Institutionen för Kultur, energi och miljö
Avdelningen för biologi
Högskolan på Gotland, SE-621 67 Visby
www.hgo.se
SWIMMING
BEHAVIOR
AND
MORTALITY
OF
THE
INDIGENOUS
AMPHIPOD
MONOPOREIA
AFFINIS
IN
PRESENCE
OF
THE
INVASIVE
POLYCHAETE
MARENZELLERIA
SPP.
IN
THE
BALTIC
SEA
Isa Wallin
Bachelor Thesis in Biology 15 credits, 2012
Tutor: Bertil Widbom
Denna uppsats är författarens egendom och får inte användas för publicering utan författarens eller dennes rättsinnehavares tillstånd. Isa Wallin
1
CONTENTS
ABSTRACT ... 2
INTRODUCTION ... 3
The ecosystem of the Baltic Sea ... 3
The benthic fauna of the Baltic Sea ... 3
Invasive species ... 4
The ecology of the amphipod Monoporeia affinis ... 4
The ecology of the polychaete Marenzelleria spp. ... 6
Competition between M. affinis and Marenzelleria spp. in the Baltic Sea ... 7
Purpose of this study ... 7
Question formulations ... 8
Premises... 8
Predictions ... 8
MATERIAL AND METHODS ... 9
Experimental design ... 9
Data analysis... 10
RESULTS ... 12
Differences in swimming behavior between treatments... 12
Differences in swimming behavior over time within treatments ... 13
Mortality ... 13
DISCUSSION ... 15
Differences in swimming behavior between treatments... 15
Differences in swimming behavior over time within treatments ... 17
Mortality ... 18
Sources of error ... 19
Conclusions and speculations ... 19
Future experiments ... 20
ACKNOWLEDGEMENTS ... 22
REFERENCES ... 22
2
ABSTRACT
The Monoporeia affinis population has declined drastically in the Baltic Sea since 1999. In the late 1980’s, an invasive polychaete, Marenzelleria spp., established in the Baltic Sea. This experiment aimed to investigate if M. affinis’ swimming behavior and mortality was affected by competition from Marenzelleria spp., and if so the competition was size- or density-dependent. One control series without Marenzelleria spp. and four series with different sizes and densities of Marenzelleria spp. were studied during 20 days, to determine potential effects of Marenzelleria spp.
No statistically significant results were found neither for differences in swimming behavior between treatments, or in amphipod mortality between treatments. However, a statistically significant difference in swimming behavior over time within treatments was found in one treatment. The experiments did not show any negative impact on M. affinis from the presence of Marenzelleria spp.
3
INTRODUCTION
The ecosystem of the Baltic Sea
The Baltic Sea is a unique ecosystem. It is the world’s second largest brackish water ecosystem, and has a species composition that exists on very few places on planet Earth, if anywhere at all (Bernes 2005). The brackish water in the Baltic Sea makes the ecosystem particularly susceptible to disturbance of any kind, since the marine and limnetic species that live there are physiologically stressed by constantly living on the verge of their capacity to handle osmosis. The number of species is also low due to the fact that the Baltic Sea is a young ecosystem, and rather few species have so far adapted to the environmental conditions that prevail there (Bernes 2005).
Irregular inflows of saline water from the Atlantic combined with run-offs from rivers and streams, abundant precipitation and low perspiration makes the salinity of the Baltic Sea much lower than in the rest of the world’s oceans (Bernes 2005). The salinity in the bottom waters of the Baltic Sea is approximately 13-22 ‰ in the Bornholm Basin, and between 10-14 ‰ in the Gdansk and Gotland basins (Nissling et al. 2002). In the Gulf of Bothnia, the bottom waters have a salinity of 2-3 ‰ (Norling 2007). As a result of the decreasing salinity, the marine species predominate in the southern parts of the Baltic Sea, while the limnetic species occur in higher numbers in the northern parts of the Baltic Sea and in the Gulf of Bothnia. The irregular inflows of saline water also sustain stratification between saline and less saline water, called a halocline. The halocline separates the upper, well-oxygenated water from the lower, oxygen-deficient waters. This means that hypoxia and anoxia in the Baltic Sea bottoms has partially natural causes (Bernes 2005).
The ongoing eutrophication in the Baltic Sea, which is largely caused by human influence, resulted in a five-fold increase of benthic fauna in the years between 1920 and 1980, and the benthic faunal biomass was tripled during the same period. These increases in numbers and biomass could take place because of the augmented emissions of nutrients during the 20th century, which in turn increased the production of organic matter in terms of food available for benthic organisms (Bernes 2005).
In recent years, the decomposition of the large quantities of organic material has resulted in severe hypoxia and anoxia in vast areas of the Baltic Sea bottoms, particularly in the deeper parts. At numerous sites below the halocline the benthic fauna has disappeared entirely, most probably because of anoxia. At sites where benthic fauna still occurs, the species composition has altered markedly. Thus, the benthic fauna in the Baltic Sea is vulnerable, mostly because of the low oxygen content in the bottom water, but also because of factors such as bottom trawling, environmental pollutants and invasive species (Bernes 2005).
The benthic fauna of the Baltic Sea
The fauna of the soft bottoms of the Baltic Sea is dominated by a few species of polychaetes, gastropods and mollusks, and a few small crustaceans (Lindegarth & Pihl 2012). According to Laine (2003) the most common species are the polychaete Harmothoe sarsi, the isopod
Saduria entomon, the amphipods Monoporeia affinis and Pontoporeia femorata and the bivalve Macoma balthica. These species, except H. sarsi, also dominate the biomass. Less common, but locally important, are the priapulid worm Halicryptus spinulosus, the crustacean
4
New data indicate that the M. affinis and P. femorata populations have declined rapidly in the past few years, while the M. balthica population has increased drastically. The invasive polychaete Marenzelleria spp. also makes an additional contribution to the benthic community in the Baltic Sea (Albertsson & Cederwall 2008).
The fauna of the deep soft bottoms in the aphotic zone, which in the Baltic Sea begins at about 20 meters depth (Dahlgren 2010), depends almost entirely on sedimented organic material (van de Bund et al. 2001 and references therein), and the diet of the benthic fauna consists primarily of diatoms (Cederwall et al. 2011).
Invasive species
For a species to be considered as invasive, a few requirements have to be met: the species needs to overcome biotic and abiotic barriers to survive in an area of introduction, it needs to be able to disperse from its area of initial introduction, and it has to have a considerable ecological and economic impact on the ecosystem (Katsanevakis et al. 2010), or apparent impact on human health (Occhipinti-Ambrogi & Galil 2010).
Over the last 30 years, world seaborne trade has more than doubled from 2490 million tonnes in 1970 to 5330 million tonnes in 2000, and today more than 12 billion tons of ballast water are shipped around the globe every year (Bax et al. 2003). At any given moment, approximately 10,000 different species are being transported between bio-geographic regions in ballast tanks alone (Carlton 1999).
Fortunately, most of these potential invaders die on their way to new destinations. Many species do not survive the dark and often dirty conditions in ballast tanks over a long voyage; for others, the environmental conditions at the port where they are released are not suitable. However, in recent years, ballast water has become cleaner, ship’s transit speed has increased and environmental management of ports has improved, and these factors may together enhance chances of survival and facilitate the establishment of marine alien species (Bax et al. 2003).
