PROGRESS REPORT
ANALYSIS OF MANTECA AND WOODLAND MOLASSES AND CORRESPONDING COLD SACCHARATE CAKE
January, 1941
BY
H.W.Daeschner & B.O.Bauer
'
-:-....'
~ (., I'\SPRECKELS SUGAR COMPANY Manteca, California
January 15, 1941
PROGRESS REPORT: ANALYSIS OF MANTECA AND WOODLAND MOLASSES AND CORRESPONDING COLD SACCHARATE CAKE.
Source
Assigned by Mr. P. W. Alston. Object
An investigation to determine the fundamental melassigenic factors and their influence upon saccharate formation involves a detailed- analysis of the molasses and saccharate made from this molasses. The object of this work was to obtain as complete an analysis ·of molasses and saccharate as was possible. Several samples with different histories were analyzed in order that a study may be made upon the constituents relative to their source.
Materials and Equipment
Eight samples of molasses and corresponding cold saccharate cakes were
saved at Woodland in 1939 and shipped to Manteca. 'According to Woodland these samples have the following histories:
Day Hour Type Molasses Analysis Cold Cake Ending Taken Mols.
-- --
RDS APC%
Sugar APC 9/17 4 PM Manteca 84.4 58.3 51.2 91.4 12 Mid. House 86.6 58.6 52.0 83.5 9/24 4 PM Manteca 85.0 58.3 52.1 89.2 9/25 12 Mid. House 86.8 59.0 52.4 81.0 10/21 4 PM Manteca 82.8 60.2 51.0 91.5 II 12 AM House 84.9 60.5 52.4 82.9 11/18 12 Mid. Manteca 84.4 60.9 51.8 92.5"
4 AM House 86.2 63.6 65.0 88.3** Estimated "raffinose" content= 3.5% on Dry Sub. Estimated true purity= 84
For the analytical work no special equipment was necessary except for the determination of glutamic acid. For this determination a special auto-clave for pressures around 45 pounds was built. A refrigerator was also required.
Method
Sugars
The usual beet sugar factory methods were employed in determining dry substance, apparent sugar, and purity. True sugar and true purity was determined by the methods of the A.O.A.C., 3d Edition; Section XX.XIV; 23a, 23b, 23c, and 26. Raffinose was calculated by means of the last method. Reducing sugars were determined by Soxhlet's Methylene blue titration method.
Ash
The ash in molasses was determined by converting to the carbonate. A.O.A.C. gravimetric procedures were employed on silica, iron and aluminum oxides, magnesium, sulphate, and phosphate. The calcium was titrated as the oxalate and iron was titrated with permanganate. Potassium was determined by the trisodium colaltinitrite method, Ind. and Eng. Chem., Anal. Edition, Vol. 29, No. 3, page 136. Sodium was determined as the difference of the weighed sodium and potassium residue. Chlorine was determined volumetrically
and carbonate was calculated from theoretical combinations. Organic (non-sugars)
The total organic (non-sugar) was calculated as the difference between dry substance and the total composed of true sugar, reducing sugar, raffinose, and ash.
Glute.mic acid was extracted according to a revised method based upon that of D. W. O'Day and Edward Bartow of the State University of Iowa.
