http://www.diva-portal.org
This is the published version of a paper published in Plant Physiology.
Citation for the original published paper (version of record): Askerlund, P., Laurent, P., Nakagawa, H., Kader, J. (1991)
NADH-Ferricyanide Reductase of Leaf Plasma Membranes: Partial Purification and Immunological Relation to Potato Tuber Microsomal NADH-Ferricyanide Reductase and Spinach Leaf NADH-Nitrate Reductase.
Plant Physiology, 95(1): 6-13
Access to the published version may require subscription. N.B. When citing this work, cite the original published paper.
Free PMC Article
Permanent link to this version:
NADH-Ferricyanide Reductase of Leaf Plasma
Membranes1
Partial
Purification and Immunological Relation
to
Potato Tuber Microsomal
NADH-Ferricyanide Reductase and
Spinach Leaf NADH-Nitrate
Reductase
PerAskerlund*2, Pascal Laurent, Hiroki Nakagawa, and Jean-Claude Kader
Department of Plant Biochemistry, University of Lund, P.O. Box 7007, S-220 07 Lund, Sweden (P.A.); Laboratoire de Biomembranes Vegetales, Unit6 de RechercheAssoci6 1180, Universit6 PierreetMarie Curie,
4Place Jussieu, 75252 Paris cedex 05, France (P.L., J. -C.K.);and FacultyofHorticulture, ChibaUniversity,
Matsudo, Chiba 271, Japan (H.N.)
ABSTRACT
Plasma membranes obtained by two-phase partitioning of mi-crosomal fractions from spinach (Spinacea oleracea L. cv Me-dania) and sugar beet leaves (Beta vulgaris L.) contained rela-tively high NADH-ferricyanide reductase and NADH-nitrate reduc-tase (NR; EC 1.6.6.1) activities. Both of these activities were latent. To investigate whether these activities were due to the same enzyme, plasma membrane polypeptides were separated withSDS-PAGE and analyzed with immunoblotting methods. An-tibodies raised against microsomal NADH-ferricyanide reductase (tentatively identified as NADH-cytochrome b5 reductase, EC 1.6.2.2), purified from potato (Solanum tuberosum L. cv Bintje) tubermicrosomes, displayed one single band at 43 kilodaltons when reacted with spinach plasma membranes, whereas IgG produced againstNRfromspinach leaves gave a major band at 110 kilodaltons together with a few fainter bands of lower
molec-ular mass. Immunoblotting analysis using inside-out and right-side-out plasma membrane vesicles strongly indicated that NR was not anintegral protein but probably trappedinside the plasma membrane vesicles during homogenization. Proteins from spin-ach plasma membranes were solubilized with the zwitterionic detergent3-[(3-cholamidopropyl) dimethylammonio] 1-propane-sulfonate and separatedon aMono Q anionexchange columnat
pH5.6 with fast protein liquid chromatography. One major peak ofNADH-ferricyanide reductase activitywasfoundafter separa-tion. Thepeak fractionwasenriched about 70-foldinthisactivity
compared to the plasma membrane. When the peak fractions wereanalyzed withSDS-PAGE the NADH-ferricyanide reductase activitystrongly correlatedwitha43kilodaltonpolypeptidewhich reacted with the antibodies against potato microsomal NADH-ferricyanide reductase. Thus,ourdata indicate thatmost,if not
all, of the truly membrane-bound NADH-ferricyanide reductase activity of leaf plasma membranes is due to an enzyme very
similar topotato tuber microsomal NADH-ferricyanidereductase (NADH-cytochromeb5 reductase).
Isolatedplasma membranesofhigh purityshowrelatively
high NAD(P)H-(acceptor) oxidoreductase
[NAD(P)H-dia-Supported inpart by grants from the Swedish Natural Science ResearchCouncil and the CarlTesdorpfFoundation.
2Present address: Department of Plant Sciences, University of
Oxford,South ParksRoad,Oxford,OX1,3RB,UK.
phorase]activities with differentelectron acceptors(1, 3,4, 7,
8, 24). It has been suggested that theseactivities are dueto
redox systems capable of transferring electrons from
cyto-plasmic donors to electron acceptors in the apoplast. One such transplasma membrane electron transport system is
thought to be induced in roots ofnongraminaceous plants
during iron deficiencyandtobeinvolvedin thereduction of
Fe3"
toFe2+
for uptake. Another system is thought to be constitutively present in all plant cells (for reviews, see 27, 28). Recently, Buckhoutetal. (7) showed that NADH- (but not NADPH-) (acceptor) oxidoreductase activities are in-duced in tomato root plasma membranes during Fe-defi-ciency, indicatingthatNADHis the donorfortheinducibletransplasma membrane electron transport system. An NAD(P)H-(acceptor) oxidoreductase has been purified from cornrootplasma membranes(24, 25).
Leaf cells also seem to possess a transplasma membrane redoxsystem,sincecarefullywashedleafsegmentsfrom both
oatandsugarbeetcanreduceaddedferricyanide3 (2, 12). By
using inside-outandright-side-out plasmamembranevesicles
from sugarbeet leaves, however, we have shown that both donor andacceptorsites oftheNADH-ferricyanidereductase
are located on the cytoplasmic surfaceof theplasma
mem-brane and that a possible transplasma membrane electron
transport wouldconstitute only a veryminorproportion of
theactivity (1). Furthermore,right-side-out vesicles ofsugar
beetleafplasma membrane loaded withan
NADH-generating
systemdo notsupportreduction of externalferricyanide (2). The location ofboth donorandacceptor sites on the
cyto-plasmic surface (1), together with spectrophotometric data (3), led us (1) to suggest that NADH-Cyt b5 reductase(EC
1.6.2.2) was responsible for the major part of the NADH-ferricyanide reductase activity in leaf plasma membranes. Another possibility is that this activity is due to a plasma
membrane-boundform ofNADH-NR(EC 1.6.6.1),sinceNR canreduceferricyanide (9, 10, 29)andwasrecently suggested tobeanintegralcomponentofbarleyandcornrootplasma
3Abbreviations: ferricyanide, K3[Fe(CN)6];NR, nitrate reductase
(EC 1.6.6.1);CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]
1-propanesulfonate; FPLC, fastproteinliquid chromatography; TLCK, N,a-p-tosyl-L-lysine chloromethylketone.
NADH-FERRICYANIDE REDUCTASE OF LEAF PLASMA MEMBRANES
membranes (33, 34). Inthe presentpaper we use antibodies against potatotubermicrosomal NADH-ferricyanide reduc-tase (tentatively identified as NADH-Cyt b5 reductase; see "Discussion" andref. 13) and NR, respectively, to determine the nature ofthe NADH-ferricyanide reductase activity of leafplasma membranes.
