I
I
E E
E
E E
I
II
E
L
E
ATOMIC ENERGY DIVISION
AMERICAN
~COMPANY
RAW MATERIALS DEVELOPMENT LABORATORY
HOLTON STREET WINCHESTER, MASS.
G. Marvin
T E L E P H O N E WINCHESTER 6-3820
Assistant Director for Process Development Division of Raw Materials
U. • Atomic Energy Commission
25, D. C.
Dr. Marvin:
July 30,
1954
Attached is a copy of Topical Report ACC0-33 entitled, "Additional Extraction and Ion Exchange Studies of Temple Mountain Ores", by
Jones and H. I. Viklund. It will be seen that the work of
• Jones and Viklund indicate a good extraction of uranium can be obtained from Temple Mountain ores by grinding the ore, acid leaching, flotation of the asphaltite, roasting the asphaltite and acid leaching the calcine.
Very truly yours,
AMERICAN CYANAMID COMPANY
TOPICAL REPORT ACC0-33
ADDITIONAL EXTRACTION AND ION EXCHANGE STUDIES OF
TEMPLE MOUNrAIN DISTRICT ORES
by Jo Q, Jones & H. I. Viklund July 30, 1954
?llc/,
1/~)
H. I., Viklund Contract AT(49-1)•533 Atomic Energy Division American Cyanamid CompanyRaw Materials Development Laboratory Holton Street
TOPICAL REPORT ACC0-33
ADDITIONAL EXTRACTION AND ION EXCHANGE STUDIES OF
TEMPLE MOUNTAIN DISTRICT ORES
by
J. Q. Jones
&
H. I. Viklund
A
Uranium extraction of
95
%
has been obtained from samples of TempleMountain ore by a cyclic test procedure in which the new feed was not roasted. This procedure involved acid leaching the ore, flotation and roasting of the carbonaceous material, and acid leaching the
calcine. A total of 100 pounds of sulfuric acid per ton of
ore and no oxidant was required for leaching.
Treatment of the ptegnant uranium liquor in a three column ion
resulted in a recovery of
99.7%
of the uraniumTABLE OF CON'l'ENTS
I. INTRODUCTION
ORIGIN AND DESCRIPTION OF SAMPLES
SUMMARY AND CONCLUSIONS
IV. TEST WORK
A. Extraetion Tests
a. Initial work reported in Letter Report L-29
b. Flowsheet Development
c., Cyclic Tests
Bo Ion Exchange Tests
Metallurgy-Raw Materials
- 4
~ 56
8 10 10 1013
14
15
TOPICAL REPORT ACC0-33
ADDITIONAL EXTRACTION AND ION EXCHANGE STUDIES OF TEMPLE MOUNTAIN DISTRICT ORES
by J. Q. Jones & H. L Viklund I. INTRODUCTION
- 5
=At the request of the Atomic Energy Commission test work was initiated on uranium ore samples from the T.em:ple Mountain District, Emery County, Utah. Commercial treatment of these ores has been difficult and this laboratory had been asked to investigate :possible methods of improving uranium recovery from such ores. -Test work of low grade material from this same district has been :previously reported in Topical Report ACC0-27 entitled, "Uranium Recovery from Temple Mountain District Ore" by F •
..Lu•::-.;;J.J.<::J.' Jr. Preliminary test work on high-grade material,
and 22-4, has also been reported in Letter Report L-29
11
Preliminary Investigations on Temple Mountain Ores" by J. W. Cole and
R. J. Woody. In that report the following conclusions were
1. Cold acid leaching extracted only 75
%
of the uranium.Roast-the ore for two~:hours at 450°C. before acid leaching extracted 94
%
of the uranium in the roasted material. However, there was an un-accountable loss during roasting which lowered the extraction from the
ore to 87
%.
2. Hot carbonate leaching at 95°C. extracted only 50
%
of theuranium. By roasting at 550°C. for two hours the extraction was in=
creased to 79
%
based on the uranium in the roasted material. Dustloss lowered the extraction from the ore to 71
%"
3. Pugging and curing the ore with sulfuric acid and sodium
fluosilicate was no better than cold acid leaching.
4. Settling tests on acid :pul~s indicate that the Temple
ore is amenable to counter-current,decantation. Less than two square feet of area is required :per ton of ore if small quantities (0.1 lb./
of flocculating agents of the Locust Bean type are used.
The object of the :present test work was to continue the work of J.
Cole and R. J. Woody, investigating :procedures, other than
of the whole ore, which would :produce 95
%
of better uranium extractions.After suitable extraction techniques had been developed a study of uranium recovery from solution by ion exchange was to be made.
