i Analysis of Tetrabromobisphenol-A in Baby Food by Gas
Chromatography-Mass Spectrometry and Liquid Chromatography-Chromatography-Mass Spectrometry
by
Kayla Marissa Allen
B.S., Fort Lewis College, 2014
A thesis for submission to the Graduate Faculty at the
University of Colorado Colorado Springs
in partial fulfillment of the
requirements for the degree of
Master of Science
Department of Chemistry
ii
This thesis for the Master of Science degree by
Kayla Marissa Allen
has been approved for the
Department of Chemistry
By
____________________________________ Dr. Janel E. Owens, Chair
____________________________________ Dr. Allen Schoffstall _____________________________________ Dr. David Weiss __________________ Date
iii
ACKNOWLEDGEMENTS
To Dr. Janel Owens, for her guidance and patience through the “re” part of research.
To Luis Lowe, for his insight and invaluable field experience.
To my parents, Patrick Yarbrough, and Ms. Kovacs for their lifelong support and encouragement of all of my pursuits.
To the National Science Foundation, for their grant which financed the LC/MS/MS. To Werner Jenkins at the El Paso County Coroner’s Office for his assistance in the development of a solid phase extraction method.
iv
TABLE OF CONTENTS
1. REVIEW OF LITERATURE…....………..……....1
Introduction………...…….…1
Modes of Exposure ……….………..….……….4
Exposure through Consumer Products……….……..…..4
Environmental Exposure………...10
Occupational Exposure………..…....11
Biological Exposure………..………...12
Human Health Risks……….……..……..….13
Immunotoxicity………..………...….14
Neurotoxicity………..……..…….15
Endocrine Disruption………...16
Thyroid Dysfunction...………...…17
Carcinogenicity………..18
Impact on Ecosystems………..………...20
Transport and Persistence………..….…...20
Environmental Fate and Toxicity……….…………...24
v
Toxicity in Mammals and Birds……….………….……..26
Toxic Effects on Plants………..……27
Green Chemistry Techniques……….….………...……29
The Twelve Principles of Green Chemistry…….…..……...…….29
Techniques for Analysis of TBBPA……….…….31
Supporting Sustainable Research..……….………....…34
Statement of Hypotheses………35
2. EXPERIMENTAL PROCEDURES……….….37
Instrumentation and Techniques.………...37
Chemicals and Reagents………37
Mass Spectrometry……….38
Dispersive Liquid-Liquid Microextraction……….……...…40
Microwave-Assisted Extraction……….……40
Solid Phase Extraction………..…….41
QuEChERS Method………..41
Enhanced Matrix Removal – Lipid………..…..42
Sample Preparation………..…..42
vi
Acidified Water Experiments………..…..44
Microwave-Assisted Extraction……….…45
SPE Spike and Recovery………..…….46
Xtract SPE………..………46
Supelco SPE………..………….47
Waters SPE………..……..…………48
Thermo Scientific SPE………..……….48
QuEChERS………49
FDA Food Emergency Response Method……...………..49
EMR – Lipid………..……50
3. DETERMINATION OF A METHOD FOR THE ANALYSIS OF TBBPA IN BABY FOOD………52
Introduction………...….52
Experimental………..53
Materials and Methods……….……..53
Sample Preparation………55
Spike and Recovery………..……….56
LC/MS………..….56
vii
Results and Discussion……….…….60
Conclusion…...………..61
4. DISCUSSION AND FURTHER WORK………..63
Context of Research………...63
Further Work……….…….65
REFERENCES………..66