The ecology of the amphipod Monoporeia affinis
Monoporeia affinis (formerly Pontoporeia affinis) can be up to 1 centimeter in length, and has
a pale yellow color (Køie & Svedberg 2004). It has black eyes, which makes it easy to distinguish from the amphipod Pontoporeia femorata, having red eyes (B. Widbom, pers. comm.). It is a deposit feeder (Sundelin et al. 1999) which swims actively at night and stays buried during daytime (Lindström & Lindström 1980). When solitary, M. affinis searches for food at various depths in the sediment, but when co-occurring with P. femorata, M. affinis forages in the uppermost layers of the sediment (Hill & Elmgren 1987). An additional study by Byrén et al. (2006) also indicates that adult M. affinis modifies feeding depth depending on food quality, since M. affinis depend mostly on fresh phytodetritus for food. The amphipods
may affect the composition of the benthic fauna through high consumption of sedimented phytoplankton (Lehtonen & Andersin 1998) and bioturbation (Ólafsson & Elmgren 1991). They are also being preyed upon by several demersal fishes and benthic invertebrate species (Hüssy et al. 1997, Hill & Elmgren 1992). M. affinis has been found to be very sensitive to hypoxia (Modig & Ólafsson 1998), and is also known to be susceptible to pollution (Cederwall et al. 2011).
The main factor regulating M. affinis’ swimming activity is light, i.e. they are swimming more extensively when light conditions are weak. The amphipods also possess an innate circadian
5
rhythm which influences their swimming behavior (Donner & Lindström 1980). Donner et al. (1987) also reported field observations of increased swimming by M. affinis as feeding conditions deteriorated in late summer, suggesting that food shortage can cause emigration from the sediment. Johansson (1997) concluded that M. affinis swims more actively at lower oxygen levels, showing an oxygen-seeking behavior.
Lindström & Fortelius (2001) were able to demonstrate that M. affinis swimming was reduced by increasing temperatures in laboratory experiments. The amphipods were found to be most active at 3o C, and least active at 18o C. The authors also found that swimming activity was highest at about one hour after light-off and lowest about one hour before the predicted time of light-on. The percentage of the population active depended on population density. As temperature was increased, leading to fewer active animals, the activity decrease was smaller in the higher population density, abundances corresponding to 16000 and 1600 animals per m2, respectively. The number of animals per sediment surface area increased the probability of nocturnal activity in M. affinis. This mechanism may, according to the authors, be an expression of physical disturbance or crowding.
Normally the M. affinis populations show cyclic fluctuations covering 6-7 years (Eriksson Wiklund et al. 2008), but as mentioned above, the populations in the Baltic Sea and the Bothnian Sea have declined drastically in recent years, starting in 1999 (Albertsson & Cederwall 2008, Cederwall et al. 2011, Karlsson & Leonardsson 2005). According to Byrén (2005), normal densities regarding M. affinis in the Askö area now are approximately 1600 individuals per m2, which is approximately 1/10 of the abundance during the 1970’s (Eriksson Wiklund et al. 2008). Several different theories exist about the cause, or causes, of the decline. One explanation suggests that the decline of the M. affinis population in the Bothnian Sea was due to abundant precipitation before and after the year 2000. The precipitation caused increased run-off from terrestrial areas, resulting in large quantities of organic matter being brought out to the sea and causing inferior light conditions. The inferior light conditions might in turn have caused a deteriorated primary production and growth of algae, leaving the amphipods to famine (Bernes 2005). Another possible explanation of food shortage might be the fact that the composition of the phytoplankton spring bloom has altered since the mid 1990’s, from a diatom based spring bloom to a dinoflagellate based spring bloom (Cederwall
et al. 2011).
M. affinis’ main predator in the Gulf of Bothnia, Saduria entomon, has decreased in line with
the amphipods (Leonardsson & Karlsson 2003), which makes predation an unlikely cause of the M. affinis decrease in abundance. Nor anthropogenic substances are likely causes of the decline, since contaminant and biological effect monitoring in the Baltic Sea do not show increasing trends (Bignert & Asplund 2005). An explanation of the decline in the M. affinis population because of lower salinity in the Baltic Sea can also be disqualified, since M. affinis is of limnetic origin (Cederwall et al. 2011). Decreasing trends regarding oxygen levels were also recorded in deep waters in the Bothnian Bay and the Bothnian Sea during the 1990’s (Bjerkebæk-Lindberg & Gorringe 2002), but the oxygen concentrations are still above levels negatively affecting M. affinis (Eriksson Wiklund & Sundelin 2001, 2004).
It is possible that the M. affinis decline in certain areas of the Bothnian Sea is related to an invasion of the polychaete Marenzelleria spp., since the M. affinis populations have decreased while Marenzelleria spp. abundance has increased during the same period (Karlsson & Leonardsson 2004). On the other hand, on some monitoring stations in the Gulf of Bothnia, the increasing abundance of Marenzelleria spp. does not correlate to decreasing abundance of
6
Eriksson Wiklund et al. (2008) suggested that the M. affinis population decline in the Gulf of Bothnia is related to food shortage and malnutrition due to an increased percentage of low quality terrestrial carbon, i.e. rich in humus, from river outflow. As a result, the bacterial food web was enhanced, which has a lower trophic efficiency. They also hypothesized that a secondary effect of malnutrition is an increased susceptibility to other types of stressors such as contaminants and parasites.
The ecology of the polychaete Marenzelleria spp.
The genus Marenzelleria consists of five species, of which three have been found in European waters; Marenzelleria viridis, Marenzelleria neglecta and Marenzelleria arctica. They look very much alike and are distinguished mainly by genetic methods (Magnusson 2008). Since the late 1980’s all three species have been found in the Baltic Sea (Bernes 2005), to where they were probably brought with ballast water (Magnusson 2008). Marenzelleria viridis prefers a salinity of around 10 ‰, and is in the Baltic Sea found along the German border in the south, in the eastern Polish waters and in the Gulf of Riga. The optimal salinity for
Marenzelleria neglecta has been shown to be between 0,5 and 10 ‰, and this species is found
in most of the bottoms of the Baltic Proper. Marenzelleria arctica seems to be the most limnetic of the three species, due to its occurrence in the northern parts of the Baltic Proper and the Bothnian Sea (Magnusson 2008).
Aside from the different preferences for salinity, the three species of Marenzelleria seem to have similar demands on their environment. They live in sandy or muddy sediments in brackish waters, such as estuaries (Magnusson 2008), and construct I- or J-shaped burrows lined with mucus, that extend down to 30 cm or more into the sediment (Essink & Kleef 1988).
The Marenzelleria species don’t seem to have any particular preferences of depth, since populations of Marenzelleria spp. in the Baltic Sea have been found in soft bottoms between 0,5 and 286 meters depth. Compared to other polychaete species, Marenzelleria spp. is tolerant to hypoxia and anoxia (Magnusson 2008). Also in terms of food, Marenzelleria spp. is non-specific. Marenzelleria spp. is a facultative suspension and deposit feeder (Dauer et al. 1981). Foraging usually takes place on the sediment surface, where it ingests sedimented dead organic matter and benthic diatoms (Magnusson 2008). Karlson et al. (2011) found that
Marenzelleria spp. incorporated and buried bloom material at rates similar to the native
species M. affinis, P. femorata and M. balthica.
