R E S E A R C H
Open Access
Mitral annular plane systolic excursion (MAPSE) in
shock: a valuable echocardiographic parameter
in intensive care patients
Lill Bergenzaun
1*, Hans Öhlin
2, Petri Gudmundsson
3, Ronnie Willenheimer
4and Michelle S Chew
5Abstract
Background: Assessing left ventricular (LV) dysfunction by echocardiography in ICU patients is common. The aim of this study was to investigate mitral annular plane systolic excursion (MAPSE) in critically ill patients with shock and its relation to LV systolic and diastolic function, myocardial injury and to outcome.
Methods: In a prospective, observational, cohort study we enrolled 50 patients with SIRS and shock despite fluid resuscitation. Transthoracic echocardiography (TTE) measuring LV function was performed within 12 hours after admission and daily for a 7-day observation period. TTE and laboratory measurements were related to 28-day mortality. Results: MAPSE on day 1 correlated significantly with LV ejection fraction (LVEF), tissue Doppler indices of LV diastolic function (é, E/é) and high-sensitive troponin T (hsTNT) (p< 0.001, p= 0.039, p= 0.009, p= 0.003 respectively) whereas LVEF did not correlate significantly with any marker of LV diastolic function or myocardial injury. Compared to survivors, non-survivors had a significantly lower MAPSE (8 [IQR 7.5-11] versus 11 [IQR 8.9-13] mm; p= 0.028). Other univariate predictors were age (p=0.033), hsTNT (p=0.014) and Sequential Organ Failure Assessment (SOFA) scores (p=0.007). By multivariate analysis MAPSE (OR 0.6 (95% CI 0.5- 0.9), p= 0.015) and SOFA score (OR 1.6 (95% CI 1.1- 2.3), p= 0.018) were identified as independent predictors of mortality. Daily measurements showed that MAPSE, as sole echocardiographic marker, was significantly lower in most days in non-survivors (p<0.05 at day 1–2, 4–6).
Conclusions: MAPSE seemed to reflect LV systolic and diastolic function as well as myocardial injury in critically ill patients with shock. The combination of MAPSE and SOFA added to the predictive value for 28-day mortality. Keywords: Echocardiography, Intensive care, Mitral annular plane systolic excursion, Outcome
Background
In intensive care patients a frequently applied method for estimating LV systolic function is left ventricular ejection fraction (LVEF) [1]. LV systolic dysfunction has been observed in critically ill patients with shock [2,3] although there is conflicting evidence that LV systolic impairment is associated with mortality [2,4,5].
Mitral annular plane systolic excursion (MAPSE) also known as left atrioventricular plane displacement (AVPD), mitral annulus excursion (MAE) or mitral ring displace-ment is an M-mode derived echocardiographic marker of LV longitudinal function [6-8]. MAPSE correlates well
with other markers of LV function [6,9,10], is easily ob-tainable [11-13] even for the untrained observer [12] and in patients with poor acoustic windows [8]. It has been suggested as a surrogate measurement for LVEF in cardiac patients [12,14]. A reduced MAPSE has been shown to correlate with age, and LV function in patients with myocardial infarction, heart failure and atrial fibrillation [15-17] and to be more sensitive than conventional echo-cardiographic markers in detecting abnormalities in LV systolic function at an early stage [7,18]. MAPSE is known to be prognostic for major cardiac events and mortality in patients with cardiovascular disease [15,19-21]. In critic-ally ill patients there are no reports of the use of MAPSE, its association with other echocardiographic parameters, myocardial injury or clinical outcome.
* Correspondence:lill.bergenzaun@skane.se
1Department of Anaesthesiology and Intensive Care, Skåne University Hospital, Institute for Clinical Sciences Malmö, Lund University, S-20502 Malmö, Sweden
Full list of author information is available at the end of the article
© 2013 Bergenzaun et al.; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The aim of this study was to investigate if MAPSE is of prognostic significance in critically ill patients with shock. Further, we wanted to examine if MAPSE correlates with other markers of LV function and myocardial injury.
Material and methods
The study was approved by the Regional Ethics Review Board, Lund, Sweden (Dnr.187/2005). Informed consent was sought from the patient or, if not possible, from the next of kin. The study design was a prospective obser-vational cohort study. Patients >18 years old admitted to the mixed-bed ICU of Skåne University Hospital, Malmö, Sweden, were screened for eligibility. We included 55 con-secutive patients with Systemic Inflammatory Response Syndrome (SIRS) and concurrent shock, where shock was defined as failure to maintain mean arterial pressure≥ 70 mmHg, despite adequate fluid resuscitation according to the surviving sepsis campaign algorithm [22]. Exclusion criteria were pregnancy, known abnormalities of coagula-tion, fibrinolytic therapy, compromised immunity or a “Do Not Attempt Resuscitation” order. Acute Physiology and Chronic Health Evaluation (APACHE) II scores [23] were calculated at admission and Sequential Organ Failure Assessment (SOFA) scores [24] were calculated daily. After the initial resuscitation period, fluids were given at the treating clinician’s discretion. Mean arterial pressure (MAP), heart rate (HR), positive end expiratory pres-sure (PEEP) and vasopressor (norepinephrine) dose was recorded at the time of the TTE examination. Blood sam-ples were taken from an indwelling arterial line within 12 h of inclusion. High-sensitive troponin T (hsTNT) and B-natriuretic peptide (BNP) were analyzed as reported previ-ously [25]. Patients were followed for 7 days or until dis-charge from ICU. Mortality was defined as 28-day all cause mortality.