Three samples of Temple Mountain ore were receivedo The in good condition, designated as Sample 22-3, arrived October
It had been stated that this sample originated from a stockpile at the plant of the Vitro Chemical Coo, Salt Lake City, Utaho The had a net weight of 418 poundso The second sample, designated as
Sample also arrived in good condition on January 23, 1953 and
was said to be from the high asphaltic stockpile at the Monticello, Utah planto It weighed 440 poundso The third sample, weighing 500
arrived in good condition on June 23, 1953o It had been
taken from the south end of Stockpile 24 at the Monticello, Utah planto
Each ore sample was staged crushed through 10 mesho After the entire sample was split into 500-gram charges with a Jones
riffleo A random charge was assayed chemically and spectrographicallyo The result of these analyses is given in Table Io
Temple Mountain ores 22-3, 22=4, and 22~5 are medium to fine
dark gray to blacksandstones with matrices of _carbonaceous material, and small amounts of carbonateo An olive green shale member is
Minor to trace amounts of pyrite, chalcopyrite,
fluorite, apatite, tourmaline and muscovite are present interstitiallyo Uranium mineralization consists essentially of carnotite and/or
tyuyamunite, a uranium-bearing hydrqcarbon, and , possibly, finely disseminated pitchblendeo
- 7
Table I
Chemical and Spectrographic Analysis
Chemical Analysis Ore No. 22-3 22-4 ajo U308 .25 .253 .355
tfo
V205 .60 .72 L2551o
Fe L90 L60 ajo P20:2 .085 .065 1o 804::: 5.27%
co
0
L27%
Ca .50 .68 1.53 ajo MgO 0 ajo Al6
o
3 3.13 ajo Si 2 82.6 8LO 1oo.r.
8.2 ~ectrographic AnalysisOre Percent Elements
10 1o Si 1-10 1o Al, .F.e .1-1
%
a.a., v, u
.01-.,1%
Be., Cr, Mg, Mn, Na, Sb, Zn, Zr Co, Ni,,001-.01 1o B, Cu, Li, Mo,
Ti, Sn, Sr
Ore Pereent Elements
)10% Si
1-10% Al, Fe
.1-1
%
ca,
v, u
.01-.1 % Ba, Cr, Ga, Mg, Mn, Na,
P, Sr, Ti, Zr
Extraction of 95
%
of the uranium in the Temple Mountain oreobtained by a combination of acid leaching, flotation and roast= techniqueso The ore was wet ground to minus'48 mesh and then leached with 100 pounds of sulfuric acid per ton of ore for 16 hours
at room temperature. Approximately 75
%
of the uranium was extracted.The remaining 25
%,
intimately associated with~ carbonaceous, is resistive to acid dissolution. After separating the liquor from the residue the carbonaceous material is
from the pulp by flotationo The flotation concentrate,~~~~'~v,
%
of the weight, is filtered and roasted at 450°Co for two hours.The uranium in the calcine, now soluble in sulfuric acid~
added back to the next raw ore leach or leached with the
acid first and the slurry added to the raw ore leach. A bench scale
test was set up using the diagramatic flowsheet as on
nine of this report.
The pregnant liquor from the cyclic tests was passed through a
3
column ion exchange system using IRA 400 resino Average column
for 11=1/3 cycles was 90.1 grams of
u
3o8 per liter of wetsettled resin. Recovery of uranium from the pregnant liquor was
71
%.
Elution efficiency was low unless the sulfate concentrationof the eluting solution was above 100 grams per liter.
The flowsheet described above, flotation of the after the
had been leached, was selected because (1) less uranium would lost on roasting the concentrate produced in this manner because
of .lower uranium content, and (2) it could be readily
into the present operation of the Vitro Chemieal.Co, plant Lake City, Utah. A single test, Table XIII, however, indicated
the of uranium would be just as good provided there was
no uranium lost in roasting if the carbonaceous material was floated roasted, and then leached with the flotation tailings.
!
TEMPLE MOUNTAIN ORE FLOWSHEET
Wet Circuit
Primar1 Leach Dewater and Wash Liquor to Uranium Product Barren. To Vanadium Recovery
t
Slimes Deslimer Sands to Tailings1
Roast- 10 =
For the purpose of reporting, the test work is divided as follows~
A. Extraction Tests
a. Initial Work Reported in
Letter Report L~29
b. Flowsheet Development c. Cyclic Tests
B. Ion Exchange Tests
A. Extraction Tests
Effect of Acid Concentration
A series of leaching tests was made, on 500=gram of
minus 10 mesh ore, using varying amounts of sulfuric acid. The ore was
leached for 16 hours, at 50
%
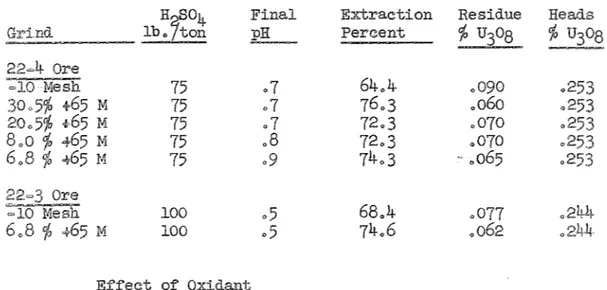
solids, on rolls in open bottles with~"v~,•u-temperatures. The results of these tests are found in Table which indicates that 75 to 100 pounds of acid per ton of ore gave extractions under the conditions of these tests.