MATERIALS AND METHODS
Plant Material
Spinach (Spinacea oleracea L. cv Medania)wasgrown in soilat 18to20°C(dark/light; 50Wm-2, 350-800nm; Power star HQI-E 400 W/DH, Osram, FRG) during a 16 h day. Spinach leaveswereharvested 3to5weeksaftersowing. Four-week-old sugar beet plants (Beta vulgaris L.) were kindly supplied byHilleshog AB, Sweden, and maintained in soil in
agreenhouse with supplementarylight (23 W m-2, 350-800 nm;PhilipsG/86/2HPLR400W, The Netherlands). Leaves of 6- to 8-week-old sugar beet plants were used. The soil (K-jord; WWeibullAB, Landskrona,Sweden)contained full nutritional requirements including iron and nitrate. Light
conditionsweremeasuredwithanIscomodel 742
spectrora-diometer (Optronics Lab., Orlando, FL) interfaced with a
Hewlett-Packard 85computer.
Preparation of Membranes
A microsomal fraction from potato (Solanum tuberosum L. cv Bintje)tubers was prepared asdescribed previously in Galleetal.(13).
Plasma membranes from spinach and sugar beet leaves
were purified from microsomal fractions (10,000-50,000 g
pellet) bypartitioninginanaqueouspolymertwo-phase sys-tem (19, 22). The homogenization medium contained 330
mm sucrose, 50 mM Mops-KOH or Mops-BTP (pH 7.5), 5
mM EDTA, 5 mm DTT, 0.5 mM PMSF, 20 uM
leupeptin,
0.2% (w/v) BSA (Sigma; protease free), 0.2% (w/v) casein
(boiledenzymatic hydrolysate, Sigmatype I), and0.6%
(w/
v) insoluble PVP. After filtration, aliquots of homogenate
were immediately withdrawn and frozen (-80°C) for subse-quent analysis ofNRactivity.The compositionofthephase
systems was asin Kjellbom and Larsson (19) and Palmgren
etal. (30) forspinachand sugarbeet,respectively, but 1 mM DTT and 0.1 mm EDTA were also included. The plasma
membranes(>90% right-side-out vesicles)wereresuspended
in5 mmK-phosphate (pH 7.8), 330mmsucrose, 1 mm DTT (= resuspension medium; DTT omittedwhen NADH-Cytc and NADH-ferricyanidereductasewere tobe measuredwith
theplasma membranes) toa protein concentration of10 to 25 mg mL-' and were stored at -80°C or inliquid N2 until
further use. Inside-out and right-side-out plasma mem-brane vesicles from sugar beet were separated as described
earlier(30).
Preparation of Antibodies
Antibodies against NADH-ferricyanide reductase purified
from potato tuber microsomes as described previously (13) wereraised in female New Zealand whiterabbits. The rabbits
wereinjected subcutaneously with 100
Ag
ofpurifiedproteinmixed 1:1 (v/v)withFreund's completeadjuvant. Following
thefirstinjection,twobooster injectionsof100 Mg ofantigen with incomplete Freund's adjuvant were given at 3 week
intervals. Therabbitswerebled 2 weeks after each injection.
The serum was collected, adjusted to 40% saturation of
(NH4)2SO4,
andtheprecipitatecontaining the antibodieswas collectedby centrifugationandwashed twicebyredissolvingin 15 mL 15 mmK-phosphate (pH 7.5), 0.15 M NaCl (PBS),
andrepeating theprecipitationstep(32). Theprecipitatewas thendissolved in 5 mL ofPBS,anddialyzed overnightat 4°C
against 10 volumes of the same buffer. Control antibodies were prepared in an identical manner using preimmune
serum.
Preparationof rabbit IgG against NR purified fromspinach
leaves was asdescribedpreviously (26).
SDS-PAGE
SDS-PAGE was run on gradient gels (concentration of
monomers, 10-22%; crosslinking, 2.7%; 5% stacking gel; gel dimensions-175- 160- 1.5 mm) in the buffer system of
Laemmli (21) with a Bio-Rad Protean II apparatus. The
samples (2.5-20 Mg protein perlane;seefigures)were solubi-lized at 22°C in 0.25 or 2% (w/v) SDS (see "Results" and
figure legends), 20
AM
leupeptin, 0.5 mm PMSF, 5% (v/v) mercaptoethanol for 10 min. Gelswere run for 15 hat4'C and 15 mA pergel. Silverstainingwasessentiallyasdescribed by Guevaraetal. (16).Western Immunoblotting Analysis
After SDS-PAGE, the wholeorpartof thegelwasincubated forabout0.5 h in 25 mMTris-HCl(pH8.3), 0.15 M glycine, 0.1%(w/v)SDS, 20%(v/v)methanol.Polypeptideswerethen transferredto anImmobilon PVDF transfer membrane (Mil-lipore, USA)at acurrent of 150mA pergel for about 1.5 h undersemidry conditions(apparatus from JKABiotech, Den-mark).Aftertransfer,themembraneswerestained forprotein (AuroDyeforte, Janssen, Belgium)orincubatedovernightin 2% (w/v) BSA in PBS. The BSA-coated membranes were
incubated with primary antibodies in PBS, then carefully
washed three times with PBS plus 0.05% (w/v) Tween 20, and incubatedwith secondary antibodies (alkaline
phospha-taseconjugatedgoatanti-rabbit IgG; Promega, USA) in PBS.
Finally,the membraneswerewashedsixtimes (three times in
PBSplus 0.015 %Tween,followed by three times inPBS) and developed (Protoblot kit; Promega, USA). Incubation with
antibodies was carried out on a shaker for 2 h at 22°C. Concentrations ofantibodieswere asfollows: control antibod-ies and antibodies against potato microsomal
NADH-ferri-cyanide reductase were diluted 2500 times; anti-NR IgG, 6
,ug
mL-';secondary
antibodieswerediluted 10,000times.The immunoblotting analysis (see "Results") was carried
outinaslightly differentway(20)thandescribedabove, using
a nitrocellulose transfer membrane and horseradish peroxi-dase-conjugated secondary antibodies (goat anti-rabbit, Bio-Rad,USA).Inthis particularexperiment the control
antibod-ies, the antibodies against potato microsomal NADH-ferri-cyanide reductase, as well as the secondary antibodies were
diluted5000times.