The introduction of Marenzelleria spp. has led to a total biomass increase in the sediments in some regions (Magnusson 2008), and considered normal densities for Marenzelleria spp. in the Askö area are 200-1100 individuals per m2 (Eriksson Wiklund et al. 2009). The polychaetes have been found to be a valuable food source for Macoma balthica (Kotta et al. 2001), and it is also possible that Marenzelleria spp. is of nutritious value for fish, though these possible relationships are poorly studied (Magnusson 2008). However, Neideman et al. (2003) claim that they have observed pelagic feeding fishes with stomachs filled with
Marenzelleria spp.
Marenzelleria viridis burrow ventilation generates water currents that irrigate the interstices
of the sediments surrounding the burrow walls, and is shown to perform two different types of ventilation. Muscular pumping extrudes anoxic water from the burrow, and cilia pumping retracts oxygen-rich water into the burrow. This means that significantly large amounts of water from deeper sediments may percolate upwards to the sediment surface. The upwelling water is rich in organic compounds and nutrients, and may affect redox conditions, organic
7
matter degradation, benthic recruitment and primary production (Quintana et al. 2011). It is possible that this mechanism is valid for all three species of Marenzelleria. The sediment remobilized through Marenzelleria bioturbation may also contain historically buried contaminants (Hedman 2008), and thus reintroduce pollutants in the Baltic Sea food web (Karlsson & Leonardsson 2005).
A three week laboratory experiment by Karlson et al. (2011) showed that Marenzelleria spp. grew faster than both M. affinis, P. femorata and Macoma balthica, both in treatments with a simulated spring bloom and in control aquaria without bloom input. According to the authors, this indicated efficient utilization of old organic matter, a trait which has likely been important for the successful colonization and establishment of Marenzelleria populations in the Baltic Sea.
Competition between M. affinis and Marenzelleria spp. in the Baltic Sea
The indigenous amphipod M. affinis and the invasive polychaete Marenzelleria spp. may compete for food as well as space in the benthic community (Neideman et al. 2003). Kotta & Ólafsson (2003) showed that M. affinis, in the presence of Marenzelleria spp., grows less well than it does otherwise. Kotta et al. (2001) showed that the growth of M. affinis was density dependent in the absence of Marenzelleria spp. but not so in the presence of the polychaetes. At densities of 2000 individuals per m2 and with food addition the amphipods grew faster in the absence of the polychaetes than in their presence. Sundelin et al. (2005) suggested competition between M. affinis and Marenzelleria spp., since M. affinis’ fecundity decreased when coexisting with the polychaete. The fact that the M. affinis population in the Baltic Sea has declined might thus be a consequence of the establishment of Marenzelleria spp.
Neideman et al. (2003) tested the hypothesis that, if given the choice, the amphipods would avoid burrowing in sediment with high polychaete abundances. The amphipods burrowed in significantly lower numbers in patches with high polychaete abundance compared to those with lower abundance during a four-day experiment. However, amphipod swimming did not increase as a reaction to higher worm densities during two 24-hour experiments.
Eriksson Wiklund et al. (2008) could not show any significant results in laboratory experiments for higher amphipod mortality in presence of Marenzelleria compared to setups without polychaetes, even though the experiment was carried out for four months. However, three times during the experiment period the individuals were provided with fresh phytodetritus. This might have caused a situation which may have prevented competition. Nor Kotta & Ólafsson (2003) or Karlson et al. (2011) were able to demonstrate any evidence of higher M. affinis mortality in the presence of Marenzelleria spp. Kotta et al. (2001) reported that the survival of M. affinis was significantly affected by food addition, though the survival was not affected by amphipod density or the presence of Marenzelleria spp.
According to Lindström & Fortelius (2001), high worm population densities will decrease the amount of food available in the surface layer, which might induce increased amphipod swimming activity in search for spots of higher nutritional value.
Purpose of this study
In this study, I attempted to investigate if Marenzelleria spp. actually has an impact on M.
affinis mortality, and if so, which factor is the most important: is the competition between the
8
affinis’ swimming behavior at varying densities and in presence of different sizes of Marenzelleria spp. Do crowding and/or competition change the M. affinis swimming
behavior?
Question formulations
1. Is M. affinis significantly more susceptible to competition from high densities of
Marenzelleria spp. than from low densities?
2. Is M. affinis significantly more susceptible to competition from large specimens of
Marenzelleria spp. than from small specimens?
3. Does M. affinis’ swimming behavior alter in presence of high densities of
Marenzelleria spp., compared to low densities and the control series?
4. Does M. affinis’ swimming behavior alter in presence of large specimens of
Marenzelleria spp., compared to small specimens and the control series?
5. Does M. affinis swimming behavior alter over time within series?
Premises
1.1. M. affinis is significantly more susceptible to competition from high densities of Marenzelleria spp. than from low densities.
1.2. M. affinis is significantly more susceptible to competition from large specimens of Marenzelleria spp. than from small specimens.
1.3. M. affinis’ swimming behavior alters in presence of high densities of Marenzelleria spp., compared to low densities and the control series.
1.4. M. affinis’ swimming behavior alters in presence of large specimens of Marenzelleria spp., compared to small specimens and the control series.
1.5. M. affinis’ swimming behavior alters over time within series with large specimens
and high abundances of Marenzelleria spp.
Predictions
1.1.1. Mortality of M. affinis in aquaria with high densities of Marenzelleria spp. will be significantly higher than in presence of low densities.
1.2.1. Mortality of M. affinis in aquaria with large individuals of Marenzelleria spp. will be significantly higher than in presence of small individuals.
1.3.1. M. affinis’ swimming increases at high densities of Marenzelleria spp., compared to low densities and the control series.
1.3.2. M. affinis’ swimming does not increase at low densities of Marenzelleria spp., compared to the control series.
1.4.1. M. affinis’ swimming increases in presence of large specimens of Marenzelleria
9
1.4.2. M. affinis’ swimming does not increase in presence of small specimens of
Marenzelleria spp., compared to the control series.
1.5.1 M. affinis’ swimming frequency increases within series towards the end of the experiment in series with large specimens and high abundances of Marenzelleria
spp.
MATERIAL AND METHODS
Marenzelleria spp., M. affinis and soft bottom sediment were collected in the Askö area (N
58° 50.822', E 17° 32.473) on the 11th of April 2012, in the beginning of the spring bloom period. The individuals and the sediment used in the experiments were collected by dredging, using a benthic sled, at depths of approximately 30 meters. The animals were then transported to the Ar Research Station on Gotland. They were kept in cooler bags with sediment from the sampling site at approximately 15o C until collected for experiments. The fine muddy sediment used was the natural sediment of both the amphipods and the polychaetes.
The collected sediment was sieved without water in a sieve with a mesh size of 1 millimeter to remove all macrofauna. Three test samples from each sediment container were examined with a stereo-microscope with tenfold magnification to ensure that no Marenzelleria spp. or
M. affinis contaminated the sieved sediment. Neither Marenzelleria spp. nor M. affinis
occurred in the test samples, but a few nematodes were found. The sediment was estimated to contain low or no levels of hydrogen sulphide, as judged from its smell and colour.