75 100 200 75 100 Effect Final pH 1.3 Ll o7 ·5 .1 Ll .9 .5 .3 .1 Table II
of Acid Concentration on Uranium Extraction
%
Residue Extraction j) U308 53.0 .119 .253 59.3 .103 .253 64.,4 .090 .253 63.6 .092 .253 63.6 .092 .253 65 .085 .244 66 .082 68.4 .077 71.3 .070 .,070= 11 =
of Particle
The effect of size on uranium extractions was
determined. Teat charges of minus 10 mesh ore were ground in a ball mill at 70 % solids for various periods of time and screen analyses were made on the discharge products. The ground ore was leached at 50 % solids under the conditions previously noted. The data, shown in Table III indicates that grinding is beneficial.
Table III
Effect o:f on Uranium Extraction
H
7
S04 Final pH Extraction Percent Residue % U308 % Headslb. ton 75 o7 64.4 .090 .5% +65 M 75 .7 76.3 .o6o 20.5% +65 M 75 o7 72.3 .070 8.0 % -t65 M 75 .8 72.3 .070 6o8 % <t65 l-1: 75 o9 74.3 - .065 100 o5 68.4 .077 .244 6.8 % ~65 M 100 o5 74.6 .062 of Oxidant
The e:ff~ct o:f an oxidant (NaClo
3) on uranium extraction was
also studied with the results as shown in Table IV. The data indicates that an oxidant has little e:f:fect on uranium extraction.
Table IV
H SO NaClo3 Final Extraction Residue Heads
lbofto~
lb,/ton pH Percent ojo U~o8
%
Ore
6.8
lfO <\' 65 M 75 0 .9 74.3 .065 6.8 ojo <\> 65 M 100 10 .6 74.3 .065 6.8 ojo .; 65 M 100 25 .6 76.7 .059 100 0 o5 68.4 .077 =10 Mesh 100 25 .5 69.7 .074 6.8 ojo ? 65 M 100 0 .5 74.6 .062 6.8 ojo + 65 M 200 25 .1 77.0 o056 Effect TimeIn order to determine the optimum leach time for uranium QV·r~c·~
tion a lOOO~gram sample of 22=4 was ground to minus 100 mesh and leached
ambienttemperatures with 200 pounds of acid-per ton of ore, at
%
in a gl~as Pachuca tank. ·Sampl€S were tak€n at various time
:for residue analysis. The results of this test, Table show
that extraction is essentially complete after 7 hours.
Table V
Effect o:f Time on Uranium Extraction
Cumulative Residue Heads
pH
%
Extraetion oj;'U308 of; u~o8
.5
hours .65 27.3 .1845
hours .65 64o8 .089 .253.5
hours .80 73.5 .067 .253 .5 hours .80 75.1 .063 o5 hours.8o
77.1 .058 .253 5.5 hours .85 75.9 .061 6 hours .90 78.6 o054 .2535
hours .90 79.4 .052 .253 hours LOO 80.2 .050b
In the
and J W,
combinationa for maximumextraction whole ore, In the following series of flowsheets and various methods of the oreo
was on extraction without
In the first series, an attempt was made to increase the
extraction -of the uranium by the aspha~tic material from
ore by flotation", and this concentrate very fine in
order to liberate the uraniumo A definite improvement in extraction resulted" Maximum extraction from the ore was 90 ·cfo as
77 ~ when the flotation concentrate was not regroundo
extraction was noted whether the float concentrate
with the float tails or leached
leacho Tables VI through XII cover the results of this test
The extraction of uranium by regrinding the ~n.~~,,+~·~+:~ was much over the straight acid
the oreo Higher extractions were anticipated
concentrate could be roasted without excessive dust losso
included in this tests in Tables XIII and
definite could be The
vuu~~~by material