Five hundred grams of molasses was mixed with 500 grams of 12 N hydrochloric acid, This was set aside for two days in a warm (37°C) oven. The carbon which had formed and the inorganic salts which has precipitated were filtered out on a large Buchner funnel. The cake was pressed as dry as possible and taken up with about 200 grams HCl and refiltered and repressed. This was
repeated again to remove all soluble material. The filtrates were combined and heated in a 3 liter balloon flask covered with an inverted beaker in an autoclave at a pressure of 45 pounds for three to four hours. After reduction in pressure and cooling, the mass of carbon and acid liquor was filtered off on a Buchner, pressed and washed with 12 N-HCl several times to remove the last traces of acid liquor. The filtrates were combined and concentrated at reduced pressure in a 3 liter flask on a steam bath at a distillation temperature of about 45-55° C until the solution was reduced to a very viscous mass. The viscous material was all transferred to a beaker and kept at a temperature of 10° C for a week when the crude crystallized glutamic acid hydrochloride mixed with large amounts of inorganic salts was removed by filtration. The dried mixture was extracted with a slight amount of water to extract the acid but not the inorganic salts. The aqueous extract was decolorized with carbon
(Darco) and filtered. The clear solution was titrated with 10 N sodium hydrox-ide to a pH of 3.28 (the isoelectric point of glutamic acid) using the Beckman
glass electrode meter. The mixture was then placed in a refrigerator for 48 hours to permit complete separation of the gluta.mic acid. It was then filtered off, dried, and weighed.
Nitrogen Analysis
Organic and Ammoniacal Nitrogen by A.O.A.C. Methods, 3d Edition, II, 20, 21, 22. .Ammoniacal and Amido Nitrogen by American Beet Sugar Co. Methods.
Harmful or Amino Nitrogen by A.B.S. Co. Methods.
Albuminoid Nitrogen - Organic and Ammoniacal less .Ammoniacal, Amido, and Harmful
Nitrogen.
Saccharate Analysis
Identioal methods were used on the cold cake where possible. The ash was run as the oxide instead of first converting it to the carbonate since it was comparatively free of sodium and potassium salts.
Discussion of Results
The results to date are included in the appended four tables. Since
both Manteca and Woodland process nearly the same quality beets any change in
the Woodland molasses would be due to the introduction of .Manteca molasses.
The saccharate process will then either build up or eliminate certain
constitu-ents from the Woodland molasses. Upon inspection of Tables I and II we find
that there is a noteworthy increase in "raffinose'', reducing sugar, calcium, and organic material which is non-sugar. There is a decrease in true purity of the molasses, ash content, potassium, chlorine, glutamic acid and total nitrogen.
Although it is not shown on the tables, the total nitrogen is also the total organic nitrogen. The results for both determinations equalled, within the limit of experimental error. This shows a lack of nitrates and nitrites.
Woodland molasses contains a larger amount of organic material not
accounted for.
Seasonal changes also seem to influence the constituents. This is
especially noticeable in "raffinose", glutamic acid in Manteca molasses, and
in nitrogen content.
As future work along this line is to be done, it is early to draw too conclusive opinions.
Physical Characteristics
No figures are included in the tables to indicate the physical characteristics of the molasses samples. A large quantity of sugar grains, some quite small, settled to the bottom upon standing in the four .Manteca
samples and in the first Woodland sample. Only a few very large grains settled
in the other three Woodland samples.
Apparent viscosity was determined by a sinking test with a glass rod supported vertically in the molasses. The time was taken for the rod to sink
an equal portion of its length in the original molasses. For the four Manteca
samples the time in seconds was 3.6, 9.2, 2.4, and 2.6, respectively, and for
the Woodland samples 3.6, 21.2, 15.2 and 28.0 respectively. Duplicate determi-nations were made which checked with each other within 0.4 second. Such a large difference in viscosity between the Manteca and Woodland samples can be attributed to difference in Brix entirely.
Future Work
Among the various problems concerning the constituents yet to be investi-gated, we propose the following list. This should take us about a month and a half.
1. Investigation of the substances remaining in solution from the glutamic acid extraction. This will cover aspartic acid, betain, some glutamic acid, and several other possible nitrogenous substances.
2. A check on what is known as harmful nitrogen by the electrometric method. (Facts About Sugar) March-1939.
3. Detection and estimation by distillation or other means of acetic, butyric, lactic, formic, succinic acids and their esters.
4. Determine if possible the cause of the greater amount of unaccounted organic material not sugar in the Woodland molasses.
