Akademin för hållbar samhälls- och teknikutveckling
Leucine intake affects brain activity
and central expression of genes
associated with food intake, energy
homeostasis and reward.
Utfört av Alina Johansson
Contents
Abstract………...…2
Introduction……….3
Materials and Methods………....4
Results……….7
Discussion………...…9
Abstract
Leucine injections directly into the brain decrease food intake whereas supplementation of this amino acid in a diet has a negligible effect on food intake. We sought to investigate why orally supplemented leucine is ineffective as an anorexigen. We found that mice consuming leucine exhibited increased cFos immunoreactivity in the ARC and PVN of hypothalamus, areas controlling energy balance. However, real time- PCR analysis of the hypothalamic tissue in mice that were exposed to oral leucine showed changes in expression of genes involved in the regulation of energy balance as well as those mediating feeding reward (TMEM18, MC4R, CRH, FTO, SLC6A15, DOR). This suggests that leucine consumption affects activity of not only brain pathways that control calorie intake, but also those that mediate eating for pleasure. Hence the lack of feeding response to leucine supplementation in a diet may stem from the simultaneous action of this amino acid at brain circuit promoting reward and energy homeostasis.
Introduction
Overeating has become a vast problem in the modern society and has received special attention from media as well as from the scientific community and pharmaceutical companies. Overeating seems to be dependent mostly on the abundance of food that people have access to, combined with the poor inherent body homeostasis control mechanisms that would reduce appetite in the obesogenic environment. "Functional foods", i.e., those rich in specific components (such as certain nutrients or water), have been developed and used as a means to decrease consumption. In general, diet supplementation is often recommended as an alternative or complementary approach to regular dieting or pharmacological interventions. The purpose is usually to reduce hunger and increase satiety. One group of substances utilized for this purpose is amino acids which are added to special dietary beverages or meal substituting bars. However, their effectiveness has been questioned. It is important to note that laboratory animal studies, typically performed to assess in depth the effect of dietary components on various physiological and behavioral parameters, including food intake and body weight, have also produced mixed data on the value of amino acid supplementation to control food intake. These studies typically utilize dietary branched-chain amino acids (BCAA), such as leucine, isoleucine and valine, as they largely escape first-pass metabolism (1). Among those, leucine has been under special scrutiny as this amino acid crosses the blood-brain barrier faster than others (2).
Several rodent studies have shown the impact of leucine on body weight and food intake. Leucine injected directly into the mediobasal hypothalamus (MBH) - omitting the paravenricular nucleus of hypothalamus (PVN) - in rats reduces food intake by decreasing the meal size by 50 % and the frequency of meals (3). However, it is unclear whether leucine intake, rather than injected leucine, influences the regulation of food intake and body weight. Studies on mice (4) show that animals on a high-fat diet and getting leucine in water for 14-15 weeks do not exhibit a significant change in body weight or in the amount of consumed food. Similar results were obtained by another group of researchers (5) who reported that mice fed for 2 weeks chow containing 5% leucine did not exhibit a different body weight or consumption profile. On the other hand, high-fat chow-fed mice that got 1.5% leucine in water for 10 weeks had a significant decrease in fat mass in comparison to the control group (6). Inconclusiveness of the results tends to point out that when leucine is administered orally, some crucial factors counteract anorexigenic effects seen with e.g., mediobasally-infused leucine.
In the current project, we hypothesized that this counteracting effect of leucine stems from leucine's simultaneous action at brain circuits that promote satiety and those that mediate feeding reward. Therefore, using real-time PCR, we examined whether intake of leucine changes central expression of genes regulating energy homeostasis and reward. We also employed immunohistostaining for cFos, the protein product of the immediate-early gene, and studied whether leucine consumption affects the number of cFos immunoreactive (thus, "activated") nuclear profiles in two hypothalamic sites known to control energy balance (7).
Materials and Methods
Experiment 1: Does leucine consumption affect cFos IR in the hypothalamic paraventricular nucleus (PVN) and arcuate nucleus (ARC)?
Animals.
The mice used in the study were males C57BL/6J mice (Scanbur, Sweden). Their weight at the beginning of the experiment was 23.5 ± 0.15 g.