contained approximately of the uranium in the
the this material would
of excessive uranium loss by dustingo
as in 'Table reduces the uranium in the
= 20 .tfv of that in the ore o Roasting this material is :rimch more
than the concentrate" A series of
-"":""'~"L<;;;C> were made to det-ermine the amount of
maximum uranium recovery and to provide
for roasting studieso by several nr-,oc~~a~:u
From the roasted concentrate uranium were to be
in Table
XV,
indicateseventeen=hour leach would were set for the
and measurement of
The ~esults of the ore leach 100 pounds of sulfuric acid
maximum These
leach
In results are shown on roasted
concentrates" In Test l the calcine was leached with 100 ~v~~o
ton of ore and in Test 2 with 100 pounds of acid
Extractions of 95 ~ of the uranium was
unaccountable loss was 3,0 ~ of the uranium
concentrate" would be a loss of
on the total uranium in the oreo Results of did not indicate this losso
After the extraction of uranium to determine a a closer measure could be obtained The results table several
3
'fo
of thecontinued to
was cut to Oo
The eluate was
seventeen column · u.L! . ..ua"'''""'
increased to 0¢5 N
was employedo The sent to precipitation and
loaded columno After
column volumeso By this time, formed in the column and high uranium final eluate fractiono One normal HCl
the remaining uranium continued in this manner and
until finally there was The final fraction of eluate was
cut to Oc3 N HCl Oo7
HCl was added to the
the to Oo3 with
well for several
Oo9 N NH4Clo This also
over 100 grams
iono It was concluded that the increase
ammonium sulfate had
after loading a column eluted with
It can be a high the 1 N chloride
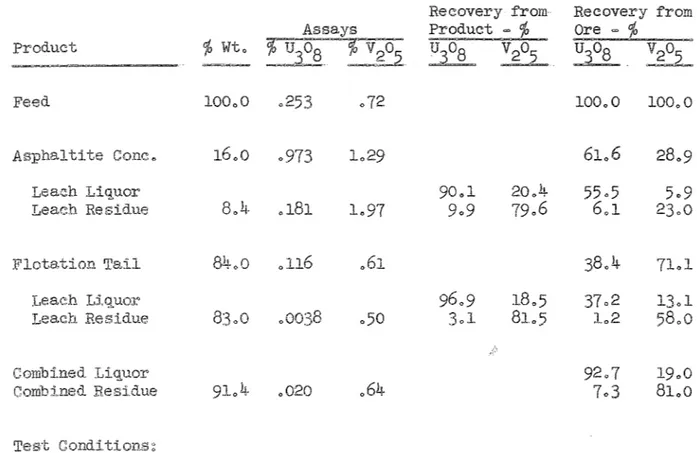
ore, float asphaltite, and leach 1 leach flotation
tailo
Test Results
Recovery from Recovery from
Assay Product - ~ Ore ~ ~
Product ~ Wt., '}I) U308
10
v25 U308 V205 U308lOOoO o259 o71 100.,0
Conco 18.,2 o958 1o40 100"0 lOOoO 67o3
Leach Liquor 80o8 29o0 54.3
Leach 17o5 .192 1.,02 19o2 71o0 13o0
Flotation Tail 8L8 o104 .56 100"0 100.,0 32o7
93.4 16o6 30o6
Leach Residue 8oo8 o007 o47
606
83.4 2olCombined 84o8
Combined Residue 98o3 .,040 o58 15o2
Test Conditions~
Ore = 1000 grams, minus 10 mesh, Sample Noo 22=4o
= labo rod mill, 70 ~ solids, minus 48 mesh"
= 0., lbo Oil, Oo07 lbo AF=25, Oo08 lb., Noo 70 Oil/
ton ore 11 min"
= 18 hours, 50 ~ solids, 3/4" Flint Balls, 100 lbs.
ton ore, 8"0 lbso Mn0
2/ton ore, pH o4, EMF =460 mv.
= 19 hours, 50 ~ solidS, 100 lbso ~604/ton ore,
mVo V205 100.,0 35.8 lOo4 25.4 64o2 10.,8 53o4 19o7 80.,3
= 17 =
Grind ore, float asphaltite, grind cone.,, leach cone", leach flotation taiL
Recovery from Recovery from
Product c{o Wt,
Product = ~ Ore = ~
u~o
8
V20;2 u~o8
V202Feed 100.,0 o275 o75 lOOcO 100,0
Asphaltite Conc" l5o3 lol40 lo39 100"0 100,0 63o6 28,1
Leach Liquor 82,2 25o3 52o3 7ol
Leach Residue l4o9 o209 L06 17,8 74o7 llo3 2LO
Flotation Tail 84o7 ,118 ,64 lOOoO 100.,0 36o4 71,9
Liquor 92,4 17,1 33o7 12o4
Residue 83,4 ,0091 o54 7o6 82o9 2o7 59o5
86oO l9o5
o039 14oO 80o5
Test
Ore = 1000 grams, minus 10
= Lab o mill, 70
%