SolubilizationandAnionExchange Chromatography
Spinach plasma membranes (12.5 mg ofprotein) was di-luted to 3.5 mL (final volume) with ice-cold resuspension medium (see above). The same volume 40mM histidine-HCl (pH5.6), 2 mm EDTA, 2mM EGTA, 20 mM CHAPS (Sigma C 3023)wasadded dropwise understirring. After about 30
min, the unsolubilized material was collected by
centrifuga-tion at 130,000g for 1 h. The solubilization procedure was
carried out at 0 to 40C. The pellet was kept on ice and resuspended with resuspension medium prior to measure-mentof NADH-ferricyanidereductase activity in all fractions. Thesolubilized proteins (6 mL; the rest was left on ice) were separated on a Mono Q HR 5/5 anion exchange column
using FPLC (Pharmacia, Sweden), which was operated at a
flow-rate of1.0 mLmin-' at22TC.ANaClgradient (0-1 M) in 20 mm histidine-HCl (pH 5.6), 1 mm CHAPS was used, andfractions of1 mLwerecollected. Proteinwasmonitored
at280nm.
Enzyme Activities
NADH-ferricyanide reductase activity was measured as A (A42o-A5oo)using an Aminco DW 2 spectrophotometer oper-ated in the dual beam mode. The assay was run at22°C in 1 mLof330 mm sucrose, 0.2 mmK3[Fe(CN)6], 25 mM Hepes-KOH (pH 7.3), 0.25 mM NADH, 40 ,ug plasma
membrane-protein or 25 to 50
gL
FPLC-eluate,
and +0.025% (w/v) Triton X-100 with plasma membranes. The reaction wasinitiatedby theaddition of NADH. Correction was made for
nonenzymaticreductionofferricyanide.NADH-Cyt c reduc-taseactivitywasmeasuredsimilarly using 40
jM
Cyt c(Sigma;C7752)asacceptorinstead of0.2 mMK3[Fe(CN)6],andwith
0.4
jLM
antimycinA(Sigma;A2006),and 1 mM KCN presentin the assay medium. The activity was recorded as A(A550-A600), andwasdetermined ±0.015% (w/v) TritonX-100 with plasmamembranes(1). The extinctioncoefficientsused were 1 and 19mM-'
cm-'
for ferricyanideandCyt c,respectively.NR activity was measuredessentially according to Naka-gawaetal.(29).The assaymedium included330mmsucrose, 25 mm Hepes-KOH (pH 7.5), 5 mm NaNO3, 50 to 70
ttg
protein,and0.25 mm NADH in 1 mL. Measurementsweredone±0.025%(w/v) TritonX-100. Thereactionwasstarted
by the addition of NADH, run for 30 min at
27°C,
andstopped by theaddition of 1 mL0.6 M HCl
containing
1% (w/v) sulfanilamide followed by 1 mLof 0.02% (w/v) N-1-naphthylethylendiamineHCl.
The red colorwhichdevelopedwas measured at 540 nm and the amountofNO2- formed wasdetermined fromastandardcurve.Subtractionwasdone for the absorbance in the absence ofNADHforeach condi-tion. Controls withoutsamplewerealsorun.
Protein
ProteinwasmeasuredessentiallyaccordingtoBearden(5),
with BSA asastandard.
RESULTS
Specificityof AntibodiesRaisedAgainstPotatoTuber MicrosomalNADH-Ferricyanide Reductase
The specificity of the antibodies raised
against
NADH-ferricyanidereductasepurified
from potato tuber microsomes(13)wasinvestigatedbyimmunoblottingwith pure
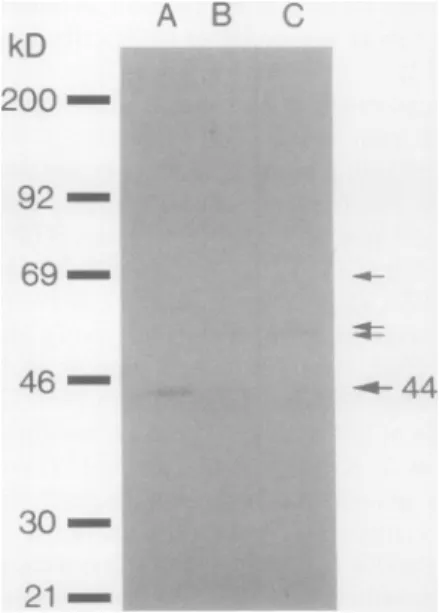
NADH-ferricyanide reductase and with a microsomal fraction from potatotubers(Fig. 1).Theantibodiesgave onebandat 44kD when reactedwith the purified protein (Fig. IA), and a band at44kDtogether with three fainter bands at higher molecular masseswhen reacted with the microsomal fraction (Fig. 1B). The 44 kD band in Figure 1, A and
B,
representNADH-ferricyanide reductase ( 13). The three fainter bands observed with the microsomal fraction were due to unspecific binding sincethey were also seen after reaction with the antibodies preparedfrom the preimmune serum (Fig. IC).
NADH-(Acceptor) OxidoreductaseActivitiesofPlasma
Membrane and Homogenate
Plasmamembranes from spinach and sugar beet leaves can use NAD(P)H to reduce ferricyanide,
phenyl-p-benzoqui-none,and Cyt c, with NADH as the preferred substrate (1,3, 4, 30). We now also report the presence ofrelatively high levels of NADH-NR activity in these plasma membranes (Table I; data not shownfor sugar beet). Similar to the NADH-ferricyanide and NADH-Cyt c reductase activities (1, 3, 4, 8, 30), the NR activity was strongly stimulated by Triton X-100, andcouldusually only be measured in the presence of deter-gent (Table I). Similar results were recentlyreported forNR
activity in barley and corn root plasma membranes (33, 34). This latent NR activity could either be due to a
membrane-A P KD
200
-92 - 69-46 - 30-21-Figure 1. Westernimmunoblotinganalysis after SDS-PAGEshowing
the specificity of the antibodies raised against the potato tuber microsomal NADH-ferricyanidereductase.A, NADH-ferricyanide
re-ductase purified from potato tuber microsomes (13) reacted with antibodiesraisedagainstthepurifiedprotein; B, potatotuber
micro-somesreacted with theantibodiesagainstNADH-ferricyanide
reduc-tase; C,potato tuber microsomesreactedwith antibodiesprepared
from the preimmuneserum. LanesA, B, andC received5,20, and 20jgofprotein,respectively.Molecularmassstandards(Amersham RPN 756 kit)inorder ofdecreasingmolecularmass were: myosin,
phosphorylaseb,BSA, ovalbumin, carbonicanhydrase,trypsin inhib-itor. Arrows indicatepositionsof different bands.Forfurtherdetails, see"Materials and Methods" and "Results."