APPENDICES………...…76
Appendix A: LC/MS conditions for TBBPA analytes and internal standards……….…….76
Appendix B: Breakdown of methods attempted to analyze TBBPA…….77
Appendix C: Matrices analyzed and their relative fat content………...…86
vi LIST OF TABLES
1. Physiochemical properties of TBBPA……….4 2. Compounds related to TBBPA and their commercial application………...5 3. Lifetime average daily dose (LADD) and cancer-based margin of exposure
(MOE) and margin of safety (MOS)………..10 4. Summary of liver tumors observed in male mice………..20 5. The Twelve Principles of Green Chemistry……….………...31
LIST OF FIGURES
1. The structure of TBBPA……….………..………...1
2. The structures of the compounds related to TBBPA……...………7
3. The structures of the derivatives of TBBPA.……….…..….……….…..7
4. The structure of 17 -estradiol………....17
5. The structure of thyroxine………..….…...18
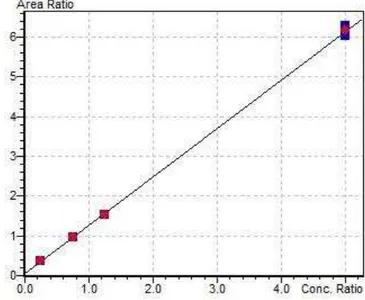
6. Plants submerged in soil with aerobic-anaerobic interface………β4 7. Calibration curve for LC/MS data……….56
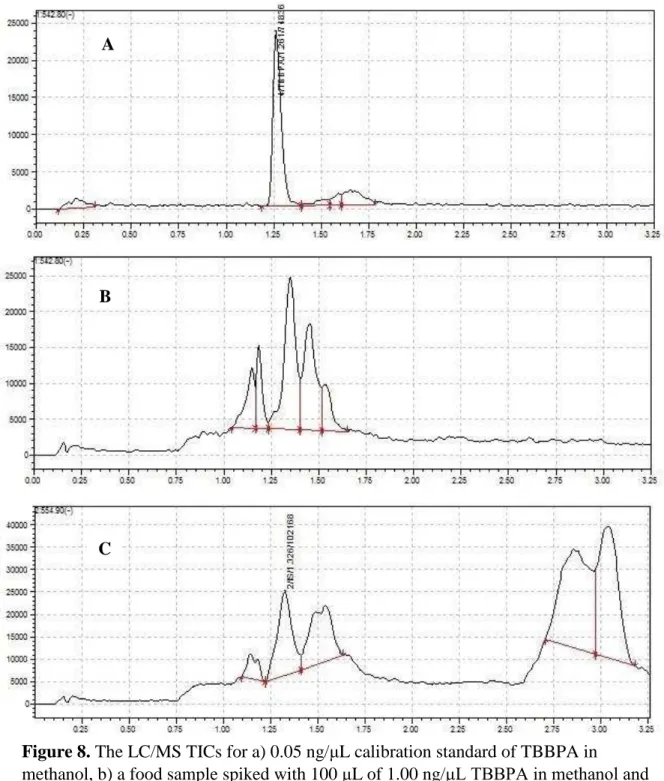
8. TIC for 0.05 ng/μL calibration standard, spiked baby food, and non-spiked baby food with an internal standard on LC/MS………57
9. TIC for 0.05 ng/μL calibration standard, spiked baby food, and non-spiked baby food with an internal standard on GC/MS……….…..59
LIST OF ABBREVIATIONS
AcN Acetonitrile
AcO Acetic anhydride
BBPA Bromobisphenol-A
BFR Brominated flame retardant
BPA Bisphenol-A
DDE Dichlorodiphenyldichloroethylene, formed from the dehydrohalogenation of DDT
DDT Dichlorodiphenyltrichloroethane, an insecticide
DI Deionized
DLLME Dispersive liquid-liquid microextraction dSPE Dispersive solvent phase extraction EFSA European Food Safety Authority
EI Electron ionization
EMR Enhanced matrix removal
GC Gas chromatography
GC/MS Gas chromatography mass spectrometry
HBCD Hexabromocyclododecane
HED Human equivalent dose
HPLC High-performance liquid chromatography HRMS High resolution mass spectrometry ISTD Internal standard
LC Liquid chromatography
LC/MS Liquid chromatography mass spectrometry
LC/MS/MS Liquid chromatography tandem mass spectrometry LOQ Limit of quantitation
MAE Microwave-assisted extraction
MDA Malondialdehyde
MeOH Methanol
MOE Margin of exposure
MOS Margin of safety
MS/MS Tandem mass spectrometry NK Natural killer (cells)
NTP National Toxicology Program
OSPAR Convention for the Protection of the Marine Environment of the North-East Atlantic (“OS” for Oslo and “PAR” for Paris) PBDD Polybrominated dibenzo-p-dioxins
PBDE Polybrominated diphenyl ether PBDF Polybrominated dibenzo-p-furans
PPAR Peroxisome proliferator-activated receptor ppb Parts per billion
ppm Parts per million
ppt Parts per trillion ppth Parts per thousand PTFE Polytetrafluoroethylene
RfD Reference dose
TBBPA Tetrabromobisphenol-A
TBBPA-BAE Tetrabromobisphenol-A bis(allyl ether)
Also called TBBPA-AE (tetrabromobisphenol-A allyl ether) or TBBPA-DAE (tetrabromobisphenol-A diallyl ether)
TBBPA-BBPE Tetrabromobisphenol-A bis(bromopropenyl ether) TBBPA-DA Tetrabromobisphenol-A diacrylate
TBBPA-DBPE Tetrabromobisphenol-A 2,3-dibromopropyl ether.
