EXPERIMENTAL INVESTIGATION ON CO2 ABSROPRTION USING ABSORBENT IN HOLLOW FIBER MEMBRANE CONTACTOR
Yuexia Lv1, Xinhai Yu1*, Shan-Tung Tu1, Jinyue Yan2,3, Erik Dahlquist2
1. School of Mechanical and Power Engineering, East China University of Science and Technology, Shanghai, 200237, China
2. School of Sustainable Development of Society and Technology, Mälardalen University SE-721 23 Västerås, Sweden
3. School of Chemial Science and Engineering, Royal Institute of Technology SE-100 44 Stockholm, Sweden
2. Dept. IST, Mälardalen University, Västerås, SE-721 23 Sweden Email address: yxhh@ecust.edu.cn
ABSTRACT
Carbon dioxide absorption from nitrogen and carbon dioxide mixture has been investigated by experiments using deionized water and low concentration amine solvents including methyldiethanolamine (MDEA) and monoethanolamine (MEA) in polypropylene (PP) hollow fiber membrane. A relatively low solvent concentration of 0.05 mol/l MEA and MDEA was used in the experiment. The dependency of CO2 removal efficiency and mass transfer rate on operating
parameters were studied which include the mixed gas flow rate, the volume concentration of CO2 at
the feed gas inlet, liquid flow rate as well as concentration of absorbents using lower absorbent concentration. It has been found that the CO2 removal efficiency increases with the increase of the
liquid flow rate and solvent concentration, while the CO2 mass transfer rate increases with the
increase of liquid flow rate, CO2 volume fraction in the feed gas, solvent concentration and gas flow
rate. It has been concluded that the absorbent concentration should be compromised between absorption efficiency and the membrane wetting to ensure a stable and efficient removal of CO2
with a long life of the hollow fiber membrane. It was observed by the experiments that a long operation period may lead to partial membrane wetting even when using relatively low solvent concentrations, resulting in a significant decrease of membrane absorption performance, which might be a main drawback for industrial applications with the studied membranes and solvents. Keywords: Carbon dioxide; Hollow fiber membrane absorption; Amine solvents; Gas separation
1. INTRODUCTION
The CO2 emissions are mainly contributed by power generation and transport sectors. CO2 capture and storage (CCS) has been recognized as one of the approaches for mitigating greenhouse gases. Current CO2 capture systems includes post-combustion capture, oxy-fuel combustion capture and pre-combustion capture, which make it possible to capture CO2 from large, centralized sources like power plants and large industries (IPCC, 2005). CO2 capture from flue gases produced by fossil fuels combustion is referred to as post-combustion capture, which is corresponding to the most widely applicable option in terms of industrial sectors and is compatible to a retrofit strategy.
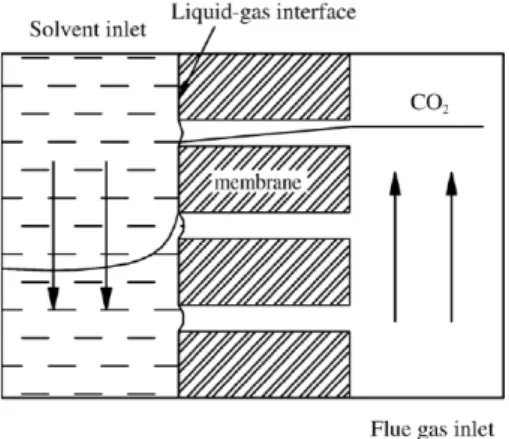
At present, a wide variety of technological options have been used to capture CO2 from flue gas of power plants, such as chemical and physical absorption, solid adsorption, cryogenic distillation, and membrane techniques (Mandal et al., 2006; Moon et al., 2006; Andrea Corti et al., 2004). This paper is focused on the membrane techniques for the CO2 capture. Membrane gas absorption technology is a hybrid technology that combines membrane separation technology and chemical absorption technology with advantages of both membrane contactor and solvent absorption processes. The principle of a membrane contactor is schematically shown in Fig. 1. Instead of depending on the membrane selectivity, the liquid flowing in a hollow fiber contactor provides the selectivity and the unselective membrane only acting as the physical barrier between the liquid and gas phases. CO2 is absorbed in the membrane contactor when the gas stream contacts with the liquid phase flowing on the opposite side of the membrane. Gabelman and Hwang (1999) studied the advantages and disadvantages of a membrane contractor in details. Compared to conventional absorption devices such as packed towers or bubble columns, the membrane contactor has advantages (Falk-Pedersen et al., 2000), such as
• the gas and fluid phase can be manipulated independently avoiding the problems such as flooding, foaming, channeling and entrainment which are commonly encountered in conventional absorption devices;
• compact structure, high specific surface area and less voluminous is less energy-consuming;
• the modularity of membrane modules makes the design simple and easy to be scaled up linearly with predictable performances .
