Bachelor thesis 15 ECTS Malmö university
CHARACTERIZATION OF
CAPSAICINOID PRODUCTION IN
RECOMBINANT
SACCHAROMYCES CEREVISIAE
CLAUDIA LENTMAIER
CHARACTERIZATION OF
CAPSAICINOID PRODUCTION IN
RECOMBINANT
SACCHAROMYCES CEREVISIAE
CLAUDIA LENTMAIER
Lentmaier, C. Characterization of capsaicinoid production in recombinant Saccharomyces cerevisiae. Degree project in Biomedical Laboratory Science 15 credits. Malmö University: Faculty of Health and Society, Department of
biomedical science, 2018.
Capsaicinoids are compounds found in chili plants and have recently gained interest as pharmaceuticals due to their analgesic, inflammatory and anti-cancer properties. A novel approach producing capsaicinoids could be synthesis in recombinant Saccharomyces cerevisiae with help of metabolic engineering. Genes from Capsicum chinensis, encoding the enzymes capsaicinoid synthase (CS) and acyl-CoA synthase (ACS), were previously inserted into S. cerevisiae. The known laboratory stain CEN.PK was modified with plasmid transformation and the novel CRISPR/Cas9 technology was used for the wild type strain ERF 5273. The aim of this project is to further characterize and compare these previously constructed strains concerning their ability to produce nonivamide or yeast specific
capsaicinoids. Furthermore, it is examined whether capsaicinoids are excreted into the broth or accumulated intracellularly. Four different strains were cultivated in bench-scale bioreactors using medium supplemented with or without different precursors (vanillylamine and nonanoic acid). Culture broth supernatants and cell pellets were extracted and analyzed by HPLC in order to identify the
capsaicinoid-producing strains. The results from this study revealed that the yeast strains harbouring both genes (ACS+CS) produced most likely nonivamide if they were cultivated in media supplemented with both precursors. Nonivamide
formation was equally observed in broth supernatant and cell pellet. Additionally it was shown that yeast specific capsaicinoid production occured, althoug the peak height was close to the limit of detection and these results have to be confirmed further. Future work needs to be done in order to ensure and improve capsaicinoid production.
Keywords: acyl-CoA synthase, capsaicinoids, capsaicinoid synthase, metabolic engineering, Saccharomyces cerevisiae, nonivamide.
KARAKTERISERING AV
KAPSAICINOID PRODUKTION I
REKOMBINANT
SACCHAROMYCES CEREVISIAE
CLAUDIA LENTMAIER
Lentmaier, C. Karakterisering av kapsaicinoid produktion i rekombinant
Saccharomyces cerevisiae. Examensarbete i biomedicinsk laboratorievetenskap 15 högskolepoäng. Malmö universitet: Fakulteten för hälsa och samhälle, Institutionen för biomedicinsk vetenskap, 2018.
Kapsaicinoider är ämnen som finns i chilifrukterna och har på senaste tiden fått intresse som läkemedel på grund av sina analgetiska, anti-inflammatoriska och anti-cancer egenskaper. Ett nytt tillvägagångssätt att producera kapsaicinoider kan vara syntesen i rekombinant Saccharomyces cerevisiae med hjälp av metabolisk engineering och rekombinant DNA-tekniker. Gener från Capsicum chinensis, som kodar för enzymerna capsaicinoid-syntas (CS) och acyl-CoA-syntas (ACS), integrerades i S. cerevisiae i tidigare projekt. Den kända laboratoriesträngen CEN.PK modifierades med plasmidtransformation och för vildtyp-stammen ERF 5273 användes den nya CRISPR/Cas9-tekniken. Syftet med detta projekt är att ytterligare karakterisera och jämföra dessa tidigare konstruerade stammar angående deras förmåga att producera nonivamid eller andra jästspecifika kapsaicinoider. Vidare undersöks huruvida kapsaicinoider utsöndras i odlings-medium eller om de ackumuleras intracellulärt. Stammarna odlades i en bioreaktor i lite laboratorieskala. Som odlingsmedium används ett definierat medium med eller utan tillsatser. Odlings-medium kompletterades med vanillyl-amin och nonanoic acid som precursor. För att identifiera de kapsaicinoid-producerande stammarna extraherades supernatanten och cellpelleten och analyserades kromatografisk med HPLC. Resultaten från denna studie visade att jäststammarna, som innehöll båda generna (ACS + CS), sannolikt producerade nonivamid om de odlades i kompletterat medium. Vidare observerades bildning av nonivamid som ackumulerades i själva cellen. Möjligtvis producerades också jästspecifika kapsaicinoider, men topphöjden är nästan inte mätbar. Därför måste dessa resultat bekräftas ytterligare. Framtida arbeten behövs för att säkerställa och förbättra produktionen av kapsaicinoider.
Keywords: acyl-CoA syntas, kapsaicinoider, kapsaicinoid syntas, metabolisk engineering, Saccharomyces cerevisiae, nonivamide
Table of content
Introduction 5
Capsaicinoids 5
Metabolic engineering of S. cerevisiae 7
Previous work at AM 8
Aim of this project 8
Methodology and Material 8
S. cerevisiae strains 8
Fermentation 9
Determination of growth 10
Determination of viability 10
Product extraction 10
High-Performance Liquid Chromatography 11
Ethical assessment 11
Results and Discussion 11
Growth during fermentation 12
Viability before harvesting 13
Nonivamide in fermentation broths 14
Nonivamide in cell pellet 15
Yeast specific capsaicinoid products 16
Conclusion 17 References 18 Acknowledgment 21 Appendix 22 SOP 1 22 SOP 2 ... 23
INTRODUCTION
The yeast Saccharomyces cerevisiae has a major economic and social impact in human culture. It has been used by humans for brewing, wine production and dough leavening as far back as the Babylonians and Egyptians (ca 5000 BC) [1]. In the 19th century, alcoholic fermentation was associated with yeast and the name Saccharomyces for “sugerfungi” and cerevisiae for “of beer” was given to the yeast. However, it can do so much more than beer brewing and bread leavening. These days, S. cerevisiae is known as a versatile cell factory [2]. In research, it serves as a eukaryotic model organism, because its genome and metabolism is well understood. Its genome was the first eukaryotic to be genome sequenced in 1996. In addition, the yeast is easy to work with because it can easily be
manipulated with novel recombinant DNA technology, e.g. CRISPR/Cas9. Other advantages are that it is generally regarded as safe and it is robust under large industrial scale production [1]. With metabolic engineering, yeasts can produce not only bioethanol but also a variety of other chemical products, such as biofuels, bioplastics, or pharmaceuticals, and nutraceuticals. Today, S. cerevisiae is already used for the commercial production of pharmaceutical protein products like insulin, lactic acid, and polyethylene [3]. Many more examples of S. cerevisiae as a cell factory are still in development. Capsaicinoids could be one of them.
