http://www.diva-portal.org
Postprint
This is the accepted version of a paper published in Langmuir. This paper has been peer-reviewed but does not include the final publisher proof-corrections or journal pagination.
Citation for the original published paper (version of record):
Yeung, S Y., Sergeeva, Y., Dam, T., Jönsson, P., Pan, G. et al. (2019)
Lipid Bilayer-like Mixed Self-Assembled Monolayers with Strong Mobility and Clustering-Dependent Lectin Affinity
Langmuir, 35(24): 8174-8181
https://doi.org/10.1021/acs.langmuir.9b01452
Access to the published version may require subscription. N.B. When citing this work, cite the original published paper.
Permanent link to this version:
Lipid bilayer-like mixed self-assembled monolayers with
1strong mobility and clustering dependent lectin affinity
23
Sing Yee Yeunga, Yulia Sergeeva, Tommy Damb, Peter Jönssonb, Guoqing Pana, Vivek 4
Chaturvedia and Börje Sellergrena* 5
a Department of Biomedical Sciences and Biofilms-Research Center for Biointerfaces 6
(BRCB), Faculty of Health and Society, Malmö University, 205 06 Malmö, Sweden.
7
b Department of Physical Chemistry, Lund University, Box 124, 22100 Lund, Sweden 8 * borje.sellergren@mau.se 9 10 Abstract 11
Glycans at the surface of cellular membranes modulate biological activity via multivalent
12
association with extracellular messengers. The lack of tuneable simplified models mimicking
13
this dynamic environment complicates basic studies of these phenomena. We here present a
14
series of mixed reversible self-assembled monolayers (rSAMs) that addresses this deficiency.
15
Mixed rSAMs were prepared in water by simple immersion of a negatively charged surface in
16
a mixture of sialic acid- and hydroxy-terminated benzamidine amphiphiles. Surface
17
compositions derived from infrared reflection absorption spectroscopy (IRAS) and film
18
thickness information (atomic force microscopy, ellipsometry) suggest the latter to be
19
statistically incorporated in the monolayer. These surfaces affinity for the lectin hemagglutinin
20
revealed a strong dependence of the affinity on the presentation, density and mobility of the
21
sialic acid ligands. Hence, a spacer length of 2 ethylene glycol and a surface density of 15%
22
resulted in a dissociation constant Kd,multi of 1.3 x 10-13 M, on a par with the best di- or tri-23
saccharide based binders reported to date, whereas a density of 20% demonstrated complete
24
resistance to hemagglutinin binding. These results correlated with ligand mobility measured by
25
fluorescence recovery after photobleaching (FRAP) which showed a dramatic drop in the same
26
interval. The results have a direct bearing on biological cell surface multivalent recognition
27
involving lipid bilayers and we believe will impact the design of model surfaces and sensors
28
for both fundamental and applied studies.
Introduction
30
Glycans, covering the surface of cellular membranes, play crucial roles in a wide range of cell
31
surface processes, such as pathogen recognition and binding, antibody recognition and cell-cell
32
interaction and signalling.1-3 As a singular glycan-protein interaction is weak, multiple 33
association between the two entities is necessary to achieve high avidity. Elucidating their
34
biological roles require understanding of the glycan’s topologies in relation to the binding
35
affinity of its corresponding ligand. The complexity and diverse nature of glycans and their
36
native environment have hampered progress in the field. In order to decode glycan’s
37
functionality for improving therapeutics and diagnostics, researchers use simplified and
38
tuneable two or three-dimensional models.1-5 39
Strategies for immobilizing glycans onto scaffolds can be either static (polymers, dendrimers,
40
self-assembled monolayers (SAMs)) or dynamic (supported lipid bilayers (SLBs), host-guest
41
based, poly rotaxanes). The latter is particularly attractive due their responsive nature and
42
biomimetic characteristics.1, 6-9 Surfaces with lateral adaptability such as SLBs or liposomes 43
spatially rearranges the glycans during the binding processes. These unique properties as
44
compared to their static counterparts enhance binding affinity and aid in the discovery of
45
secondary mechanisms.10-13 Despite their importance, apart from lipid assemblies, 2D interfaces 46
with long-range mobility resembling biological membranes suitable for molecular recognition
47
are rare.
48
We have recently reported on an air-stable and adaptable biosensing platform, reversible
self-49
assembled monolayers (rSAMs), featuring strongly enhanced affinity and sensitivity towards
50
viruses and lectins.13 This new sensing system utilizes noncovalent amidinium-carboxylate ion 51
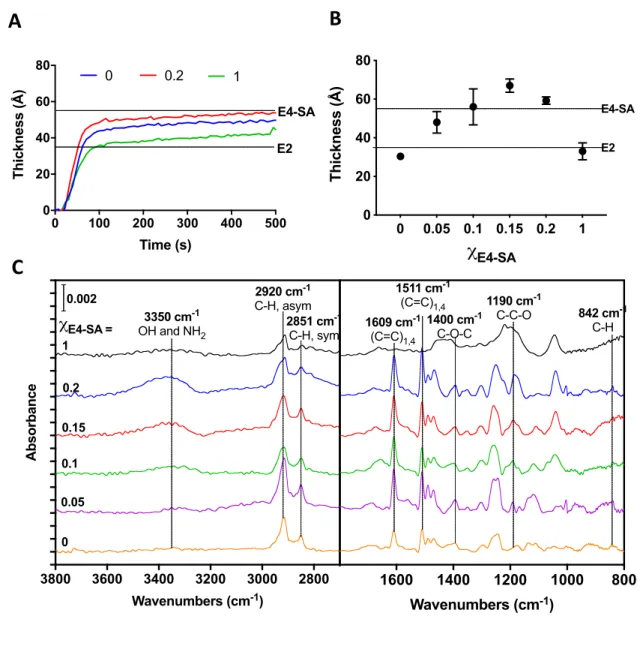
pairs for building up stable two-dimensional assemblies, akin to lipid bilayers but with a simple
52
preparation process and fast on/off rates. Thus, benzamidine-terminated amphiphiles
thiol SAMs to form ordered monolayers with a tunable pH responsiveness. Layer thicknesses
55
and order correlate with the molecular length of the amphiphile, which beyond a certain length
56
feature liquid crystalline-like order and an odd even chain length related tendency to form
57
bilayers. These layers are stable towards rinsing and resist exchange by common plasma
58
proteins and charged surfactants, while resisting non-specific protein adsorption.13-17 59
Here we demonstrate the use of mixed rSAMs as a tunable platform to control surface density
60
and presentation of glycans using different mole fractions of sialic acid amphiphiles (E2-SA
61
and E4-SA) in ω-(ethylene glycol)2,α-(4-amidinophenoxy)decanes (E2-OH) solutions (Fig. 1). 62
The mixed rSAMs surfaces demonstrated tunable surface density, while retaining fluidity with
63
the formation of distinct saccharide clusters akin to gangliosides in lipid bilayers.12 Glycan 64
surface density thus modulates the dynamics and affinity of these surfaces towards its
65
corresponding lectins.