~:>u.~...~...,,.,Flotation = o lb., , o08 lbo Noo 70 oil/ton
ore ll mino
Carbon Cone, = ~ hours, 50
%
solids, 3/4" Flint Balls.,Carbon = 19 hours, 50 t:fo solids, 100 lbs., H2so4/ton ore,
8,0 lbo Mn02/ton ore, pH = Oc01 EMF =~)0 IDVo
flotation tail = 19 hours, 50 t:fo solids, 100 lbs., H~so
4
/tonFlowsheet~
Grind ore, float
Results~ Product Feed Conco Leach Liquor Leach Residue Combin.ed ~-'"'~~·-~ Combined Residue Test Conditions~
%
Wto oO o7 86oO o3 Ore = 1000 minus 10 o265 lo093 o417 , leach flotation o75L24 lOOoO lOOoO 57o8
o8 28o4 36o3
o91 7L,6 2L5
o67 lOOoO 100"0
97ol 20o5 40o8
0 2o9 79o5
77ol 22o9
Grind = Labo rod 70
%
solids,Flotation = " lbo Fuel Oil, o07
ton ore, 11 mino
Conco = None
Carbon conco = hours
BoO lbso
flotation tail =
%
1 100 lbso ore,pH OoO, EMF mvo
50
%
ov.1..J..u."' 100 lbso H2so4/ton ore,·0 mvo o2 6o6 16,6 76o8 15o 7 6L,l 3 77o7
Flowsheet~
ore, float asphaltite, tite, leach
leach flotation tail,
Product
'fo
wt,Feed ( 0) 100.0 100,0 100,0
Cone. 2L3 o720 L 100,0 100,0 65.6 39.5
Liquor 8L5 53o5 8.0
Leach Residue 20o5 .139 lolO l8o5 79o7 12,1 5
Flotation Tail 78o7 ol02 0 100.0 60,5
Leach 97 19,4 o4 lL7
Leach Residue 77o7 o003Q 8 8o,6 LO 48o8
86,9 l9o7
Combined Residue 98o2 o031 o59 13,1 80o3
Ore = 1000 grams minus 10
= Lab. rod 70
'fo
minuslbo Fuel Oil, .07 lb. , ,08 lb,;No., 70 oil/ton ore,
min,
Cone. ~ 2
'fo
Carbon Cone, = 19 50 solids, 100 lbso ore
8 lbs" Mn02/ton ore, 0 EMF =570 rnv,
Flotation Tail = 19 50
'fo
.100 lbs, H2so4/ton'{o Wto o) 0 0 1 " o6 0 1 oO 9 o9 4 9 0 lo
5
= 21 =
ore:;1 grind and leach , leach flotation
Resultsg
Recovery from
P.roduc:t = ~ _
cjo Wto U308 V205
100.,0 o239 o73
19.,6 o800 lo36 lOOoO 100,0 65 ,lt- 36o6
86oO 20o7 56o2 7o5
19o0 oll7 loll 14.,0 79o3 9o2 29,1
8o.,4 ol03 100,0 100.,0 34o6 63o4
97o3 20o6 33o6 .,1
Leach Residue 79o4 o o0030 o46 7 79o4 LO 50o3
89.,8 20o6
98.,4 o025 o59 l0o2 79o4
Conditions
Ore 1llinus 10 No"
Mill3 70
%
minus 48 mesholbo o07 lbo AF=25, .,08 lbo No., 70 oil/ton
11 Min,
carbon = 5 50
%
1/4" steel ballsocarbon conco = 19 hours9 50
%
100 lbs., H2so4/ton ore,8 " Mn.02/ton ore pH 0"0, EMF mv"
flotation = 19 50 ~ 100 lbsh H
2so
4
/tonore,
Results~
c{o Wto
Feed (Calco) lOOoO
Leach Liquor
Primary Leach Residue 0
Conco l7o0
Conco Leach
Conco Leach Residue l6o7
Flotation oO Test Ore = 11 mino Conco = 19 Gone, = 19 Table ,2~9 0 72 100,0 lOOoO
78o8 20o8 78o8
o057 o58 2L2 79o2 2L2
o295 14 lOOoO 100"0 18.,7 51o8 1L6 9o7 48 88o4 9,0 o0082 o46 5 88o5 1L5 Noo •·.t"'"•"'""lli' 48 mesho
100 lbso ~804/ton ore, 20 lbso
= Oo51 EMF =605 mv"
o07 lbso , o08 lbo Noo 70 oil/ton
:floato 50
%
o8 79o2 8 ol o7 9 ol BoO lbBo}
%
""'-'.!...!..'-''"'float roast , leach roasted calcine, leach flotation tail.
Results~
from Recovery from
·· ~;::oduct = ~ Ore = ~
ojo Wt. U308 V205 U308 V205
Feed ) 100.0 .72 100.0 100.0 Cone. 17.0 .921 100.0 100.0 62.6 29.3 Leach .2 22.8 57.7 6.7 Leach Residue 9.3 0 L73 7.8 77.2 4.9 .6 Tail 83.0 0 100.0 100.0 37.4 70.7 97.2 18.,9 36.4 13 0 .50 2.8 81.1
LO
Combined 94ol .,1Combined Residue 9lo .016 5.9 79.9
Test
Ore = '"'""'ll!J.J·"' No.
minus 48 mesh •
• 07 lb. » .o8 lbs. No. 70 Oil/ton
/ton ore) mv.