Capsaicinoids
Capsaicinoids are compounds found in chili plants (Capsicum spp.) [4]. Traditionally, chili pepper is used for food seasoning, but recently the
capsaicinoids have gained interest as pharmaceuticals [5]. The most common capsaicinoid is capsaicin; see Figure 1 [6]. It is identified as agonist for a transient receptor potential vanilloid 1 (TrpV1), whose activation leads to the painful, burning sensation (pungency) when chili pepper fruits are consumed [4].
However, prolonged exposure causes desensitization leading to pain relief. Moreover, TrpV1 is also expressed in neoplastic tissue [7]. It is discussed that capsaicin could induce growth inhibition and apoptosis of these cancer cells [8]. Also the capsaicin analogue nonivamide is described to exhibit anti-inflammatory properties [9]. Therefore, it is desirable to increase its production and to generate analogs with similar characteristics but without its pungency [10]. This can be done by enhancing biosynthesis in plants or by synthetic production of
capsaicinoids. However, a novel approach is synthesis of capsaicinoids in recombinant S. cerevisiae with help of metabolic engineering.
The capsaicinoid biosynthetic pathway in chili fruits is well understood and the precursors, key enzymatic steps and the associated genes are identified [4,11]. It is described that the enzymes Acyl-CoA synthase (ACS) and Capsaicin synthase (CS) are required for the last steps in capsaicinoid-synthesis. The genes encoding these enzymes were inserted into S. cerevisiae in previous projects at the division for Applied Microbiology (AM) [12-13].
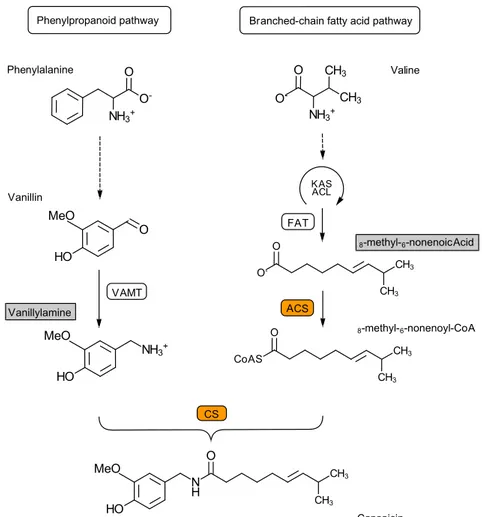
Capsaicinoids consist of an aromatic vanillylamine moiety linked to a fatty acid by an amide bond [4,10]. The formation of capsaicinoids in chili plants is described as a two-pathway reaction. Figure 2 represents the formation of capsaicin, the most predominant capsaicinoid in chili plants.
The precursor vanillylamine derives from the phenylpropanoid- and benzenoid metabolism and the branched-chain fatty acid pathway delivers various fatty acids, among others 8-methyl-6-nonenoic acid. The enzyme Acyl-CoA synthase (ACS) is required in order to activate these fatty acids. Capsaicinoid synthase (CS) is the last enzyme responsible for the condensation between vanillylamine and a fatty acid-CoA. Other capsaicinoids have been reported [11]. The aromatic vanillylamine moiety is paired with various acyl groups, mostly medium-length with 9 to 11 carbon atoms. If nonanoic acid is used as a precursor then
nonivamide as a capsaicin analog is formed as product; see Figure 3.
Figure 3. Condensation reaction between vanillylamine and nonanoic acid to nonivamide catalyzed by CS Figure 2. Capsaicin biosynthetic pathway in chilli pepper. Phenylalanine is converted into vanillin in
sequential steps (not shown) in the phenylpropanoid pathway. Vanillin is converted into vanillylamine by vanillin aminotransferase (VAMT). The branched-chain fatty acid pathway includes several steps with ketoacyl-ACP synthetase (KAS), acyl carrier protein ACL and FAT acyl-ACP thioesterase FAT. Valine is finally transformed into 8-methyl-6-nonenoic acid. Acyl-CoA synthase (ACS) catalyzes the conversion into 8-methyl-6-nonenoyl-CoA. A condensation reaction catalyzed by capsaicin synthase (CS) leads to the formation of capsaicin out of vanillylamine and 8-methyl-6-nonenoyl-CoA. The enzymes and precursors are highlighted in orange and gray. Figure modified from [4].
Metabolic engineering of S. cerevisiae
Metabolic engineering is a new way to harvest natural or novel products [3,14]. In recent years some very powerful tools have been developed, both for analyzing cellular function and for introducing directed genetic changes. It is expected that the use of these novel techniques and metabolic engineered products will increase in the future.
The process of metabolic engineering should be done iteratively [15]. In the first step, the genes encoding enzymes for product formation must be selected and a suitable host organism must be chosen. The next step is to construct the
recombinant strain with the desired properties. This can be done by recombinant DNA technology, e.g. CRISPER/Cas9 or plasmid transformation. The resulting recombinant strain has to be analyzed and characterized, especially its
performance compared with the original strain (control stain). Analytical techniques are e.g. DNA expression analysis, metabolite, protein and flux analysis. The results are used as base for identifying future engineering targets. The cycle is performed iteratively until objectives are fulfilled.
The laboratory S. cerevisiae CEN.PK strain family was developed in Germany in the 1990’s and is now widely used for metabolic engineering in both fundamental research and industrial applications [3,16]. In research, these strains are popular when studying rates of growth and product formation. Within the CEN.PK family the strains can be prototrophic or bearing one or more auxotrophies [17-18]. Auxotrophy is the inability of an organism to synthesize a particular organic compound required for its growth.