5. Complete the saccharate analysis.
6. Investigate the pectin substances by solvent action upon dried and pulverized molasses. cc - P. W. Alston E. T, Winslow W. J. Resch L. Lewan R. E. Briggs E. M. Hartmann A. A. Norman Experimental file. 4. H. W. DAESCHNER B. O. BAUER
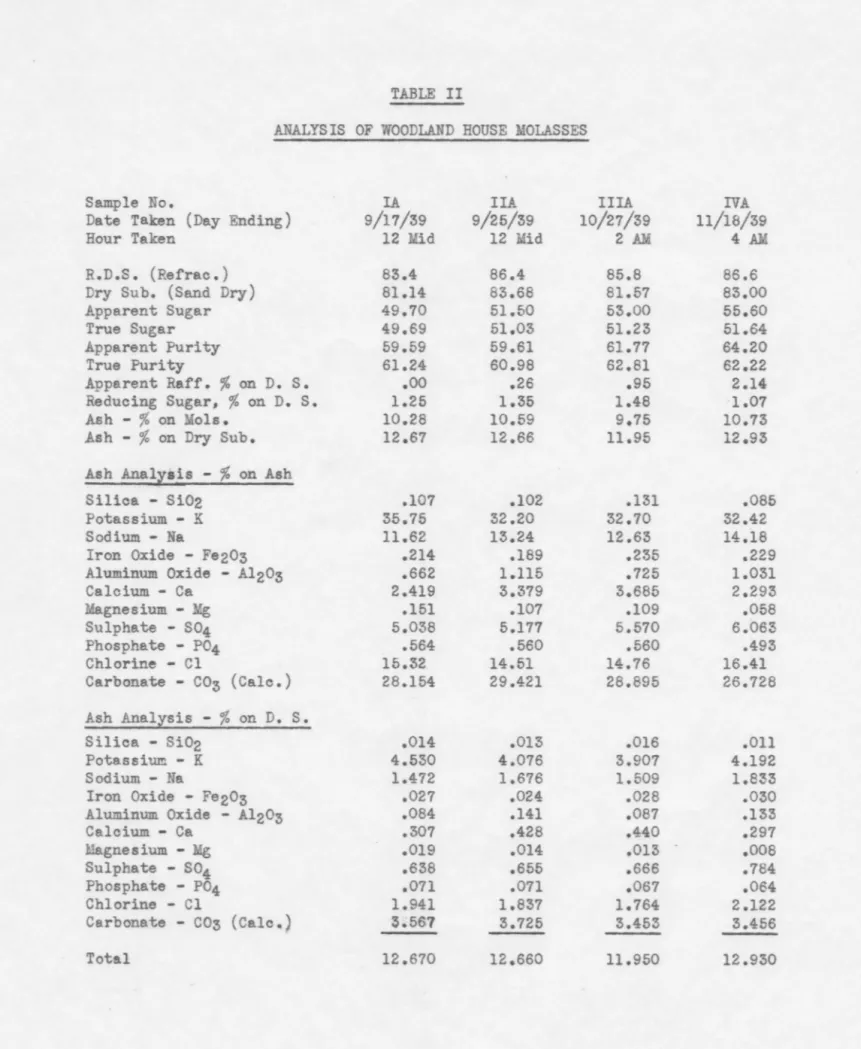
TABLE I
ANALYSIS OF MANTECA MOLASSES
Sample No. I II III
rv
Date Taken (Day Ending) 9/17/39 9/24/39 10/21/39 11/18/39
Hours Taken 4 PM 4 PM 4 PM 12 M
R.D.S. {Refrac.) 84.8 86.0 83.6 83.0
Dry Sub. (Sand Dry) 81.47 83.36 80.62 80.23
Apparent Sugar 52.50 51.70 53.50 50.90
True Sugar 53.15 51.79 53.54 51.04
Apparent Purity 61.91 60.12 64.00 61.33
True Purity 65.24 62.13 66.41 63.62
Apparent Raff.% on D.S.
.oo
.oo
oOO.oo
Reducing Sug.
