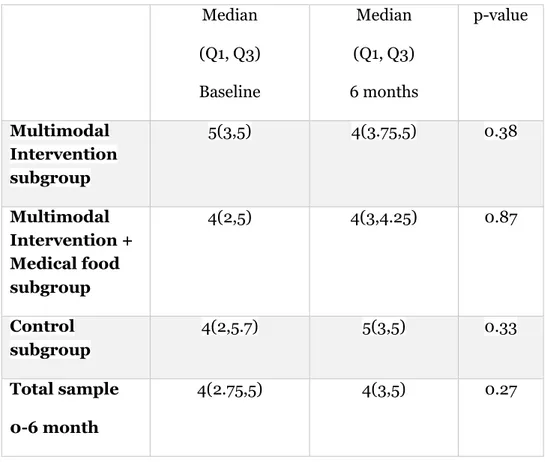
There were no significant differences in PA (p=0.48) or SB (p=0.65) between the subgroups at 6-month follow-up.
There were no significant differences in PA in the Multimodal Intervention subgroup (z=-0.870, two-tailed), in the Intervention + Medical food subgroup (z=-0.159, two-tailed) or in the control subgroup (z=-0.965, two-tailed) between baseline and the 6 month follow-up (Table 5).
No significant differences were observed in SB in the Multimodal Intervention subgroup (z=-1.634, tailed), in the Multimodal Intervention + Medical food subgroup (z=-.707, two-tailed) or in the control subgroup (z=-0.225, two-two-tailed), (Table 6).
Due to no significant differences in the subgroups over time in either PA or SB, differences in the total sample over time were analysed between baseline and the 6-month follow-up. These analyses showed no significant difference in PA (z= -1.100, two-tailed), (Table 5) or in SB (z-0.930, two-tailed), (Table 6).
Table 3: Physical activity measured by Actigraph
Table 4: Sedentary behaviour measured by Actigraph Median (Q1, Q3) Baseline Median (Q1, Q3) 6 months
p-value Effect size η2 Multimodal Intervention subgroup 174(127,205) 221(186,293) 0.002 0.54 Multimodal Intervention + Medical food subgroup 220.5(148.7, 251.7) 236(138,309) 0.05 0.25 Control subgroup 194(133,227) 194(126.7,231.2) 0.52 Median (Q1, Q3) Baseline Median (Q1, Q3) 6 months p-value Multimodal Intervention subgroup 525(459,596) 607(551,648) 0.10 Multimodal Intervention + Medical food subgroup 528(457.5,556.7) 576(491,623) 0.17 Control subgroup 542.5(460.7,603,2) 534(476,632.2) 0.59
Table 5: Physical activity measured by questionnaire
Table 6: Sedentary behaviour measured by questionnaire Median (Q1, Q3) Baseline Median (Q1, Q3) 6 months p-value Multimodal Intervention subgroup 5(3,5) 4(3.75,5) 0.38 Multimodal Intervention + Medical food subgroup 4(2,5) 4(3,4.25) 0.87 Control subgroup 4(2,5.7) 5(3,5) 0.33 Total sample 0-6 month 4(2.75,5) 4(3,5) 0.27 Median (Q1, Q3) Baseline Median (Q1, Q3) 6 months p-value Multimodal Intervention subgroup 4(3,5) 4(4,4) 0.10 Multimodal Intervention + Medical food subgroup 4(3,4) 4(3,4) 0.48 Control subgroup 4(3,4.75) 4(3.2,5) 0.82 Total sample 0-6 month 4(3,4) 4(4,4) 0.35
5.3 Self-efficacy and outcome expectancies
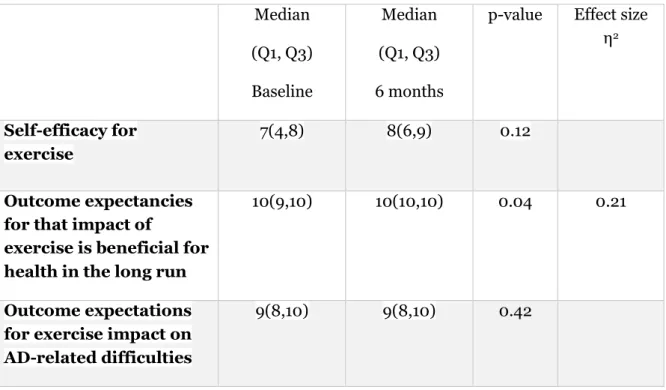
For the two intervention subgroups together, there was no significant change in self-efficacy for exercise between baseline and the 6-month follow-up (z = -1.539, two-tailed). There was no significant change in outcome expectancies for the impact of exercise on AD-related difficulties between baseline and 6-month follow-up (z = -0.797, two-tailed). Outcome expectancies for that impact of exercise is beneficial for health in the long run, significantly increased between baseline and the 6 months follow up (z = -2.099, N = 21, two-tailed), (Table 7). Effect size (η2)= 0.21 indicates a large effect size (Table 7).