For auxotrophic strains, plasmid transformation can be used as a way for
metabolic engineering. In S. cerevisiae endogenously circular 2 µm plasmids are found [1]. They are very useful as cloning vectors. For instance the yeast episomal plasmid (YEp) replicates independently extrachromosomally at 50 -100 copies per cell. However, plasmid instability could be a drawback in this recombinant DNA technology. Therefore, plasmid stabilization techniques are required to prevent plasmid loss [19-20]. This is achieved by using auxotrophic yeast strains with episomal plasmids carrying corresponding marker genes. In addition, selective marker genes are used to complement specific nutritional requirements. For instance, the marker gene LEU2 encodes the essential enzyme for the amino acid L-leucin biosynthesis in S. cerevisiae. This changes the auxotrophic yeast to prototrophy. The idea behind this approach is that the interaction between plasmid and the host are substantial [20]. Plasmid maintaining is then necessary for
survival of the otherwise auxotrophic yeast in synthetic, minimal medium [19]. S. cerevisiae wild type strains are also interesting candidates for metabolic engineering because they could harbor some beneficial phenotypic traits [21], for instance robustness of a strain towards inhibitors like vanillin [22]. A wild-type yeast cell is often prototroph, which means that it has the ability to synthesize its own nutritional requirements. Therefore, plasmid transformation is no option, but the novel CRISPR/Cas9 technology enables adding or removing of genetic material into the chromosomal genome [23].
Problems within metabolic engineering could occur when the novel pathway gene expression causes a burden for the host cell [14]. For instance, siphoning away of host resources or via the build-up of toxic pathway intermediate products. The goal of metabolic engineering is to balance and maximize the pathway yield.
Previous work at AM
In previous projects at AM, several genetically modified strains of S. cerevisiae were constructed to express genes encoding the enzymes ACS and/or CS needed to synthesize capsaicinoids [12-13]. Capsaicin analogues production was detected for the CEN.PK strain with inserted genes coding for ACS+CS [12]. It was found that the capsaicinoid nonivamide was synthesized if vanillylamine and nonanoic acid were present as precursors in the medium. However, it remains unclear whether S. cerevisiae can synthesize yeast specific capsaicinoids if only
vanillylamine is used as precursor. Furthermore, it was never researched whether the yeast cell capsaicinoids are accumulated intracellularly or only excreted into the fermentation broth. The long-term objective (not in this project) is to develop a S. cerevisiae strain as whole-cell biocatalyst for large scale production of
capsaicinoids and later on to investigate the TRPV1 activity of these capsaicinoid-products.
Aim of this project
The aim of this project is to further characterize and compare these previously constructed strains concerning their ability to produce capsaicinoids. In particular, the targeted production of nonivamide is investigated, as well as yeast specific capsaicinoid synthesis. This is done by qualitative assessment with HPLC. It is further investigated where capsaicinoids accumulate by comparing extracted broth supernatant to the extract of cell pellet.
METHODOLOGY AND MATERIAL
The laboratory work included preparation of media, fermenter assembling, inoculation, harvest, extraction and product analysis. The selected strains were cultivated in bench-scale bioreactors. As medium the Central Bureau of Fungal Cultures (CBS) medium was used with either no substrate or vanillylamine / nonanoic acid as precursors. It has been reported that the precursors vanillylamine and nonanoic acid inhibit growth and viability of the yeast cells [12-13].
Therefore, culture growth is determined by optical density (OD) and cell viability is analyzed with flow cytometry (FCM). Measurement of optical density was also used to compare the increase of biomass between different yeast strains. After harvest, the yeast cell product had to be extracted in order to isolate the product. This was carried out by liquid-liquid extraction and rotary evaporation. The product characterization was then performed by High-performance liquid chromatography (HPLC). The workflow is illustrated in Figure 4.
S. cerevisiae strains
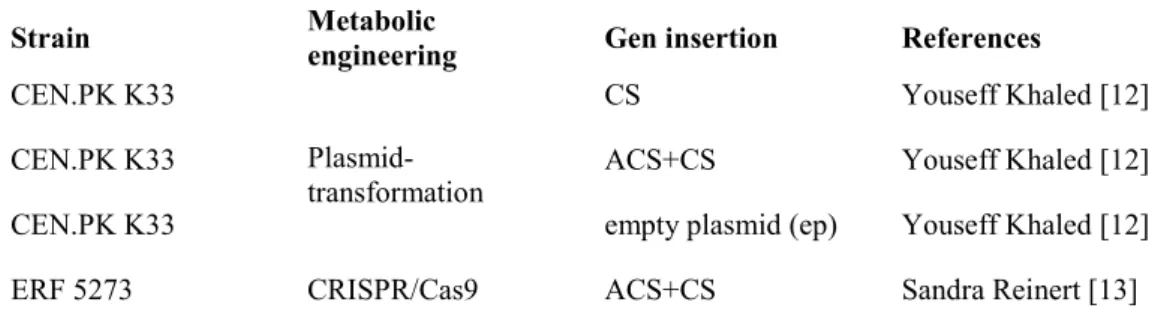
Four different strains of S. cerevisiae were investigated in this project, see Table 1. CEN.PK K33 was used as one parental strain. This strain is a known
auxotrophic laboratory strain, purchased from the EUROpean Saccharomyces Cerevisiae ARchive for Functional analysis (EUROSCARF). The second strain is ERF 5273 from the Ljubljana yeast collection in Slovenia. This wild-type strain has been found more tolerant to vanillin, a precursor for vanillylamine [13,22].
In previous projects, the genes coding for the enzymes CS and ACS/CS from Capsicum chinense were transformed into these two different S. cerevisiae strains in two fundamentally different ways. Sandra Reinert (2016) used the novel recombinant DNA technique CRISPR/cas9 to modify the strain ERF 5273 [13]. This means that genes encoding the enzymes ACS and CS were inserted into the chromosomal genome of S. cerevisiae. Youseff Khaled (2016), on the other hand, used plasmid transformation to construct three new strains [12]. The genes for either CS or ACS+CS were injected into yeast episomal plasmid yEP181 (AM, plasmid collection) with additional genes for leucine (LEU2). An empty plasmid (ep) strain without genes for CS and ACS was used as control strain. All yeast strains were restreaked from the –80°C glycerol stock and maintained on yeast nitrogen bas (YNB) agar plates in 4 °C.