%
on D.S. 1.09 .68.as
.72Ash -
%
on Mols. 9.76 11.08 10.62 11.41Ash -
%
on Dry Sub. 11.97 13.29 13.17 14.22Ash Analysis -
%
on AshSilica - Si02 .126 .131 .llS .147
Potassium - K 35.11 36.03 33.90 33.99
Sodium - Na 13.37 13.36 14.17 14.49
Iron Oxide - Fe203 .146 .140 .166 .169
Aluminu.~ Oxide - Al203 .474 .392 .594 .535
Calcium - Ca 1.132 .858 1.295 .989 Magnesium - Mg .140 .089 .122 .117 Sulphate - S04 5.081 5.167 5.479 5.631 Phosphate - P04 .595 .554 .573 .535 Chlorine - Cl 19.32 14.80 17.82 17.03 Carbonate - C03 (Cale.) 24.506 28.479 25.763 26.347 Ash Anallsis -
%
on D.S. Silica - Si02 .016 .011 .015 .021 Potassium - K 4.203 4.788 4.465 4.833 Sodium - Na _ 1.600 1.776 1.866 2.060Iron Oxide - Fe203 .017 .018 .022 .021
Aluminum Oxide - Al203 .057 .052 .078 .076
Calcium - Ca .135 .114 .111 .141 Magnesium - Mg .017 .012 .016 .017 Sulphate - S04 .609 .687 .722 .801 Phosphate - P04 .071 .074 .075 .076 Chlorine - Cl 2.313 1.967 2 .347 2.422 Carbonate - C03 (Cale.) 2.933 3.785 3.393 3.746 Total 11.970 13.290 13.170 14.220
Sample No.
Organic (Non-Sugar) Glutamic Acid
Nitrogen Analysis .Ammoniacal & Amido Album.inoid Harmful or Amino Total Nitrogen Protein Calculated (Total N2 x 6.24) Organic (Non-Sugar) Glutamic Acid Nitrogen }..nalysis .Ammoniacal & Amido Albuminoid Harmful or Amino Total Nitrogen Protein as Calculated (Total Nitrogen x 6.24) TABLE I (Cont.) MANTECA MOLASSES I II III
PER CENT ON TOTAL MOLASSES 17.68 19.92 15.75 1.73 1.89 1.88 .993 1.075 1.075 .402 .427 .375 .631 .675 .480 2.026 2.177 1.930 12.64 13.58 12.04