~ail = Thickened to
5.5
hours, 100 lbs.Table XIV
Conditions
Ore minus 10 .Noo o
Rod 70
%
solids, minus 48 mesholbo Fuel Oil9 o07 lbo , oOB lbo Noo 70 Oil/ton ore,
Flotation Tail =
0 0 0 c 0 0 0
Product
Calcine
Calcine
Table
~ Distribution
Wto r{o U308 V205
Conco lOOoO o335 Ll7 lOOoO lOOoO
Leach Liquor 87o4 35o9
Leach 56oO o030 L45 5o0 68,1
56o7 o548 2ol8 92o4 l04oO
7o6 +4oO
from Tests 11, 12 & 13 = roasted 2
19 hours at 30°Co3 50 % solids, 100 lbso
1020 lb" calcine), 8 lb, .lvln02/ton ore ( 82 lb" /ton
EMF =910 mvo
~ Distribution
Wto r{o U308 V205
100"0 17 lOOoO . 100"0
95o0 6o5
o8 o039 L93 606 90o5
o5 "603 2o04 lOL6 97o0
·+
lo6 3o0fromTests 11, 12
&
13- roastedleached for 19 at 30°Co, 50% solids,
ore ( lbs,/ton calcine), o76 lDo
Mno
2 tonTable XVII
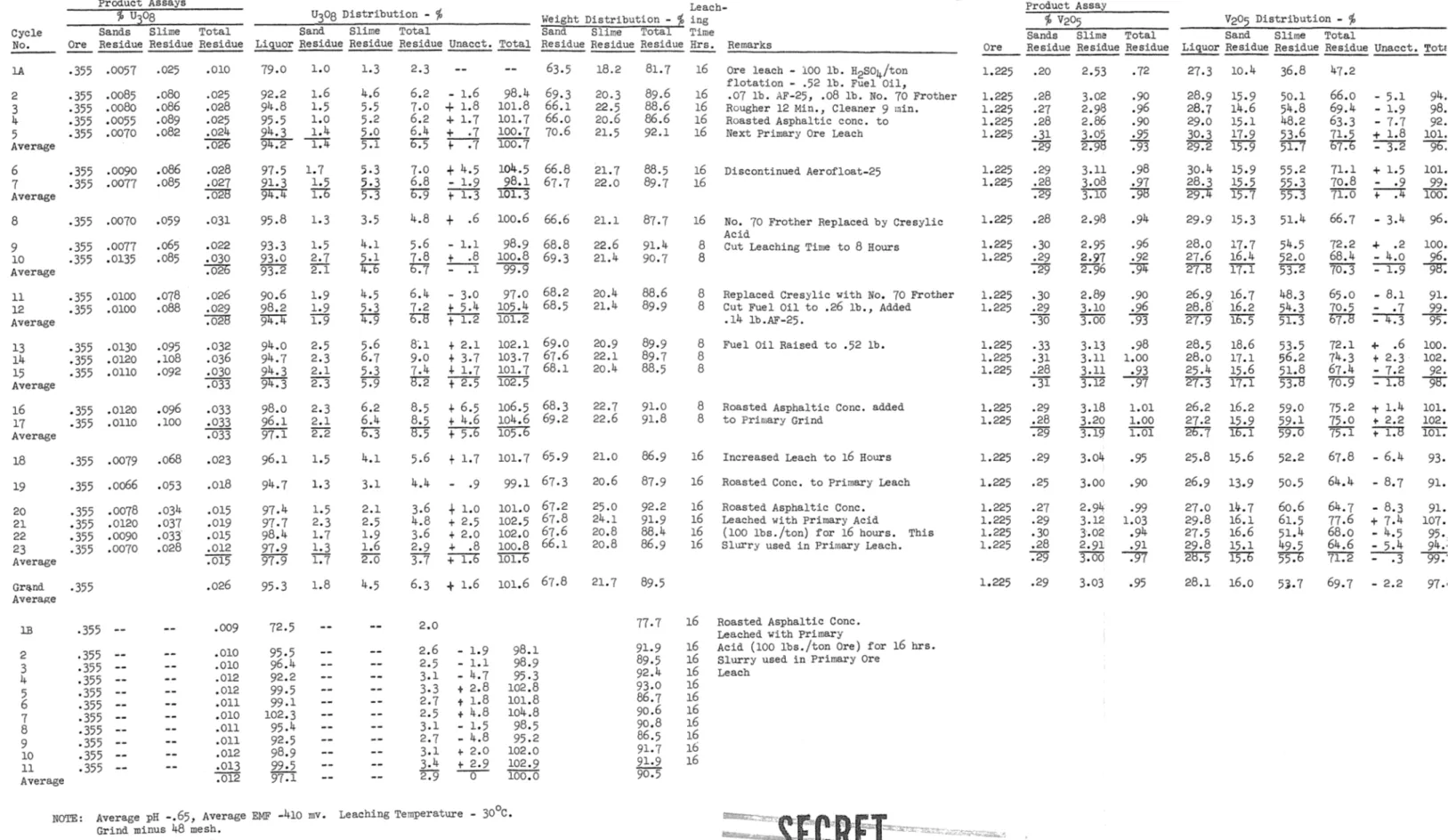
Cyclic Leaching Tests - Temple Hountain Ore - Sample 22-5
Product Assays
'1l U308 U308 Distribution - %
Sands Slime Total Sand Slime Total
Cycle
No. Ore Residue Residue Residue Liquor Residue Residue Residue Unacct.