Even though the mass-transfer coefficient of the membrane contactor is inferior to the conventional devices for the flow of gas and liquid are normally laminar where the turbulent flow is power-consumption, the membrane gas absorption due to its large interfacial area, is still considered as one promising alternative to conventional and potential large scale application technology for CO2 recovery and removal (Li et al., 2005).
Fig. 1. Schematic drawing of CO2 absorption in a hollow fiber membrane contactor.
Zhang and Cussler (1985) were the pioneers who proposed the idea of CO2 absorption by sodium hydroxide in a hollow fiber membrane contactor. In recent years, hollow fiber membrane technology has been extensively studied. Various liquid absorbents including pure water and aqueous solutions of NaOH, KOH, monoethanolamine (MEA), diethanolamine
(DEA), 2-Amino-2-mechyl-1-propanol (AMP), N-methyldiethanolamine (MDEA), CORAL and the potassium salt of glycine and taurine were used as absorption liquids in polyethylene (PE) or polypropylene (PP) or polytetrafluoroethylene (Teflon) microporous hydrophobic hollow fiber membrane contactors, in which the MDEA and MEA aqueous solutions in PP hollow fiber membrane contactor are the most widely used for CO2 absorption (Kumar et al., 2002; Kim et al., 2000; Mandal et al., 2001). Compared with other fibers, PP fibers are less expensive and commercially available in a wider size range. Wang et al. (2004) performed a theoretical simulation to study CO2 absorption using three typical alkanolamine solutions of AMP, DEA and MDEA in a hollow fiber membrane contactor. The effects of operating parameters, membrane configuration, module structures and different solvents on absorption flux and removal efficiency were investigated. Lu et al., (2007) studied the effects of activators 2-Amino-2-mechyl-1-propanol (AMP) and piperazine (PZ) on mass-transfer enhancement using MDEA alkanolamine solutions in a hollow fiber contactor and concluded that the mass transfer fluxes of the activated MDEA solutions are significantly higher than that of MDEA solution and effects of operation conditions on mass-transfer enhancement are limited. Theoretical simulations and corresponding experiments were carried out to describe the CO2 absorption by distilled water and aqueous diethanolamine (DEA) solutions for better understanding of CO2 absorption in a hollow fiber membrane contactor (Zhang et al., 2006). A theoretical model was developed based on CO2 absorption simulation by water and DEA under the wetted and the non-wetted operation modes to study the influence of membrane wetting on CO2 capture in microporous hollow fiber membrane contactors (Zhang et al., 2007). Matsumiya et al. (2005) developed a novel facilitated transport membrane system where the feed gas and aqueous diethanolamine solutions were supplied to the lumen side of the hollow fiber ultrafiltration membrane module with an upward flow. In addition, they evaluated the energy consumption and compared with conventional separation processes. Liu et al. (2005) examined the mass transfer performances using coiled hollow fiber membrane modules. Their results showed that coiled hollow fiber modules can remarkably enhance the mass transfer compared with conventional straight module. Most researches were limited to atmospheric pressure applications using aqueous absorption solvents, so CO2 absorption in propylene carbonate at elevated pressure using hollow fiber membrane contactor was investigated by Dindore et al. (2004). The results showed that the decrease in the binary gas phase diffusivity and hence the membrane mass transfer coefficient due to gas pressures increase does not have a significant impact on the overall mass transfer coefficient.
As mentioned above, a variety of experiments and simulations have been carried out to study the membrane absorption performance using different kind of solvents in the hollow fiber membrane contactor. The solvent concentration used by most researches was varied from 1mol/l to 3mol/l. However, it was reported that the surface tension of most absorbent solutions significantly decreases with increasing solution concentration (Vázquez et al., 1997; Rinker et al., 1994), which resulted in PP membrane wetting according to the Laplace-Young equation (Franken et al., 1987), thus reducing the membrane performance. According to Laplace-Young equation, lower solvent concentration can reduce membrane wetting and prolong the membrane service life.