66
67
Figure 1. Schematic illustration of preparation and evaluation of sialic acid-functionalized mixed reversible self-assembled monolayers (rSAMs). i. 16-mercaptohexadecanoic acid
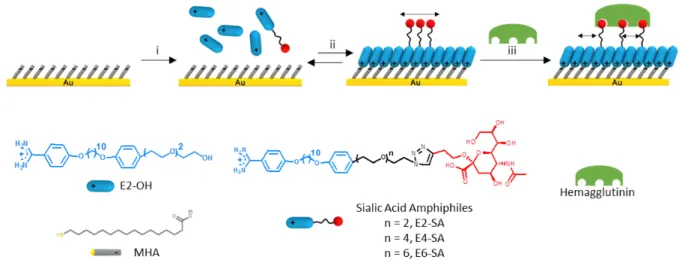
(MHA) modified surface placed in 50 µM mixed sialic acid amphiphiles, E2-SA, E4-SA or E6-SA mixed with E2-OH in pH 8 HEPES buffer solution. ii. Ordered mixed rE6-SAMs formed after 18 hrs of incubation and rinsing with pH 8 HEPES buffer. iii. Hemagglutinin binding isotherms on mixed rSAMs obtained. Images are not drawn to scale.
Results and discussion
69 70
Design of ligands and rSAMs: Optimization of ligand decorated SAMs demands attention to
71
multiple factors governing the multivalent interactions with the receptor. Two key parameters
72
to consider are the nature and length of the linker group and the surface density of ligands. For
73
instance, accessibility to lectin binding is enhanced by inserting linkers containing 2-3
74
ethyleneglycol repeat units between the glycan and the mesogen unit (Fig. 1) whereas lateral
75
spacing requires mixed SAMs to contain less than 20 % of ligand decorated amphiphile to
76
promote strong multivalent interactions.18-24 We have here compared sialic acid terminated 77
amphiphiles with two, four and six EG repeats in the sialic acid tether (E2-SA, E4-SA, E6-SA)
78
in combination with an OH terminated amidine with 2 EG repeats (E2-OH). The amphiphiles
79
were synthesised as described in the Supporting Information with the final step being the click
80
coupling of alkyne-modified sialic acid and azide-terminated amidine.
81
Mixed rSAM formation and stability: Our first objective was to study the formation and nature
82
of mixed rSAMs of ligand and OH terminated amphiphiles. For this purpose, we first chose the
83
shorter OH terminated amphiphiles E2-OH and the medium size ligand terminated amphiphile
84
E4-SA. Based on previous reports demonstrating optimal sialic acid coverages with respect to
85
hemagglutinin binding of 20 % or below 19-21, 23-24 a series of mixed rSAMs was prepared by 86
immersing 16-mercaptohexadecanoic acid (MHA) modified gold surfaces (MHA-SAMs) in pH
87
8 HEPES buffer containing varying mole fractions of sialic acid amphiphile, E4-SA in filler
88
E2-OH,
c
E4-SA = 0 to 0.2 (50 µM). Layer composition and molecular order and orientation of 89the rSAM amphiphiles were evaluated by in situ (ISE) and ex situ (ESE) ellipsometry, quartz
90
crystal microbalance (QCM-D), infrared reflection-adsorption spectroscopy (IRAS) and atomic
91
force microscopy (AFM) (Fig. 2 and 3).
A comparison of the assembly kinetics by ISE and limiting equilibrium thickness for the
93
homogenous, E4-SA and E2-OH and mixed,
c
E4-SA = 0.2 systems suggested incorporation of 94both amphiphiles in the latter layer (Fig. 2A).
95 96 97 98 99 100
A
B
C
0 0.05 0.1 0.15 0.2 1 0 20 40 60 80 χE4-SA Thickness (Å) E4-SA E2 0 100 200 300 400 500 0 20 40 60 80 Time (s) Thickness (Å) 0 0.2 1 E4-SA E2 2920 cm-1 C-H, asym 2851 cm-1 C-H, sym 2800 3000 3200 3400 3600 3800 Wavenumbers (cm-1) Absorbance 3350 cm-1 OH and NH2 0 0.05 0.1 0.15 0.2 χE4-SA = 1 0.002 842 cm-1 C-H 1511 cm-1 (C=C)1,4 800 1000 1200 1400 1600 Wavenumbers (cm-1) 1400 cm-1 C-O-C 1190 cm-1 C-C-O 1609 cm-1 (C=C)1,4Figure 2. Surface characterization of mixed rSAMs. A. Real-time change in film thickness
upon exposure of an MHA-SAM to pH 8 HEPES buffer containing 50 µM cE4-SA = 0, 0.2 or 1.
B. Ex situ ellipsometric thickness of rinsed MHA-SAMs surfaces modified with cE4-SA = 0 - 1.
C. Baseline-corrected IRAS spectra of MHA-SAMs modified with cE4-SA = 0 – 1. Dotted lines
in A and B indicate calculated length of the corresponding amphiphiles. Assignments of the IR bands were based on positions reported in literature.13-17
All three systems demonstrated a rapid initial adsorption upon amphiphile exposure with a close
101
to monolayer thickness attained after ca. 100 s. This was followed by a considerably slower
102
phase reflecting continued amphiphile incorporation or ordering of the existing layer
103
components. Interestingly, the mixed rSAM with
c
E4-SA = 0.2 displayed the largest thickness as 104compared to the homogenous systems of pure E2-OH or E4-SA.