lA 2 3 4 5 Average -355 .0057 .355 .0085 .355 .oo8o .355 .0055 -355 .0070 6 .355 .0090 7 .355 .0077 Average 8 .355 .0070 9 .355 .0077 10 -355 .0135 Average n • 355 • 0100 12 .355 .0100 Average 13 .355 .0130 14 .355 .0120 15 .355 .0110 Average 16 .355 .0120 17 .355 .ono Average 18 .355 .0079 19 .355 .oo66 20 .355 .0078 21 .355 .0120 22 .355 .0090 23 • 355 .0070 Average .025 .080 .o86 .089 .082 .o86 .085 .059 .065 .085 .078 .088 .095 • 108 .092 .096 .100 .o68 .053 .034 .037 .033 .028 .010 • 025 .028 .025 .024 .026 .028 • 027 .028 .031 .022 .030 .026 .026 .029 .028 .032 .036 .030 .033 .033 .033 .033 .023 .018 .015 .019 .015 .012 .015 79.0 92.2 94.8 95.5 94.3 '94.2 97.5 91.3 ~ 95.8 93.3 93.0 93.2 90.6 98.2 ~ 94.0 94.7 94.3 94.3 98.0 96.1 97.1 96.1 94.7 97.4 97.7 98.4 97.9 97.9 1.0 1.6 1.5 1.0 1.4 ---r:-4 1.7 1.5 l.b 1.3 1.5 2.7 2.1 1.9 1.9 1.9 2.5 2.3 2.1 2.3 2.3 2.1 2.2 1.5 1.3 1.5 2.3 1.7 1.3 1.7 1.3 4.6 5-5 5.2 5.0 5:1 5.3 5.3 5.3 3.5 4.1 5.1
u
4.5 5.3 "4:9 5.6 6.7 5.3 5.9 6.2 6.4 b.3 4.1 2.1 2.5 1.9 1.6 2.0 2.3 6.2 7.0 6.2 6.4 '6:5 7.0 6.8 o.9 4.8 5.6 7.8 "6":7 6.4 7.2 b."8 8·.1 9.0 7.4 B:2 8.5 8.5F.5
5.6 4.4 3.6 4.8 3.6 2.9 3.7 - 1.6 + 1.8 + 1.7 + .7 -r:-7+
4.5 - 1.9 t 1.3+
.6 - 1.1 ~ - .1+
2.1+
3.7+
1. 7 + 2.5+
6.5 + 4.6 rr.ti+
l. 7 .9+
1.0 + 2.5 + 2.0 + .8 +I:O Leach-Weight Distribution - '/; ingSand Slime Total Time
Total Residue Residue Residue Hrs. Remarks
98.4 101.8 101.7 100.7 100.7 63.5 69.3 66.1 66.0 70.6 104.5 66.8 98.1 67.7 101.3 100.6 66.6 98.9 68.8 100.8 69.3 99.9 97 .o 68.2 105.4 68.5 101.2 102.1 69.0 103.7 67.6 101.7 68.1 102.5 106.5 68.3 104.6 69.2 105.6 101.7 65.9 99.1 67.3 101.0 67.2 102.5 67.8 102.0 67.6 100.8 66.1 101.6 18.2 20.3 22.5 20.6 21.5 21.7 22.0 21.1 22.6 21.4 20.4 21.4 20.9 22.1 20.4 22.7 22.6 21.0 20.6 25.0 24.1 20.8 20.8 81.7 89.6 88.6 86.6 92.1 87.7 91.4 90.7 88.6 89.9 91.0 91.8 86.9 87.9 92.2 91.9 88.4 86.9 16 16 16 16 16 16 16 16 8 8 8 8 8 8 8 8 8 16 16 16 16 16 16
---Ore leach - 100 lb. H2so4/ton flotation - .52 lb. Fuel Oil,
.07 lb. AF-25, .08 lb. No. 70 Frother Rougher 12 Min., Cleaner 9 min. Roasted Asphaltic cone. to Next Primary Ore Leach
Discontinued Aerofloat-25
No. 70 Frother Replaced by Cresylic Acid
Cut Leaching Time to 8 Hours
Replaced Cresylic with No. 70 Frother Cut Fue 1 Oil to • 26 lb., Added .14 lb .AF-25.
Fuel Oil Raised to .52 lb.
Roasted Asphaltic Cone. added to Primary Grind
Increased Leach to 16 Hours Roasted Cone. to Primary Leach Roasted Asphaltic Cone.
Leached with Primary Acid
(100 lbs./ton) for 16 hours. This Slurry used in Primary Leach.
Gr~nd .355 .026 95.3 1.8 6.3
+
1.6 101.6 67.8 21.7 Average lB -355 2 .355 3 .355 4 .355 5 .355 6 .355 7 .355 8 .355 9 .355 10 • 355 n .355 Average .009 .010 .010 .012 .012 .on .010 .on .on .012 .013 .012 72.5 95.5 96.4 92.2 99-5 99.1 102.3 95.4 92.5 98.9 99-5 97.1 2.0 2.6 - 1.9 2.5 - 1.1 3.1 - 4.7 3.3 + 2.8 2.7 t 1.8 2.5 t 4.8 3.1 - 1.5 2.7 - 4.8 3.1 + 2.0 3.4 + 2.9 2.9 0 -98.1 98.9 95.3 102.8 101.8 104.8 98.5 95.2 102.0 102.9 100.0NOTE: Average pH -.65, Average El~ -410 mv. Leaching Temperature - 30°C.
Grind minus 48 mesh.