Transthoracic echocardiography
Transthoracic echochardiography (TTE) was performed within 12 hours after admission and daily for a 7-day ob-servation period or until discharge from ICU by one of four experienced echocardiographers (LB, MC, PG, MD). Images were acquired using a Hewlett- Packard Sonos 5500 (Andover, Mass, U.S.A) scanner and a 3 MHz trans-ducer. Two-dimensional (2D) imaging examinations were performed in the standard apical four- and two- chamber views (2C- and 4C views). Tissue harmonic imaging was used to enhance 2D image quality. LV ejection fraction (LVEF) was assessed by visual estimation of EF, based on “eyeball” ejection fraction. M-mode images were obtained at the LV septal, lateral, anterior, and posterior borders of the mitral ring [18] in the apical 2C- and 4C views, and an average mitral annular plane systolic excursion (MAPSE) value was calculated. Pulsed-wave (PW) tissue Doppler recorded the peak systolic velocity (TDIs) of the LV septal
wall at the level of the mitral annulus in the apical 4C view [26]. Transmitral velocities were measured with PW Dop-pler in the 4C view. For LV diastolic function, we used the mitral inflow profile, the E- and A-velocity and calculated the E/A ratio. PW tissue Doppler recorded the diastolic velocities (é) of the LV septal wall at the level of the mitral annulus in the apical 4C view. The E/é ratio, an index of LV filling pressure, was calculated and é (septal) < 8cm/s indicated diastolic dysfunction [27]. All TTE studies were recorded over three consecutive cardiac cycles independ-ently of the respiratory cycle and averaged. In patients with non-sinus rhythm measurements were collected over 5–10 heartbeats. Analyses of the measurements were made in Phillips digital storing and analysis software Xcelera (Best, the Netherlands) offline.
Statistical analysis
Data are presented as median (lower quartile: upper quar-tile), percentages or absolute values. For not normally dis-tributed variables we used non-parametric test exclusively. For correlation between two variables, Spearman’s rank correlation was used and for differences between two groups we used Mann-Whitney’s U-test. Categorical data were analyzed with Fisher’s exact test. HsTNT and BNP were log transformed with natural logarithm due to skewed distribution. Discrimination analysis was performed using receiver operating characteristics (ROC) curve under the area using multiple logistic regression predictions. Our aim was to investigate how 28-day mortality can be predicted by more than one explanatory variable measured early dur-ing ICU stay. Since we did not have any censored data during this period and odds ratio was the outcome of inter-est, logistic regression was chosen to be the most suitable method [28]. Multivariate (backward stepwise selection method with probability for the removal of 0.10) logistic regression analyses were used to determine the associa-tion of variables with 28-day mortality. The Hosmer and Lemeshow test of goodness of fit was used to indicate if the model provides an adequate fit to the data. Odds ratios (OR) were calculated. We have used the method described by Hoaglin and Iglewicz [29] to test for outliers. The intra-and inter-observer variability of echocardiographic parame-ters was measured by the coefficient of variation (CV). CV was defined as the ratio of the standard deviation to the mean multiplied by 100. All probability values are two-tailed and significance was set at p < 0.05. The analyses were performed using SPSS 18.0 (SPSS, Chicago, IL, U.S.).
Results
Patient characteristics
The original study included 55 consecutive patients. Two patients were excluded due to lack of written consent. In two patients TTE examinations were not recorded (death before possible TTE and morbid obesity
respectively) and one patient was incorrectly registered in the echocardiography database. These five patients were excluded resulting in a total of 50 analyzed patients. Of 350 expected echocardiographic examinations, 96 were missing because of death or discharge from the ICU before Day 7. Another 23 examinations were lost during the installation of a new offline storing and analysis sys-tem. Thus, in total, 231 examinations were available for analysis. An additional file shows this in more detail see (Additional file 1). Two-thirds of the population suffered from septic shock and one-third from shock due to other