The mice had been exposed to leucine to avoid neophobia. Before the experimental trials, they had a 3 day-long wash-out phase to ensure cFos immunoreactivity would not be affected by earlier leucine exposure. The night before the study the animals were food- and water-deprived for 16 h. To study the effects of leucine intake on cFos immunoreactivity, the animals were randomly divided into 3 groups: leucine group (n=6) - 1.5% leucine solution was administered for 15 minutes, water group (n=5) - water was administered for 15 minutes, deprived group (n=5) - no fluid was given. Chow (Lactamin,Sweden) (a standard, solid nutrient composition, caloric value = 3.6 kcal/gram) was not returned to cages to avoid induction of cFos immunoreactivity by chows consumption.
Ninety minutes after the end of consummatory behavior (i.e., at the time corresponding to the maximum cFos immunoreactivity, as this maximum value occurs ca. 90 minutes after the actual neuronal activity has occurred), the animals were anaesthetized with pentobarbital (100mg/kg) and perfused transcardially with 0.9% saline solution and then with 4% paraformaldehyde (50mL). Brains were removed and post-fixed in 4% paraformaldehyde overnight as in (8).
c-Fos immunohistochemistry.
The brains were sliced to 60µm thick sections using the Leica VT 1000 S vibratome.
The forebrain sections containing the PVN and ARC were incubated 10 minutes in 10%methanol 3-4%hydrogen peroxide in TBS and then incubated in goat c-Fos (Vector lab) antibody diluted in a mixture of TBS, gelatin and Triton X-100 1:3000 overnight at 4˚C at constant agitation. The sections were incubated in the secondary antibody (rabbit anti-goat IgG) (Novastin) diluted in a mixture of TBS, gelatin and Triton X-100 1:400 for 75 minutes and then incubated in ABC (Vectastain ABC kit PK-6100 standard) for 75 minutes. The
staining was visualised with diaminobenzidine (DAB, Sigma Diagnostics, St. Louis)solution
(0.05% DAB, 0.01% hydrogen peroxide, and 0.3% nickel sulfate) as in (9).
The sections were transferred onto gelatinized slides and air-dried overnight. They were dehydrated in EtOH 70% for 5 minutes, 95% 5 minutes, 100% 10 minutes, soaked in xylene for 20 minutes and coverslipped in DPX (BDH, UK) as in (10).
Experiment 2: PCR studies Animals.
Male C57BL/6J (Taconic, Denmark) mice were used in the study. Their weight at the beginning of the experiment was 25.3 ± 0.60g.
The animals were randomly divided into 2 groups: leucine (n=8) - 1.5% leucine solution during 48 hours was given as the only liquid, water (n=8) - water during 48 hours was offered. The mice had free access to standard chow.
The animals were sacrificed by cervical dislocation directly after the treatment. Hypothalami were isolated from the brain tissue as in (11). The samples were kept in RNA-later at 4˚C for 2 hours and then stored at -20˚C.
RNA purification for RT-PCR studies
The hypothalami were homogenized by sonication in TRIzol (Invitrogen, Sweden) with sonifier (BransonUltrasonics Corp., Germany).The homogenate was centrifuged at 12.000 g for 10 min at 4˚C and separated from the excess of lipids and other cellular waste. Additional TRIzol was added to increase the volume of liquid. Chloroform was added, the samples were incubated for 5 min at room temperature and centrifuged at 12.000 g for 15 min at 4˚C. The aqueous phase was transferred to a new tube and RNA was precipitated with isopropanol. The sample was incubated at -20˚C for 2h and then centrifuged at 12.000 g for 10 min at 4˚C. The supernatant was removed. The pellet was washed with 75% EtOH 2 times. It was left to air dry and the RNA was dissolved in RNAse free water and 10x DNAse buffer and left to incubate for 15 min at 75˚C (12).
DNA contamination was removed with DNAse I treatment overnight (16 hours) at 17˚C and the reaction was stopped by incubation for 15 min at 75˚C. Conformation of genomic DNA removal by the DNAse treatment was done by PCR, primer for mouse tub b5 513bp. Nanodrop ND-1000 Spectrophotometer (NanoDrop Technologies, USA) was used to determine RNA concentration. For cDNA synthesis, random hexamers were used as primers and they were processed with MMLV reverse transcriptase (GE, Sweden). The cDNA synthesis was confirmed with PCR (13).
RT-PCR analysis Relative expression
Levels of mRNAs housekeeping genes (HKGs) GAPDH, RPL19, H3b were determined for relative expression analysis. Concentration of the template was 5ng and less. Reactions were
run with total 25µl volume (5µl template, 9.52µl H2O, 2.00µl 10xDNA polymerase buffer,
0.2µl nM dNTP, 1.60µl MgCl2, 1.00µl DMSO (1:20), 0.50µl SYBR green (1:50000), 0.10µl
primers, 0.08µl Taq polymerase (Bitools, Sweden)). Primers concentration was 5ng/µl (Thermo Scientific, Sweden). The PCR program was started with denaturation at 95˚C for 3 minutes and followed by 50 cycles of given procedure: denaturation 95˚C for 20 seconds, annealing each primer pair for 30 seconds, extension 72˚C for 30 seconds (14). The annealing temperatures for the used primers are given in Table 1.