105
Ex-situ air ellipsometry was then used to estimate the layer thickness of rinsed homogenous
106
and mixed rSAMs after incubation in the amphiphilic solutions overnight (Fig. 2B). Starting
107
with the rSAM of E2 a thickness slightly beneath the molecular length of E2 (Fig. S1) was
108
measured suggesting a nearly complete layer of E2 oriented perpendicular to the surface.
109
Monitoring the self-assembly by QCM-D confirmed this result, indicating the formation of a
110
relatively rigid film of a mass agreeing with the ISE results (ca 2.3 mg/m2) (Fig. S13). With 111
increasing E4-SA the thickness then continuously increased to reach a value of nearly 70 Å at
112
a ligand density of
c
E4-SA = 0.15. Beyond this ligand density the thickness dropped to reach a 113value corresponding to sub-monolayer at
c
E4-SA = 1. As we previously argued13, this suggests 114that the steric repulsion of the bulky sialic acid head groups (10 Å of the sialic acid head group
115
as compared to 4 Å of the benzene ring (see Fig. S1) precludes close packing of the layers in
116
the homogenous system. With the inclusion of filler E2-OH in the mixed systems (3 Å across
117
the ethylene glycol head group) the sialic acid head groups are brought further apart leading to
118
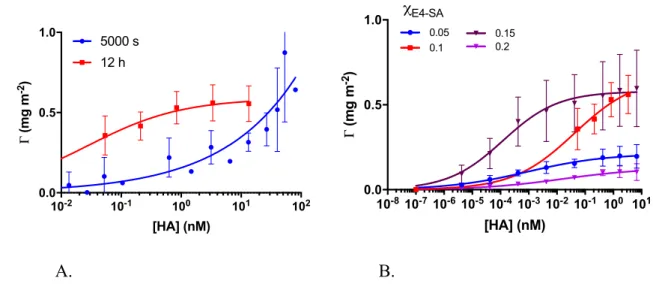
an improved lateral packing.25 Although qualitatively the behaviour above makes sense, the 119
quantitative changes in thickness are puzzling. The two-fold increase in thickness observed by
120
increasing the ligand density from
c
E4-SA = 0 toc
E4-SA = 0.15 way exceeds the theoretical value 121expected for statistically mixed monolayers. Formation of bilayered structures is one possible
122
explanation for this behaviour that we put forward in our first report. Although refractive index
123
effects could be another contributing factor, the increased IRAS signal intensity of the C=C
stretch band at 1609 cm-1 (Fig. 2C) supports the former explanation. To give further clues 125
regarding these structural features IRAS, goniometry and AFM were used to study the layers.
126
rSAM characterisation by IRAS and goniometry: Although the layer formed by homogenous
127
sialic acid amphiphiles,
c
E4-SA = 1 appeared partly unstable to rinsing (Fig. 2C), the layers 128formed using mixed sialic acid and filler amphiphiles,
c
E4-SA = 0.05 – 0.2 exhibit relative band 129intensities and bandwidths corresponding to well-ordered layers. Important structural detail is
130
revealed considering the intensities of the (C=C)1,4 stretch at 1609 cm-1 and 1511 cm-1 that have 131
transition dipole vectors oriented along the 1,4-axis of the benzene ring relative to the intensities
132
of the aromatic C–H out-of-plane bending mode at 841 cm-1 and the amidine N-C-N asymmetric 133
stretch at 1650 cm-1, that have transition dipole vectors perpendicular to the 1,4-axis. The 134
pronounced increase of the former and the concomitant decrease of the latter signals with
135
reference to the transmission spectra of the neat compounds point to a near upright position of
136
the layer amphiphiles.
137
In the high frequency region, the CH2 stretch vibrations at 2918 cm-1 (asym) and 2850 cm-1 138
(sym) and the sharpness of these bands of the layer spectra indicate the presence of trans
139
extended closely-packed amphiphiles. It is interesting to note that layers with
c
E4-SA = 0.2 and 1400.1 feature a slight shift to lower wavenumbers (2919 cm-1 (asym); 2850 cm-1 (sym) and 2918 141
cm-1 (asym); 2849 cm-1 (sym) respectively) as compared to
c
E4-SA = 0.15 (2920 cm-1 (asym); 1422852 cm-1 (sym)). This suggests an enhanced molecular order at the former sialic acid 143
densitiesand coincides with the decrease in amphiphile mobility (vide infra).
144
To obtain information regarding the percentage of sialic acid amphiphiles in the mixed rSAMs,
145
we compared the IRAS spectra of the homogenous filler and sialic acid layers, focusing on the
146
prominent signals from sialic acid at ca. 3350 cm-1 (bonded OH and mono substituted amide), 147
1400 cm-1 (C-O-C) and 1190 cm-1 (C-OH) in the E4-SA spectra. These signals corresponded 148
well with literature observations.27 Although a visual comparison of the intensities of the signals 149
characteristic for sialic acids correlated with an increased mole fraction of E4-SA in the
150
adsorption solution, a quantitative analysis was avoided due to overlapping signals and
151
inconsistency in the fingerprint regions between replicas.
152
Water contact angle measurements were performed to gain insight into surface hydrophilicity
153
(see Table S1). The highest value (Q = 50°) was measured for cE4-SA=0, showing that the rSAM 154
of neat E2-OH featured a less hydrophilic character. This value is higher than those measured
155
for PEG-SAMs on gold (Q ≈ 36°)31 which can be explained by the larger number of EG repeats 156
(> 3) in the latter compared to E2-OH. Lower values were measured for the mixed rSAMs
157
reflecting the introduction of E4-SA with its more hydrophilic head group. The most
158
hydrophilic surface (Q = 22°) was measured for cE4-SA=1, confirming the partial destabilization 159
of the rSAM and exposure of the MHA thiol SAM (vide supra).