Table 1. Overview of used recombinant S. cerevisiae strains
Strain Metabolic engineering Gen insertion References
CEN.PK K33
Plasmid-transformation
CS Youseff Khaled [12]
CEN.PK K33 ACS+CS Youseff Khaled [12]
CEN.PK K33 empty plasmid (ep) Youseff Khaled [12] ERF 5273 CRISPR/Cas9 ACS+CS Sandra Reinert [13]
Fermentation
All yeast cultures were carried out in CBS medium, which is a defined mineral medium (see standard operating protocol (SOP) 1 and SOP 2 in the appendix). A single colony was transferred from an agar-plate to 50 mL CBS pre-culture medium (SOP1) in a baffled shake flask. This pre-culture was performed in an incubator at a temperature of 30 °C and 180 rpm for at least 24 hours.
The batch cultures were carried out under controlled conditions in three
bioreactors (1.4 L bioreactor, Multifors, Infors HT, Bottmingen, Switzerland) for
Figure 4. Workflow within the project for cultivation, harvest and extraction of the fermentation broth (excreted capsaicinoids)
48 +/-1 hours. The working volume was 500 mL. A base (potassium hydroxide 3M) was required to adjust the pH to 6.5. CBS medium for fermentation contains 50 g/L glucose; see SOP 2. The strains were cultivated in CBS medium with either no supplement or vanillylamine (0.5 g/L) or vanillylamine combined with nonanoic acid (0.1 g/L). The inoculation volume was calculated to start with an OD620 of 0.2. It should not be greater than 50 mL (10 % of the working volume), so that the medium is not diluted too much. If this was not possible due to poor growth in the pre-culture, then the pre-culture was centrifuged and the whole cell pellet was inoculated. All fermentations were carried out in duplicates. The parameters for bioreactor setup were: temperature: 30 °C; stirring: 300 rpm; 6.5 pH and airflow: 500 mL/min.
Determination of growth
The OD was measured with a spectrophotometer (Absorbance Ultrospec 2100 pro, Biochrom Ltd.) at 620 nm to determine cell culture growth. Measurements were carried out after inoculation at the beginning of the growth phase (t = 0h) and after 48 +/-1 hours when the cells entered the stationary phase. Start OD ought to be 0.2 to ensure that growth started in the lag-phase. The final-OD (in technical duplicates) were compared to each other in order to analyze differences between strains and as well as the influence of supplemented media.
Determination of viability
Flow cytometry (FCM) is a practical method for simple and rapid assessment of the viability of microorganisms such as S. cerevisiae [24]. Viability is defined as the percentage of live cells in a whole population. To determine cell viability, the cells were analyzed by FCM using Propidium iodide (PI) as a viability marker. PI is a charged molecule and can penetrate only into dead cells due to the
permeabilized membrane [25]. Therefore, PI-positive cells are defined as dead. The fermentation broth was diluted in PBS buffer pH 7.4 to an OD620 of 0.1 – 0.5. An Eppendorf tube containing 500 µL diluted fermentation broth was stained with 5 µL PI (Sigma-Aldrich, Darmstadt, Germany). After 10 minutes dark incubation in room temperature, the cells were analyzed with flow cytometry.
In this project flow cytometry was performed with BD Accouri ™ C6 flow
cytometer (BD Biosciences, Becton, Dickinson and Company, New Jersey, USA). The excitation wavelength for the laser was set to 488 nm. Fluorescence emission levels were measured using a 670 nm long pass filter. The raw data was analyzed with FlowJo® 10.4.2 (LLC, Ashland, OR, USA). As gating strategies were used background noise reduction and live/dead determination. At first, the cells were separated from background by their side- and forward scatter characteristics. Subsequently PI-fluorescence was used as a gating parameter to separate viable from dead cells. This was done by combining the noise reduction FSC gate with signals from FL3-H.
Product extraction
The fermentation broth was harvested by centrifuging the broth at 5000 rpm for 10 minutes. The supernatant was gently poured over to a flask and the cell pellet was discarded or saved for further investigation. The next step was to extract the product from the supernatant. This was done by liquid-liquid extraction. Ethyl acetate was used as the organic solvent. The expected product (the solute) prefers this solvent due to its similar polarity. The supernatant (aqueous phase) and roughly the same volume ethyl acetate (organic phase) were poured together in a
separatory funnel. Phase separation occurred and the organic phase with the product was collected and ready for distillation in a rotary vacuum evaporator. The cell pellet of each batch was collected in one Falcon tube, centrifuged at 5000 g for 10 min and the supernatant was discarded. The wet weight (ww) of the cell pellet was measured. A dialyzable yeast protein extraction reagent (Y-PER™ Plus, Thermo Fisher Scientific Inc. Rockford USA) was used to extract
capsaicinoid content from inside the cell. Approximately twice the volume (mL) was added for 1 g (ww) cell pellet and incubated for 1 hour at 30°C. Initially no cell lysis appeared; therefore Dithiothreitol (DTT) was added according to the instruction manual of Y-PER™ Plus [26]. Cell debris was centrifuged to pellet and the resulting supernatant was extracted. The extraction was performed twice due to strong formation of an emulsion as a result of high protein content. Further separation was done with a rotary vacuum evaporator. Its purpose is to separate a volatile solvent from a non-volatile solute. In other words, ethyl acetate as the solvent was removed to isolate the highly concentrated product. The
remaining product appeared like an oily substance in the boiling flask. It was then dissolved in 2 mL methanol with a bulb pipette. The walls of the boiling flask were rinsed multiple times with these 2 mL to ensure that all product was collected. Finally, the methanol was pipetted to a small sample vial.