The data was analysed with Bio-Rad software, primer efficiency with use of LinRegPCR (15), Grubbs' test was used for elimination of outliers (16), Genorm was used to calculate the normalisation factor (17). GraphPad prism 5 was used to visualize the results (18).
Table 1. Real -time PCR primers for mice from Thermo Scientific.
___________________________________________________________________________
Name Annealing Forward primer Reverse primer
temperature ___________________________________________________________________________
GAPDH 55˚C gccttccgtgttcctacc gcctgcttcaccaccttc RPL19 55˚C aatcgccaatgccaactc ggaatggacagtcacagg H3b 55˚C ccttgtgggtctgtttga cagttggatgtccttggg
SLC6A15 58.2˚C gcatcggaagaatttctgagc agcgacgaatgatgaacacc NPY 60.1˚C cccttccatgtggtgatg gacaggcagactggtggc Oxytocin R 58.2˚C atggatctacatgctcttcac acgaaggtggaggagttg Oxytocin 60.1˚C acgctgcttcggaccaag gcgaaggcaggtagttctcc KappaR (KOR) 60.1˚C caccttgctgatcccaaac ttcccaagtcaccgtcag MUOpiodR (MOR) 60.1˚C cctgccgctcttctctgg cggactcggtaggctgtaac DeltaR (DOR) 60.1˚C gctggtggacatcaatcg gctggtggacatcaatcg FTOb 60.1˚C gatgtcagagcgtcagagag aaggtcatggagtgagtgc CRH 60.1˚C taccaagggaggagaagagag ggacgacagagccaccag MC4R 58.2˚C cgctccagtaccataacatc gaagaggacgcctgacac POMC 58.2˚C gaacgccatcatcaagaac ctaagaggctagaggtcatc TMEM18 60.1˚C gctctgtgaatggcttatctg ctctgtcacctcaaatctctaaag
Results
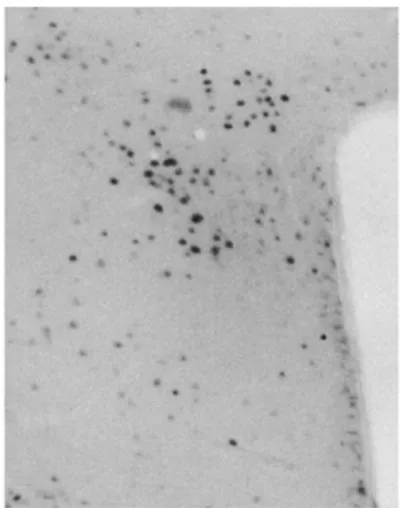
The immunohistochemical staining for cFos (Fig.1) showed a significant change in the level of cFos immunoreactivity in the paraventricular nucleus (PVN) and arcuate nucleus (ARC). There was a significant decrease of the cFos expression level in the leucine group both in the PVN and ARC.
Fig. 1 Immunohistochemical staining for cFos in the PVN.
There was also a significant decrease of the cFos expression level from leucine group to water group in the ARC, unlike the PVN (Fig. 2).
Fig.2 Changes in cFos immunoreactivity following the consumption of leucine-spiked water (leu), water alone (wat) or in the absence of fluid (depr). Animals had been water-deprived overnight prior to the experiment. *, p<0.05 in mice brain parts PVN and ARC.
The RT-PCR analysis of the hypothalamic of mice used in the second experiment showed a significant increase in the leucine group, in the expression of food intake- and energy control- associated genes: TMEM18, SLC6A15, CRH, FTO, and an opioid receptor gene DOR. One of the genes, MC4R, showed decrease in the expression in the leucine group compared to controls (Fig. 3).