causes (pancreatitis, post-major non-cardiac surgery, in-toxication and multiorgan failure, gastrointestinal bleeding and portal hypertension or unknown cause). Pre-existing cardiac disease was present in 12 (24%) patients defined as severe arrhythmia, heart failure or ischemic heart disease. Forty-five (90%) patients were mechanically ventilated at inclusion. Acute kidney failure was present in 14 (28%) pa-tients requiring continuous renal replacement therapy (CRRT), including one patient with chronic kidney failure (Table 1). Twelve patients (24%) received dobutamine and one (2%) adrenaline at inclusion. The median ICU length
Table 1 Baseline and echocardiographic characteristics of studied patients at day 1
All n=50 Survivors n=37 Non-survivors n=13 p
Clincal data Median age, y 65 (54–74) 60.5 (49.5–72.0) 72.0 (69.0–76.0) 0.033 Female sex, n (%) 14 (28) 12 (32) 2 (15) >0.1 Diabetes mellitus, n (%) 6 (12) 5 (14) 1 (8) >0.1 Hypertension, n (%) 12 (24) 11 (30) 1 (8) >0.1 Cardiac disease, n (%) 12 (24) 8 (22) 4 (31) >0.1 APACHE II score 24 (19–29) 24 (17–28) 28 (21–34) 0.060 SOFA score 12 (9–14) 10 (9–13) 13 (13–14) 0.007 HR, beats/min 99 (85–115) 99 (85–116) 98 (83–107) >0.1 MAP, mmHg 76 (70–88) 76 (70–90) 76 (73–79) >0.1 NE dose,μg/kg/min 0.09 (0.05-0.14) 0.08 (0.04-0.13) 0.1 (0.09-0.18) >0.1 CRRT, n (%) 14 (28) 11 (30) 3 (23) >0.1 Fluids, ml/kg/24h 43 (22–84) 38 (20–81) 46 (28–77) >0.1 Mechanical ventilation, n (%) 45 (90) 35 (95) 10 (77) >0.1 Peep, cmH2O 8 (5–10) 8 (5–10) 10 (9–13) 0.083 Biochemical data Creatinine,μmol/L 156 (94–243) 138 (86–221) 182 (118–246) >0.1 Lactate mmol/L 2.2 (1.6-3.2) 2.2 (1.6-3.2) 2.6 (1.8-4.6) >0.1 hsTNT, ng/L 79.5 (23–182) 77.5 (18.6-125.3) 152 (80–501) 0.014 BNP, pmol/L 187 (98–375) 157 (84–356) 262 (183–440) >0.1 Echocardiographic data n=47 n=34 n=13 LVEF, % 49 (40:55) 50 (40–50) 45 (35–65) >0.1 LVEF, %, mean (±SD) 47 (±14.8) 47 (±12.8) 48 (±19.7) MAPSE, mm 10 (8.0-12.6) 11 (8.9-13) 8 (7.5-11) 0.028 MAPSE, mm, mean (±SD) 10.3 (±2.8) 10.9 (±2.9) 8.7 (±2.5) TDIs cm/s 8.9 (7.1-10.0) 8.9 (7.2-9.9) 7.5 (6.6-10) >0.1 E/A 1.2 (0.9-1.5) 1.2 (0.9-1.5) 1.3 (0.9-1.6) >0.1 é, cm/s 8.3 (7–10) 8.4 (7.5-10.5) 7.2 (6.3-8.6) >0.1 é, cm/s, mean (±SD) 8.7 (±2.2) 8.9 (±2.0) 8.0 (±2.6) E/é 10.2 (8.5–12) 10.0 (8.2–11.5) 13.0 (9.6–15.2) 0.055 E/é, mean (±SD) 10.7 (±3.7) 9.8 (±2.8) 12.7 (±5.0)
Data are presented as median (lower quartile- upper quartile), as mean (± SD) or in numbers (%). APACHE II (Acute Physiology and Chronic Health Evaluation), CRRT (Continuous Renal Replacement Therapy), SOFA (Sequential Organ Failure Assessment), HR (heart rate), MAP (mean arterial pressure), NE (norepinephrine), Peep (positive end expiratory pressure).
of stay was 8 (IQR 4–13) days. Eight patients died within seven days and thirteen patients after 28 days with an all cause 28-day mortality of 26%.
Mitral annular plane systolic excursion (MAPSE) and relation to other echocardiographic parameters
On day 1 a total of 47 echocardiographic examinations were available for analysis, since 3 examinations were lost during the installation of a new offline storing and analysis system. Results on day 1 showed that MAPSE was signifi-cantly lower in non-survivors (median 8 [IQR 7.5-11] mm) than in survivors (median 11 [IQR 8.9-13] mm) of 28-day mortality (p= 0.028). No other echocardiograohic parameter showed any significant difference (Table 1). In 14 (30%) patients ejection fraction was preserved (LVEF≥
55%) and in 33 patients (70%) impaired (LVEF ≤ 50%)
with no significant difference in mortality between these two groups. Six patients had severely reduced LV systolic function (LVEF < 30%). MAPSE was 11 [11–12.8] mm in patients with preserved EF and 9 [7.3-12.3] mm in those with reduced EF (p= 0.069). MAPSE correlated signifi-cantly with LVEF, é, E/é and hsTnT whereas LVEF did not correlate significantly with markers of LV diastolic func-tion, filling pressure or cardiac biomarkers (Table 2). MAPSE showed a negative correlation with age (r=−0.411, p=0.003) but was not associated with previous hyper-tension or cardiac disease. LV diastolic dysfunction (é < 8 cm/s) showed a significant negative association with MAPSE (p= 0.047) whereas there was none with LVEF.
The intra- and inter-observer variability for MAPSE was 4.4% and 5.3% respectively, and for other echocar-diographic markers as reported previously [13,25].