NADH-FERRICYANIDE REDUCTASE OF LEAF PLASMA MEMBRANES
bound (integral) form of NR with its NADH-binding site locatedonthecytoplasmic surface of the plasma membrane
as suggested by Ward et al. (33, 34), or it could be due to
soluble NR trapped inside the plasma membrane vesicles during homogenization. Alternatively, the NR could be loosely boundtothecytoplasmic surface. The NR activity in the homogenate was not significantly stimulated by Triton X-100(Table I).
Western ImmunoblottingAnalysisofLeafPlasma Membranes
Polyclonalantibodies, raisedagainsttwodifferentenzymes
capableoftransferringelectronsfromNADH toferricyanide,
namelyNR(9, 10, 29) andpotatotuber microsomal
NADH-ferricyanidereductase(tentativelyidentifiedasNADH-Cytb5
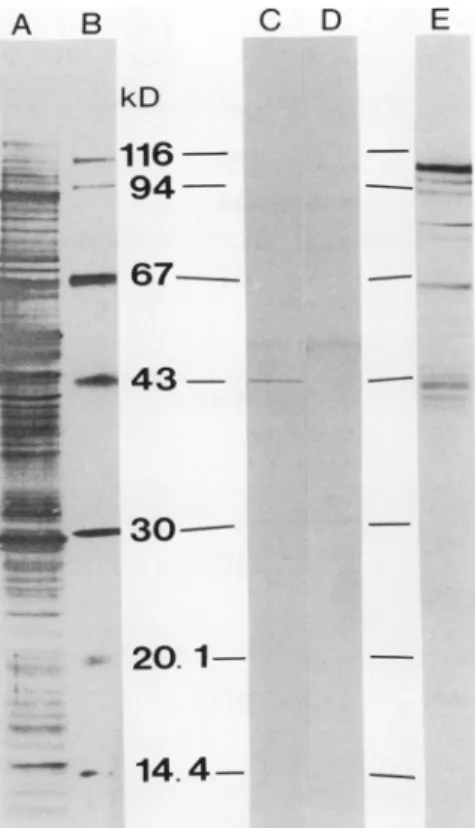
reductase; see "Discussion" and ref. 13), were reacted with polypeptidesofspinach leafplasma membrane (Fig. 2). The
antibodies against NADH-ferricyanide reductase purified from potato tubermicrosomes revealed one strong band at
43 kD(Fig.2C).Afewadditionalfaint bandswerealsoseen,
but they were also found after reaction with the antibodies preparedfromthepreimmuneserum(Fig. 2D)andwerethus duetounspecific binding.Amolecularmassof 43 kD isvery
closeto the 44 kD found for NADH-ferricyanide reductase frompotatotubermicrosomes(13;Fig. IA). The 43 kD band
wasalsotheonlybandseenwhen theantibodieswerereacted withan intracellular membrane fraction depleted in plasma membranes (the lower phase remaining after extraction of plasma membranes [19, 22]), although it was fainter than with the plasma membranes (thesame amountofproteinwas appliedtothegel;resultsnotshown).Thereasonfor thiswas
probably that endoplasmic reticulum, outer mitochondrial membrane, Golgi, etc., which could be expectedto contain NADH-Cyt b5 reductase (6, 11) constituted only a small
proportion ofthe membranes in the intracellular membrane fraction, whichconsisted mainly ofthylakoids (19).
Antibodies againstNR purified from spinach leaves (26) displayedone majorband atabout 110 kD together with a
few fainter bands at lower molecular masses when reacted
TableI. NRActivity inSpinachPlasma Membrane and
Homogenate,and theEffectof Anti-NRIgG
Latency isdefinedas percentageof latent activity(difference in
activitymeasured ± Triton X-100) oftotal activity(activity measured
+TritonX-100). Dataare means ±SDof 3-4independent prepara-tions. NRActivity Assay Latency -Triton X-100+0.025%Triton X-1 00 nmolN02-/h-1 % (mgprotein)` ° Homogenate 292 ± 88 315 ± 68 7 Plasmamembrane 0 94± 66 100
Plasmamembrane + NDa 50 ± 41 ND
0.6jiganti-NR IgG
Plasma membrane + ND 0 ND 2.5Aganti-NR IgG aNot determined. A B C D E kD
--116-4; 94- -, -67--3
20.1
14.4
Figure 2. Western immunoblotting analysis ofspinach leaf plasma
membranepolypeptidesafterSDS-PAGE.A, Protein-stain (AuroDye forte)ofblottingmembranereflectingtotaltransferredpolypeptides;
B, protein-stainof transferred standard molecularmassmarkers; C,
immunoblot with antibodies raisedagainst potatotubermicrosomal
NADH-ferricyanide reductase; D, immunoblot with antibodies
pre-paredfrompreimmuneserum; E, immunoblot with antinitrate
reduc-tase IgG. Lanes A, C, D, and E received 20 Mg of protein. SIDS concentrationduringsolubilizationwas0.25%(w/v).Molecularmass
standards in orderofdecreasingmolecularmasswere: fl-galactosid-ase (Sigma G-8511), phosphorylase b, BSA, ovalbumnin, carbonic
anhydrase, soybean trypsin inhibitor anda-lactalbumnin(the latterall from a Pharmacia kit). For further details, see "Materials and Methods."
with spinachleafplasmamembranes(Fig. 2E). A molecular massof110kDisconsistentwith the molecularmassfor the intactsinglesubunit ofNR,which isahomodimer of210to
230kDinhigher plants (9, 29).The bands atlower molecular
masses were probably due to proteolytic breakdown ofthe 110kD subunit, sinceNR isextremelysensitiveto protease activity(9, 29, and references therein). Supporting thisview wastheobservationthat a lowconcentrationof SDS (0.25%
[w/v]), and the presence of leupeptin and PMSF in the
solubilization medium, in combination witha low solubiliza-tion temperature (22°C), minimizedtheappearanceof bands
with Mr < 110 kD and intensified the band at 110 kD.