TMEM18 ctrl Leuc ine 0.0 0.5 1.0 1.5 *** CRH ctrl Leuc ine 0.0 0.5 1.0 1.5 * FTO ctrl Leuc ine 0.0 0.5 1.0 1.5 * DOR ctrl Leuc ine 0.0 0.5 1.0 1.5 * MC4R ctrl leuc ine 0.0 0.2 0.4 0.6 * SLC6A15 ctrl Leucine 0.0 0.5 1.0 1.5 *
Discussion
Immunohistochemical staining was crucial in order to determine which areas of the brain would show response to the administration of 1.5% leucine solution. The purpose was to examine whether ingestion of leucine in water had a different effect on cFos immunoreactivity than intake of water alone or no fluid intake. It was expected that the increased activity would occur in the hypothalamus as it is one of the areas responsible for the energy control (19). cFos immunoreactivity was measured because cFos is a marker determining the level of neuronal activity in response to any stimulation, process or event involving neurons, including food intake (20). The immunohistochemical staining of different proteins is often used to determine a change in response to diet manipulation (21, 22, 23, 24, 25). The experiment showed that cFos immunoreactivity was evident in the PVN and ARC independently of the treatment. However, a significant change in cFos immunoreactivity in the leucine-supplemented and fluid-deprived group was observed in both sites. Also, the change in leucine-supplemented and plain water group was significant in the ARC. All the changes have the same direction and show a strong influence of the leucine supplementation on the cFos response.
cFos immunostaining is not a selective method and cannot answer the question of which genes are activated and which molecules are synthesised in response to leucine intake. The staining method can only show whether neuronal activity is affected by a given stimulus (26). Changes in cFos levels indicate, however, that a particular area in the brain responds to, e.g., leucine treatment, and that hence this area should be studied further to define molecular phenotypes of affected. Our cFos experiment revealed the hypothalamic sites are sensitive to leucine.
The expression of studied genes: TMEM18, CRH, SLC6A15, FTO, DOR, MC4R reveals that there is a link between leucine consumption and multiple aspects of energy homeostasis regulation, including food intake, hunger, satiety and reward. The function of these genes has been determined in earlier studies (27, 28, 29, 30, 31, 32, 33). DOR has important role in feeding reward, MC4R and CRH promote satiety responses and SLC6A15 may act as an amino acid transporter. TMEM18 and FTO may function as mediators of energy balance and, especially FTO, may play a role in development-related processes. Those studies are interesting but they have not given all the answers of their importance to food intake related subjects. By examining the expression of genes in question we cannot get a simple answer on how to regulate homeostasis and reward by diet supplementation. The problem lies in that we do not know the exact function of all the genes in the organism and the regulative processes are complicated and involve many bodily mechanisms apart from gene expression. Lots of basic studies need to be done on the mechanisms and the correlations between them.
The comparison of the data obtained in the studies where leucine was injected directly into MBH resulting with reduction of food intake (3) versus those where leucine was supplemented orally and had no anorexigenic effect (4,5) indicates that the difference in the observed feeding response likely stems from how the leucine-derived signalling is relayed from the digestive system to the brain. Our RT-PCR studies of gene expression in the hypothalamus in response to the oral leucine supplementation showed that the expression levels of genes controlling both feeding for energy and reward were affected, likely counteracting each other's effects. That can explain why no significant overall change in food intake was observed when leucine was administered orally. The genes found to be affected were MC4R, TMEM18, CRH, FTO, DOR, SLC6A15.
As shown before, influence of leucine injected directly into the brain caused significant increase of POMC gene expression in the ARC (3). In our studies the level of POMC gene expression was also measured but showed no significant change. The difference was mainly the lack of signalling pathway from the digestive system to the brain in the first case. This shows that the hypothalamic response is altered by the route of leucine's administration. It would be of great interest to search more in that direction and compare more genes and their activity depending on the way leucine was administered. This could give us information on possibilities to design more effective ways of diet supplementation. Since gene expression does not provide a definitive answer regarding the peptidergic activity within signalling pathways, it would be also of interest to measure the level of peptides coded by the affected genes.
Although many studies on food intake and food supplementation have been carried out, we do not understand all the mechanisms regulating it. The organism seems to be capable of "disregarding" supplementation strategies aimed at reducing calorie intake. As it has already been described, our molecular background geared toward conserving energy does not serve us well in the environment of free access to high-calorie foods and minimised the physical effort to gain it (33). Our environmental changes have been occurring a lot faster than any evolutionary adaptations to food abundance could take place.
The studies showed that leucine diet supplementation increases the c-Fos activity in the ARC
and PVN of the hypothalamus controlling energy balance. Another effect of leucine's oral administration was the simultaneous change in expression of TMEM18, MR4C, CRH, FTO, SLC6A15 and DOR genes which indicates counteracting effects of leucine on activating central pathways responsible for the regulation of feeding for reward and those promoting satiety.