M. affinis specimens and Marenzelleria spp. specimens were picked with a pipette, sorted and
counted, and large and small Marenzelleria spp. specimens were separated from each other. As considered small were specimens of 1 cm or less in length, and large specimens were of more than 1 cm in length. The largest Marenzelleria individuals were around 4 cm in length. The M. affinis specimens were also examined to make sure that they indeed belonged to the species by verifying that they had black eyes. The animals were then kept without food for 24 hours.
30 aquaria with a bottom area of 269 cm2 each were filled up with sieved sediment to a depth of 5 cm. They were then filled with 3 liters each of brackish water, with a salinity of 7 ‰. The aquaria were placed randomly in a thermo-constancy room with a temperature varying between 7,1-7,4o C.
Experimental design
The experiment setup consisted of 5 series, each with 6 replicates. Normal densities for M.
affinis, i.e. 1600 individuals per m2 (Byrén 2005), were found to correspond to 45 individuals per aquarium, and normal densities of Marenzelleria spp., i.e. 1100 individuals per m2 (Eriksson Wiklund et al. 2009), were found to correspond to 30 individuals per aquarium. For
Marenzelleria, considered as high densities were the double amount of normal abundances,
i.e. 60 individuals per aquarium. Considered as low densities were half the amount of normal abundances, i.e. 15 polychaetes per aquarium. The series with high and low densities of
10
Control series: 6 aquaria containing 45 specimens of M. affinis each
Series with high densities of Marenzelleria spp.: 6 aquaria containing 45 specimens of M.
affinis and 60 specimens of Marenzelleria spp.
Series with low densities of Marenzelleria spp.: 6 aquaria containing 45 specimens of M.
affinis and 15 specimens of Marenzelleria spp.
Series with large specimens of Marenzelleria spp.: 6 aquaria containing 45 specimens of M.
affinis and 30 specimens of Marenzelleria spp.
Series with small specimens of Marenzelleria spp.: 6 aquaria containing 45 specimens of M.
affinis and 30 specimens of Marenzelleria spp.
A majority of the aquaria were provided with oxygen pumps, but due to lack of equipment, seven aquaria were left without pumps in the beginning of the experiment. These aquaria were instead oxygenated at a 12-hour interval, and were provided with oxygen pumps 36 hours after the experiment started. The light in the thermo-constancy room was switched on at 8 am, and switched off at 7 pm, to ensure that the amphipods had time to acclimatize to night conditions before being monitored.
The experiments were performed between April 15th and May 4th 2012. Swimming behavior, i.e. counting the number of visible amphipods swimming, was monitored every 12 hours, at 8 am and 8 pm. This procedure was performed every day except the morning of April 19th and the morning of May 1st, due to a power failure. At 8 pm, the light was switched on temporarily to enable monitoring. When swimming behavior was surveyed, dead amphipods were also removed and counted. The controls took approximately 30-45 seconds per aquarium. On the first night of the experiment, an extra monitoring round was performed at 9 pm to make sure that 1 hour was enough for the amphipods to acclimatize to night conditions. The measured swimming activity at 8 pm and 9 pm indicated that one hour of darkness was enough, since swimming activity was higher at 8 pm than at 9 pm. If the situation had been reversed, with higher swimming activity at 9 pm than at 8 pm, that would have indicated that one hour was not sufficient because of the increasing levels of swimming.
The experiment was terminated after 20 days, on May 4th, with a total of 37 observations of swimming behavior for each replicate. At the end of the experiment, the sediment in the aquaria was sieved carefully with water in a sieve with 1 mm mesh size to collect surviving amphipods and polychaetes, and sort out dead animals. The sieve was rinsed with water between each sieving, to ensure that no amphipods or polychaetes contaminated the sediment of other aquaria. Both amphipods and polychaetes were counted and added to the number of animals that had died during the experiment. It was then possible to confirm how many amphipods that had died in each aquarium.
Data analysis
The average swimming frequencies of each replicate were calculated for the different treatments: for the entire experimental period (observations 1-37), for the first, second and third week of the experiment (observations 1-12, 13-24 and 25-37, respectively), and for the first and second half of the experiment (observations 1-18 and 19-37, respectively).
11
Kruskal-Wallis tests (Fowler et al. 1998) were performed to examine whether there was a significant difference in swimming behavior between series, with the intention of performing Mann-Whitney U-tests (Fowler et al. 1998) if significant differences were found. In the different treatments, the average number of swimming amphipods was calculated for each replicate. Thereafter, the means for different time periods were calculated by using the means of the replicates. The tests were performed in sets of control series combined with series with high and low densities of Marenzelleria spp. individuals, and in sets of control series combined with series with large and small Marenzelleria spp. specimens. The null hypothesis was that there would be no difference in swimming behavior between series, or between different time periods.
To examine possible differences in swimming behavior over time within series, Wilcoxon tests for matched pairs (Fowler et al. 1998) were performed. In this case, the means of the replicates of each series for the first and second half of the experimental period were tested against each other. The null hypothesis was that there would be no difference in swimming within series between the first and second half of the experimental period.
R*c contingency table tests (Fowler et al. 1998) were used when testing mortality between series. The mortality was calculated by adding the number of surviving amphipods of each replicate, and by adding the numbers of dead amphipods of each replicate. The numbers of surviving and dead amphipods were then used in the r*c contingency tables when testing the replicates of each treatment against each other, in order to detect diverging mortality within treatments. The total number of surviving amphipods for each series was then used when performing tests between all treatments. Tests were also performed for sets of control series combined with series with high and low densities of Marenzelleria spp. individuals, and in sets of control series combined with series with large and small Marenzelleria spp. specimens. The tests were also repeated when excluding replicate number 5 in the series with large
Marenzelleria spp., due to extremely high mortality. The null hypothesis was that there would
12
RESULTS
Differences in swimming behavior between treatments
The means indicated that there were differences in swimming behavior between the control series and the series with low densities of Marenzelleria spp., since those were the two highest means, respectively, compared to the series with high densities of Marenzelleria spp. and the series with large and small Marenzelleria spp. specimens (Table 1).
Table 1. Mean ± standard error of swimming amphipods (N=45) in the different treatments (N=5) and during different time periods.
Observations 1-37 1-12 13-24 25-37 1-18 19-37
Control series 1,9±0,65 1,81±0,4 1,83±0,69 2,08±0,94 1,9±0,5 1,92±0,83
High densities of Marenzelleria
spp. 1,49±0,22 2,31±0,3 1,11±0,27 1,08±0,48 2,51±0,37 0,98±0,38
Low densities of Marenzelleria
spp. 2,02±0,43 2,36±0,42 1,7±0,33 2,04±0,68 2,05±0,38 1,99±0,56
Large Marenzelleria spp.
specimens 1,09±0,21 1,81±0,25 0,94±0,2 0,58±0,33 1,65±0,27 0,57±0,27
Small Marenzelleria spp.
specimens 1,41±0,29 1,71±0,36 1,54±0,44 1,03±0,18 1,72±0,38 1,12±0,23
Kruskal-Wallis tests were then performed in order to detect differences in swimming behavior between treatments. No statistically significant differences were found regarding swimming behavior (Table 2). The null hypothesis could therefore not be rejected.