In this paper, the effects of operating parameters on membrane absorption performance using solutions of relative low concentration were investigated in the pilot-scale hollow fiber
membrane module. The wetting phenomenon of PP membrane, which is often neglected in reported studies, was also observed in prolonged operation. In addition, the optimal operating parameters were obtained for the specified membrane module.
2. EXPERIMENTAL
The experiments were performed to obtain the operating parameters effect on the absorption using deionized water and MEA and MDEA aqueous solutions of low concentration as the absorbent in a PP hollow fiber membrane contactor.
Table 1. Specifications of the hollow fiber membrane module Parameter Value
Module outer diameter (mm) 50
Module inner diameter (mm) 42
Module length (mm) 360
Fiber inner diameter (μm) 380
Fiber outer diameter (μm) 500
Fiber length (mm) 300
Number of fibers 3200 Fiber porosity 0.65 Pore size (μm) 0.16 Contact area (m2) 1.5
A sketch of CO2 absorption in hollow membrane contactor is shown in Fig. 2. And the photo of experimental setup is shown in Fig. 3. The HDMF-100-1 type microporous polypropylene hollow fiber membrane module (Tianjin Blue Cross Membrane Technology Co., Ltd.) was used as the contactor in this study. The characteristics of the membrane module are listed in Table 1. A gas mixture containing CO2, N2 with various volume ratios was selected as the feed gas. Deionized water, aqueous solutions of Monoethanolamine (MEA), methyldiethanolamine (MDEA) were chosen as absorption liquids. The 99.5% grade MEA and MDEA (Shanghai Bangcheng Chemical Co., Ltd.) were dissolved in deionized water to make aqueous solutions of 0.05mol/l-0.25mol/l. The gas phase and liquid phase flowed countercurrent through the module (the gas passed through the shell side, and the absorbent flowed countercurrent through the lumen side of the hollow fibers).
In the experiment, the feed gas was introduced into the system from compressed gas cylinders and the flow rate was adjusted by Mass Flow Controller (Sevenstar Electronics Co., Ltd. MFC D07) which can precisely control the gas flow rate. Then the gas was introduced into the static mixer where the mixtures can be mixed uniformly. Pressure gauges at the inlet and outlet of the membrane module measure the gas pressures, and outlet gas flow rate was measured by a mass flow meter (Sevenstar Electronics Co., Ltd.). The inlet and outlet gas compositions were analyzed on-line by a 9790 Ⅲ gas chromatograph (FuLi Analytical Instrument Co., Ltd.) using a thermal conductivity detector (TCD). A stainless steel peristaltic pump (Tian Li Liquid Industrial Equipment Factory) was used to pump the liquid into the lumen side of the hollow fibers from solvent container, and the flow rate of liquid was controlled by a rotational flow meter. The concentrations of the inlet and outlet absorption liquids were
measured by a chemical titrimetric method. Gas out N CO Sampling MFC MFC Static mixer Pressure gauge Liquid out Flow meter Gas in contactor Membrane Liquid in Pressure gauge Pump absorbent Liquid MFM Sample analysis Gas chromatograph Venting
Fig.2. Sketch of experimental system.
Fig.3. Experimental setup.
Before each run of an experiment, the system was operated for 15 min by deionized water to eliminate the influence of the former experiment. All data were collected at steady state, after at least 20 min of operating time. Steady state will be indicated by a constant CO2 concentration in the outlet gas stream. Under the same operating conditions, five samples of the gas and absorbent samples were taken and the average value was calculated. All experiments were carried out at atmospheric pressure (0.1MPa) and at room temperatures.
Removal efficiency (η) and mass transfer rate of CO2 ( 2
CO
J
) were used to describe the separation properties of hollow fiber membrane module using low concentration absorbents, which can be calculated by Eq.(1) and Eq.(2) (Kumar et al., 2002; Yeon et al., 2005):in in out out in in Q C Q C Q C η= × − × × (1)
(
)
2 273.15 0.0224 in in out out CO g Q C Q C J T S × − × × = × × (2)Where η denotes the CO2 removal efficiency, %; is the CO2 mass transfer rate, mol/ (m2·h); and represent the inlet and outlet gas flow rate respectively, m3/h; and
are the CO2 volumetric fraction in the gas inlet and outlet respectively, %; 2 CO
J
inQ
Q
outC
in outC
T
g is the gastemperature, K; S represents the gas-liquid mass transfer area and herein equals to the effective membrane area, m2.