References
1. John T. Brosnan and Margaret E. Brosnan (2006) Branched-Chain Amino Acids: Enzyme and Substrate Regulation. American Society for Nutrition J. Nutr. 136:207S-211S
2. Keiko Kanamori, Bran D. Ross, Richard W. Kondrat (1998) Rate of Glutamate Synthesis from Leucine in Rat Brain Measured In Vivo by N NMR. Journal of Neurochemistry 70:1304–1315
3. Clémence Blouet, Young-Hwan Jo, Xiaosong Li, and Gary J. Schwartz (2009) Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamic-brainstem circuit. J Neurosci. 29(26): 8302–8311.
4. Ali Nairizi, Pengxiang She, Thomas C. Vary, and Christopher J. Lynch (2009) Leucine Supplementation of Drinking Water Does Not Alter Susceptibility to Diet-Induced Obesity in Mice. J Nutr. 139(4): 715–719.
5. Bassil MS, Hwalla N, Obeid OA (2007) Meal pattern of male rats maintained on histidine-, leucine-, or tyrosine-supplemented diet. Obesity (Silver Spring) 15(3):616-23.
6. Yiying Zhang1, Kaiying Guo, Robert E. LeBlanc, Daniella Loh, Gary J.
Schwartz, Yi-Hao Yu (2007) Increasing Dietary Leucine Intake Reduces
Diet-Induced Obesity and Improves Glucose and Cholesterol Metabolism in Mice via Multimechanisms. Diabetes 56 :6 1647-1654
7. Pawel K Olszewski, Robert Fredriksson, Agnieszka M Olszewska, Olga
Stephansson, Johan Alsiö, Katarzyna J Radomska, Allen S Levineand Helgi B
Schiöth (2009) Hypothalamic FTO is associated with the regulation of energy intake
not feeding reward. BMC Neuroscience 10:129
8. Pawel K. Olszewski , Robert Fredriksson , Jenny D. Eriksson , Anaya Mitra ,
Katarzyna J. Radomska,Blake A. Gosnell , Maria N. Solvang , Allen S. Levine , Helgi B. Schiöth (2011) Fto colocalizes with a satiety mediator oxytocin in the brain
and upregulates oxytocin gene expression. Biochem Biophys Res Commun. 408 (2011) 422–426
9. Anaya Mitra, Blake A. Gosnell, Helgi B. Schiöth, Martha K. Grace, Anica
Klockars, Pawel K. Olszewski, Allen S. Levine (2010) Chronic sugar intake
dampens feeding-related activity of neurons synthesizing a satiety mediator, oxytocin. Peptides 31 (2010) 1346–1352
10. Pawel K. Olszewski, Katarzyna J. Radomska, Kedar Ghimire, Anica Klockars,
Caroline Ingman, Agnieszka M. Olszewska, Robert Fredriksson, Allen S. Levine, Helgi B. Schiöth (2011) Fto immunoreactivity is widespread in the rodent brain and
abundant in feeding-related sites, but the number of Fto-positive cells is not affected by changes in energy balance. Physiology & Behavior 103 (2011) 248–253
11. Tatjana Haitina, Fredrik Olsson, Olga Stephansson, Johan Alsiö, Erika Roman,
Ted Ebendal, Helgi B Schiöth and Robert Fredriksson (2008) Expression profile of
the entire family of Adhesion G protein-coupled receptors in mouse and rat. BMC Neuroscience 2008, 9:43
12. Malin C. Lagerström, Nadine Rabe, Tatjana Haitina, Ineta Kalnina, Anders R.
Hellström, Janis Klovins, Klas Kullander and Helgi B. Schiöth (2007) The
evolutionary history and tissue mapping of GPR123: specific CNS expression pattern predominantly in thalamic nuclei and regions containing large pyramidal cells. Journal of Neurochemistry, 2007, 100, 1129–1142
13. Smitha Sreedharan, Jafar HA Shaik, Pawel K Olszewski, Allen S Levine, Helgi B
Schiöth, Robert Fredriksson (2010) Glutamate, aspartate and nucleotide transporters
in the SLC17 family form four main phylogenetic clusters: evolution and tissue expression. BMC Genomics 2010, 11:17
14. R.H. Lekanne Deprez, A.C. Fijnvandraat, J.M. Ruijter, A.F.M. Moorman, (2002) Sensitivity and accuracy of quantitative real-time PCR using SYBR green I depend on cDNA synthesis conditions, Anal. Biochem. 307 (2002) 63–69.