Also called TBBPA-BDBPE (tetrabromobisphenol-A bis(2,3-dibromopropyl ether))
TBBPA-DME Tetrabromobisphenol-A dimethyl ether TBBPA-GE Tetrabromobisphenol-A bis(diglycidyl ether) TBBPA-OHEE Tetrabromobisphenol-A bis(2-hydroxyethyl ether)
Also called TBBPA-DHEE (tetrabromobisphenol-A dihydroxyethyl ether)
TCS Triclosan
TDI Total daily intake
TIC Total ion chromatogram
TTR Transthyretin
SPE Solid-phase extraction STW Sewage treatment works
sWEEE Small waste electric and electronic equipment WEEE Waste electric and electronic equipment
CHAPTER 1
I. REVIEW OF LITERATURE
INTRODUCTION
Flame retardants are substances that are added to products to prevent or deter the spread of fire. Brominated flame retardants (BFRs) in consumer products have been of growing concern since the turn of the 20th century.1 There are several types of flame retardants currently in use, including halogenated paraffin,2 organophosphorus
compounds,3 and brominated compounds such as polybrominated diphenyl ethers (PBDEs),
hexabromocyclododecane (HBCD) isomers, and tetrabromobisphenol-A (TBBPA) (Figure 1).2 As of December 2006, plastics containing BFRs must be separated from waste electric and electronic equipment (WEEE) prior to recovery and recycling.4In the early 1990’s, the most common BFRs worldwide were PBDEs, which accounted for 40,000 metric tons, or 30%, of the global BFR consumption.1 However, by the late 1990’s, worldwide
consumption of PBDEs had decreased to 11% because of concern for the high levels of PBDEs being discovered in the environment.1 PBDEs were added to products such as textiles, car seats, and computer components as an additive (rather than a reactive component), which means that leaching from the product into the environment was common.1 The toxicity of PBDEs with fewer bromines, such as penta-DBE, is of concern when widely distributed in the environment because of resistance to environmental degradation processes.5 Tetra and penta-DBE congeners are the main forms detected in
Figure 1. The structure of TBBPA.