3.1. Effect of liquid flow rate on CO2 removal efficiency and CO2 mass transfer rate
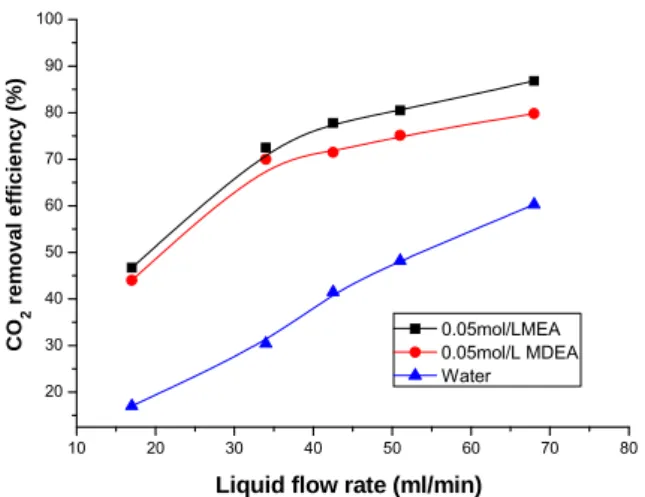
In Fig. 4 and Fig. 5, the CO2 removal efficiency and mass transfer rate are plotted against liquid flow rate using 0.05 mol/l MEA, 0.05 mol/l MDEA and deionized water as the absorbent respectively. The CO2 volume fraction in feed gas is 40 % and the gas flow rate is 150 ml/min. As shown in Fig. 4 and Fig. 5, the liquid flow rate has an important influence on both CO2 removal efficiency and CO2 mass transfer rate. As the liquid flow rate increases, CO2 removal efficiency and CO2 mass transfer rate increase. Even though physical absorption using deionized water, the CO2 removal efficiency could reach 60.3% as the liquid flow rate is 68 ml/min. With the increase of liquid flow rate, the liquid disturbance is enhanced, which results in a higher speed of CO2 diffusing into the liquid. The consumed absorbents at the membrane boundary layer could diffuse into the liquid phase at a higher speed due to the increase of liquid flow rate. Therefore, the gas-liquid interface could be maintained at a higher solvent concentration, which increases the CO2 removal efficiency. In addition, as the increase of liquid flow rate, the thicknesses of gaseous and liquid-phase boundary layers decrease, leading to enhancement of the mass transfer rate. However, CO2 removal efficiency and mass transfer rate increase slowly at higher liquid flow rate due to the limited boundary layer thickness, which are also shown in the Fig. 4 and Fig. 5.
10 20 30 40 50 60 70 80 20 30 40 50 60 70 80 90 100 CO 2 re mov a l e ffi cie n cy (% )
Liquid flow rate (ml/min)
0.05mol/LMEA 0.05mol/L MDEA Water
Fig. 4. Influence of liquid flow rate on CO2 removal efficiency.
10 20 30 40 50 60 70 80 1 2 3 4 5 6 7 8 9 10
Liquid flow rate (ml/min)
JCO 2 E2 ( mo l/m 2 .h ) 0.05mol/L MEA 0.05mol/L MDEA Water
Fig. 5. Influence of liquid flow rate on CO2 mass transfer rate.
3.2. Effect of CO2 volume fraction in feed gas on CO2 removal efficiency and mass
transfer rate
The influence of inlet CO2 volume fraction at the inlet of feed gas on the removal efficiency and mass transfer rate is shown in Fig.6 and Fig.7, respectively. Deionized water, 0.05 mol/l MEA and 0.05 mol/L MDEA were used as the absorbent. The gas flow rate is 150 ml/min and the liquid flow rate is 17 ml/min. The CO2 volume fraction in the feed gas varied from 10 % to 40%. Fig. 6 demonstrates that the CO2 removal efficiency decreases with the increase of CO2 volume fraction in the feed gas. As the CO2 is absorbed in the liquid through physical absorption or chemical absorption, more liquid is consumed with higher CO2 concentration at the liquid membrane. The liquid will be insufficient relative to higher CO2 concentration at a constant liquid flow rate, which results in a decrease of CO2 removal efficiency.