Table 2. Results of Kruskal-Wallis tests for differences in swimming behavior between treatments.
Observations df x2crit 0,05 K p-value
1
Controls, high and low densities of Marenzelleria
spp. 1-37 2 5,99 0,85 >0,05
2 Controls, large and small Marenzelleria spp. 1-37 2 5,99 0,59 >0,05
3
Controls, high and low densities of Marenzelleria
spp. 1-12 2 5,99 0,50 >0,05
4 Controls, large and small Marenzelleria spp. 1-12 2 5,99 0,02 >0,05
5
Controls, high and low densities of Marenzelleria
spp. 13-24 2 5,99 1,22 >0,05
6 Controls, large and small Marenzelleria spp. 13-24 2 5,99 0,83 >0,05
7
Controls, high and low densities of Marenzelleria
spp. 25-37 2 5,99 1,63 >0,05
8 Controls, large and small Marenzelleria spp. 25-37 2 5,99 3,69 >0,05
9
Controls, high and low densities of Marenzelleria
spp. 1-18 2 5,99 0,55 >0,05
10 Controls, large and small Marenzelleria spp. 1-18 2 5,99 0,09 >0,05
11
Controls, high and low densities of Marenzelleria
spp. 19-37 2 5,99 2,37 >0,05
13
Differences in swimming behavior over time within treatments
The average number of swimming amphipods in the different treatments was calculated for the first and second half of the experimental period, to assess whether or not swimming behavior changed over time within treatments. The calculated means indicated that a decrease in swimming frequency occurred towards the end of the experiment in the series with high
Marenzelleria spp. densities and large and small Marenzelleria spp. specimens (Table 3). Table 3. Mean ± standard error of swimming amphipods (N=45) in the different treatments (N=5) during the first and second half of the experiment.
Observations 1-18 19-37
Control series 1,90 ± 0,50 1,92 ± 0,83
High densities of Marenzelleria spp. 2,51 ± 0,37 0,98 ± 0,38
Low densities of Marenzelleria spp. 2,05 ± 0,38 1,99 ± 0,56
Large Marenzelleria spp. specimens 1,65 ± 0,27 0,57 ± 0,27
Small Marenzelleria spp. specimens 1,72 ± 0,38 1,12 ± 0,23
Wilcoxon tests for matched pairs were performed in order to detect any differences in swimming behavior within treatments during the experimental period (Table 4). Statistical significance at the 0,05 level was found in the series with large Marenzelleria spp. specimens (N=6, T=0, Tcrit 0,05=0), showing that there was a significantly higher swimming frequency during the first half than during the second half of the experimental period (Table 4). This means that the null hypothesis could be rejected for the series with large Marenzelleria specimens.
Table 4. Wilcoxon test for matched pairs, comparing swimming frequencies of Monoporeia affinis between the first and second half of the experimental period.
Observations Tcrit 0,05 N T p-value
1 Control series 1-18 vs 19-37 0 6 6 >0,05
2 High densities of Marenzelleria spp. 1-18 vs 19-37 0 6 2 >0,05
3 Low densities of Marenzelleria spp. 1-18 vs 19-37 0 5 8 >0,05
4 Large Marenzelleria spp. 1-18 vs 19-37 0 6 0 <0,05
5 Small Marenzelleria spp. 1-18 vs 19-37 0 6 2 >0,05
Mortality
The mortality of Monoporeia affinis was low in almost all aquaria during the experimental period. In the control series, the number of surviving amphipods of the originally 45 specimens was 43, 41, 43, 44, 45 and 45 in the different replicates. In the series with high densities of Marenzelleria spp., the number of surviving amphipods in the different replicates was 40, 43, 43, 44, 37 and 41, respectively. The number of amphipods surviving in the treatment with low densities of Marenzelleria spp. was 44, 41, 45, 44, 43 and 43. In the series with large Marenzelleria spp. specimens, the number of surviving amphipods was 41, 42, 42, 43, 2 and 43. In the series with small Marenzelleria spp. specimens, the number of surviving amphipods was 42, 44, 41, 43, 44 and 42, respectively.
14
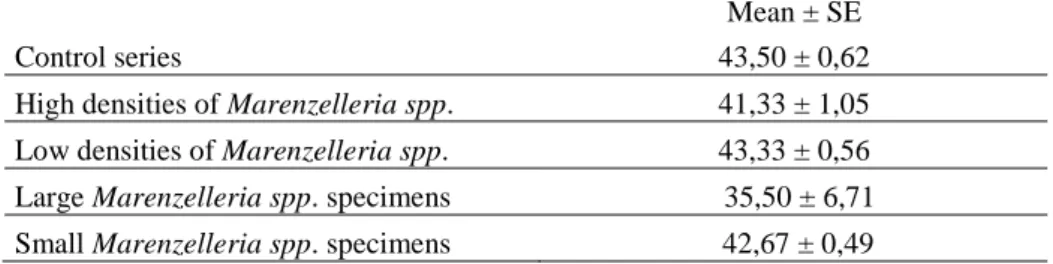
The calculated means indicated that there was a tendency towards higher mortality in the series with high densities and large specimens of Marenzelleria spp., even though the number of surviving amphipods was still high (Table 5).
Table 5. Mean number ± standard error of surviving amphipods in each treatment. Mean ± SE
Control series 43,50 ± 0,62
High densities of Marenzelleria spp. 41,33 ± 1,05
Low densities of Marenzelleria spp. 43,33 ± 0,56
Large Marenzelleria spp. specimens 35,50 ± 6,71
Small Marenzelleria spp. specimens 42,67 ± 0,49
R*c contingency table tests were performed between all replicates of each series, in order to detect differences in mortality between replicates within treatments. No differences were found (p>0,05) except in the series with large Marenzelleria spp., where the result was highly significant (p<0,01).
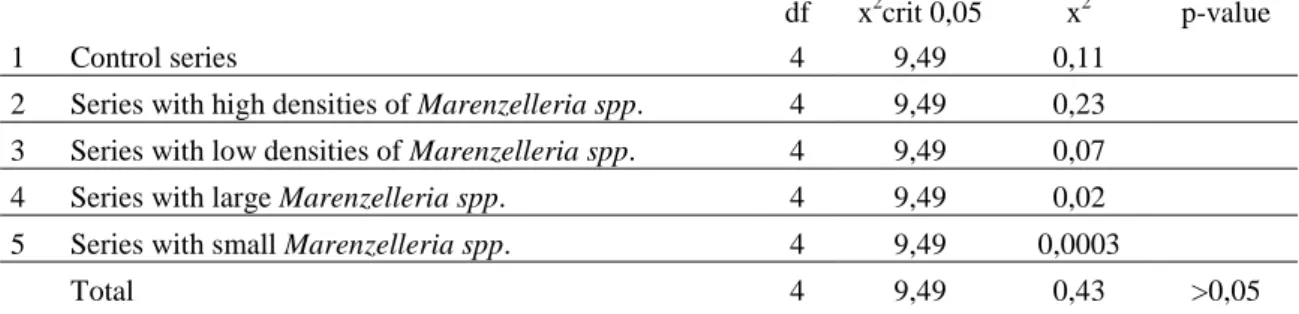
When testing for differences in mortality between all treatments, using a r*c contingency table, the result was shown to be highly significant (p<0,01) (Table 6). When reviewing the result of each treatment, it could be concluded that the series with large Marenzelleria spp. differed from the four other treatments. The null hypothesis could therefore be rejected.