Mortality
MAPSE (p= 0.028), SOFA score (p= 0.007), age (p= 0.033) and hsTNT (p= 0.014) were identified as univariate pre-dictors of 28-day mortality (Table 3). A multivariate logis-tic regression analysis including these variables identified
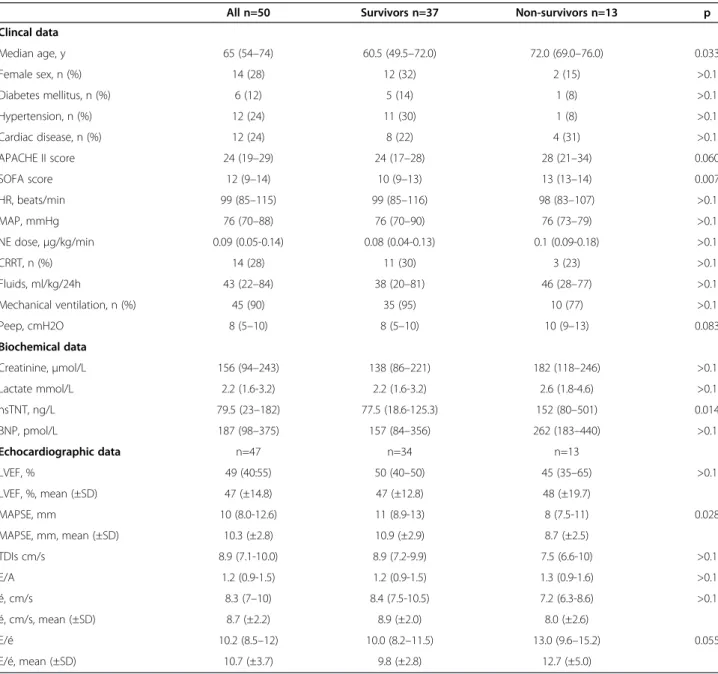
a model with MAPSE (p= 0.015) and SOFA (p= 0.018) as independent predictors of 28-day mortality. The Hosmer and Lemeshow test for the model was non-significant (p=0.998) indicating adequate fit to the data. The adjusted OR for MAPSE and SOFA score were 0.6 (95% CI 0.5-0.9) and 1.6 (95% CI 1.1- 2.3) respectively (Table 3). With regards to 28-day mortality the area under the curve (AUC) at day 1 for MAPSE was 0.709 (95% CI 0.548- 0.870, p = 0.028) with 69% sensitivity and 68% specificity for a cut-off value of 8 mm and AUC for SOFA score was 0.733 (95% CI 0.592- 0.874, p = 0.014) with 69% sensitivity and 68% specificity for a cut-off value of 12. When adding MAPSE to SOFA score, the AUC for the combined predictor in-creased to 0.831 (95% CI 0.711- 0.952, p <0.001) (Figure 1).
Cardiac biomarkers
Results at day 1 showed that hsTNT was significantly higher in non-survivors (152 [IQR 80–501] ng/L) than in survivors (77.5 [IQR 18.6-125.3] ng/L) (p= 0.014) whereas there was no difference in BNP (Table 1). In patients with diastolic dysfunction (é< 8cm/s) both hsTNT and BNP were significantly higher in non-survivors compared to survivors (p= 0.020 and p= 0.039 respectively); this was not seen in those with systolic dysfunction (LVEF≤ 50%). hsTNT showed a significant negative correlation with MAPSE but not with LVEF or TDIs (Table 3). In patients with diastolic dysfunction (é< 8cm/s) hsTNT and BNP showed a significant negative correlation with MAPSE (r= −0.478, p= 0.033; borderline for BNP r= −0.441, p= 0.051) but there was none with TDIs.
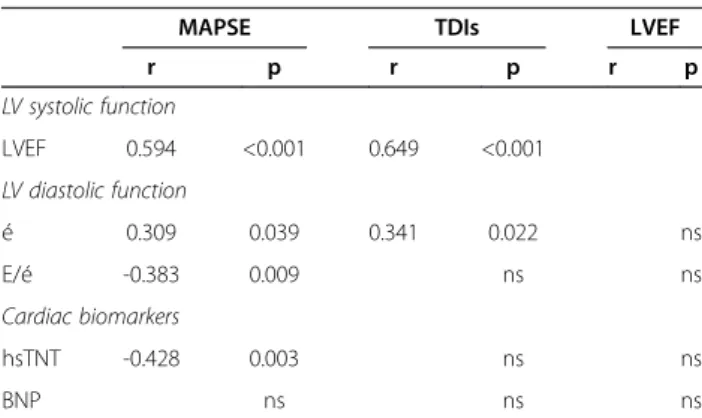
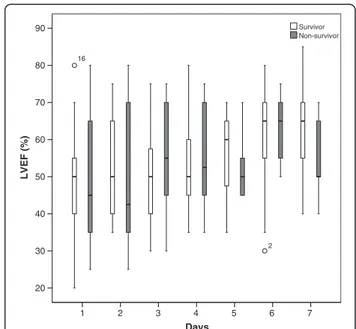
Daily measurements over 7 days showed that in non-survivors MAPSE was significantly lower in most days (day 1 p=0.028, day 2 p=0.003, day 3 p=0.060, day 4 p= 0.036, day 5 p=0.026, day 6 p=0.017, day 7 p=0.075) (Figure 2) whereas LVEF was not significant different (p>0.1) at any day (Figure 3).
Discussion
Our main findings are that MAPSE was an independent predictor of 28-day mortality in critically ill patients with shock and systemic inflammation. Combining MAPSE with SOFA increased the predictive value for mortality. MAPSE correlated with markers of LV systolic and dia-stolic function as well as myocardial injury, whereas LVEF did not.