Neither outcome expectations for that impact of exercise is beneficial for health in the long run (p=0.52), or self-efficacy for exercise (p=0.94) at baseline correlated to physical activity measured by Actigraph at six months follow-up. There was a significant positive correlation between outcome expectations for exercise impact on AD-related difficulties (p=0.01) at baseline on physical activity at the 6 months follow-up (Spearman’s rho =0.464) with a moderate correlation.
A simple linear regression was calculated to predict physical activity measured by Actigraph at 6 months based on outcome expectations for exercise impact on AD-related difficulties at baseline. The results of the regression suggested that outcome expectations for exercise impact on AD-related difficulties at baseline explained 15% of the variance in PA at the 6 months follow-up, R2=0.15, F(1,27)=5.9, p=0.22. Outcome expectations for exercise impact on AD-related difficulties at baseline significantly predicted PA at the 6-month follow-up, β=0.42, t=2.42, p=0.22.
Table 7: Self-efficacy and outcome expectancies between baseline and 6-month follow-up Median (Q1, Q3) Baseline Median (Q1, Q3) 6 months
p-value Effect size η2
Self-efficacy for exercise
7(4,8) 8(6,9) 0.12
Outcome expectancies for that impact of
exercise is beneficial for health in the long run
10(9,10) 10(10,10) 0.04 0.21
Outcome expectations for exercise impact on AD-related difficulties
6
DISCUSSION
6.1 Summary of Results
In this sub-study, the aim was to study how sedentary behaviour and physical activity change during a multimodal lifestyle intervention for people with prodromal AD and to explore the predictive value of self-efficacy and outcome expectancies for exercise on level of physical activity. The results showed that objectively measured PA increased significantly in the Multimodal Intervention subgroup with a large effect size. For the Multimodal intervention + Medical food subgroup, the difference between baseline and 6 months follow up was not significant but the effect size was large. SB did not change significantly in the subgroups or in the total sample during the 6-month intervention. In the two intervention subgroups together, outcome expectancies for that impact of exercise is beneficial for health in the long run was significantly higher at the 6-month follow-up compared to the baseline with a large effect size. A simple linear regression suggested that outcome expectations for exercise impact on AD-related difficulties at baseline explained 15% of the variance in PA at the 6 months. Self-efficacy for exercise or outcome expectancies for the impact of exercise on AD-related difficulties did not change during the intervention period.
6.2 Results discussion
PA measured by Actigraph was significantly higher in the Multimodal Intervention subgroup at the 6-month follow-up compared to the baseline measurement. PA levels were not significantly higher in the Multimodal Intervention + Medical food subgroup but the difference had a large effect size. This increase in PA-level in the multimodal intervention subgroup could be the result of the intervention that included individually tailored physical therapy led exercise sessions. This result is confirmed by a previous study (Streber et al., 2017) that investigated the effect of a multimodal program combining PA with cognitive and social interventions in people over the age of 60 at risk for developing dementia in which the multimodal intervention resulted in increased level of average number of steps in the intervention group after 3 month but not in the control group (Streber et al., 2017). It is therefore possible that the multimodal lifestyle intervention in the MIND-AD study had a positive effect on the participants physical activity level.
SB did not change significantly in the subgroups or in the total sample during the multimodal intervention. A possible explanation for this result can be that decreasing SB can be more challenging if the intervention program does not include interventions to decrease SB. To reduce SB, Dillon and Prapavessis, (2021) has tested a prompting device in people with mild-to moderate cognitive impairment over the age of 65, with a significant effect on reducing SB. According to the description of the intervention in the main study, there were no parts of the intervention targeting to reduce sedentary behaviour which can have resulted in either unchanged or increased SB for the participants during the intervention.
There was a significant increase in outcome expectancies for that impact of exercise is beneficial for health in the long run. This result is comparable to the findings from a previous study of Tochel et al. (2019) in which outcome expectancies, what people with mild cognitive
impairment or AD consider important, were physical health and mobility (Tochel et al., 2019). Outcome expectancies for the impact of exercise on AD-related difficulties at baseline had a positive predictive value PA at the 6-month follow-up. This could indicate that the participants initial outcome expectations to be able to deal with everyday tasks was an essential factor in their intention and decision making to reach higher levels of PA.