Inclusion ofthe additional protease inhibitors
p-aminoben-zamidine (2 mM) and TLCK (65 MM) in the solubilization medium (as well asin the homogenization medium during
preparation ofplasmamembranes)had no additional effect,
however.A 110kD band wasseen also with the homogenate,
but the problem with proteolytic breakdown was more pronounced with this fraction (results not shown). Thus,
in. -.jl p Drft' 1-n- :) 116 __-- 94S A. ~ 6.7 ;4, :I:.. :. _LI!. 30--2() _ _--_ 144
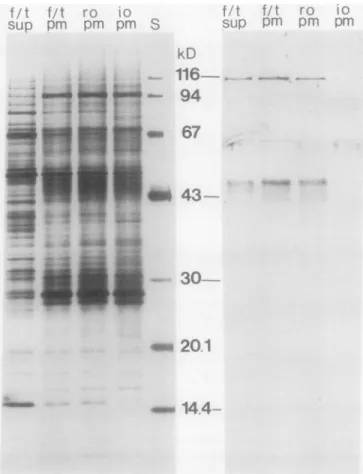
Figure3. Silverstaining(left)andWeste
*U-;
. ' Part ofthese vesicles(about
25%)
can be turned inside-outi- Drew 98 at, 355
by freezing
andthawing,and sealedinside-outandright-side-outvesicles can subsequently be separated by repeating the
phase partition step (30). The rationale was that a soluble
enzyme trapped inside the right-side-out vesicles would be released upon formation of inside-out vesicles and
subse-quently lost. Thus, if NR was trapped inside the plasma
membranevesicles, or was only weakly boundto the
cyto-plasmic
surface,
it would not be observed with inside-out plasma membrane vesicles inanimmunoblotanalysis.Asshown in Figure 3, antibodies against NR reactedwith
unseparatedplasmamembranes (f/t pm)andtoaslightly less extentwithright-side-outplasma membranes (ro pm).
Bind-ing was also observed with a supernatant fraction obtained
after pelleting the plasma membranes after the freeze/thaw
procedure(f/tsup;containingsolubleproteinsreleasedfrom
thevesicles). In contrast, anti-NR IgG hardly reacted atall
with inside-out plasma membranes (io pm). These results
strongly suggest that soluble NR was enclosed inside the
plasma membrane vesicles. Other soluble proteins, trapped
inside the plasma membrane vesicles, were also excluded
during preparation of inside-out vesiclesasjudged from the
polypeptide patterns(Fig. 3, left): For example, BSA (added
tothehomogenization buffer)at67 kD,andtwopolypeptides
at about 53 and 14.5 kD tentatively identified as the large
and small subunits ofribulose-1,5-biphosphatecarboxylase/ oxygenase, respectively, were found to less extent with the
inside-out plasma membrane vesicles comparedtotheother plasmamembranefractionsandwereenriched in the
freeze/
arnimmunoblottinqanalysis thaw supernatant (Fig. 3,left).with anti-nitrate reductase IgG (right) of sugar beet leaf plasma
membrane polypeptides after SDS-PAGE. The fractions analyzed
were: plasmamembranes that had been frozen and thawed
repeat-edly (f/t pm= startingmaterial for separation ofright-side-out and
inside-out plasma membrane vesicles); supernatant obtained after pelleting f/tpm(f/t sup) containing soluble proteins released fromthe
plasma membrane vesicles; right-side-out (ro pm) and inside-out(io
pm) plasma membrane vesicles, both obtained from f/t pmas
de-scribed in Palmgren, et al. (30). The lanes received 5 ug (silver
staining)or20Ag(immunoblotting) ofplasmamembraneprotein or 2.5 tig f/t sup protein (same amount for both silver staining and
immunoblotting, correspondingtotheprotein released from 50,g f/
tplasma membraneprotein). S, Standard molecularmassmarkers
(as in Fig. 2). SDS concentration during solubilization was 0.25%
(w/v).
SolubilizationandAnionExchange Chromatographyof NADH Ferricyanide ReductaseActivity
The zwitterionic detergent CHAPS has previously been
successfully used for the purification ofNADH-ferricyanide
reductase from potato tuber microsomes (13), and for the
same or related enzymes from endoplasmic reticulum and
glyoxysomes ofcastorbean(23). Under the conditions used in the present study, CHAPS solubilized 74 ± 4% (n = 3
preparations) of total recoveredNADH-ferricyanide reductase
activity, and about 75% of total protein with spinach leaf plasma membranes (Table II). All the activity seemedtobind
to a Mono Qanion exchange column atpH 5.6, indicating
the plasma membrane-associated NR seemed to be better
protected against protease activity than the NR in the
homogenate.
Antibodies raised against NRfrom spinach leavesreacted
with polypeptides ofsugarbeet plasma membranes (Fig. 3, right; f/t pm).Amajor bandwasfoundat 114kD,whichwas
less intense than the 110 kD band ofspinach leafplasma membranes (cf. Figs. 2 and 3). Since we have developed a
techniqueforpreparinginside-outplasmamembranevesicles
fromsugarbeet leaves(30),thisspecieswasusedtoinvestigate whetherNRwas firmlyboundtotheplasma membrane, or
only trapped inside the vesicles during homogenization. Plasmamembranes obtainedbystandard phase partitioning proceduresareabout 95%right-side-out(apoplasticsideout).
TableII. SolubilizationandPartialPurificationofNADH-Ferricyanide
Reductase Activity fromSpinach Leaf Plasma MembranesbyFPLC
Datafrom atypical experiment of a total of three (sameseparation asshown inFigures4 and5).
Assay Total Total Specific Purifi- Yield
Protein Activitya
Activityb
cationPlasmamembrane 10.7 12 1.1 1 100
CHAPSsupernatant 7.6 7.6 1.0 0.9 63 Mono0 eluate
Total 5.5 48
Fraction 27 0.05 4.1 78 70 34
agmol ferricyanide reduced min-'. b
Mmol
ferricyanide reducedNADH-FERRICYANIDE REDUCTASE OF LEAF PLASMA MEMBRANES
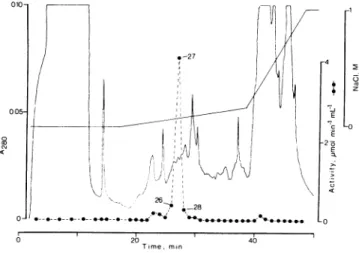
an isoelectric point below pH 5.6 for the enzyme (Fig. 4).
Mostoftheactivity applied(71 ±2%; n =2)was recovered
after elution with a gradient ofincreasing concentration of NaCI. This resulted inonemajorpeakofNADH-ferricyanide reductaseactivityatabout 70mmNaCl in threeindependent separations. Intheseparationshown inFigure4, the activity
waspurified about 70-fold relativetotheplasma membrane (TableII). This partlypurifiedenzymecouldalso reduce Cyt
cat a ratethatwas 10%of that withferricyanide.Withplasma membranes therate of Cytcreductionis about 20% of that
with ferricyanide, the difference probably being due to the
presenceof Cytb5in the latter(3). No other electronacceptors were testedwiththe partlypurifiedenzyme.