Fig.7 shows that the increase of CO2 volume fraction could effectively enhance the mass transfer rate. With the increase of CO2 volume fraction, the CO2 concentration gradient at the liquid-gas boundary layer increases. That is, the CO2 driving force of mass transfer in the gas is enhanced, which leads to the increase of CO2 diffusion mass transfer rate. Therefore, more CO2 is absorbed in the liquid by permeating the membrane module.
10 15 20 25 30 35 40 45 20 30 40 50 60 70 80 90 100 0.05mol/L MEA 0.05mol/L MDEA Water
CO2 volume fraction in feed gas (vol %)
CO 2 re mov al e fficiency (% )
Fig. 6. Influence of CO2 volume fraction at the feed gas inlet on the CO2 removal efficiency.
10 15 20 25 30 35 40 45 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0
CO volume fraction in feed gas (vol %) JCO 2 E2 ( mo l/m 2 .h ) 2 0.05mol/L MEA 0.05mol/L MDEA Water
Fig. 7. Influence of CO2 volume fraction at the feed gas inlet on CO2 mass transfer rate.
3.3. Effect of solvent concentration on CO2 removal efficiency and mass transfer rate
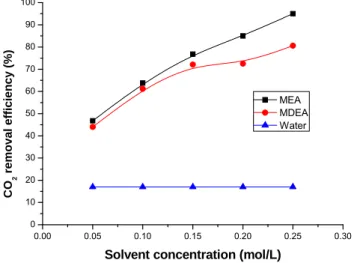
The influences of the absorbent concentration on the CO2 removal efficiency and mass transfer rate are plotted in Fig. 8 and Fig. 9. Deionized water, MEA and MDEA with the liquid concentration varying from 0.05 mol/l to 0.25 mol/l were used as the absorbent. The gas flow rate is 150 ml/min with 40 vol.% CO2 in the feed gas and the liquid flow rate is 17 ml/min. As shown in Figs. 8 and 9, the CO2 removal efficiency and mass transfer rate obviously increase with the increase of the concentration of MEA and MDEA. With the increase of absorbent concentration, the effective component absorbing CO2 in the liquid boundary layer increases, resulting in higher CO2 transfer rate into the liquid. As CO2 enters the liquid and reacts with the corresponding solvent, the CO2 concentration decreases in liquid-gas boundary layer. It enhances the CO2 solubility rate and increases the CO2 removal efficiency. The CO2 removal efficiency can be as high as 95 % with the MEA concentration of 0.25 mol/l.
As shown in Fig. 8, the CO2 removal efficiency of MEA and MDEA is much higher than that of deionized water under the same operating conditions. This might be caused by more CO2 consumed in the presence of MEA and MDEA by the chemical reaction. In addition, Fig. 8 also indicates that the CO2 removal efficiency of MEA is higher than that of MDEA especially at high absorbent concentration due to the higher rate of MEA reacting with CO2.
As discussed above, a higher removal efficiency and mass transfer rate can be effectively achieved by increasing the solvent concentration. However, with the increase of concentration of the solvent, the PP membrane is more likely to be wetted and its performance is deteriorated. It can be explained by that the surface tension of aqueous MEA and MDEA solution decreases with the increase of the amine concentration according to the Laplace-Young equation. The membrane is successfully operated for about 60 h without being wetted by using MEA and MDEA at the concentration of 0.05 mol/l. Therefore, the absorbent concentration should be compromised between removal efficiency and the wetting to ensure a stable and efficient absorption of CO2 over a long life of the hollow fiber membrane. Many researches on improving non-wettability have shown promising results. Studies have shown that the performance of polyethylene (PE) membranes could be greatly improved by a hydrophobic treatment to its surface using fluorocarbonic materials (Nishikawa et al., 1995). The wetting problem can also be achieved by coating the membrane with a very thin permeable layer (Kreulen et al., 1993). However, a hollow fiber membrane with non-wettability is still required to be further investigated and developed.