Table 6. Results of r*c contingency table tests for differences in mortality between all treatments, showing the result of the respective individual components and the overall result.
df x2crit 0,05 x2 p-value
1 Control series 4 9,49 0,73
2 Series with high densities of Marenzelleria spp. 4 9,49 0,0006
3 Series with low densities of Marenzelleria spp. 4 9,49 0,62
4 Series with large Marenzelleria spp. 4 9,49 12,03
5 Series with small Marenzelleria spp. 4 9,49 0,73
Total 4 9,49 14,12 <0,01
When testing the control series against the treatments with high and low densities of
Marenzelleria spp., the result was not significant (p>0,05). The r*c contingency table test
result for control series against treatments with large and small Marenzelleria spp. was shown to be highly significant (p<0,01) (Table 7), and the null hypothesis could be rejected.
Table 7. Results of r*c contingency table tests for differences in mortality for two different sets of treatments.
df x2crit 0,05 x2 p-value
1 Controls, high and low densities of Marenzelleria spp. 2 5,99 1,35 >0,05
2 Controls, large and small Marenzelleria spp. 2 5,99 13,48 <0,01
When excluding replicate number 5 in the series with large Marenzelleria spp., where amphipod survival was very low, a r*c contingency table test showed no difference in mortality between all treatments (p>0,05) (Table 8). The null hypothesis could therefore not be rejected.
15
Table 8. Results of r*c contingency table tests for differences in mortality between all treatments, showing the result of the respective individual components and the overall result, excluding replicate number 5 in the series with large Marenzelleria spp.
df x2crit 0,05 x2 p-value
1 Control series 4 9,49 0,11
2 Series with high densities of Marenzelleria spp. 4 9,49 0,23
3 Series with low densities of Marenzelleria spp. 4 9,49 0,07
4 Series with large Marenzelleria spp. 4 9,49 0,02
5 Series with small Marenzelleria spp. 4 9,49 0,0003
Total 4 9,49 0,43 >0,05
When excluding replicate number 5 in the series with large Marenzelleria spp. and testing the control series against the treatments with high and low densities of Marenzelleria spp., and the control series against the series with large and small Marenzelleria spp., the results were shown to be non-significant (p>0,05) (Table 9).
Table 9. Results of r*c contingency table tests for differences in mortality between two different sets of treatments, excluding replicate number 5 in the series with large Marenzelleria spp.
df x2crit 0,05 x2 p-value
1 Controls, high and low densities of Marenzelleria spp. 2 5,99 0,41 >0,05
2 Controls, large and small Marenzelleria spp. 2 5,99 0,13 >0,05
DISCUSSION
Differences in swimming behavior between treatments
The amphipod swimming behavior was expected to differ significantly between control series and series with high abundances and large Marenzelleria spp. specimens. This assumption was based on the fact that M. affinis swims more frequently at higher abundances, due to increased levels of social facilitation or other expressions of physical disturbance and crowding (Lindström & Fortelius 2001). If the mechanism is valid also in presence of polychaetes, the swimming frequency should elevate at least in the series with high abundances of Marenzelleria spp. One plausible outcome of interactions between the two species is that the amphipod, the more mobile species, would avoid areas where the more sessile polychaetes are present in high numbers. Chemical substances involved in communication between interacting species are believed to be an important feature in interference competition and predation among benthic invertebrates (Neideman et al. 2003), and may cause amphipod migration from the sediment in presence of Marenzelleria spp. Also food shortage, which was supposed to emerge from competition with the polychaetes, can cause migration from the sediment (Donner et al. 1987). The only factor which was supposed to change in the aquaria during this experiment was food levels, which in turn was supposed to affect M. affinis swimming behavior, so that they would be swimming more extensively in aquaria where food levels were supposed to decline more rapidly.
There are several possible explanations for the fact that no significant differences were found when testing swimming behavior between series, and for the fact that there was a tendency towards decreasing swimming behavior over time in some treatments.
16
Food shortage may not have played a particularly important role in the results of this experiment, since the amphipods in nature starve during spring (Lehtonen 1994). Competition might not have occurred due to this mechanism, and the amphipods would in that case have no reason to swim more frequently in search for food. The amphipods would therefore have no particular reason to forage to a greater extent in the series with high densities and large
Marenzelleria spp. specimens. On the other hand, it has been stated that the amphipods feed on old organic matter if their preferred food, fresh diatoms, is not available (B. Widbom, pers. comm.).
The non-significant results between series may also be a consequence of performing the experiments during spring. According to Lindström & Lindström (1980), the amphipods are least active during this time of the year. It is therefore worth noticing that only a low number of amphipods were swimming during each observation episode in this experiment, according to the calculated means.
Temperature affects amphipod swimming behavior (Lindström & Fortelius 2001), and may have had an effect on the results. It is established that swimming is most frequent at temperatures around 3° C (Lindström & Fortelius 2001), but the temperature in the thermo-constancy room was between 7,1-7,4° C. If the higher temperatures during the experiment lowered the levels of amphipod swimming, this may have reduced the chances of getting significant results. A further important factor may have been that a few aquaria from each series were placed in the middle of the thermo-constancy room, beneath the fan where the coldest air was blown out. This could, theoretically, have elevated the levels of swimming in these aquaria compared to the aquaria placed along the walls. On the other hand, this elevation would have been equally high in all series and should therefore not have affected the results in any particular direction.
A tendency towards a difference in swimming behavior between series could be seen in the experiments based on observations 19-37, both for treatments with controls and large and small Marenzelleria spp. specimens, and for treatments with controls and high and low densities of Marenzelleria spp. The K-values for those treatments were closer to the level of significance, and by looking at the means, lower swimming frequencies could be detected towards the end of the experiment in the series with high densities and large and small
Marenzelleria spp. specimens. At least in treatments with high densities and large Marenzelleria spp. specimens, decreasing swimming behavior can be partially explained by
slightly higher mortality in those series, since dead amphipods do not contribute to swimming frequencies.
Combined with the mortality, the non-significant results in the treatments with high densities and large Marenzelleria spp. specimens may be due to the fact that the polychaetes in those aquaria may have oxygenated the sediment more effectively than in treatments with smaller or fewer polychaetes. This assumption is based on the argument that all amphipod swimming in all series is a reaction to low oxygen levels, which is rather far-fetched.
Two factors possibly affecting the results of the swimming experiment are the length of the experiment and the number of replicates. It is possible that the results would have been significant if the experimental period was prolonged, or performed with a larger number of replicates.
17
Differences in swimming behavior over time within treatments
M. affinis’ swimming behavior was predicted to increase significantly over time in series with
high abundances and large Marenzelleria spp. specimens. This assumption was based on the notion that food levels would be lower in those aquaria because of the polychaetes ingesting organic material, with increased need for foraging and therefore increased amphipod swimming as a result.