160
rSAM characterisation by AFM: Atomic force microscopy (AFM) of the rSAMs was
161
performed in the peak force tapping mode (Fig. 3A, Fig. S2). The image of the rSAM of pure
162
OH-terminated amphiphile (E2-OH), cE4-SA = 0 was relatively featureless as expected from the 163
monolayer thickness recorded by ISE, with a roughness RRMS = 0.41 nm. Comparing this 164
surface with the rSAM formed from the lowest E4-SA concentration in the adsorption solution,
165
cE4-SA = 0.05, the roughness value had increased to RRMS = 0.54 nm. rSAMs formed from 166
higher E4-SA concentrations, cE4-SA = 0.1 – 0.2 featured distinct nano-sized domains protruding 167
ca. 20 Å from the shorter domains. This height difference roughly equates to the calculated
168
length difference (17 Å) between E4-SA and filler E2-OH suggesting that the tall domains are
169
primarily E4-SA and the shorter domains are primarily the filler. Similar phase separations were
170
observed in mixed carbohydrate self-assembled monolayers over a 28 day period28 and 171
gangliosides, GM3 in SLB membranes29 supporting our observations with long-range lateral 172
mobility in the rSAMs (vide infra).
173 174 175 176 177 178 179 180 Χ = 0 Χ = 0.1 Χ = 0.15 Χ = 0.2
Increasing mole fraction of E4-SA, χE4-SA
A
B
C
Χ = 0.05D
0.00 0.05 0.10 0.15 0.20 0.25 0 5 10 15 20 χE4-SA Percentage Coverage by T all Domains (%) R2 = 0.9623 0.00 0.05 0.10 0.15 0.20 0.0 0.5 1.0 1.5 χE4-SA D ( µm 2/s) 0 100 200 300 400 0 5 10 15 x (nm) z (nm) 0 0.05 0.1 0.15 0.2 Δ = 2.4 nm Δ = 1.3 nm Δ = 2.3 nm Δ = 2.0 nm χE4-SA =Figure 3. Atomic force microscopy (AFM) topography and profile and fluorescence recovery after photobleaching (FRAP) of layers formed by cE4-SA = 0-0.2 in the adsorption
solution. A. AFM topographic image of layers formed using cE4-SA = 0-0.2. B. Height profile
of AFM topographic images of layers in A. C. Plot of area covered by taller domains as a function of cE4-SA . D. Diffusivity of E2-FAM in different rSAMs measured with FRAP. The
rSAMs were doped with 1 % fluorescein-tagged amidine E2-FAM in the adsorption solution as reported in the experimental section. In the case of cE4-SA=0.2 no recovery was discernable
Both the mean size and the percent area coverage by the taller domains increased as a function
181
of mole fraction of E4-SA in the adsorption solution (Fig. 3A and C). Whereas the domain size
182
increased from ca. 25 to 100 nm in the range cE4-SA = 0.1-0.2 the coverage of the tall domains 183
correlated linearly with cE4-SA. Based on the slope of the line in Fig. 3C we conclude that at 184
least 70% of E4-SA should reside in the tall regions whereas 30% are either not incorporated
185
or reside in the thin regions. The nearly linear increase in average ISE layer thickness (Fig. 2B)
186
and the intensities of the E4-SA characteristic bands (Fig. 2C) in the interval cE4-SA=0-0.15 187
support the latter explanation. This moreover agrees with studies of mixed carbohydrate-based
188
self-assembled monolayers28 and indicate that mixed rSAMs of E4-SA and E2-OH form in a 189
near stochiometric manner. We then turned to investigate their affinity for the lectin
190
hemagglutinin.
191
rSAM binding affinity for hemagglutinin: The influence of the rSAM parameters (e.g. ligand
192
density, presentation, adsorption time) on the binding affinity of a trimeric sialic acid binding
193
protein, hemagglutinin (HA, dimensions (Å): 135 x (15-40) x (15-40)) was evaluated. After
194
formation and rinsing of the rSAMs in pH 8 buffer, HA was added and the film thickness
195
followed in real time by ISE until a stable reading was obtained followed by another rinsing
196
step. We first studied the adsorption of HA on mixed rSAMs of E2-SA and E2-OH at different
197
ligand densities (Fig. S3, Table S2). In agreement with our previous report,13 all curves display 198
positive cooperativity (h > 1) with a steep initial portion followed by clear saturation at
199
concentrations exceeding 20 nM. The cooperative Hill equation gave in all cases the highest
200
goodness of fit with the corresponding binding parameters listed in Table S2.
201
Highest affinity and capacity were measured for the rSAM with the lowest ligand density (c E2-202
SA=0.01) followed by slightly lower affinities at higher densities with exception of cE2-SA=0.2 203
(vide infra) (Fig. S3). This general trend agrees with previous literature reports on glycolipid
204
doped SLBs.11,12 Moreover, we observed that the EG terminated E2-monolayer generally 205
displayed a poor protein resistance and that also the 100% E2-SA rSAM bound HA with high
206
affinity. These results are supported by our previous findings.13,17 The latter rSAM was then 207
shown to display weaker affinity for the competing lectin (Concanavalin A) and human serum
208
albumin whereas the complementary lectin HA could be inhibited by the heavily sialylated
209
protein mucin. This showed that the recognition was driven by sialic acid-lecin interactions.
210
In view of the 2-phase kinetic profiles of the amphiphile adsorption (vide supra) we suspected
211
the time of rSAM adsorption to be a critical parameter. A comparison of two rSAMs (c E4-212
SA=0.1) prepared using a short (5000 s) and long (12 h) adsorption time confirmed this 213
assumption (Fig. 4A). Fig. 4 and Table 1 show that an extended adsorption time leads to a
214
dramatic boost of the rSAM binding affinity for HA (see also Supporting Information Figs
S4-215
S10). In addition, contrasting with the behaviour in Fig. S3, the binding affinity depended now
216
critically on the ligand density peaking at cE4-SA=0.15. 217
218
219
A. B.
220
Figure 4. HA binding isotherms on mixed rSAMs of E2-OH and E4-SA (A) for different
221
adsorption times (cE4-SA=0.1) and (B) for different mole fractions E4-SA (cE4-SA) after 12h 222
rSAM equilibration.