High-Performance Liquid Chromatography
The unknown product was analyzed in two replicates with high-performance liquid chromatograhphy (HPLC), using Reversed-Phase Chromatography. In this project the HPLC was performed with Waters HPLC system (Waters Binary HPLC pump 1525, UV/Vis detector 2489, Auto sampler 2707) (all Waters Corporation, Milford, USA) equipped with Kinetex 2.6 EVO C18 column (50 ×2.1). The mobile phase in this system was a polar solvent containing an isocratic mixture of 65% acetonitrile (solvent A) and 35% Millipore water with 0.1% TFA (trifluoroacetic acid) (solvent B). The flow rate was determined to be 0.13 for A and 0.07 for B. The injection system injected 30 µl of sample into the mobile phase. The nonivamide and yeast specific capsaicinoid content was determined qualitatively. This means the peaks in the chromatograms are identified by their retention time by comparing with the retention time of a nonivamide standard [27].
Ethical assessment
In this project, the yeast S. cerevisiae is used and therefore no ethical assessment is required.
RESULTS AND DISCUSSION
The main goal of this study is to further characterize the four previously genetically engineered S. cerevisiae strains regarding their ability to produce capsaicinoids; see Table 1. The strains were cultivated in bench scale bioreactors for 48 +/-1 hours in order to reach the stationary phase. All strains were grown in CBS medium (CBS only) as well as in media supplemented with two different precursors. The supplemented media contained either vanillylamine (va) (0.5 g/L), or vanillylamine was combined with nonanoic acid (0.1 g/L) (va+noa). The
capsaicinoid formation in general and the nonivamide formation in particular was then evaluated by HPLC. For the CEN:PK strain is reported that precursors vanillylamine and nonanoic acid inhibit growth and viability of the yeast cells. Therefore, culture growth is determined by optical density (OD) and cell viability is analyzed with flow cytometry (FCM).
Growth during fermentation
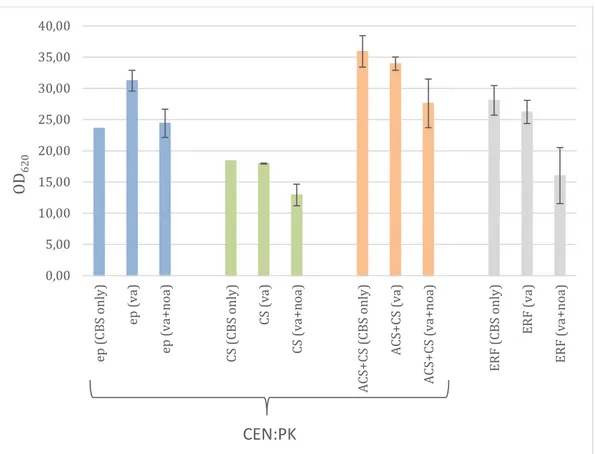
Capsaicinoid production in engineered yeast is associated with cell growth, biomass and cell viability. OD measurements were used to compare the final biomass between the different engineered strains as well as an indicator of their ability to grow in media containing putative inhibitors like vanillylamine and nonanoic acid. The results are shown in Figure 5.
Figure 5. Optical density of four different metabolic engineered strains (CEN.PK and ERF) in different media (CBS without precursors and supplemented with vanillylamine (va) and nonanoic acid (noa). The strains were cultivate in duplicates, except ep and CS (CBS only). The error bars indicate the variation in OD620.
The OD620 was measured directly before harvesting. The batches were cultivated in duplicates except the batches with CEN.PK ep and CS in CBS medium without precursors that were cultivated only once. The highest OD620 with an average of 35.92 was reached by the CEN.PK ACS+CS strain without precursors. In general it can be seen that the CEN.PK CS strain has lower growth in all three medium setups compared to the other strains. The maximum OD reached in CEN.PK ACS+CS is about twice as large as that reached in CEN.PK CS. Furthermore, this strain did not in three of five cases reach the required start OD coming from the pre-culture. Therefore, the whole cell pellet was inoculated and consequently the start OD was lower than 0.2. A possible reason behind the poor growth may be plasmid loss [19]. This phenomenon is also described as plasmid instability [20] and is a recurring issue in plasmid transformation, where plasmid maintenance is necessary for survival of the otherwise auxotrophic yeast in synthetic, minimal medium. For instance, uneven partitioning during cell budding can result in
0,00 5,00 10,00 15,00 20,00 25,00 30,00 35,00 40,00 ep (C BS o nl y) ep (v a) ep (va +n oa ) CS (CB S o nl y) CS (v a) CS (v a+ no a) ACS+ CS (CB S on ly ) ACS+ CS (v a) AC S+ CS (v a+ no a) ER F ( CB S on ly ) ER F (v a) ER F ( va +n oa ) OD 62 0 CEN:PK
plasmid-free cells [28]. Further genetic and environmental influences that may be responsible for plasmid instability are described in Zhang et al (1996) [20].
However, the recombinant cell loses its engineered prototrophic property, because the number of the marker gene LEU2 is decreased. Consequently, the biosynthesis of the amino acid L-leucin is also reduced, which can result in poor growth. This could be tested by adding the amino acid leucine to the medium and comparing the growth of the cell cultures with and without leucine. Nevertheless, the
question remains why the CEN.PK ACS+CS and CEN.PK ep is seemingly not or less affected. This leads to the conclusion that the plasmid system in the CEN.PK CS strain may be compromised. For this reason, it should be considered to redo the plasmid transformation.
In general, the results for CEN.PK strains and ERF 5273 reveal a trend that growing on vanillylamine and nonanoic acid leads to a slightly reduced growth in comparison to CBS medium without precursors. The only exception is the
CEN.PK ep (control strain) growing on CBS only. Even though this result is only one replicate, it may be explained by the use of only one restreaked plate from the glycerol stock during the whole project. The batch with CEN.PK ep was
cultivated more than 12 weeks after the initial restreak from the original glycerol stock and the cultivation of CEN.PK CS and ACS+CS in the same medium setup was performed 11 weeks earlier. Therefore, it can be suspected that this long storage time may affect the agar culture negatively and the strain becomes attenuated in its ability to regrow. Whether this phenomenon affected the other strains is not known. To prevent this problem in the future, it is recommended to restreak the agar cultivation from the glycerol stock more frequently. Sherman (2002) recommends that yeast strains can be stored for short periods at 4°C on YPD medium in petri dishes [29]. A common recommendation is a restreak every two to four weaks.