Thefraction with highest activity (fraction 27) aswell as
neighboring fractions (fractions 26 and 28) were analyzed with SDS-PAGE (Fig. 5, left). In this particular run, silver-stainedpolypeptideslocatedatabout 120, 43, 40, 37, 30,and 22 kD(the 120, 30, and 22 kDbands werevery faint)were enriched in fraction 27 relative to fractions 26 and 28 and thuswere all correlatedfairlywell with the
NADH-ferricya-nide reductase activity
(cJf
Figs. 4 and 5). However, thepolypeptide at43 kD(one of the more prominentbands in
fraction 27) had a distribution between the fractions that coincided best with theactivity in thethree separationsthat
wereanalyzedwith SDS-PAGE. Moreover,antibodiesagainst
potato microsomal NADH-ferricyanide reductase reacted with the 43kDpolypeptide only(Fig. 5,right). These results clearly indicate that an enzyme identical or very similar to
potato tuber microsomal NADH-ferricyanide reductase is responsible for the major part, if not all, of the NADH-ferricyanide reductase
activity
ofspinach
leaf plasmamembranes.
Effects of Antibodieson NADH-(Acceptor) OxidoreductaseActivities
AntibodiesagainstpotatomicrosomalNADH-ferricyanide
reductase hadnoeffectonNADH-Cytcreductase or
NADH-Solub. Fractions | S 26 27 281 kD Fractions 26 27 28 kD -116-- - ~--94
43-..
~--30
up
~
in.20.1-14.4-Figure 5. Silverstaining (left) and Western immunoblotting analysis
withantibodies raised against potatotuber microsomal
NADH-ferri-cyanide reductase (right) of total CHAPS-solubilized spinach leaf
plasmamembraneproteins(Solub)andFPLC fractions 26to28,from theseparation shown in Figure4. Theamountsofprotein (samefor
silverstaining as for immunoblotting) applied to the
SDS-polyacryl-amidegelwere: 5 and2.5,4g for solubilized protein(Solub), left and
right lane, respectively; and 1.2, 1.5, and 2.5 gg, corresponding to
identical volumes of fractions 26, 27, and 28, respectively. SDS concentrationduringsolubilizationwas2%(w/v).5,Standard molec-ularmassmarkers(asinFig. 2).
~~~~~~I ;1_, v, ww 26* 28 _ _ @** ^~*O O-e , 20 Time, min 4 4 -2 E >S -2 K -Q z -0 Lo 40
Figure 4. Purification of NADH-ferricyanide reductase activity of
CHAPS-solubilizedspinach leaf plasma membraneswithFPLCusing
a Mono Q anion exchange column. (@), Total NADH-ferricyanide reductase activity of collected fractions(1 mL). Fractions analyzed
withSDS-PAGE (26-28; Fig. 5)areindicated. The flow-ratewas1.0
mLmin-'.
ferricyanidereductaseactivity withspinachleaf plasma
mem-branes, even at averyhigh concentration (antibodies diluted 50 timesonly, 3 minincubationat22°C; resultsnotshown).
This indicatedthat theantibodiesboundto asiteotherthan theactive site of theenzyme. Anti-NRIgG (10
jig
mL-', 10min incubation) inhibited NADH-Cyt creductase by about 20% but no further effectwas obtained with higher
concen-trationof IgG (50 ,ugmL-'). This could indicatethat partof the NADH-Cyt c reductase activity was catalyzed by NR.
Morelikely, however,this inhibition reflectsstructural simi-larities between NR and related enzymes (10). The NADH-ferricyanide reductase activity was not affected by anti-NR IgG(50,ugmL-';results notshown). By contrast, the NADH-NR activity of spinach leaf plasma membranes was com-pletelyinhibitedbyanti-NR IgG, even at a very low
concen-tration (TableI).
DISCUSSION
The aim ofthe present work was to determine what en-zymes areresponsiblefortherelatively high
NADH-ferricya-1 NADH-ferricya-1
0 a) c\l
nide reductase (NADH-diaphorase) activities obtained with leaf plasma membranes. Especially interesting was the possi-bility thataplasmamembrane-bound (integral) form ofNR
catalyzedthis activity (33, 34). Antibodies against NRfrom
spinachleaveswerefoundto reactwith polypeptides of spin-ach and sugarbeet leaf plasma membranes (Figs. 2 and 3).
The molecularmasses, 11Oand 1 14 kDfor spinachand sugar
beet plasma membrane, respectively, are identical to those
reported for the subunits of the soluble enzyme (9, 29), indicating that the size of the plasma membrane-associated formwasthesame.
Asjudgedfrom theimmunoblottingdata(Fig. 3), the NR
associatedwith sugar beet plasma membranes could be
re-movedfromthevesiclesbyfreezingandthawing (also found with spinach plasma membranes;results notshown),and the inside-outplasma membranevesiclesweretherefore depleted in the 114 kD polypeptide in comparison to right-side-out plasma membrane vesicles andunfractionatedplasma
mem-branevesicles (Fig. 3).This strongly suggests that solubleNR was enclosed inside the right-side-out vesicles, or possibly weakly bound to thecytoplasmic surface. Since IgG against
solubleNRcompletelyinhibitedthe NRactivity with spinach plasma membranes (Table I), thepossibilitythat theplasma membranescontained animmunologically different form of
NRcanberuled out.
The NR reportedtobeassociated with plasma membranes
purified by phase partitioning from barley and corn roots
could be removedfromtheplasma membranes by Triton X-100 (0.1%) but not by sonication in the presence of 1.0 M
NaCl (33, 34). The authorssuggestedthat thedetergent solu-bilizedanintegral NRprotein in the plasma membrane.An
alternative explanation fortheeffect ofTriton X-100 is that the plasma membrane vesicles were disrupted so that en-closed, soluble NR wasreleased. Whereas the 114 kD
poly-peptidereacting withthe anti-NR IgGwasalmostcompletely lost from the inside-out vesicles (Fig. 3, right), the
NADH-ferricyanide
reductase and NADH-Cytcreductaseactivities areratherenrichedininside-outsugarbeetplasmamembrane vesicles comparedtoright-side-out plasma membranevesicles (1, 30).Therefore, thepredominatepartoftheseactivitiesinsugarbeet(andspinach) plasmamembranescannotbe
cata-lyzed by enclosed NR. Indeed, wefound that apolypeptide of43 kD rather than 110 kD
copurified
with the NADH-ferricyanidereductaseactivity
ofspinach plasmamembranes,
and that antibodies against
NADH-ferricyanide
reductasepurified from potato tuber microsomes reacted with this
polypeptide
(Figs.