223 224
Fitting the curve assuming a cooperative Hill equation resulted in a dissociation constant
225
Kdmulti of 1.3 (± 0.1) x 10-13 M, on a par with the best binders reported to date based on higher 226 10-2 10-1 100 101 102 0.0 0.5 1.0 [HA] (nM) Γ (mg m -2) 5000 s 12 h 10-810-710-610-510-410-310-210-1 100 101 0.0 0.5 1.0 [HA] (nM) Γ (mg m -2) 0.05 0.1 0.2 0.15 χE4-SA
saccharide ligands. The optimal surface coverage matches well with literature observations of
227
both adaptable and non-adaptable sialic acid systems.19-21,23 Questions remained concerning the 228
origin of the strongly enhanced affinity observed.
229
230
Table 1. Dissociation constant (Kd) and surface saturation capacity (Γmax) of HA binding
231
to mixed rSAMs of E4-SA and E2 with 12h adsorption time
232
c
E4-SA Kd (M) Γmax (mg m-2) h0.05 1.3 (± 0.9) x 10-12 0.22 ± 0.06 0.3 ± 0.1
0.1 3.1 (± 1.4) x 10-11 0.59 ± 0.09 0.6 ± 0.1
0.15 1.3 (± 0.1) x 10-13 0.58 ± 0.15 0.5 ± 0.03
a) Data for cE4-SA=0 and 0.2 have been left out due to weak curvature and poor adherence to the Hill
233
equation.
234
b) The large errors of the data in Fig. 4B are due to a systematic variability of Γmax. This is clearly seen
235
from plots of normalized values (see Fig. S5).
236 237 238
The low amount of adsorbed protein at cE4-SA = 0.2 for rSAMs prepared for both short and long 239
adsorption times (Fig. 4, S3-S4), suggests the lateral distribution of sialic acid head groups to
240
be an important factor for modulating the binding affinity for hemagglutinin (Fig. 5). The
241
drastic drop in binding affinity coincides with the increase in cluster size from 50 nm to 100
242
nm, as seen in the AFM image of the layer formed from cE4-SA = 0.2 (Fig. 3B). It has been 243
reported that formation of larger clusters in ganglioside GM1 containing supported lipid
244
bilayers decreases binding affinity of cholera toxin.12 Hydrogen bonding between the sialic acid 245
head groups was inferred as a likely cause for the segregation of ganglioside, GM3 or GM1 in
246
those membranes.29-30 However, the binding affinity was highest for the lowest ligand density 247
(as low as 0.02% GM1) and then dropped gradually in the interval 0.02% - 10%. This contrasts
248
with the results in Fig.4 and Table 1 where the affinity peaked at an intermediate ligand density
249
whereas it agrees with the gradual decrease in affinity observed for rSAMs prepared with
shorter adsorption times (Fig. S3, Table S2). Hence, in spite of the similar clustering behaviour
251
in the mixed rSAMs in the former case, this is unlikely to be the main cause for the lack of
252
binding of hemagglutinin at cE4-SA = 0.2. 253
254
255
Study of rSAM dynamics by FRAP: We then turned to investigate the ligand lateral mobility
256
as this was inferred as a key factor accounting for the enhanced rSAM affinity.17 To gain insight 257
into this phenomenon, fluorescence recovery after photobleaching (FRAP) was performed on
258
the layers. Dye doped rSAMs were made by including 1 mol% fluorescein-tagged amidine
E2-259
FAM (Fig. S11) in the mixed amidine solution prior to rSAM formation.
260
After rinsing in HEPES buffer pH 8, the rSAMs were examined by fluorescence
TIRF-261
microscopy in order to follow the recovery process in the bleached regions. First, we observed
262
that a significant portion of the total fluorescence (ca 10-35%) stemmed from immobile
263
fluorophores (Fig. S11-S12). In view of the lack of obvious correlation between the latter and
264
the type of sample (Fig. S12) we attribute this to substrate variability defects in the anchor layer
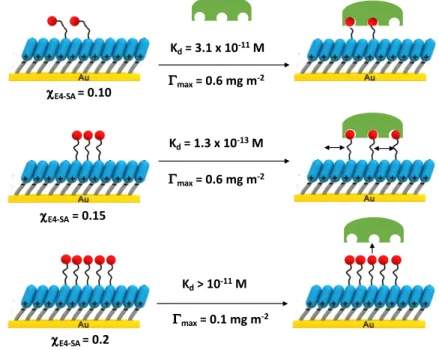
265 cE4-SA = 0.10 cE4-SA= 0.15 cE4-SA = 0.2 Kd= 3.1 x 10-11M Kd= 1.3 x 10-13M Kd> 10-11M Gmax= 0.1 mg m-2 Gmax= 0.6 mg m-2 Gmax= 0.6 mg m-2
Figure 5. Sialic acid density effect on binding affinity of HA. Schematic illustration of
leaving room for amidines (including the dye tagged amidine) to enter and bind irreversibly. In
266
this context, we have previously shown that benzamidines have a strong affinity for bare gold.15 267
Assuming this explanation, we decided to calculate the diffusion constant while correcting for
268
the immobile fraction using Eq. 1 (see experimental section) (Fig. 3D). The lowest diffusivity
269
was displayed for the pure E2 rSAM (cE4-SA=0) with a D = 0.51 µm2/s was lower than that of 270
E4-SA rSAMs which appeared more mobile. The highest diffusivity was observed for rSAMs
271
with cE4-SA=0.05 and cE4-SA=0.15. With a D ≈ 1 µm2/s these surfaces display diffusivities in the 272
order of supported lipid bilayers (Fig. S11).33 The results in Table S3 show that the diffusivities 273
of the rSAMs withcE4-SA=0.05-0.15 are significantly higher than for the pure E2 rSAM (c E4-274
SA=0). Most striking is the lack of mobility at cE4-SA=0.20. At this SA density, no recovery 275
was observed indicating all fluorophores to be immobile. The larger clustering observed at this
276
ligand density apparently stiffens the rSAM and inhibits ligand diffusion (Fig. 5). This lack of
277
mobility we believe can explain the concomitant drop in HA affinity and capacity when
278
comparing rSAMs with cE4-SA=0.15 and cE4-SA=0.20. Moreover, the binding affinities (Table 279
1) and rSAM order, inferred from the CH-stretch frequencies (Table S1, vide supra), seem in
280
part to correlate with the ligand mobility. The rSAMs featuring the lowest ligand mobility (c E4-281
SA=0.10 and cE4-SA=0.20) also display the lowest affinities. This explanation contrasts with that 282
of Cremer et al. where the decreasing affinity was attributed to ligand clustering alone.12 283
284
Conclusions
285
We have demonstrated that mixed rSAMs featuring ligand terminated amhiphiles form in a near
286
statistical manner resulting in adaptable surfaces that are optimal for multivalent receptor
287
binding. This leads to a strong boost in overall affinity as here, exemplified by the sialic
acid-288
hemagluttinine ligand receptor pair. The layers are simple to prepare, they demonstrate lateral
mobility and can be reversibly assembled in response to pH. These features we believe render
290
these systems attractive as models for studies of lipid bilayer membrane dynamics and
291
multivalent molecular recognition.