Table 2 Correlation (r) between markers of LV systolic function with LV diastolic function and cardiac biomarkers
MAPSE TDIs LVEF
r p r p r p LV systolic function LVEF 0.594 <0.001 0.649 <0.001 LV diastolic function é 0.309 0.039 0.341 0.022 ns E/é -0.383 0.009 ns ns Cardiac biomarkers hsTNT -0.428 0.003 ns ns BNP ns ns ns
Spearman rank correlation was used. Statistical significance at p< 0.05.
Table 3 Multivariate analysis for predictors of death in patients with shock
Multivariate analysis
Odds Ratio (95%CI) Wald statistics p
MAPSE (mm) 0.666 (0.5-0.9) 5.915 0.015
MAPSE and prognosis
In critically ill patients echocardiography has gained popu-larity as a tool for assessing LV function [5,30]. LVEF is probably the most commonly used and accepted method of measuring LV systolic function in this setting [1] how-ever its usefulness in predicting mortality has produced conflicting results [2,5,31]. In patients with septic shock studies measuring the LV longitudinal function by tissue Doppler imaging (TDI) have moved into focus during the recent years identifying mainly diastolic TDI indices as prognostic markers whereas systolic TDI parameters seem to be less consistently related to mortality [31-34]. Inter-estingly in none of these studies MAPSE was used as a marker of LV systolic function.
MAPSE is a simple, easily obtained parameter and may contribute to the evaluation of systolic function. MAPSE was obtainable in all patients, and showed inter-and intra-observer variability of 4.4% inter-and 5.3% [13]. It is less well investigated than its right ventricular counter-part, tricuspid annular plane systolic excursion (TAPSE), and has received considerably less attention than TDI variables. In critical care settings where acoustic win-dows are often suboptimal, MAPSE seems to be an at-tractive parameter. A decreased MAPSE is known to be associated with conditions affecting LV function such as myocardial infarction, heart failure, atrial fibrillation and age [15-17] and its relation to mortality has been de-scribed by several studies in patients with cardiovascular disease [15,19-21]. 1 - Specificity 1,0 0,8 0,6 0,4 0,2 0,0 Sensitivity 1,0 0,8 0,6 0,4 0,2 0,0 ROC Curve Reference Line MAPSE+ SOFA MAPSE
Figure 1 Receiver operating characteristic (ROC) for MAPSE and for a combined predictor consisting of MAPSE and SOFA score. With regards to 28-day mortality the area under the curve (AUC) for MAPSE was 0.709 (95% CI 0.548- 0.870, p= 0.028) and for the combined predictor 0.831 (95% CI 0.711- 0.952, p<0.001). Days 7 6 5 4 3 2 1 MAPSE (mm) 18 16 14 12 10 8 6 4 2 Non-survivors Survivors
Figure 2 Boxplots of daily measurements show that MAPSE is significantly lower in non-survivors (grey) of 28-day mortality compared to survivors (white) in most days (day 1 p=0.028, day 2 p=0.003, day 3 p=0.060, day 4 p= 0.036, day 5 p=0.026, day 6 p=0.017, day 7 p=0.075). ° Value and case number within 1.5 boxplot length (SPSS 18.0). Days 7 6 5 4 3 2 1 LVEF (%) 90 80 70 60 50 40 30 20 2 16 Non-survivor Survivor
Figure 3 Boxplots of daily measurements show that there is no significant difference (p>0.1) at any day in LVEF between survivors (white) and non-survivors (grey) of 28-day mortality. ° Value and case number within 1.5 boxplot length (SPSS 18.0).
We found that MAPSE on day 1 was significantly lower in non-survivors compared to survivors and could to-gether with SOFA score be identified as independent pre-dictors of 28-day mortality. Further, combining MAPSE and SOFA score seemed to increase the risk of death. These results are strengthened by the finding that MAPSE was significantly decreased in non-survivors compared to survivors in most days of the 7-day observation whereas LVEF was not. LVEF, on the other hand, being near nor-mal could not be identified as a prognostic marker and we speculate if this, like in patients with cardiovascular disease and preserved ejection fraction, could be due to LVEF being less sensitive in uncovering subtle myocardial changes [35,36].
MAPSE in relation to other markers of LV function and myocardial injury
Previous studies in patients with cardiovascular disease have suggested MAPSE as a surrogate measurement for LVEF with both normal and reduced LV function [12,14]. A mean value for MAPSE of > 10 mm was linked with preserved EF (≥ 55%) and values < 8 mm with reduced EF (< 50%) [6,19,37]. Our results are in line with this, with MAPSE correlating significantly with LVEF. MAPSE was 11 [11–12.8] mm in patients preserved EF and in those with reduced EF slightly higher than 8 mm (MAPSE 9 [7.3-12.3] mm).
Although MAPSE and LVEF may be related, they are not entirely interchangeable [13,17]. MAPSE is sug-gested to be primarily representative of subendocardial, longitudinally oriented, myocardial fibres compared to the subepicardial, circumferential fibres measured by LVEF, and is known to detect more subtle abnormalities of LV function [7,18]. This is seen in patients with in-creasing age, myocardial hypertrophy or diastolic dysfunc-tion with preserved ejecdysfunc-tion fracdysfunc-tion (HFpEF) where long axis function of the heart is already impaired while the radial function can be preserved or even increased [18,35]. Thus by using LVEF the long axis function of the heart is not necessarily considered.