Self-efficacy for exercise and outcome expectancies for the impact of exercise on AD-related difficulties did not change between baseline and the 6-month follow-up. To change a person´s behaviour, the determinants for behaviour change need to be addressed. Changing a behaviour requires that the person understands what the behaviour change means as the determinants for behaviour change are based on thoughts and associations (Kok et al., 2016). It is therefore not certain that the participants in the study received interventions targeting determinants for behaviour change such as self-efficacy and outcome expectancies for exercise. Resnick et al. (20002) suggests that increasing both self-efficacy and outcome expectations to exercise can be done by interventions including efficacy-based interventions, such as teaching about the benefits of exercise, being exposed to other elderly people who succeed with exercise as role models, and reinforcing the benefits of exercise (Resnick et al., 20002). The MIND-AD trial was mainly a clinical trial with an addition of questionnaires about self-efficacy and outcome expectancies for exercise. The intervention did not explicitly include any BCTs but had certain components that could serve as BCTs. These components were social support from a study partner, support from personal trainer, physiotherapist, psychologist, nutritionist as well as modelling as the participants participated in group activities there, they could meet other participants as their role models. These components were however not designed as parts of an efficacy-based intervention. In previous research, outcome expectancies and self-efficacy for exercise have been studied in an elderly population after stroke (Groeneveld, et al., 2018) and in elderly over the age of 60 without any specific inclusion criteria for cognitive status (Gellert et al., 2012). This sub-study has therefore a unique contribution to the research field by exploring self-efficacy and outcome expectancies for exercise for people with prodromal AD.
6.3 Method discussion
It has been shown in previous research (Herbolsheimer et al.; 2018; Tudor-Locke & Myers, 2001; Watts et. al., 2013) that people with cognitive decline or AD have difficulties making proper subjective estimation of their PA by either over- or underestimating their PA levels when compared to objective measurements of PA. According to Watts et al. (2013), this could be due to the floor effect, which, according to the author, can be found in most of the self-report measures of PA designed to elderly, sedentary people with chronic health problems or disability (Watts et. al., 2013). In the cross-sectional study of Herbolsheimer et al., (2018) the results suggested that cognitive decline was one factor that could explain the differences between objective and subjective measurements of PA. Therefore, no attempts were made in this substudy to make any correlation analysis between the subjective and objective measurements of PA.
The MIND-AD trial was mainly a clinical study without the incorporation of physiotherapy with a behavioural medicine approach. Data about physical activity, sedentary behaviour and questionnaires about self-efficacy and outcome expectancies were added to the study by a researcher in order for these variables to be analysed in a separate sub-study, making it possible to carry out a secondary-analysis in a behavioural medicine perspective. The
theoretical background of Social Cognitive theory was added and the results from the analyses were put in a context of behavioural medicine after data was collected. Detailed description of the method and the multimodal intervention was necessary to be able to draw relevant conclusions from the results as the author of the secondary analysis did not participate in the main study.
Inter-instrument reliability of accelerometer was investigated in a sample of adults (mean age 31 years) for wear time, overall physical activity (counts per minute), sedentary behaviour, light, moderate and vigorous physical activity, moderate-to-vigorous physical activity and the overall physical activity level based on the vector magnitude. The results show that inter-instrument reliability was higher with increased accumulation of data which suggested at least a 3-4 days period of valid wear time to obtain valid results. (Aadland & Ylvisåker, 2015). In order to get a reliable estimate of physical activity and sedentary behaviour in older adult (65-75 years), Saaski et al. (2018), has examined the number of days needed to monitor physical activity and sedentary time in elderly people living in senior centre without any significant cognitive disorders. The results showed that at least five consecutive days of monitoring were required to estimate sedentary behaviour and physical activity from accelerometer data in older adults (Sasaki et al., 2018). This confirms the reliability of Actigraph measurements in the MIND-AD trial as participants wore the Actigraph on their waist in periods of 7 days. Both the BHW-PA and SED-GIH questions were tested in cognitively healthy adults with a mean age below 64 years (Olsson et al., 2016; Kallings et al., 2019). Cognitive impairment can make it difficult for a person to answer a questionnaire. To ta find out more about the connection between cognition and the ability to answer a questionnaire in people with dementia, Kutschar et al. (2019) has examined the quality of responses to a survey about pain. The participants were nursing home residents (≥65 years of age) of which 32% had dementia. The purpose of the study was to analyse how different factors, such as cognitive impairment affects data quality from a survey by assessing item nonresponse (missing information for a certain variable for a certain participant). The participants were divided into two groups: no/mild impairment group (MMSE 18-30) and a moderate impairment group (MMSE 10-17). The results show that the likelihood for item nonresponse was significantly higher for participants with moderate impairment with 11.9% item nonresponse compared to those with no/mild impairment where the proportion was 4.1% (Kutschar et al., 2019). These results indicate that people with prodromal AD (MMSE score ≥ 24 scores) should be equally able to answer a questionnaire as people with moderate dementia. Therefore, questionnaires were a suitable way of measuring efficacy beliefs, physical activity and sedentary behaviour in the MIND-AD trial and should be considered as an adequate method of data collection in future studies.
The OOE questions in the MIND-AD study were designed for people with prodromal AD and were not the same in the MIND-AD trial as the nine questions in the original OOE scale. Also, the range of the answers differed with a 5-point Likert scale in the original questions and a 10-point Likert scale in the MIND-AD trial. Thus, the OOE questions reliability and validity cannot be generalized from the original scale to the scale used in the study. On the other hand, the questions needed to be adjusted for the participants in the MIND-AD trial to people with prodromal AD as the original questions included question about bone strength and mental alertness, that would not have been appropriate questions to the study