292
293
Experimental section
294 295
Preparation of amphiphile and protein stock solutions. E4-SA was synthesised as described
296
in the Supporting Information whereas E2-OH, E2-FAM and E2-SA were synthesized as
297
previously reported.13,17 2.5 mM amphipihile stock solutions were prepared in 0 - 5 % ethanolic 298
pH 8 HEPES buffer. Stock solutions of 42 µM hemagglutinin were prepared in Milli-Q water
299
and unused fractions were stored at −80 °C.
300
rSAM preparation. SAMs of mercaptohexadecanoic acid (MHA) on gold were prepared as
301
previously reported17 and was fully immersed into a pH 8 HEPES buffer solution (0.01 M) 302
containing the amidines E4-SA or E2-SA and filler E2, (total concentration=50 µM)at ambient
303
conditions. After 5000 s or 12-18 hrs, the rSAM- modified surfaces were removed from
304
solution, rinsed with pH 8 HEPES buffer and dried under a stream of nitrogen before
305
characterisation by spectroscopic ellipsometry, infrared reflection-adsorption specotroscopy
306
(IRAS) and atomic force microscopy (AFM) measurements. Samples for in situ ellipsometry
307
(ISE) were not dried under a stream of nitrogen and kept wet throughout the entire process.
308
Spectroscopic ellipsometry. Ex situ ellipsometric measurements were taken using a UVISEL
309
HORIBA spectroscopic ellipsometer covering a wavelength range of 200 – 820 nm and
310
incidence angle of 70° at room temperature in air. Optical constants of substrates (MHA) were
311
determined before adsorption of the amphiphiles and each surface was sampled randomly at
3-312
4 points. rSAM thicknesses were modelled based on a homogenous 3-layer model
rSAM-air) using a Cauchy layer, with an extinction coefficient, k = 0 and an assumed refractive
314
index, n = 1.45 to allow systematic comparison. To verify the accuracy of the ellipsometric
315
measurements, the thickness of MHA on gold was determined. An experimental thickness of
316
19±1 Å corresponded well with literature values.32 317
IRAS. The measurements were made using a Nicolet 6400 instrument, equipped with a liquid
318
nitrogen-cooled MCT-A detector, operating at a resolution of 4 cm-1. Data was collected with 319
a Smart SAGA™ accessory operating at an angle of incidence of 80°. The instrument was
320
purged with nitrogen before and during measurements. Each spectrum is the sum of 512 scans
321
on the modified surfaces using an unreacted, cleaned gold substrate as reference. Each spectrum
322
was processed using OMNIC software and baseline corrected.
323
Atomic Force Microscopy (AFM) Measurements. The surfaces were modified as described
324
above using freshly deposited gold on mica as reported in supporting information. The surfaces
325
were examined with a commercial Atomic Force Microscope (AFM) (MultiMode 8 SPM with
326
a NanoScope V control unit, Bruker AXS) in air at room temperature in PeakForce Tapping®
327
mode. Cantilevers with nominal spring constant 0.5819 N m−1 were employed. Analysis and 328
processing of AFM images were performed using the WSxN 5.0 Develop 8.2.5 Each substrate
329
was scanned randomly at min. 3 points.
330
Fluorescence recovery after photobleaching (FRAP). The rSAMs were prepared as
331
described above with the exception that 1 mol% of fluorescein-tagged amidine (E2-FAM) was
332
added to the amidine solution. After rinsing with HEPES buffer of pH 8, the surfaces were dried
333
using nitrogen gas and concealed in the dark. The rSAMs were again washed in HEPES buffer
334
pH 8 before examination with a fluorescence microscope. Samples were observed using
TIRF-335
microscopy in a Nikon Eclipse Ti fluorescence microscope with a 100x oil objective for all
336
FRAP measurements. The FRAP measurements were done using a 15-mW laser emitting at
The recovery process in the bleached region was observed by taking images of the sample with
339
five seconds apart. The diffusion constant (D) and immobile fractions (g0) were derived using 340
a MATLAB-programme specially developed for this purpose (Fig. S11-S12, Tables S3).34 341
Compensating for the influence of the immobile fraction the diffusion is given by35: 342
(1) 𝐷 = 𝐷#$%∙'()'+)*
*
343
Where D = diffusion constant corrected for the immobile fraction and Dexp = experimentally 344
measured diffusion constant with FRAP. Applying expression (1) on the above-presented
345
diffusion constants yielded the results shown in Fig. 3D. Performing the Students t-test now
346
gave the values presented in Table S3 showing that there is a significant difference between the
347
diffusion constants of all rSAMs except between cE4-SA = 0.05 and cE4-SA = 0.15. 348
In situ ellipsometry. Saturation plots were obtained by sequential addition of proteins as
349
previously reported.17 Additional amounts of protein were added once the limiting steady state 350
had been attained where adsorbed amount change is <0.035 mg m-2 per 30 data points. 351
Statistical methods. Error bars are standard deviation of observed values unless stated
352
otherwise. Molecular lengths of the compounds were estimated from energy minimized models
353
obtained by molecular mechanics using the MM2 force field (ChemDraw 3D, CambridgeSoft).