Similar to MAPSE, tissue Doppler imaging is de-scribed to be superior to conventional echocardiography in detecting abnormalities of LV function [38] and its correlation with MAPSE has been described previously [39]. In a recent study (Wenzelburger), TDI indices of both LV diastolic and systolic function correlated sig-nificantly with MAPSE in patients with HFpEF [35] il-lustrating the close relationship between systolic and diastolic LV function. LV systolic torsional (twisting) de-formation is one mechanism by which potential energy is stored. After systole the heart relaxes or untwists, an energy releasing process, and aids to early LV filling by suctioning [40]. Thus a decrease in atrioventricualar
plane motion will result in less energy stored during sys-tole and hence reduced LV diastolic mechanics.
The relationship between MAPSE and TDI indices is supported by our results where MAPSE correlated signifi-cantly with é, a diastolic marker, and showed a negative significant correlation with E/é, a surrogate marker for LV filling pressure. Additionally we found a significant as-sociation between diastolic dysfunction (é< 8cm/s) and MAPSE, but none with LVEF. Of note, in our previous study we showed a significant correlation between MAPSE and the systolic marker of tissue Doppler imaging (TDIs) (r= 0.427, p< 0.01) [13].
Notably, MAPSE and TDI are not interchangeable. The two parameters describe different vector compo-nents of longitudinal systolic motion. Although TDIs has been described to correlate well with MAPSE in studies with healthy individuals [39,41], they differ in some im-portant aspects [42] as MAPSE measures the entire sys-tolic phase including isovolumetric contraction [15] in contrast to TDIs. In addition a blurred TDI signal can be a confounding factor when measuring the velocity [43] and in these situations MAPSE can be a valuable. Overall we believe that MAPSE and tissue Doppler mea-surements are important echocardiographic parameters in assessing LV function.
Finally, we sought to investigate if there was a relation-ship between cardiac biomarkers (hsTNT and BNP) and MAPSE and found that hsTNT but not BNP was cantly higher in non-survivors and correlated signifi-cantly with MAPSE but not LVEF. This is in line with a recent study in septic neonates were hsTNT was signifi-cantly higher in non-survivors and correlated with longi-tudinal LV systolic function measured by TDI but not with fractional shortening [44]. Landesberg et al. [32] found that hsTNT was significantly higher in patients with decreased é and LVEF. This is similar to our find-ings where hsTNT in patients with diastolic dysfunction showed a significant negative correlation with MAPSE. Although we found no relationship between LVEF and cardiac troponin T our findings are generally in support of these studies and we speculate if MAPSE may be more sensitive than LVEF in detecting early myocardial changes in critically ill patients with shock.
Limitations
Firstly, we used eyeballing to measure LVEF. Simpson’s bi-plane method is the currently accepted standard. We and others have previously shown that eyeball EF was as good as the Simpson’s method [13,45], and was more easily obtained in ICU patients [13]. Although unlikely, we can-not exclude that using Simpson’s method for measuring LVEF could have influenced our results. Secondary ana-lysis (data not shown) in 44 patients where good-quality Simpson’s EF could be obtained showed no relationship
to mortality. Secondly, we did not screen our patients for specific conditions affecting MAPSE measurements such as localized wall motion abnormalities or mitral an-nular calcifications. Thirdly, no dynamic fluid responsive-ness tests were used limiting our results, as MAPSE in analogy with tissue Doppler measurements, is affected by changing fluid conditions. Finally, the multivariate analysis was limited by the small number of patients in this study, and we cannot exclude other confounding factors. Never-theless, we clearly demonstrate a relationship between MAPSE and 28-day mortality.
Conclusions
In this study we showed that MAPSE but not LVEF cor-related with other markers of LV function and myocar-dial injury and could be identified as an independent predictor of 28-day mortality. Its predictive value in-creased when added to SOFA score. A reduced MAPSE persisted in non-survivors throughout the ICU stay. Fu-ture prospective studies should evaluate the advantages and weaknesses of MAPSE in critically ill patients with shock where vasoactive drugs and positive pressure ven-tilation are commonly used, all influencing echocardio-graphic measurements.
Additional file
Additional file 1: Flow diagram showing number of patients, echocardiographic examinations and hsTNT measurements.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LB, RW, MC, PG have made substantial contributions to conception and design of the study. LB, MC and HO participated in interpretation of data, helped to draft the manuscript. PG and LB made substantial contributions in acquisition and analysis of data. All authors have made substantial intellectual contributions to the manuscript and have given final approval of the version to be published.
Acknowledgements
The authors thank Nuray Güner and Magnus Dencker for their assistance. Supported by grants from Anna-Lisa and Sven Eriks Lundgren´s Foundation, Acta Foundation and the Region Skane County Council, Sweden. None of the funding agents were involved in study design, data collection, analysis and interpretation, and in writing and submitting the manuscript. Author details
1Department of Anaesthesiology and Intensive Care, Skåne University Hospital, Institute for Clinical Sciences Malmö, Lund University, S-20502 Malmö, Sweden.2Department of Cardiology, Skåne University Hospital, S-22185 Lund Lund University, Sweden.3Department of Biomedical Science, Malmö University, S- 20506 Malmö, Sweden.4Heart Health Group, Lund University, Geijersg. 4C, 21618 Limhamn, Sweden.5Department of Anesthesia and Intensive Care, Hallands Hospital Halmstad and Institute for Clinical Sciences Malmö, S-30185 Halmstad, Sweden.