The prediction of increased swimming over time in series with high abundances and large
Marenzelleria spp. specimens was not met in these experiments. The situation was reversed,
and lower swimming frequencies towards the end of the experiment were detected; non-significant in the series with high densities and small specimens of Marenzelleria spp., but significant in the series with large Marenzelleria spp. specimens.
There are several possible explanations for these results. One of them might be the fact mentioned earlier, that the amphipods naturally starve during spring (Lehtonen 1994) and would have no reason to swim during the experiment. It is also possible that the experimental period was too short for serious competition to occur. A contributing explanation for the low amphipod activity is that they normally reach swimming minimum during spring (Lindström & Lindström 1980). In this experiment, only a low number of amphipods were swimming during each observation episode according to the calculated means, which is in line with the results of Lindström & Lindström (1980).
The lower amphipod activity towards the end of the experiment may be due to weakness from food shortage in the aquaria. However, this contradicts previous studies such as Donner et al. (1987), and Lindström & Fortelius (2001), who claim that the amphipods swim more extensively when food is scarce. The explanation might be that the experiment by Donner et
al. (1987) was performed in the field, where the amphipods actually had a chance of finding
spots with higher nutritional value if they swam more extensively when food was scarce. The experiments by Lindström & Fortelius (2001) were performed in the laboratory for 20 days, but in the autumn, when the amphipods should have been well-nourished from the spring bloom and possibly also an autumn bloom. The amphipods may then, as a consequence, have had more energy to forage than the amphipods in this experiment, which was performed in the spring when nutrient levels are naturally low.
The more extensive amphipod swimming in the previous studies may thus be a result of the amphipods being in a healthier condition when the experiment started (Lindström & Fortelius 2001), or an instinct to forage more actively when there are good opportunities to actually find food (Donner et al. 1987). When food is scarce during a prolonged period it is likely that the activity decreases, which was seen in this experiment. This indicates that the experiment was long enough to make the amphipods starve and become stagnant in the aquaria with high densities and large and small Marenzelleria spp., but was obviously too short to enhance amphipod mortality. Another possibility is that food was not scarce at all, and the decreasing swimming in the aquaria with high densities and large and small Marenzelleria spp. has an alternative explanation that I have not yet found out.
The significant difference in swimming behavior in one series, and a tendency to difference in two series, could partly be a result of higher amphipod mortality in those series, since dead amphipods would not be able to contribute to the swimming statistics. At least this could help explaining the results in the series with high densities and large specimens of Marenzelleria
18
The decreasing swimming activity in the series with high densities and large and small
Marenzelleria spp. specimens may be due to efficient oxygenation in the sediment, performed
by the polychaetes. It is possible that the polychaetes in those aquaria oxygenated the sediment more effectively than in control series or the series with low polychaete density. This assumption, however, is based on the argument that all amphipod swimming is caused by low oxygen levels, which is not very likely.
The result of this experiment may indicate that competition between M. affinis and
Marenzelleria spp. is more density-dependent than size-dependent. This conclusion is based
on the fact that swimming decreased to a larger extent in series with high densities and large and small Marenzelleria spp. specimens, but was unaffected in series with few Marenzelleria
spp. specimens and the control series. However, since a significant difference was found only
in the series with large Marenzelleria spp. specimens, it is not advisable to put too much credence into this conclusion. Further studies are needed to confirm whether the competition between the two species is density-dependent or size-dependent.
Maybe the results would have met the prediction, or been significant in more than one series, if the experimental period was prolonged, or performed with a larger number of replicates.
Mortality
The non-significant results of amphipod mortality in these experiments are not consistent with the predictions, but are well in line with previous studies in the field (Kotta & Ólafsson 2003, Eriksson Wiklund et al. 2008, Karlson et al. 2011). However, the mortality of the amphipods was expected to be significant, since they utilize the same resources as the polychaetes (Neideman et al. 2003). Also the fact that Marenzelleria spp. incorporates bloom material at rates similar to native species, but grows more rapidly than these species (Karlson et al. 2011), was believed to play an important part in the mortality experiments. The organic content of the sediment was probably low, and the sediment was also stirred and sieved, which most probably resulted in a homogenous distribution of organic matter throughout the sediment in the aquaria. This was also supposed to affect amphipod mortality, since the mechanisms of competition were believed to be more powerful when food supply was low. If competition would actually emerge, the chance of it would be greater during the period before the spring bloom, when the amphipods were believed to be more vulnerable because of depleted food supplies.
There are several possible explanations for the unexpected results of the mortality experiments. One of them might be the period during the year when the experiment was performed. The amphipods naturally starve in the spring (Lehtonen 1994), regardless of if the polychaetes are present or not. It is therefore possible that the amphipod mortality was not affected by the presence of the polychaetes, at least not during the time of the year when the experiment was performed.
An interesting observation when the experiment was terminated was the fact that the amphipods were flushed out from the aquaria before the polychaetes, with almost no exceptions. This may indicate that the amphipods, in the presence of Marenzelleria spp., forage primarily in the uppermost layers of the sediment, instead of in the entire sediment. The mechanism of niche separation, i.e. M. affinis feeding primarily in the uppermost layers of the sediment in presence of the competitor P. femorata, is well known (Hill & Elmgren 1987). If this mechanism is applicable on competition with the polychaetes, it may provide an explanation of reduced competition, and ultimately reduced mortality.
19
In the data from the experiments, a tendency towards higher mortality can be seen in the series with high abundances and large Marenzelleria spp. specimens. It may be possible that intraspecific competition in combination with interspecific competition plays a part in the slightly higher amphipod mortality in those series, since the total number of animals differed greatly between series. On the other hand, the amphipods can under natural conditions exist in very high abundances, up to 27600/m2 (Sarvala 1986). This fact makes the intraspecific competition-theory rather implausible.
It is difficult to find a plausible answer to the question why almost all amphipods died in replicate number 5 in the series with large Marenzelleria spp. The conditions for that specific aquarium should have been the same as for the rest of the aquaria, regarding light regime, temperature, salinity, oxygen levels etcetera. When the experiment was terminated, nothing unusual was noted when the sediment was flushed out.
Two factors that might affect the results of the mortality experiment are the length of the experiment and the number of replicates. It is possible that the results would have been significant if the experimental period was prolonged, or performed with a larger number of replicates.
Sources of error
The sieve used for removing macrofauna and unwanted M. affinis and Marenzelleria spp. specimens had a mesh size of 1mm which was too large, and made it possible for polychaetes to slip through the sieve. Three polychaetes were found in the control series, where there should have been none. If polychaetes occurred in larger numbers than they should have in several aquaria, it could have influenced the results. However, it is not possible to determine if this was the case, and since almost no significant results were found, this source of error probably did not contribute to any particularly strange results.
M. affinis feeds primarily on fresh phytodetritus (Byrén et al. 2006), which has not been
provided for the animals during the experiment. The decision of not providing the amphipods with their preferred food may have prevented competition from occurring, if M. affinis rather eats nothing at all than feeds on older phytodetritus or organic material of any other origin. An additional source of error may be the light regime during the experiment. Since the light affects swimming behavior (Donner & Lindström 1980), the light during the experiment should be as similar as possible to natural conditions. Unfortunately, this was not the case during the experiments. The light in the thermo-constancy room came from fluorescent lamps, and was extremely intense compared to the light conditions at 30 meters depth in the Askö area (Elmgren et al. 2001). This may have affected the amphipod swimming behavior and thus influenced the result, and the hours of light-on and light-off may also have played a role in the results.