354
355
ACKNOWLEDGMENTS
356
This work was supported by grants from the Swedish Research Council (contract number
357
C0296601) and Marie Skłodowska-Curie Actions (H2020-MSCA-IF-2017, 794999 – Y.
358
Sergeeva and H2020-MSCA-IF-2014-EF, 658953 – G. Pan). PJ was supported by a grant from
359
the Swedish Research Council (number: 2018-03872). Giuliana Grasso (Malmö University) is
360
acknowledged for experimental assistance. We are grateful to Javier Soutres and Juan Francisco
Gonzales (Malmö University) for assistance and helpful discussion concerning the AFM 362 experiments. 363 364 Supporting Information 365
Experimental procedures and data from goniometry, IRAS, ISE, AFM, QCM and FRAP
366
supporting the main findings of the paper.
367
368
Keywords: self-assembled monolayers, carbohydrates, lectins, lipid bilayer, adaptable.
369
370
References
371
1. Delbianco, M.; Bharate, P.; Varela-Aramburu, S.; Seeberger, P. H., Carbohydrates in
372
Supramolecular Chemistry. Chemical Reviews 2016, 116 (4), 1693-1752.
373
2. Kiessling, L. L.; Gestwicki, J. E.; Strong, L. E., Synthetic multivalent ligands in the
374
exploration of cell-surface interactions. Current Opinion in Chemical Biology 2000, 4 (6),
375
696-703.
376
3. Bertozzi, C. R.; Kiessling, L. L., Chemical Glycobiology. Science 2001, 291 (5512),
377
2357-2364.
378
4. Horlacher, T.; Seeberger, P. H., Carbohydrate arrays as tools for research and
379
diagnostics. Chemical Society Reviews 2008, 37 (7), 1414-1422.
380
5. Hushegyi, A.; Tkac, J., Are glycan biosensors an alternative to glycan microarrays?
381
Analytical Methods 2014, 6 (17), 6610-6620.
382
6. Muller, C.; Despras, G.; Lindhorst, T. K., Organizing multivalency in carbohydrate
383
recognition. Chemical Society Reviews 2016, 45 (11), 3275-3302.
7. Evans, S. V.; MacKenzie, C. R., Characterization of protein–glycolipid recognition at
385
the membrane bilayer. Journal of Molecular Recognition 1999, 12 (3), 155-168.
386
8. Boullanger, P., Amphiphilk carbohydrates as a tool for molecular recognition in
387
organized systems. In Glycoscience Synthesis of Substrate Analogs and Mimetics, Driguez,
388
H.; Thiem, J., Eds. Springer Berlin Heidelberg: Berlin, Heidelberg, 1998; pp 275-312.
389
9. Hyun, J. Y.; Pai, J.; Shin, I., The Glycan Microarray Story from Construction to
390
Applications. Accounts of Chemical Research 2017, 50 (4), 1069-1078.
391
10. Zhu, X. Y.; Holtz, B.; Wang, Y.; Wang, L.-X.; Orndorff, P. E.; Guo, A., Quantitative
392
Glycomics from Fluidic Glycan Microarrays. Journal of the American Chemical Society
393
2009, 131 (38), 13646-13650.
394
11. Jung, H.; Robison, A. D.; Cremer, P. S., Multivalent ligand–receptor binding on
395
supported lipid bilayers. J Struct Biol 2009, 168 (1), 90-94.
396
12. Shi, J.; Yang, T.; Kataoka, S.; Zhang, Y.; Diaz, A. J.; Cremer, P. S., GM1 Clustering
397
Inhibits Cholera Toxin Binding in Supported Phospholipid Membranes. Journal of the
398
American Chemical Society 2007, 129 (18), 5954-5961.
399
13. Yeung, S. Y.; Mucha, A.; Deshmukh, R.; Boutrus, M.; Arnebrant, T.; Sellergren, B.,
400
Reversible Self-Assembled Monolayers (rSAMs): Adaptable surfaces for enhanced
401
multivalent interactions and ultrasensitive virus detection. ACS Central Science 2017, 3 (11),
402
1198-1207.
403
14. Auer, F.; Nelles, G.; Sellergren, B., Odd-Even Chain Length-Dependent Order in
pH-404
Switchable Self-Assembled Layers. Chem. Eur. J. 2004, 10 (13), 3232-3240.
405
15. Auer, F.; Sellergren, B.; Swietlow, A.; Offenhäuser, A. Self-Assembled Layers of
406
Bisbenzamidines on Gold. Langmuir 2000, 16, 5936-5944.
407
16. Sellergren, B.; Swietlow, A.; Arnebrant, T.; Unger, K., Consecutive Selective
408
Adsorption of Pentamidine and Phosphate Biomolecules on a Self-Assembled Layer:
Reversible Formation of a Chemically Selective Coating. Analytical Chemistry 1996, 68 (2),
410
402-407.
411
17. Yeung, S. Y.; Ederth, T.; Pan, G.; Cicėnaitė, J.; Cárdenas, M.; Arnebrant, T.;
412
Sellergren, B., Reversible Self-Assembled Monolayers (rSAMs) as Robust and Fluidic Lipid
413
Bilayer Mimics. Langmuir 2018, 34 (13), 4107-4115.
414
18. Bhatia, S.; Camacho, L. C.; Haag, R., Pathogen Inhibition by Multivalent Ligand
415
Architectures. Journal of the American Chemical Society 2016, 138 (28), 8654-8666.
416
19. Kingery-Wood, J. E.; Williams, K. W.; Sigal, G. B.; Whitesides, G. M., The
417
agglutination of erythrocytes by influenza virus is strongly inhibited by liposomes
418
incorporating an analog of sialyl gangliosides. Journal of the American Chemical Society
419
1992, 114 (18), 7303-7305.