PER CENT ON DRY SUBSTANCE 21.70 23.90 19.54 2.12 2.27 2.33 1.218 1.290 1.333 .494 .512 .465 .775 .810 .596 2.487 2.612 2.394 15.52 16.30 14.94 IV 17.20 2.82 1.091 .362 .500 1.953 12.19 21.44 3.52 1.360 .461 .623 2.434 15.19
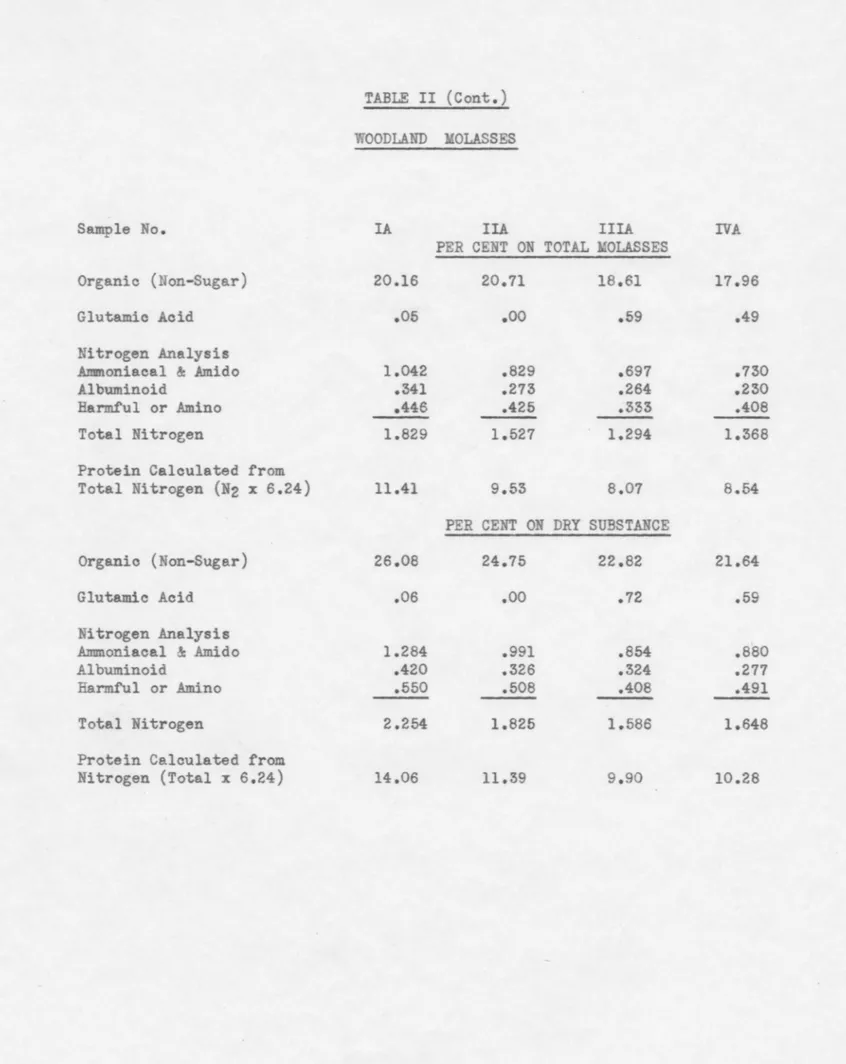
TABLE II
ANALYSIS OF WOODLAND HOUSE MOLASSES
Sample No. IA IIA IIIA IVA
Date Taken (Day Ending) 9/17/39 9/25/39 10/21/39 11/18/39
Hour Taken 12 Mid 12 Mid 2 AM 4 AM
R.D.S. (Refrac.) 83.4 86.4 85.8 86.6
Dry Sub. (Sand Dry) 81.14 83.68 81.57 83.00
Apparent Sugar 49.70 51.50 53.00 55.60
True Sugar 49.69 51.03 51.23 51.64
Apparent Purity 59.59 59.61 61.77 64.20
True Purity 61.24 60.98 62.81 62.22
Apparent Raff.% on D.S.
.oo
.26 .95 2.14Reducing Sugar,% on D.
s.
1.25 1.35 1.48 1.07Ash - % on Mola. 10.28 10.59 9.75 10.73
Ash -
%
on Dry Sub. 12.67 12.66 11.95 12.93Ash Anallsis -
%
on AshSilica - Si02 .107 .102 .131 .085
Potassium - K 35.75 32.20 32.70 32.42
Sodium - Na 11.62 13.24 12.63 14.18
Iron Oxide - Fe203 .214 .189 .235 .229
Aluminum Oxide - Al203 .662 1.115 .725 1.031
Calcium - Ca 2.419 3.379 3.685 2.293 Magnesium - Mg .151 .107 .109 .058 Sulphate - S04 5.038 5.177 5.570 6.063 Phosphate - P04 .564 .560 .560 .493 Chlorine - Cl 15.32 14.51 14.76 16.41 Carbonate - C03 (Cale.) 28.154 29.421 28.895 26.728 Ash Analysis -
%
on D.S. Silica - Si02 .014 .013 .016 .011 Potassium - K 4.530 4.076 3.907 4.192 Sodium - Na 1.472 1.676 1.509 1.833Iron Oxide - Fe203 .027 .024 .028 .030
Aluminum Oxide - Al203 .084 .141 .087 .133
Calcium - Ca .307 .428 .440 .297 Magnesium - Mg .019 .014 .013 .008 Sulphate -
so
4 .638 .655 .666 .784 Phosphate - P04 .071 .071 .067 .064 Chlorine - Cl 1.941 1.837 1.764 2.122 Carbonate - C03 (Cale.) :5.567 3.725 3.453 3.456 Total 12.670 12.660 11.950 12.930Sample No.