Conclusions and speculations
Since almost no statistically significant differences could be shown, all predictions should be rejected. However, premise 1.5, “M. affinis’ swimming behavior alters within series with large specimens and high abundances of Marenzelleria spp.”, could not be rejected. The swimming behavior did alter in those series, although not in the predicted direction.
The polychaetes were expected to influence the amphipods both directly and indirectly. The polychaete presence, their chemical and/or tactile signals and their space-demanding tubes would have made a direct impact on M. affinis’ living space. Indirect impact would have been
20
the amphipod reaction to food shortage: a change in swimming behavior and/or enhanced mortality.
The results of these experiments indicate that M. affinis is not significantly susceptible to competition from Marenzelleria spp., neither regarding size nor abundance, since mortality and swimming behavior did not differ significantly between treatments. It also appears that food shortage can be ruled out as a cause of increased swimming behavior, at least in these experiments. This conclusion can be drawn because the concentration of organic material in the sediment should have been low in the beginning of the experiment, and decreasing towards the end of the experiment,while the swimming frequency was constant or decreasing over time within treatments.
It is debatable if the amphipods actually suffered from food shortage during this experiment.
M. affinis has, in the absence of fresh phytodetritus, been shown to feed on old organic
material during spring (B. Widbom, pers. comm.), which was available in the aquaria. The low mortality in the experiment also indicates that food levels were sufficient for the amphipods to survive on, even in the presence of Marenzelleria spp. When the experiment was terminated, the amphipods were kept in jars for 24 hours before they were released. During those 24 hours the amphipods produced visible amounts of feces, which indicates that they had been feeding on old organic material during the experiment.
If possible in the future, it would be interesting to investigate polychaete mortality. Can their mortality be somehow affected by the presence of M. affinis? Unfortunately, some polychaetes were able to slip through the sieve when the experiment was terminated, which made it impossible to determine the mortality of Marenzelleria spp. during the experiment. However, if the polychaete mortality was high during the experiment, the worms would have decomposed, which in turn would have increased the amount of organic matter in the sediment (Kotta et al. 2001). If polychaetes actually died during the experiment, and if the amphipods were starving and in need of nutrients, the decaying polychaetes would in that case have provided an extra nutrient source and possibly reduced amphipod mortality.
From the results of these experiments, it is not advisable to draw the conclusion that
Marenzelleria spp. has played a significant role in the decline of the M. affinis. population.
The experiment indicates that both species can co-exist at normal densities as long as they have access to a sufficient food supply.
However, since Marenzelleria spp. grows fast (Karlson et al. 2011) and affects both M.
affinis’ fecundity (Sundelin et al. 2005) and growth (Kotta & Ólafsson 2003), it may be an
important structuring factor for the benthic community in general and for M. affinis in particular. If Eriksson Wiklund et al. (2008) are correct about the M. affinis population decline, which means that the amphipods are stressed from pollutants and parasites as a result of malnutrition, the amphipods may in turn be extra susceptible to competition from
Marenzelleria spp.
Future experiments
If these experiments are to be repeated sometime in the future, it would be a good idea to measure the organic content of the sediment before and after the experiment. If this procedure is conducted, it would be possible to assess with greater certainty whether or not it is decreasing food amounts that induce altered swimming behavior.
If the experiments are repeated, it should also be arranged for the light regime to correspond with natural conditions.
21
Another idea would be to investigate polychaete mortality when co-existing with M. affinis. Such an experiment could address two question formulations: whether or not the polychaetes are somehow affected by amphipod presence, and if it is possible that the polychaetes actually die and decompose in the presence of the amphipods, and thereby provide M. affinis with an additional food source.
It would also be interesting to investigate if it is true that the amphipods forage primarily in the uppermost layers of the sediment in presence of Marenzelleria spp., which I thought was indicated when the experiment was terminated and the aquaria were rinsed out. The mechanism of niche separation is well worth to study further in the future, and may provide some answers to the questions about competition between M. affinis and Marenzelleria spp.
22
ACKNOWLEDGEMENTS
I want to thank my tutor, Bertil Widbom, for help and guidance during the time I have been working on this experiment. I would also like to thank Jennie Ljungberg, my field assistant, and the Askö laboratory with staff, for helping me to collect the animals, as well as Anders Nissling for the help and support I received during the time on Ar Research Station. Finally, I would like to thank Kerstin Kempe and Karl Kihlberg for help, support and inspiration during my work on this experiment.
REFERENCES
Albertsson, J. & Cederwall, H. 2008. Känslig bottenfauna tappar mark. Havet. http://www.havet.nu/dokument/Havet2008-bottenfaunaostersjon.pdf Published 2008-12-03, downloaded 2012-03-27.
Bax, N., Williamson, A., Aguero, M., Gonzalez, E. & Geeves, W. 2003. Marine invasive alien species: a threat to global biodiversity. Marine Policy 27: 313-323.
Bernes, C. 2005. Förändringar under ytan – Sveriges havsmiljö granskad på djupet. Monitor 19, Naturvårdsverket. ISBN 91-620-1245-2.
Bignert, A. & Asplund, L. 2005. Miljögifter i biota. Umeå Marine Research Center.
http://www.havet.nu/dokument/Bv2004miljogift.pdf Published, downloaded 2012-05-15. Bjerkebæk-Lindberg, A. E & Gorringe, P. 2002. Hydrografi/hydrokemi. In: Wiklund, K.,
Bottniska viken 2002. Umeå Marine Research Center, pp. 7–9.
Byrén, L. 2005. Sammanställning av de marina värdena i Fifångs naturreservat i Stockholms
län. Miljöinformationsenheten, länsstyrelsen i Stockholms län, 1–9.
Byrén, L., Ejdung, G. & Elmgren, R. 2006. Uptake of sedimentary organic matter by the deposit-feeding Baltic amphipods Monoporeia affinis and Pontoporeia femorata. Marine
Ecology Progress Series, 313: 135-143.
Carlton, J. T. 1999. The scale and ecological consequences of biological invasions in the
world’s oceans. In: Sandlund, O.T., Schei, P.J. & Viken, Å., editors. Invasive species and biodiversity management. Dordrecht: Kluwer Academic Publishers; p. 195–212.
Cederwall, H., Albertsson, J., Raymond, C. & Gunnarsson, J. 2011. Långtidsförändringar av bottenfauna i Östersjön. Havet.
http://www.havsmiljoinstitutet.se/digitalAssets/1350/1350770_havet_2011_bottenfauna.pd f Published 2011-11-17, downloaded 2012-03-27.
Dahlgren, T. (2010). Manual för uppföljning av skyddade marina miljöer. Rapport från Naturvårdsverket.
http://www.naturvardsverket.se/upload/04_arbete_med_naturvard/Skydd_och_skotsel_var defull_natur/Uppfoljning/Manual-uppfoljning-hav.pdf. Downloaded 2012-03-01.