0.00 0.05 0.10 0.15 0.20 0.25 0.30 0 10 20 30 40 50 60 70 80 90 100 CO 2 r e mo va l ef fic ien cy (%)
Solvent concentration (mol/L) MEA MDEA Water
Fig. 8. Influence of solvent concentration on CO2 removal efficiency. 0.00 0.05 0.10 0.15 0.20 0.25 0.30 1 2 3 4 5 6 7 8 9 10 MEA MDEA Water
Solvent concentration (mol/L)
JCO 2 E2 ( mo l/ m 2 .h )
3.4. Effect of gas flow rate on CO2 removal efficiency and CO2 mass transfer rate
Effect of gas flow rate on the CO2 removal efficiency and mass transfer rate are shown in Figs. 10 and 11. Deionized water, 0.05 mol/l MEA and 0.05 mol/l MDEA were used as the absorbent. The gas flow rate was varied from 75 ml/min to 200 ml/min with 40 vol. % CO2 in the feed gas and the liquid flow rate is 17 ml/min. It is clearly shown in Fig.11 that there is a difference in the effects of gas flow rate on CO2 mass transfer rate of MEA, MDEA aqueous solutions and deionized water due to some uncontrolled factors. With the decrease of gas retention time, the CO2 concentration at the gas-liquid interface increases, resulting in an increase of the mass transfer rate for MEA and MDEA aqueous solutions. Although increase of gas flow rate can reduce the thickness of gas boundary layer and enhance the gas mass transfer, which is favorable for the CO2 removal. However, it simultaneously decreases the residence time of gas in the membrane contactor, which is unfavorable for the CO2 removal. The combined effects result in the tendency as shown in Fig. 10 and Fig. 11. It indicates that the residence time plays an important role in the removal of CO2.
60 80 100 120 140 160 180 200 220 10 20 30 40 50 60 70 80 CO 2 r emo val e ffi ci en cy (%)
Gas flow rate (ml/min)
0.05mol/L MEA 0.05mol/L MDEA Water
Fig. 10. Influence of gas flow rate on CO2 removal efficiency.
60 80 100 120 140 160 180 200 220 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 5.0 5.5 JCO 2 E2 ( mol /m 2 .h )
Gas flow rate (ml/min)
0.05mol/L MEA 0.05mol/L MDEA Water
4. CONCLUSIONS
Experiments have been carried out to investigate the effect of operating parameters on the efficiency of carbon dioxide removal using deionized water and low concentration MDEA and MEA in polypropylene hollow fiber membrane. It has been found that the CO2 removal efficiency increases with the increase of the liquid flow rate and solvent concentration, while the CO2 mass transfer rate increases with the increase of liquid flow rate, CO2 volume fraction in the feed gas, solvent concentration and gas flow rate. It is concluded that the absorbent concentration should be compromised between absorption efficiency and the membrane wetting to ensure a stable and efficient removal of CO2 with a long life of the hollow fiber membrane. It has also been observed by the experiments that a longer operation period render partial membrane wetting even using relative lower solvent concentration, resulting in significant decrease of membrane absorption performance. It is indicated that the decrease of residence time plays more important role in the removal of CO2 than the intensification of the gas mass transfer.
ACKNOWLEDGEMENTS
The work described in this paper has been carried out with the financial support of the Swedish Research Links Programme and the China Natural Science Foundation (contract No. 20606011) for which due acknowledgement is given. We also would like to thank Mr. H. L. Li and Ms. Y. H. Huang for the help in the experimental setup.
REFERENCES
IPCC 2005. IPCC Special Report on Carbon Dioxide Capture and Storage. Chapter 3. http://www.ipcc.ch/ipccreports/special-reports.htm
B.P. Mandal, S.S. Bandyopadhyay. 2006. Absorption of carbon dioxide into aqueous blends of 2-amino-2-methyl-1-propanol and monoethanolamine. Chemical Engineering Science 61: 5440-5447
S.H. Moon, J.W. Shim. 2006. A novel process for CO2/CH4 gas separation on activated carbon
fibers-electric swing adsorption. Journal of Colloid and Interface Science 298: 523-528.
A. Corti, D. Fiaschi, L. Lombardi. 2004. Carbon dioxide removal in power generation using membrane technology. Energy 29: 2025-2043.
A. Gabelman, S.T. Hwang. 1999. Hollow fiber membrane contactors. Journal of Membrane Science 159: 61-106.
O. Falk-Pedersen, M.S. Grønvold, H. Dannström, D.B. Stuksrud. Gas treatment using membrane gas/liquid contactors, Proceedings of the Fifth International Conference on Greenhouse Gas Control Technologies, August 13-16, 2000. Cairns Convention Centre, Collingwood, Australia. J.L. Li, B.H. Chen. 2005. Review of CO2 absorption using chemical solvents in hollow fiber
membrane contactors. Separation and Purification Technology 41: 109-122.