The ERF strain is a S. cerevisiae wild type that was previously found to be more tolerant to vanillin, a precursor to vanillylamine [13,22]. The results of OD-measurement show a similar trend compared to the CEN.PK CS and ACS+CS strains; see Figure 5. However, the results show clearly that the ERF strain has lower growth in all setups compared to the CEN.PK ACS+CS strain. It should be pointed out that the biomass growth was hardly reduced when vanillylamine was added but it drops to almost half if the medium was supplemented with
vanillylamine and nonanoic acid. On the other hand, these two batches have a quite large variation between the duplicates, so that these results should be interpreted carefully.
Viability before harvesting
Cell viability was assessed by flow cytometry in order to examine the influence of vanillylamine and nonanoic acid on growth of S. cerevisiae strains. After staining with PI, a viability of 95 % and above was distinguished for the CEN.PK strains; see Figure 6. These results confirm that the previously determined optimal concentration of precursors was not cidal to the cells and can be recommended in further experiments regarding the CEN.PK strains.
Unfortunately, the viability was not determined for the ERF 5273 strain. It should be noted that the toxicity of nonanoic acid for this strain has never been
investigated previously. Nonanoic acid may have an inhibitory effect on the cells of the ERF 5273, because the OD measurement within ERF in va+noa
supplemented media drops to nearly one half compared to CBS only; see Figure 5. As stated above this result should be interpreted carefully due the larger standard deviation. Nevertheless, the influence of different nonanoic acid concentrations on growth and viability should be investigated in order to optimize the substrate level for this specific strain.
Nonivamide in fermentation broths
One purpose of this study is to investigate the differences in strain ability of producing the capsaicinoid nonivamide when growing in media supplemented with vanillylamine and nonanoic acid. The broth supernatant was extracted as described and analyzed with HPLC. The chromatograms show that a product is formed in the CEN.PK ACS+CS and ERF 5273 strains around a retention time of 8.4 minutes; see Figure 7A and B.
95,3 99,3 97 97,3 97 99,3 99,4 99,4 50 60 70 80 90 100 ep (va+noa) ep (va) CS (va+noa) CS (va) CS (CBS only) ACS+CS (va+noa) ACS+CS (va) ACS+CS (CBS only)
Figure 6. Viability in percent (%) for CEN:PK strains measured by flow cytometry.
Figur 7. HPLC profiles with retention time [min] of (A) CEN:PK ACS+CS and (B) ERF 5273 in extracted broth supernatant in va+noa supplemented media
A
The appearance of these peaks corresponding to the retention time of the nonivamide standard in Figure 8A (8.4 min). This indicates the production of nonivamide. On the other hand, no peak for capsaicinoid production is observed in the control strain; see Figure 8B. The products formed by the CEN.PK CS strain in Figure 8C is most likely not nonivamide, because the retention time does not clearly comply with the 8.4 min of the nonivamide standard. Other peaks, especially before 6 minutes, are considered as medium substrates or yeast specific products.
The obtained results show that the production of nonivamide strongly depends on the presence of both genes for enzymes CS and ACS. The CEN.PK strain
containing genes for only CS might produce other capsaicinoids with yeast specific fatty acids; see Figure 8C. However, the identity of the peaks should be confirmed by Liquid chromatography–mass spectrometry (LC-MS/MS) analysis or by spiking the sample with a known analyte to establish peak identity [27].
Nonivamide in cell pellet
Another purpose of this study is to investigate the accumulation site of
nonivamide. As stated above and described by Khaled 2016 [12] nonivamide was found in the broth supernatant, but the intracellular content was never
investigated. Therefore, the cell pellet of the batch cultivation from CEN.PK ACS+CS and ERF 5273 with va+noa medium was collected, lysed with the Y-PER™ Plus and extracted. Additionally the cell pellet of CEN.PK empty plasmid in CBS only was also analyzed as control. The results show a peak around seven minutes in all three chromatograms; see Figure 9. Only the strains containing the genes coding for ACS and CS reveal a second peak around 8.4 minutes; see Figure 9A and B. This retention time is similar to the nonivamide standard (Figure 8A), indicating that nonivamide is also accumulated inside the cells. The peak (around 6,7 minutes) found in all three chromatograms could be caused by the used extraction reagent Y-PER™ Plus reagent or an unknown compound within the cells but unrelated to nonivamide production.
Figure 8. HPLC profiles with retention time [min] of (A) standard nonivamide (5mg/L), retention time 8,4 min. (B) control strain CEN.PK empty plasmid, (C) CEN:PK CS, all in extracted broth supernatant in va+noa supplemented media.
A B
Figure 9. HPLC profileswith retention time [min] of extracted cell pellet in va+noa supplementd media (A) CEN. PK ACS+CS (B) ERF 5273 and (C) CEN.PK empty plasmid in CBS only.
Another extraction method for the cell pellet might lead to even better results. It was observed under the microscope that the cell walls were still intact. Y-PER™ Plus is a protein extraction reagent that is used as a mild detergent to extract soluble proteins from the cytosol [26]. Standard glass bead disruption method or sonication could increase cell lysis and thus the yield.
Even though the HPLC analysis is done qualitatively in order to establish the identity of an unknown component, the peak height gives a hint on the titer of nonivamide production [27]. The broth supernatant is approximately concentrated 400 fold, while the cell pellet is concentrated around 20 fold or less. In this order it could be expected that more product is accumulated in the cell biomass, but further studies have to confirm that. With an external standardization, it would be possible to quantify the product.
Yeast specific capsaicinoid products
Fatty acids produced in S. cerevisiae are primarily C16 and C18 in length [30]. They can be activated by yeast specific ACS and could in theory form a novel capsaicinoid product, if the medium is supplemented with vanillylamine only. The HPLC results show a peak near the retention time of nonivamide standard (8,4 min); see Figure 9B. Therefore, it could be concluded that yeast specific capsaicinoids might be synthesized. However, neither the CEN.PK ACS+CS strain nor the CEN:PK empty plasmid strain show product formation, which was expected; see Figure 9A and C. The peak height is very low and close to the limit of detection. However, further studies are needed to confirm the formation of yeast specific capsaicinoids.