Received: 28 March 2013 Accepted: 24 May 2013 Published: 30 May 2013
References
1. Dittoe N, Stultz D, Schwartz BP, Hahn HS: Quantitative left ventricular systolic function: from chamber to myocardium. Crit Care Med 2007, 35(8 Suppl):S330–S339.
2. Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, Damske BA, Parrillo JE: Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med 1984, 100(4):483–490. 3. Poelaert J, Declerck C, Vogelaers D, Colardyn F, Visser CA: Left ventricular
systolic and diastolic function in septic shock. Intensive Care Med 1997, 23(5):553–560.
4. Pulido JN, Afessa B, Masaki M, Yuasa T, Gillespie S, Herasevich V, Brown DR, Oh JK: Clinical spectrum, frequency, and significance of myocardial dysfunction in severe sepsis and septic shock. Mayo Clin Proc 2012, 87(7):620–628. 5. Vieillard-Baron A: Septic cardiomyopathy. Ann Intensive Care 2011, 1(1):6. 6. Alam M, Hoglund C, Thorstrand C: Longitudinal systolic shortening of the
left ventricle: an echocardiographic study in subjects with and without preserved global function. Clin Physiol 1992, 12(4):443–452.
7. Jones CJ, Raposo L, Gibson DG: Functional importance of the long axis dynamics of the human left ventricle. Br Heart J 1990, 63(4):215–220. 8. Hu K, Liu D, Herrmann S, Niemann M, Gaudron PD, Voelker W, Ertl G, Bijnens B,
Weidemann F: Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Imaging: Eur Heart J Cardiovasc; 2012. 9. Hu K, Liu D, Herrmann S, Niemann M, Gaudron PD, Voelker W, Ertl G, Bijnens B,
Weidemann F: Clinical implication of mitral annular plane systolic excursion for patients with cardiovascular disease. Eur Heart J Cardiovasc Imaging 2013, 14(3):205–212.
10. Willenheimer R, Israelsson B, Cline C, Rydberg E, Broms K, Erhardt L: Left atrioventricular plane displacement is related to both systolic and diastolic left ventricular performance in patients with chronic heart failure. Eur Heart J 1999, 20(8):612–618.
11. Elnoamany MF, Abdelhameed AK: Mitral annular motion as a surrogate for left ventricular function: correlation with brain natriuretic peptide levels. Eur J Echocardiogr 2006, 7(3):187–198.
12. Willenheimer R: Assessment of left ventricular dysfunction and remodeling by determination of atrioventricular plane displacement and simplified echocardiography. Scand Cardiovasc J Suppl 1998, 48:1–31. 13. Matos J, Kronzon I, Panagopoulos G, Perk G: Mitral annular plane systolic
excursion as a surrogate for left ventricular ejection fraction. J Am Soc Echocardiogr 2012, 25(9):969–974.
14. Bergenzaun L, Gudmundsson P, Ohlin H, During J, Ersson A, Ihrman L, Willenheimer R, Chew M: Assessing left ventricular systolic function in shock: evaluation of echocardiographic parameters in intensive care. Crit Care 2011, 15(4):R200.
15. Alam M, Hoglund C, Thorstrand C, Philip A: Atrioventricular plane displacement in severe congestive heart failure following dilated cardiomyopathy or myocardial infarction. J Intern Med 1990, 228(6):569–575.
16. Willenheimer R, Cline C, Erhardt L, Israelsson B: Left ventricular
atrioventricular plane displacement: an echocardiographic technique for rapid assessment of prognosis in heart failure. Heart 1997, 78(3):230–236. 17. Emilsson K, Wandt B: The relation between ejection fraction and mitral
annulus motion before and after direct-current electrical cardioversion. Clin Physiol 2000, 20(3):218–224.
18. Emilsson K, Wandt B: The relation between mitral annulus motion and ejection fraction changes with age and heart size. Clin Physiol 2000, 20(1):38–43.
19. Höglund C, Alam M, Thostrand C: Atrioventricular Valve Plane Displacement in Healthy Persons. Acta Med Scand 1988, 224:557–562. 20. Rydberg E, Arlbrandt M, Gudmundsson P, Erhardt L, Willenheimer R: Left
atrioventricular plane displacement predicts cardiac mortality in patients with chronic atrial fibrillation. Int J Cardiol 2003, 91(1):1–7.
21. Brand B, Rydberg E, Ericsson G, Gudmundsson P, Willenheimer R: Prognostication and risk stratification by assessment of left atrioventricular plane displacement in patients with myocardial infarction. Int J Cardiol 2002, 83(1):35–41.
22. Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, Gea-Banacloche J, Keh D, Marshall JC, Parker MM, et al: Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med 2004, 32(3):858–873.
23. Knaus WA, Draper EA, Wagner DP, Zimmerman JE: Prognosis in acute organ-system failure. Ann Surg 1985, 202(6):685–693.
24. Vincent JL, De Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S: Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med 1998, 26(11):1793–1800.
25. Bergenzaun L, Ohlin H, Gudmundsson P, During J, Willenheimer R, Chew MS: High-sensitive cardiac Troponin T is superior to echocardiography in predicting 1-year mortality in patients with SIRS and shock in intensive care. BMC Anesthesiol 2012, 12(1):25.
26. Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, et al: Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol 1997, 30(2):474–480.
27. Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A:
Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography. J Am Soc Echocardiography 2009, 22(2):107–133.
28. Bewick V, Cheek L, Ball J: Statistics review 14: Logistic regression. Crit Care 2005, 9(1):112–118.
29. Hoaglin DC: Performance of some resistant rules for outlier labeling. J Am Stat Assoc 1986, 81:991–999.
30. Vieillard-Baron A, Slama M, Cholley B, Janvier G, Vignon P: Echocardiography in the intensive care unit: from evolution to revolution? Intensive Care Med 2008, 34(2):243–249.
31. Weng L, Liu YT, Du B, Zhou JF, Guo XX, Peng JM, Hu XY, Zhang SY, Fang Q, Zhu WL: The prognostic value of left ventricular systolic function measured by tissue Doppler imaging in septic shock. Crit Care 2012, 16(3):R71.
32. Landesberg G, Gilon D, Meroz Y, Georgieva M, Levin PD, Goodman S, Avidan A, Beeri R, Weissman C, Jaffe AS, et al: Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur Heart J 2012, 33(7):895–903. 33. Sturgess DJ, Marwick TH, Joyce C, Jenkins C, Jones M, Masci P, Stewart D,
Venkatesh B: Prediction of hospital outcome in septic shock: a prospective comparison of tissue Doppler and cardiac biomarkers. Crit Care 2010, 14(2):R44.
34. Furian T, Aguiar C, Prado K, Ribeiro RV, Becker L, Martinelli N, Clausell N, Rohde LE, Biolo A: Ventricular dysfunction and dilation in severe sepsis and septic shock: relation to endothelial function and mortality. J Crit Care 2012, 27(3):319–315.
35. Wenzelburger FW, Tan YT, Choudhary FJ, Lee ES, Leyva F, Sanderson JE: Mitral annular plane systolic excursion on exercise: a simple diagnostic tool for heart failure with preserved ejection fraction. Eur J Heart Fail 2011, 13(9):953–960.
36. Svealv BG, Olofsson EL, Andersson B: Ventricular long-axis function is of major importance for long-term survival in patients with heart failure. Heart 2008, 94(3):284–289.
37. Simonson JS, Schiller NB: Descent of the base of the left ventricle: an echocardiographic index of left ventricular function. J Am Soc Echocardiogr 1989, 2(1):25–35.
38. Bolognesi R, Tsialtas D, Barilli AL, Manca C, Zeppellini R, Javernaro A, Cucchini F: Detection of early abnormalities of left ventricular function by hemodynamic, echo-tissue Doppler imaging, and mitral Doppler flow techniques in patients with coronary artery disease and normal ejection fraction. J Am Soc Echocardiogr 2001, 14(8):764–772.
39. Study Group of Echocardiography of the Italian Society of C, Mondillo S, Galderisi M, Ballo P, Marino PN: Left Ventricular Systolic Longitudinal Function: Comparison Among Simple M-Mode, Pulsed, and M-Mode Color Tissue Doppler of Mitral Annulus in Healthy Individuals. J Am Soc Echocardiography 2006, 19(9):1085–1091.
40. Notomi Y, Popovic ZB, Yamada H, Wallick DW, Martin MG, Oryszak SJ, Shiota T, Greenberg NL, Thomas JD: Ventricular untwisting: a temporal link between left ventricular relaxation and suction. Am J Physiol Heart Circ Physiol 2008, 294(1):H505–H513.
41. Alam M, Wardell J, Andersson E, Samad BA, Nordlander R: Characteristics of mitral and tricuspid annular velocities determined by pulsed wave Doppler tissue imaging in healthy subjects. J Am Soc Echocardiogr 1999, 12(8):618–628.
42. Ballo P, Bocelli A, Motto A, Mondillo S: Concordance between M-mode, pulsed Tissue Doppler, and colour Tissue Doppler in the assessment of
mitral annulus systolic excursion in normal subjects. Eur J Echocardiogr 2008, 9(6):748–753.
43. Chen QM, Li W, O'Sullivan C, Francis DP, Gibson D, Henein MY: Clinical in vivo calibration of pulse wave tissue Doppler velocities in the assessment of ventricular wall motion. A comparison study with M-mode echocardiography. Int J Cardiol 2004, 97(2):289–295.
44. Abdel-Hady HE, Matter MK, El-Arman MM: Myocardial dysfunction in neonatal sepsis: a tissue Doppler imaging study. Pediatr Crit Care Med 2012, 13(3):318–323.
45. Gudmundsson P, Rydberg E, Winter R, Willenheimer R: Visually estimated left ventricular ejection fraction by echocardiography is closely correlated with formal quantitative methods. Int J Cardiology 2005, 101(2):209–212.
doi:10.1186/1476-7120-11-16
Cite this article as: Bergenzaun et al.: Mitral annular plane systolic excursion (MAPSE) in shock: a valuable echocardiographic parameter in intensive care patients. Cardiovascular Ultrasound 2013 11:16.
Submit your next manuscript to BioMed Central and take full advantage of:
• Convenient online submission
• Thorough peer review
• No space constraints or color figure charges
• Immediate publication on acceptance
• Inclusion in PubMed, CAS, Scopus and Google Scholar
• Research which is freely available for redistribution
Submit your manuscript at www.biomedcentral.com/submit