Q. Zhang, E.L. Cussler. 1985. Microporous hollow fibers for gas absorption: Mass transfer in the liquid. Journal of Membrane Science 23: 321-332.
Q. Zhang, E.L. Cussler. 1985. Mocroporous hollow fibers for gas absorption: Mass transfer across the membrane. Journal of Membrane Science 23: 333-345.
P.S. Kumar, J.A. Hogendoorn, P.H.M. Feron, G.F.Versteeg. 2002. New absorption liquids for the removal of CO2 from dilute gas streams using membrane contactors. Chemical Engineering
Science 57: 1639-1651.
S.-H. Yeon, K.-S. Lee, B. Sea, Y.-I. Park, K.-H. Lee. 2005. Application of pilot-scale membrane contactor hybrid system for removal of carbon dioxide from flue gas. Journal of Membrane Science 257: 156-160.
Y.S. Kim, S.M. Yang. 2000. Absorption of carbon dioxide through hollow fiber membranes using various aqueous absorbents. Separation and Purification Technology 21: 101-109.
B.P. Mandal, M. Guha, A.K. Biswas, S.S. Bandyopadhyay. 2001. Removal of carbon dioxide by absorption in mixed amines: modeling of absorption in aqueous MDEA/MEA and AMP/MEA solutions. Chemical Engineering Science 56: 6217-6224.
R. Wang, D. F. Li, D. T. Liang. 2004. Modeling of CO2 capture by three typical amine solutions in
hollow fiber membrane contactors. Chemical Engineering and Processing 43: 849-856
J.G. Lu, Y.F. Zheng, M.D. Cheng, L.J. Wang. 2007. Effects of activators on mass-transfer enhancement in a hollow fiber contactor using activated alkanolamine solutions. Journal of Membrane Science 289: 138-149.
H.Y. Zhang, R. Wang, D.T. Liang, J.H. Tay. 2006. Modeling and experimental study of CO2
absorption in a hollow fiber membrane contactor. Journal of Membrane Science 279: 301-310. H.Y. Zhang, R. Wang, D.T. Liang, J.H. Tay. 2007. Theoretical and experimental studies of membrane wetting in the membrane gas-liquid contacting process for CO2 absorption. Journal of
Membrane Science. Doi: 10.1016/j. memsci. 2007.09.050
N. Matsumiya, M. Teramoto, S. Kitada, H. Matsuyama. 2005. Evaluation of energy consumption for separation of CO2 in the gas by hollow fiber facilitated transport membrane module with permeation
of amine solution. Separation and Purification Technology 46: 26-32.
L.Y. Liu, L.J. Li, Z.W. Ding, R. Ma, Z. Yang. 2005. Mass transfer enhancement in coiled hollow fiber membrane contactor. Journal of Membrane Science 279: 301-310.
V.Y. Dindore, D.W.F. Brilman, P.H.M. Feron, G.F. Versteeg. 2005. CO2 absorption at elevated
pressures using a hollow fiber membrane contactor. Journal of Membrane Science 235: 99-109. G. Vázquez, E. Alvarez, J.M. Navaza, R. Rendo, E. Romero. 1997. Surface tension of binary mixtures of water + monoethanolamine and water + 2-amino-2-methyl-1-propanol and tertiary mixtures of these amines with water from 25 to 50 . ℃ ℃ Journal of Chemical Engineering 42: 57-59. E.B. Rinker, D.W. Oelschlager, A.T. Colussi, K.R. Henry, O.C. Sandal. 1994. Viscosity, density and surface tension of binary mixtures of water and mixtures of these amines with water over the temperature range 20-100 . ℃ Journal of Chemical Engineering 39: 392-395.
A.C.M. Franken, J.A.N. Nolten, M.H.V. Mulder, D. Barrgeman, C.A. Smolders. 1987. Wetting criterion for the applicability of membrane distillation. Journal of Membrane Science 33: 315-328. N. Nishikawa, M. Ishibashi, H. Ohta, N. Akutsu, H. Mastsumoto, T. Kamata, H.Kitamura. 1995. CO2 removal by hollow fiber gas liquid contactor. Energy Conversion and Management 36:
415-418.
H. Kreulen, C.A. Smolders, G.F. Versteeg, W.P.M. van Swaaij. 1993. Microporous hollow fiber membrane modules as gas-liquid contactors Part 2. Mass transfer with chemical reaction. Journal of Membrane Science 1993: 217-238.