4and5).Usingaffinity bindingtoCibacron blueagarose, Lusterand Buckhout(24,25)purifiedan
NAD(P)H-(acceptor)
oxidore-ductasefromcornrootplasma
membraneswhichwascapable
ofreducing, for
example,
ferricyanide
andduroquinone.
Al-thoughpolypeptidesof both44,40,
and 28kDwereassociated with these activities, further purification indicated that a polypeptide of27 kD was responsible for theactivity (25).
Threemore or lessstronglysilver-stained
polypeptides
of 27to 28 kD
copurified
with theNADH-ferricyanide
reductasealso in this study (Fig. 5, left), but they did not seem to
correlatewith the
activity
(cf Figs.
4and5). Notably,
several molecularmasses have been reportedfor membrane-bound(microsomal) NADH-(acceptor)oxidoreductases from
differ-entplantsources( 13, 15, 17,
23).
The potatomicrosomalNADH-ferricyanide reductase(13)
hasproperties very similartothatof ratliver NADH-Cyt
b5
reductase(31),althoughit hasnotyetbeendefinitely proven
that thepotato enzymeisanNADH-Cytb5 reductase. How-ever, the finding that antibodies raised against the potato microsomal NADH-ferricyanide reductase reacts with a 43
kDpolypeptide in spinach leafplasma membranes(Fig.
2),
and that thispolypeptide correlates with the NADH-ferricy-anidereductaseactivity (Figs.4and5), supports theview(1, 3) thatthe major part ofthe NADH-ferricyanide reductase
activity ofleafplasma membranes isdueto the presenceof NADH-Cyt b5 reductase. This was also supported by the
localizationofthedonor and acceptorsites of this activityto the same (cytoplasmic) surface of sugar beet leaf plasma membranes(1),aknownpropertyof NADH-Cytb5reductase
(11),andbythepresence ofacomponentsimilar to Cytb5in
lowtemperature spectra(3).
Theflavoprotein NADH-Cytb5 reductase and itselectron acceptorCytb5havearelativelywide subcellulardistribution
inanimalcells(6, 11, 14).This is probably related to thefact
that they,in contrast to most otherintegral membrane pro-teins, are synthesized on free polysomes and inserted post-translationallyintomembranes(6, 11). Alsoinplants
NADH-Cyt b5 reductase and Cyt b5 may have a wide subcellular
distribution, since inaddition to theirmore wellestablished compartments,theendoplasmic reticulum and the outer mi-tochondrial membrane, NADH-Cytcreductase activity has beenreportedin glyoxysomes, tonoplast, and plasma
mem-brane (for a review, see ref. 28). In animal endoplasmic
reticulum,Cyt b5 andits reductase functionasintermediary linksforelectronsfromNADH to a fatty acid desaturase(1 1)
and thisisprobablyalso the case inplants (18).Itremainsto be established whether the plasma membrane NADH-ferri-cyanidereductase has asimilaror adifferent function.
Although the plasmamembranes used in this studyare90 to95%pure(19, 22, 30),itcould beargued that the
NADH-ferricyanide reductase may be due to a small amount of
contaminating endoplasmic reticulumhavingamuchhigher specificNADH-ferricyanide reductase activity thanwasfound for theplasma membrane fractions(1,3, 4, 30). Itseemsvery unlikely, however,that such acontaminationwasresponsible
for amajor part ofthe NADH-ferricyanide reductase, since
vesiclesoriginating from the endoplasmic reticulum wouldbe
expected to partition in the lower, dextran-rich phase like other intracellular membranes (22). Furthermore, since the
donor and acceptor sites for NADH-ferricyanide reductase
activity of the endoplasmic reticulum are located on the
cytoplasmic surface (1 1), and vesicles with opposite orienta-tion areunlikelytoform(11),nolatencyofthisactivitywould
be observed. Theoretically, however,the latent NADH-ferri-cyanide reductase activity could be catalyzed by enclosed
endoplasmic reticulum. This latter possibility was recently investigated by separating two-phase partitioned, inside-out sugar beet leaf plasma membrane vesicles (prepared from
originally right-side-outvesicles;30)on acontinuoussucrose
gradient (4). After separation, the NADH-ferricyanide and
NADH-Cyt c reductase activities correlated perfectly with
NADH-FERRICYANIDE REDUCTASE OF LEAF PLASMA MEMBRANES
wellas with theprotein profile, which seems toexclude the
possibilitythat theformertwoactivitiesweredueto contam-inating membranes originally enclosed inside the plasma membranevesicles(4).
Inconclusion, ourdataindicate thatmost,ifnotall,of the truly membrane-boundNADH-ferricyanidereductase of leaf
plasmamembranesis duetoanenzyme verysimilartopotato tubermicrosomal NADH-ferricyanide reductase (NADH-Cyt b5 reductase), and that the NRassociated with plasma
mem-branevesicles isnotanintegral protein but probably trapped
inside the vesiclesduring homogenization, orpossiblyloosely
boundtothecytoplasmic surface.
ACKNOWLEDGMENTS
Wewishtothank Mrs.Ann-Christine Holmstrom and Mrs. Adine
Karlsson forhelpwith preparation of plasma membranes, and Pro-fessor Christer Larsson (Department ofPlant Biochemistry, Lund,
Sweden)for valuablediscussions.
LITERATURE CITED
1. AskerlundP,LarssonC,WidellS (1988) Localization of donor
and acceptor sites ofNADH dehydrogenase activities using
inside-outandright-side-out plasma membranevesicles from
plants. FEBS Lett239:23-28
2. AskerlundP,LarssonC(1989)Redox activitiesmeasured with
inside-out and right-side-outplasmamembrane vesicles from
sugar beet leaves. In J Dainty, MI De Michelis, E Marre, F
Rasi-Caldogno,eds,Plant MembraneTransport: The Current
Position. Elsevier, Amsterdam,pp43-47
3. Askerlund P, Larsson C, Widell S (1989) Cytochromes of plantplasmamembranes.Characterizationbyabsorbance
dif-ference spectrophotometry and redoxtitration. Physiol Plant 76: 123-134
4. AskerlundP(1990) Redoxprocessesof plant plasmamembranes.
Studieswith isolated plasma membrane vesicles. PhD thesis.