Organic (Non-Sugar) Glutamio Aoid
Nitrogen Analysis .A:mmoniacal & Amido
.Albuminoid
Harmful or Amino Total Nitrogen
Protein Calculated from Total Nitrogen (N2 x 6.24)
Organic (Non-Sugar) Glutamio Acid
Nitrogen Analysis Annnoniacal & Amido Albuminoid
Harmful or .Amino Total Nitrogen
Protein Calculated from Nitrogen (Total x 6.24)
TABLE II (Cont.)
WOODLAND MOLASSES
IA IIA IIIA PER CENT ON TOTAL MOLASSES 20.16 20.71 18.61 .05
.oo
.59 1.042 .829 .697 .341 .273 .264 .446 .425 .333 1.829 1.527 1.294 11.41 9.53 8.07 PER CENT ON DRY SUBSTANCE 26.08 24.75 22.82 .06.oo
.72 1.284 .991 .854 .420 .326 .324 .550 .508 .408 2.254 1.825 1.586 14.06 11.39 9.90 I:VA 17.96 .49 .730 .230 .408 1.368 8.54 21.64 .59 .880 .277 .491 1.648 10.28TABLE III
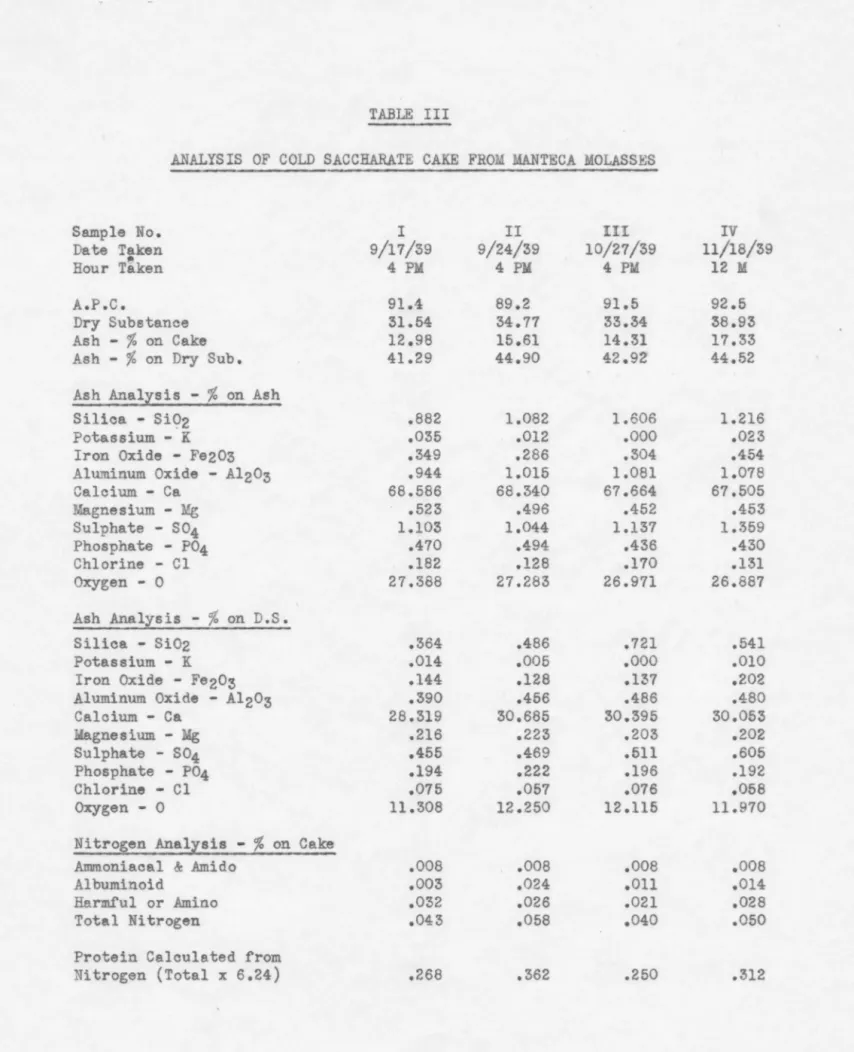
ANALYSIS OF COLD SACCHARATE CAKE FROM MANTECA MOLASSES
Sample No. I II HI IV Date Taken 9/17/39 9/24/39 10/27/39 ll/18/39 ti!' Hour Taken 4 PM 4 PM 4 PM 12 M A.P.C. 91.4 89.2 91.5 92.5 Dry Substance 31.54 34.77 33.34 38.93 Ash -
%
on Cake 12.98 15.61 14.31 17.33Ash -
%
on Dry Sub. 41.29 44.90 42.92 44.52Ash Analrsis -
%
on AshSilica - Si(?2 .882 1.082 1.606 1.216