77.7 91.9 89.5 92.4 93.0 86.7 90.6 90.8 86.5 91.7 91.9 90.5
Roasted Asphaltic Cone. Leached with Primary
Acid (100 lbs./ton Ore) for 16 hrs. Slurry used in Primary Ore
Leach Ore 1.225 1.225 1.225 1.225 1.225 1.225 1.225 1.225 1.225 1.225 1.225 1.225 1.225 1.225 1.225 1.225 1.225 1.225 1.225 1.225 1.225 1.225 1.225 Product Assay
Sands Slime Total
Residue Residue Residue .20 .28 .27 .28 .31 .29 .29 .28 -:29 .28 .30 .29 .29 .30 .29 730 .33 .31 .28 731 .29 .28 .29 .29 .25 .27 .29 .30 .28 .29 2.53 3.02 2.98 2.86 3.05 2.98 3.11 3.08 3.10 2.98 2.95 2.97 2.96 2.89 3.10 3.00 3.13 3.11 3.11 3.12 3.18 3.20 3.19 3.04 3.00 2.94 3.12 3.02 2.91 3.00 .72 .90 .96 .90 .95 .93 .98 .97 :98 .94 .96 .92 ~ .90 .96 -:93 .98 1.00 .93 .97 1.01 1.00 1.01 • 95 .90 .99 1.03 .94 .91 .97 1.225 .29 .95
Sand Slime Total
ACC0-33
-27-Liquor Residue Residue Residue Unacct, Tot! 27.3 28.9 28.7 29.0 30.3 29.2 30.4 28.3 29.4 29.9 28.0 27.6 27.8 26.9 28.8. 27.9 28.5 28.0 25.4 '27.3 26.2 27.2 2b.7 25.8 26.9 27.0 29.8 27.5 29.8 28:5 10.4 15.9 14.6 15.1 17.9 15.9 15.9 15.5 15.7 15.3 17.7 16.4 17.1 16.7 16.2 16:5 18.6 17.1 15.6 17.1 16.2 15.9 16.1 15.6 13.9 llJ..7 16.1 16.6 15.1 I'5.b 28.1 16.0 36.8 50.1 54.8 48.2 53.6 51.7 55.2 55.3 55.3 51.4 54.5 52.0 53.2 48.3 54.3 51.3 53.5 56.2 51.8 53."8' 59.0 59.1 59.0 52.2 50.5 6o.6 61.5 51.4 49.5 ~ 5~.7 47.2 66.0 69.4 63.3 71.5 b'7:'b 71.1 70.8 71.0 66.7 72.2 68.4 70.3 65.0 70.5 "6"DJ 72.1 74.3 67.4 70.9 75.2 75.0 75.1 67.8 64.4 64.7 77.6 68.0 64.6 71.2 - 5.1 - 1.9 - 7.7 + 1.8 - 3.2 + 1.5 - .9 ~ - 3.4 + .2 - 4.0 - 1.9 - 8.1 - • 7
:-n
+ .6 + 2.3 - 7.2 -:-r:-8 -t 1.4 + 2.2 +1."8 - 6.4 - 8.7 - 8.3 + 7.4 - 4.5 - 5.4 ::---:3 69.7 - 2.2 94 . 98. 92. 101. 9b. 101. 99 • 100. 96. 100. 96. 9B: 91. 99. 95. 100. 102 • 92. 98: 101. 102. IOT:" 93 • 91. 91. 107. 95. 94.· 99.' 97 •'Table XVIII o1 = LO g/L oOl = o1 g/1 oOOl = oOl g/1
<
o001 g/1 L03 L06 Oo20 0.38 Oo21 0.138o54
Ca MnA1~ Mg, Na, Si, Ti
Cr, Cu, Ga, Co, Ni, P, Zr
Table
Two columns on exhaustion, one column on elution~ 3 minute retention
time on exhaustion, 10 minute retention time on elution, 25 mlo of
.>-.ru .... ~-.~'<' wet settled resin per column"
Feed Barren Samples Backwash Eluate Unaccount Balance for go 79o49 Oo23 0()31 loll 76o57 1.27 Uranium recovery from Leach
(computed from barren
Loading (computed from eluate
Lead Distribution lOOoOO ojo Oo29 ojo Oo39
%
L4o
ojo 96.,32%
1.60 1o 99.,71 '{o 90"1 g u3o8/lo WSROo0029 g"u3o8/L
0.,0083 go U3Qa/L Oo804 golJ:30-8/lo
lo03 goU308/lo
11 1/3 resin column loadings, 3088 column volumes~
77o200 column volumes loading, pH lo4,
Table Effect of on Elution Resin Solution Fresh .1
!
HCL = .9!
0 LO 1=11 ( ) 7.79 21 (Spot .151 N HCl wash started (Spot) 5.87
(Spot) 2.4o
(Spot) o75
(Spot) 85.0
Eluate 100 LO 1=11 (Composite) 8.39
21 (Spot) .012
1 N HCl wash started (Spot) .007
(Spot} .005 96.0 Fresh .1 N HCl = .9