A B
Figure 7. HPLC profileswith retention time [min] of extracted broth in va supplemented media (A) CEN.PK ACS+CS, (B) CEN.PK CS, (C) CEN.PK ep.
CONCLUSION
Three different strains and one control strain of S. cerevisiae were genetically engineered for capsaicinoid production. Two genes from Capsicum chinensis were inserted into S. cerevisiae in two fundamentally different ways. The results show that nonivamide formation was achieved in the S. cerevisiae strains
containing ACS+CS by supplying the medium with vanillylamine and nonanoic acid. The results also show that nonivamide is accumulated in the yeast cells. However, very low product formation was observed in the CEN.PK strain with the enzyme for capsaicinoid synthase (CS) only. Therefore, it could be concluded that S. cerevisiae might be able to synthesize yeast specific capsaicinoids, but to what extent remains unclear. Furthermore, it was confirmed that the previously determined optimal concentration of precursors were not cidal to the cells and can be recommended in further experiments regarding the CEN.PK strains. However, the strain ERF 5273 should be investigated for the influence of different nonanoic acid concentrations on its growth and viability.
The work presented in this project contributes to the aim of using S. cerevisiae as a whole cell biocatalyst for capsaicinoid production. This was never done before and therefore the results, especially the yeast specific capsaicinoids, are new territory in metabolic engineering of S. cerevisiae. However, further engineering and optimization is needed to improve the targeted nonivamide production or other novel yeast specific capsaicinoids.
C
REFERENCES
1. Walker G M, (2011) Yeast Physiology and Biotechnology. Chichester, John Wiley & Sons Ltd.
2. Li M, Borodina I, (2015) Application of synthetic biology for production of chemicals in yeast Saccharomyces cerevisiae. FEMS yeast research, 15(1), 1-12.
3. Borodina I, Nielsen J, (2014) Advances in metabolic engineering of yeast Saccharomyces cerevisiae for production of chemicals. Biotechnology Journal, 9(5), 609-620.
4. Aza-Gonzalez C, Nunez-Palenius H, Ochoa-Alejo N, (2010) Molecular biology of capsaicinoid biosynthesis in chilli pepper (Capsicum ssp.). Plant Cell Reports, 30(5), 695-706.
5. McCarty M F, DiNicolantonio J J, O'Keefe J H, (2015) Capsaicin may have important potential for promoting vascular and metabolic health. OpenHeart, 2.
6. Arora R, Gill N S, Chauhan G, Rana A C, (2011) An Overview about Versatile Molecule Capsaicin. International Journal of Pharmaceutical Sciences and Drug Research, 3(4), 280-286.
7. Díaz-Laviada I, (2010) Effect of capsaicin on prostate cancer cells. Future oncology, 6(10).
8. Surh Y-J, (2002) More Than Spice: Capsaicin in Hot Chili Peppers Makes Tumor Cells Commit Suicide. Journal of the National Cancer Institute, 94(17), 1263–1265.
9. Walker J, Ley J P, Schwerzler J, Lieder B, Beltran L, Ziemba P M, Hatt H, Hans J, Widder S, Kramer G, Somoza V, (2017) Nonivamide, a capsaicin analogue, exhibits anti‐inflammatory properties in peripheral blood mononuclear cells and U‐937 macrophages. Molecular Nutrition & Food Research, 61(2).
10. Rayes-Escogido M L, Gonzalez-Mondragon E, Vazquez-Tzompantzi E, (2011) Chemical and Pharmacological Aspects of Capsaicin. Molecules, 16, 1253-1270.
11. Mazourek M, Pujar A, Borovsky Y, Paran I, Mueller L, Jahn M M, (2009) A Dynamic Interface for Capsaicinoid Systems Biology. Plant Physiology, 150, 1806-1821.
12. Khaled Y, (2016) Capsaicin biosynthesis in baker’s yeast Saccharomyces cerevisiae. Master thesis, Division of Applied Microbiology, Lund
13. Reinert S, (2016) Towards the synthesis of capsaicinoids and capsinoids in baker's yeast. Project report. Division of Applied Microbiology, Lund University.
14. Awan A R, Shaw W M, Ellis T, (2016) Biosynthesis of therapeutic natrual products using synthetic biology. Advanced Drug Delivery Reviews, 105, 98-106.
15. Nielsen J, (2001) Metabolic engineering. Applied Microbiology and Biotechnology, 15(3), 263–283.
16. Nijkamp J, van den Broek M, Datema E, (2012) De novo sequencing, assembly and analysis of the genome of the laboratory strain
Saccharomyces cerevisiae CEN.PK113-7D, a model for modern industrial biotechnology. Microbial Cell factories, 11(36).
17. Paciello L, Zueco J, Landi C, (2014) On the fermentative behavior of auxotrophic strains of Saccharomyces cerevisiae. Electronic Journal of Biotechnology, 17, 246–249.
18. J. P. van Dijken, J. Bauer, L. Brambilla och P. Duboc, ”An interlaboratory comparison of physiological and genetic properties of four Saccharomyces cerevisiae strains,” Enzyme and Microbial Technology, vol. 26, p. 706– 714, 2000.
19. Pronk J, (2002) Auxotrophic Yeast Strains in Fundamental and Applied Research. Applied and environmantal microbiology, 68(5), 2095-2100. 20. Zhang Z, Moo-Young M, Chisti Y, (1996) Plasmid stability in
recombinant Saccharomyces cerevisiae. Biotechnology Advances, 14(4), 401-435.
21. Liti G, (2015) The fascinating and secret wild life of the budding yeast S. cerevisiae. eLife, 4.
22. Klintner C-F, (2011) Robust yeast for whole cell biocatalysis of ligning derived phenolic monomers. Master thesis, Division of Applied
Microbiology, Lund University.
23. Stovicek V, Holkenbrink C, Borodina I, (2017) CRISPR/Cas system for yeast genome engineering: advances and applications. FEMS Yeast Research, 17(5), 1-16.
24. Alvarez-Barrientos A, Arroyo J, Canton R, Nombela C, Sanchez-Perez M, (2000) Applications of Flow Cytometry to Clinical Microbiology. Clinical Microbiology reviews, 13(2), 167-195.