15. Christian Ramakers, Jan M. Ruijter , Ronald H. Lekanne Deprez, Antoon F.M.
Moorman (2003) Assumption-free analysis of quantitative real-time polymerase
chain reaction (PCR) data. Neuroscience Letters 339 (2003) 62–66
16. Ram B. Jain (2010)A recursive version of Grubbs' test for detecting multiple outliers
in environmental and chemical data.Clinical Biochemistry 43 (2010) 1030–1033
17. Jo Vandesompele, Katleen De Preter, Filip Pattyn, Bruce Poppe, Nadine Van
Roy, Anne De Paepe and Frank Speleman Accurate normalization of real-time
quantitative RT-PCR data by geometric averaging of multiple internal control genes Genome Biology 2002, 3(7):research0034.1–0034.11
18. J. Alsiö, P. K. Olszewski, A. H. Norbäck, Z. E. A. Gunnarsson, A. S. Levine, C.
Pickering, and H. B. Schiöth (2010) Dopamine D1 receptor gene expression
decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience 171 (2010) 779–787
19. Harvey J. Grill (2006) Distributed Neural Control of Energy Balance: Contributions from Hindbrain and Hypothalamus. Obesity 14, 216S–221S
20. Dragunow M, Faull R. (1989)The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. Sep;29(3):261-5
21. Lohmeier TE, Warren S, Cunningham JT. (2003) Sustained activation of the central baroreceptor pathway in obesity hypertension. Hypertension. Jul;42(1):96-102 22. Maciejewska I, Spodnik JH, Wójcik S, Domaradzka-Pytel B, Bereznowski Z.
(2006) The dentin sialoprotein (DSP) expression in rat tooth germs following fluoride treatment: an immunohistochemical study. Arch Oral Biol.;51(3):252-61
23. Razny U, Polus A, Kiec-Wilk B, Wator L, Hartwich J, Stachura J, Tomaszewska
R, Dyduch G, Laidler P, Schmitz G, Goralczyk R, Wertz K, Riss G, Franssen-van Hal NL, Keijer J, Dembinska-Kiec A. (2010) Angiogenesis in Balb/c mice under
beta-carotene supplementation in diet. Genes Nutr.;5(1):9-16
24. Polkowska J, Gładysz A. (2001) Effect of food manipulation on the neuropeptide Y neuronal system in the diencephalon of ewes. J Chem Neuroanat.;21(2):149-59.
25. Jin Y, Yan EZ, Li XM, Fan Y, Zhao YJ, Liu Z, Liu WZ. (2008) Neuroprotective effect of sodium ferulate and signal transduction mechanisms in the aged rat hippocampus. Acta Pharmacol Sin.;29(12):1399-408.
26. Bullitt E. (1990) Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 22;296(4):517-30.
27. Pawel K Olszewski, Robert Fredriksson, Agnieszka M Olszewska, Olga
Stephansson, Johan Alsiö, Katarzyna J Radomska, Allen S Levineand Helgi B Schiöth(2009) Hypothalamic FTO is associated with the regulation of energy intake
29. Drgonova J, Liu QR, Hall FS, Krieger RM, Uhl GR. (2007) Deletion of v7-3 (SLC6A15) transporter allows assessment of its roles in synaptosomal proline uptake, leucine uptake and behaviors. Brain Res. Dec 5;1183:10-20.
30. Señarís RM, Trujillo ML, Navia B, Comes G, Ferrer B, Giralt M, Hidalgo J. (2011) Interleukin-6 regulates the expression of hypothalamic neuropeptides involved in body weight in a gender-dependent way. J Neuroendocrinol. Aug;23(8):675-86. doi: 10.1111/j.1365-2826.2011.02158.x.
31. Holzapfel C, Grallert H, Baumert J, Thorand B, Döring A, Wichmann HE,
Hauner H, Illig T, Mielck A. (2011) First investigation of two obesity-related loci
(TMEM18, FTO) concerning their association with educational level as well as income: the MONICA/KORA study. J Epidemiol Community Health. 2011 Feb;65(2):174-6. Epub 2010 Jul 13.
32. Marczak ED, Jinsmaa Y, Myers PH, Blankenship T, Wilson R, Balboni G,
Salvadori S, Lazarus LH. (2009) Orally administered H-Dmt-Tic-Lys-NH-CH2-Ph
(MZ-2), a potent mu/delta-opioid receptor antagonist, regulates obese-related factors in mice. Eur J Pharmacol. 2009 Aug 15;616(1-3):115-21.