420
20. Papp, I.; Sieben, C.; Sisson, A. L.; Kostka, J.; Böttcher, C.; Ludwig, K.; Herrmann,
421
A.; Haag, R., Inhibition of Influenza Virus Activity by Multivalent Glycoarchitectures with
422
Matched Sizes. ChemBioChem 2011, 12 (6), 887-895.
423
21. Spevak, W.; Nagy, J. O.; Charych, D. H.; Schaefer, M. E.; Gilbert, J. H.; Bednarski,
424
M. D., Polymerized liposomes containing C-glycosides of sialic acid: Potent inhibitors of
425
influenza virus in vitro infectivity. Journal of the American Chemical Society 1993, 115 (3),
426
1146-1147.
427
22. Kwon, S.-J.; Na, D. H.; Kwak, J. H.; Douaisi, M.; Zhang, F.; Park, E. J.; Park, J.-H.;
428
Youn, H.; Song, C.-S.; Kane, R. S.; Dordick, J. S.; Lee, K. B.; Linhardt, R. J., Nanostructured
429
glycan architecture is important in the inhibition of influenza A virus infection. Nature
430
Nanotechnology 2016, 12, 48-54.
431
23. Hushegyi, A.; Pihíková, D.; Bertók, T.; Adam, V.; Kizek, R.; Tkac, J., Ultrasensitive
432
detection of influenza viruses with a glycan-based impedimetric biosensor. Biosensors &
433
bioelectronics 2016, 79, 644-649.
24. Charych, D.; Nagy, J.; Spevak, W.; Bednarski, M., Direct colorimetric detection of a
435
receptor-ligand interaction by a polymerized bilayer assembly. Science 1993, 261 (5121),
436
585-588.
437
25. Fyrner, T.; Lee, H.-H.; Mangone, A.; Ekblad, T.; Pettitt, M. E.; Callow, M. E.;
438
Callow, J. A.; Conlan, S. L.; Mutton, R.; Clare, A. S.; Konradsson, P.; Liedberg, B.; Ederth,
439
T., Saccharide-Functionalized Alkanethiols for Fouling-Resistant Self-Assembled
440
Monolayers: Synthesis, Monolayer Properties, and Antifouling Behavior. Langmuir 2011, 27
441
(24), 15034-15047.
442
26. Lin-Vien, D.; Colthup, N. B.; Fateley, W. G.; Grasselli, J. G., APPENDIX 3 - A
443
Summary of Characteristic Raman and Infrared Frequencies A2 - Lin-Vien, Daimay. In The
444
Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules,
445
Academic Press: San Diego, 1991; pp 477-490.
446
27. Khajehpour, M.; Dashnau, J. L.; Vanderkooi, J. M., Infrared spectroscopy used to
447
evaluate glycosylation of proteins. Analytical Biochemistry 2006, 348 (1), 40-48.
448
28. Tantakitti, F.; Burk-Rafel, J.; Cheng, F.; Egnatchik, R.; Owen, T.; Hoffman, M.;
449
Weiss, D. N.; Ratner, D. M., Nanoscale clustering of carbohydrate thiols in mixed SAMs on
450
gold. Langmuir 2012, 28 (17), 6950-6959.
451
29. Cantu, L.; Corti, M.; Brocca, P.; Del Favero, E., Structural aspects of
ganglioside-452
containing membranes. Biochimica et Biophysica Acta (BBA) - Biomembranes 2009, 1788
453
(1), 202-208.
454
30. Gu, R.-X.; Ingólfsson, H. I.; de Vries, A. H.; Marrink, S. J.; Tieleman, D. P.,
455
Ganglioside-Lipid and Ganglioside-Protein Interactions Revealed by Coarse-Grained and
456
Atomistic Molecular Dynamics Simulations. The Journal of Physical Chemistry B 2017, 121
457
(15), 3262-3275.
31. Cerruti, M.; Fissolo, S.; Carraro, C.; Ricciardi, C.; Majumdar, A.; Maboudian, R.
459
Poly(ethylene glycol) Monolayer Formation and Stability on Gold and Silicon Nitride
460
Substrates. Langmuir 2008, 24, 10646-10653.
461
32. Bain, C. D.; Troughton, E. B.; Tao, Y. T.; Evall, J.; Whitesides, G. M.; Nuzzo, R. G.,
462
Journal of the American Chemical Society 1989, 111 (1), 321-335.
463
33. Deng, Y.; Wang, Y.; Holtz, B.; Li, J.; Traaseth, N.; Veglia, G.; Stottrup, B. J.; Elde, R.;
464
Pei, D.; Guo, A.; Zhu, X. Y. Fluidic and Air-Stable Supported Lipid Bilayer and
Cell-465
Mimicking Microarrays. Journal of the American Chemical Society 2008, 130, 6267-6271
466
34. Jönsson, P., M. P. Jonsson, J. O. Tegenfeldt, and F. Hk. 2008. A Method Improving the
467
Accuracy of Fluorescence Recovery After Photobleaching Analysis. Biophys J
95:5334-468
5348." (doi: 10.1529/biophysj.108.134874).
469
35. Jönsson, P.; Jonsson, M. P.; Höök, F. Sealing of Submicrometer Wells by a Shear-Driven
470
Lipid Bilayer. Nano Letters 2010, 10, 1900-1906.
471 472 473
Table of Contents Graphics
475 476 477 E2-OH MHA O O HSSialic Acid Amphiphiles n = 4, E4-SA O O H2N H2N 10 2 O OH N N N O O OH OH HO NH O OH O HO O O H2N H2N 10 n O Hemagglutinin pH 8 Dissociation constant, Kd,multi ΧE4-SA = 0.15 ΧE4-SA = 0.2 1.3 x 10-13 M Minimal binding
Sialic acid-functionalized reversible self-assembled monolayers (rSAMs) with lipid bilayer like fluidity exhibit pM range binding affinity towards hemagglutinin. A pronounced dependence of the affinity on ligand density, clustering and mobility was observed.