25. Kwolek-Mirek M, Zaddrag-Tecza R, (2014) Comparison of methods used for assessing the viability and vitality of yeast cells. FEMS Yeast
26. Thermo Fisher Scientific Inc, (2012) Instructions: Y-PER TM Plus, Dialyzable yeast protein extraction reagent.
27. D. MW, Modern HPLC for practicing scientists, Hoboken, New Jersey: John Wiley & Sons, 2006.
28. Kilonzo P M, Margaritis A, Bergougnou M A, (2009) Plasmid stability and kinetics of continuous production of glucoamylase by recombinant Saccharomyces cerevisiae in an airlift bioreactor. Journal of Industrial Microbiology and Biotechnology, 36, 1157–1169.
29. Sherman F, (2002) Getting started with yeast. >https://instruct.uwo.ca< PDF (2018-03-25).
30. C. Leber, B. Polson, R. Fernandez-Moya och D. N. A, ”Regular article: Overproduction and secretion of free fatty acids through disrupted neutral lipid recycle in Saccharomyces cerevisiae,” Metabolic Engineering , vol. 28, pp. 54-62, 2015.
ACKNOWLEDGMENT
I would like to thank Tove Sandberg at Malmö University for the contact and opportunity to do this kind of project in our program.
This project would not have been possible without my supervisor Magnus
Carlquist at Applied Microbiology, Lund University. I would like to thank you for your guidance through a completely new topic for me, your support and patience when I came with new thoughts and questions and the really helpful discussions and drawings on the white board. I really appreciated your enthusiasm towards yeast and capsaicinoids, it truly was contagious.
Thank you to all members and students at TMB for a pleasant working
atmosphere and a helping hand when needed. Especially I would like to thank Anette Ahlberg for our nice fika talks and your support with all the little things. Special thanks to Lise-Lotte Kure for all the discussions on and off topic, fika breaks, and that you convinced me of the advantages of a microwave.
I would like to thank my parents for their interest in my work and their earlier support in my twenties. Without that, I wouldn’t be here.
Finally, I would like to thank my wonderful husband Michael (comma:) who has been a constant source of love, support and encouragement. Thank you, that you believe in me.
APPENDIX
SOP 1
PREPARATION OF CBS-MEDIUM FOR INOCULUM FLASKS
This SOP describes the preparation of CBS-medium for precultivation of
Saccharomyces cerevisiae in 0,5 L baffled shake flasks. Each shake flask should contain 50 mL CBS-medium.
Equipment
Baffled shake flasks (0,5 L) Sterile syringe
Sterile pipette
Procedure
Preparation of 1 L CBS-medium from stock solutions: (NH4)2SO4 75 mL
KH2PO4 120 mL
MgSO4* 7H2O 10 mL
Trace metals 2 mL
Are poured together in a measuring cylinder. Water is added up to 800 mL and the pH is adjusted to 6,5.
As carbon source is used glucose with a concentration of 20 g/L. It is dissolved in 80 mL distilled water. If glucose monohydrate (C6H12O6* H2O is used, then see calculation beneath.
Autoclave these two solutions separately.
Work in the LAF-bench: Pour the solutions together, adjust to 1 Liter. Add 1 mL vitamins with a sterile pipette into each flask. Store cold and dark. The medium is ready for inoculation.
Preparation of 1 L glucose solution (20 g/L) m = n * M
Molar mass for glucose: 180,16 g*mol-1
ng = 20 g / 180,16 g*mol-1
ng = 0,11 mol
Molar mass for glucose monohydrate: 198,16 g*mol-1
mgm = 198,16 g*mol-1 * 0,11 mol
SOP 2
PREPARATION OF CBS-MEDIUM FOR YEAST CULTIVATION IN BIOREACTORS
This SOP describes the preparation of CBS-medium for cultivation of
Saccharomyces cerevisiae in 1,4 Liter bioreactors. Each vessel contains 500 mL CBS-medium.
Equipment
Vessel of bioreactor Sterile syringe
Procedure
Preparation of 500 mL CBS-medium from stock solutions for each vessel: (NH4)2SO4 25 mL
KH2PO4 12,5 mL
MgSO4* 7H2O 5 mL
Trace metals 0,5 mL
Are poured together in a measuring cylinder. Water is added up to 350 mL. Adjust the pH to 6,5. Add water up to 400 mL.
Pour the medium into the vessel of the bioreactor and sterilize it by autoclaving. As carbon source is used glucose with a concentration 50 g/L. See calculation beneath if glucose monohydrate is used.
The carbon source is dissolved in 100 mL distilled water (27,5 g for 500 mL medium) and autoclaved separately in flasks. Then add this glucose solution into the bioreactor by sterile filtration. Work in the LAF-bench to fill the glucose solution into a sterile syringe.
Add 0,5 ml vitamins (1 mL/L) to the vessel by sterile filtration. Work in the LAF-bench to prepare the syringe.
![Figur 7. HPLC profiles with retention time [min] of (A) CEN:PK ACS+CS and (B) ERF 5273 in extracted broth supernatant in va+noa supplemented media](https://thumb-eu.123doks.com/thumbv2/5dokorg/4156913.89582/14.892.149.471.708.1090/figur-hplc-profiles-retention-extracted-broth-supernatant-supplemented.webp)
![Figure 8. HPLC profiles with retention time [min] of (A) standard nonivamide (5mg/L), retention time 8,4 min](https://thumb-eu.123doks.com/thumbv2/5dokorg/4156913.89582/15.892.156.747.273.621/figure-hplc-profiles-retention-time-standard-nonivamide-retention.webp)
![Figure 9. HPLC profiles with retention time [min] of extracted cell pellet in va+noa supplementd media (A) CEN](https://thumb-eu.123doks.com/thumbv2/5dokorg/4156913.89582/16.892.146.719.82.409/figure-hplc-profiles-retention-extracted-pellet-supplementd-media.webp)
![Figure 7. HPLC profiles with retention time [min] of extracted broth in va supplemented media (A) CEN.PK ACS+CS, (B) CEN.PK CS, (C) CEN.PK ep](https://thumb-eu.123doks.com/thumbv2/5dokorg/4156913.89582/17.892.146.746.80.430/figure-hplc-profiles-retention-extracted-broth-supplemented-media.webp)