University ofLund,Sweden. ISBN 91-628-0063-9
5. BeardenJC Jr(1978)Quantitation of submicrogram quantities
ofprotein byanimproved protein-dye bindingassay.Biochim BiophysActa533:525-529
6. Borgese N,PietriniG (1986) Distribution of the integral
mem-brane protein NADH-cytochrome b5 reductase in rat liver cells, studied with aquantitative radioimmunoblottingassay.
Biochem J 239: 393-403
7. BuckhoutTJ, BellPF, LusterDG,ChaneyRL(1989)Iron-stress induced redox activity in Tomato (Lycopersicon esculentum Mill.) islocalized ontheplasmamembrane. PlantPhysiol 90:
151-156
8. BuckhoutTJ,Hrubec TC (1986) Pyridine nucleotide-dependent ferricyanide reduction associated with isolated plasma
mem-branes of maize (Zea maFvs L.) roots. Protoplasma 135:
144-154
9. Campbell WH (1988) Nitrate reductase and its role in nitrate assimilation inplants. PhysiolPlant 74: 214-219
10. Crawford NM, Smith M, Bellissimo D, Davis RW (1988)
Se-quenceandnitrateregulation oftheAradopsisthalianamRNA
encoding nitrate reductase, a metalloflavoprotein with three
functional domains.ProcNatl AcadSci USA 85: 5006-5010
11. DePierre JW, Andersson G, Dallner G (1988) Endoplasmic reticulum and golgi complex. In IM Arias, WB Jacoby, H Popper, DSchachter,DAShafritz,eds, TheLiver.Biologyand
Pathobiology, Ed 2.Raven Press,New York,pp165-187
12. DharmawardhaneS, Stern Al,Rubinstein B (1987) Light-stim-ulated transplasmalemma electrontransportinoat mesophyll
cells. PlantSci 51: 193-201
13. GalleA-M,BonnerotC,JolliotA,Kader J-C(1984)Purification of a NADH-ferricyanide reductase from plant microsomal
membranes with a zwitterionic detergent. Biochem Biophys
ResCommun 122: 1201-1205
14. Goldenberg H (1982) Density gradient fractionation of digitonin-treatedratliverplasma membranes and subcellular localization of NADH-oxidoreductase and b-type cytochromes. Enzyme 27: 227-238
15. Guerrini F, Valenti V, PupilloP(1987)Solubilization and puri-fication ofNAD(P)H dehydrogenaseof Cucurbita microsomes. PlantPhysiol 85: 828-834
16. Guevara J Jr,Johnston DA, Ramagali LS, Martin BA, Capetillo S, Rodriguez LV (1982) Quantitative aspects of silver deposi-tion in proteins resolved in complex polyacrylamide gels. Elec-trophoresis 3: 197-205
17. Jollie DR, Sligar SG, SchulerM (1987)Purification and char-acterization of microsomal NADH cytochrome b5 reductase from Pisum sativum. Plant Physiol 85: 457-462
18. KaderJ-C (1977) Cyanide sensitivityandinduction of the mi-crosomal oleoyl-CoA desaturase ofpotato tuber. Biochim Bio-phys Acta 486:429-436
19. Kjellbom P, LarssonC(1984) Preparation andpolypeptide
com-position of chlorophyll-free plasma membranes from leaves of light-grownspinach and barley.Physiol Plant 62: 501-509 20. Kyhse-Andersen J (1984) Electroblotting of multiple gels: a
simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods10: 203-209
21. Laemmli UK (1970) Cleavage of structural proteins during
the assembly of the head ofbacteriophage T4. Nature 227: 680-685
22. Larsson C(1985)Plasma membranes. In HFLinskens, JF Jack-son, eds, CellComponents. Methods of Plant Analysis (New Series), Vol 1. Springer-Verlag, Berlin, pp 85-104
23. LusterDG, Bowditch MI, EldridgeKM, Donaldson RP(1988) Characterization ofmembrane-bound electron transport
en-zymesfrom castorbean glyoxysomes andendoplasmic reticu-lum.ArchBiochemBiophys256: 50-61
24. Luster DG, Buckhout TJ (1988) Characterization and partial
purification ofmultiple electron transport activities inplasma membranesfrommaize (ZeamaysL.)roots.PhysiolPlant73: 339-347
25. LusterDG, Buckhout TJ (1989)Purification and identification ofa plasma membrane associated electron transportprotein from maize (Zea mays L.) roots. PlantPhysiol91: 1014-1019 26. Maki H, Yamagishi K,Sato T, Ogura N, Nakagawa H (1986)
Regulation of nitrate reductase activity in cultured spinach
cells as studied byan enzyme-linked immunosorbent assay. PlantPhysiol 82:739-741
27. M0ller IM, CraneFL(1990) Redox processes in the plantplasma membrane. InC Larsson, IMM0ller, eds, The Plant Plasma Membrane-Structure, Function and Molecular Biology. Springer-Verlag,Berlin,pp93-126
28. M0ller IM, LinW(1986)Membrane-bound NAD(P)H dehydro-genases in higher plant cells. Annu Rev Plant Physiol 37: 309-334
29. Nakagawa H, Yonemura Y, Yamamoto H, Sato T, Ogura N, SatoR(1985)Spinach nitrate reductase. Purification, molec-ular weight, and subunit composition. Plant Physiol 77: 124-128
30. PalmgrenMG,AskerlundP,FredriksonK, Widell S, Sommarin M, Larsson C (1990) Sealed inside-out and right-side-out plasmamembrane vesicles. Optimal conditions for formation andseparation. Plant Physiol 92: 871-880
31. Spatz L, Strittmatter P(1973)Aformof reduced nicotinamide adenine dinucleotide-cytochrome b5 reductase containing both the catalytic site and an additional hydrophobic membrane-binding segment. J Biol Chem 248: 793-799
32. Thomas PE, Lu AYH, Ryan D, West SB, Kawalek J, Lewin W (1976) Multipleforms of rat liver cytochrome P-450. Immu-nologicalevidence with antibody against cytochrome P-448. J Biol Chem 251: 1385-1391
33. Ward MR, Grimes HD, Huffaker RC (1989) Latent nitrate reductase activity is associated with the plasma membrane of
cornroots. Planta177:470-475
34. Ward MR, Tischner R, Huffaker RC(1988) Inhibition of nitrate transport by anti-nitrate reductase IgG fragments and the identification of plasma membrane associated nitrate reductase in rootsof barley seedlings. Plant Physiol 88: 1141-1145