Potassium - K .035 .012
.ooo
.023Iron Oxide - Fe203 .349 .286 .304 .454
Aluminum. Oxide - Al203 .944 1.015 1.081 1.078
Calcium - Ca 68.586 68.340 67.664 67.505 Magnesium - Mg .523 .496 .452 .453 Sulphate - S04 1.103 1.044 1.137 1.359 Phosphate - P04 .470 .494 .436 .430 Chlorine - Cl .182 .128 .170 .131 Oxygen - 0 27.388 27.283 26. 971 26.887 Ash Analysis -
%
on D.S. Silica - Si02 .364 .486 .721 .541 Potassium - K .014 .005.ooo
.010Iron Oxide - Fe203 .144 .128 .137 .202
Aluminum Oxide - Al203 .390 .456 .486 .480
Calcium - Ca 28.319 30.685 30.395 30.053 Magnesium - Mg .216 .223 .203 .202 Sulphate - S04 .455 .469 .511 .605 Phosphate - P04 .194 .222 .196 .192 Chlorine - Cl .075 .057 .076 .058 Oxygen - 0 11.308 12.250 12.115 11.970
Nitrogen .Analzsis -
%
on CakeAmm.oniacal & .Am.ido .008 .008 .008 .008
Albuminoid .003 .024
.on
.014Harmful or .Amino .032 .026 .021 .028
Total Nitrogen .043 .058 .040 .050
Protein Calculated from
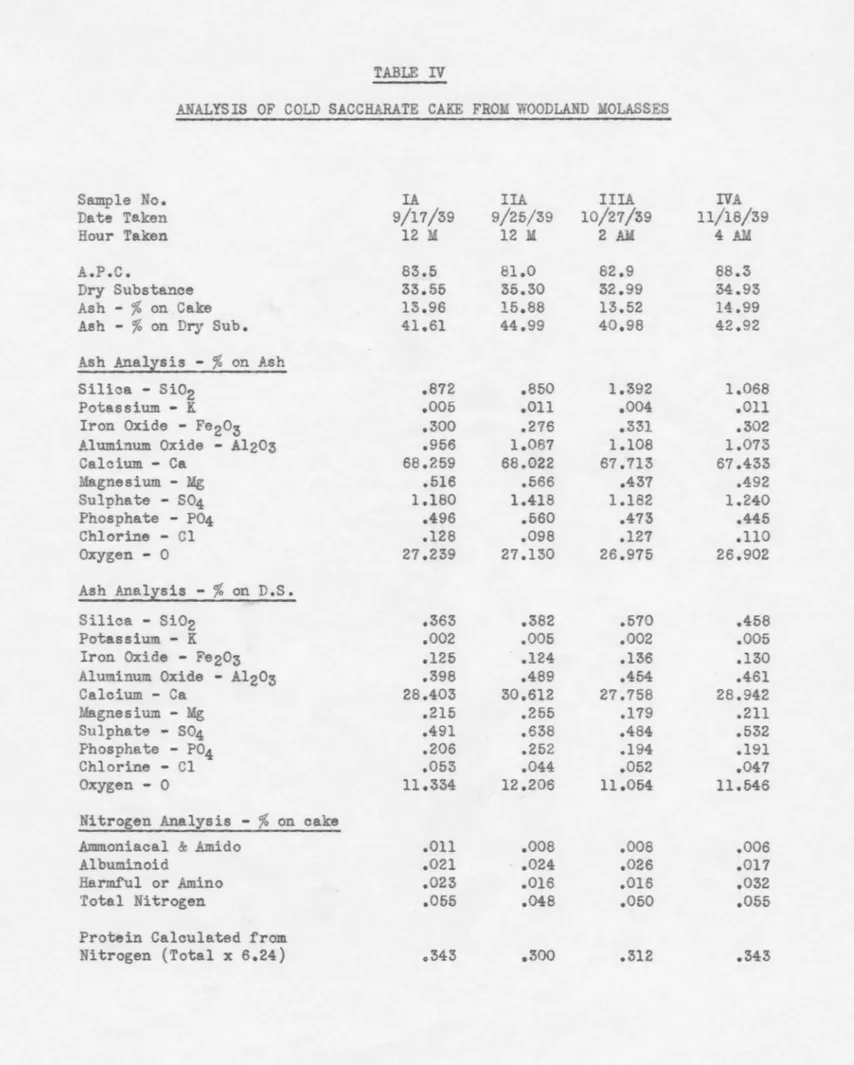
TABLE IV
ANALYSIS OF COLD SACCHARATE CAKE FROM WOODLAND MOLASSES
Sample No. IA IIA IIIA IVA
Date Taken 9/17/39 9/25/39 10/21/39 11/18/39
Hour Te.ken 12 M 12 M 2 AM 4 AM
A.P.C. 83.5 81.0 82.9 88.3
Dry Substance 33.55 35.30 32.99 34.93
Ash -
%
on Cake 13.96 15.88 13.52 14.99Ash -
%
on Dry Sub. 41.61 44.99 40.98 42.92Ash Analisis -
%
on AshSilica - Sio2 .872 .850 1.392 1.068
Potassium - K .005 .011 .004 .011
Iron Oxide - Fe203 .300 .276 .331 .302
Aluminum Oxide - Al203 .956 1.087 1.108 1.073
Calcium - Ca 68.259 68.022 67. 713 67.433 Magnesium - Mg .516 .566 .437 .492 Sulphate - S04 1.180 1.418 1.182 1.240 Phosphate - P04 .496 .560 .473 .445 Chlorine - Cl .128 .098 .127 .110 Oxygen - 0 27.239 27.130 26.975 26.902 Ash Analzsis -
%
on D.S. Silica - Si02 .363 .382 .570 .458 Potassium - K .002 .005 .002 .005Iron Oxide - Fe203 .125 .124 .136 .130
Aluminum Oxide - Al203 .398 .489 .454 .461
Calcium - Ca 28.403 30.612 27.758 28.942 Magnesium - Mg .215 .255 .179 .211 Sulphate - S04 .491 .638 .484 .532 Phosphate - P04 .206 .252 .194 .191 Chlorine - Cl .053 .044 .052 .047 Oxygen - 0 11.334 12.206 11.054 11.546
Nitrogen Analysis -
%
on cakeAmmoniacal & .Amido .011 .008 .008 .006
Albuminoid .021 .024 .026 .017
Harmful or Amino .023 .016 .016 .032
Total Nitrogen .055 .048 .050 .055
Protein Calculated from