the environment as well as in human tissues.5 Penta-DBE was the major contributor to environmental and human body burdens (concentrations detected in humans).6 The bioaccumulation of lower brominated PBDEs is likely due to partitioning and retention in lipid-rich tissues, whereas deca-DBE has a higher rate of metabolism and elimination, and is less likely to partition.5 The lower bromine count PBDEs (penta-DBE, octa-DBE, etc.) exhibited greater toxicity, especially toward the reproductive system and fetal development, in rats.5 PBDEs with higher bromine counts such as deca-DBE could degrade into lower bromine count PBDE,1 and so bans were placed on penta- and octa-DBE formulations worldwide, as well as on the deca-octa-DBE for use in computers and electronics. PBDEs are now included on the Stockholm Convention’s list of Persistent Organic Pollutants7 and their production was scheduled for elimination by the end of 2013.8
Today, tetrabromobisphenol-A (TBBPA) is the BFR produced in the greatest quantities worldwide, with annual worldwide production values exceeding 200,000 tons.9 TBBPA is a novel, reactive BFR, which means that it is not expected to migrate from the product to which it is applied, unlike PBDEs.10 Under basic conditions, the hydroxyl groups of TBBPA can react with epichlorohydrin to give diglycidyl ether, commonly used in epoxy resin formulations.11 In this way, TBBPA is used in flame retardant epoxy resins for laminated computer circuit boards, industrial controls, and automotive
electronics.10 TBBPA can also be used as an additive in the circuit boards of remote controls and video recorders, and for the plastic housing of electrical and electronic equipment.10 In these latter applications, where TBBPA has not been applied to the product in a reactive way, there is the possibility for it to migrate from the product and
enter the surrounding environment. TBBPA and its derivatives have been positively identified in dust of indoor microenvironments,2,3,12-21 the dust of electronic production facilities22 and recycling or waste facilities,4,23,24 sediment,25,26 sewage sludge,26-28 and also in human breast milk10,29-31 and other food products.29,32-34 The growing concern over the levels of PBDEs in fish and human breast milk was ultimately part of its eventual disuse,1,24 but there is now concern that TBBPA is showing bioaccumulation in the same places.
The official opinion of the European Food Safety Authority (EFSA) as of 2011 regarding TBBPA in human breast milk was that the presence of TBBPA “does not raise a health concern” and that levels of TBBPA in household dust were also “unlikely to raise a health concern.”35 In both cases, EFSA cited that TBBPA did not currently show any carcinogenic or genotoxic behavior. However, TBBPA has been shown to have neurotoxic effects on rats, as well as endocrine disrupting behaviors,36 toxicity on human natural killer (NK) cells,37 and was shown to have effects on the germination of plants.38 When used as a flame retardant, TBBPA contains <0.01 to 0.05 parts per billion (ppb) polybrominated dibenzo-p-dioxins (PBDDs) and <0.01 to 0.02 ppb polybrominated dibenzo-p-furans (PBDFs).11 As of today, these are levels that are higher than those seen in samples from human populations, but they may become more relevant as the use of TBBPA continues and increases. TBBPA has not been shown to degrade into its
metabolites under ideal environmental conditions, it has instead demonstrated stability in natural systems due to low volatility, solubility, and bioavailability.27 This chapter will discuss the modes of exposure to TBBPA and the potential toxic effects of TBBPA on humans, animals, and the environment. Current studies involving baby food specifically
will then be examined, although there are only a few, because consumption of baby food represents exposure to a particularly sensitive human population.
Table 1. Physiochemical properties of TBBPA39,40
Property Value (±SD)
Molecular weight g/mol 543.87
Acid dissociation constant (pKa) 7.5, 8.5
Aqueous solubility (Sw) at 298 K, mol/L 3.19 ± 0.07 × 10-7 Enthalpy of solution (ΔsolH), kJ/mol 24.1 ± 1.2
Melting point (Tm), K 451.5 ± 0.5
Enthalpy of fusion (ΔfusH), kJ/mol 29.1 ± 1.5 1-octanol – water partition coefficient (logKow) 6.53 ± 0.12
Enthalpy of sublimation (ΔsubH), kJ/mol 153 ± 3 Henry’s Law constant (Hw) at 298K, Pa/m3
/mol 1.47 × 10-5
MODES OF EXPOSURE
Exposure through Consumer Products
The indoor dust found in homes and professional buildings is a common matrix for TBBPA because this kind of dust acts as a sink for flame retardants2 and other semi-volatile compounds.41 TBBPA specifically has a tendency to adsorb onto suspended particulate matter and dust because of its phenolic character.22 As was previously mentioned, TBBPA often finds its way into this indoor dust as a result of leaching from products to which TBBPA was applied as a non-reactive additive (although, reactive TBBPA can also experience leaching into the environment, especially during combustion
or disposal).32 Derivatives of TBBPA and their uses and methods of application can be found in Table 2. Specific consumer products that can be a source of non-reactive TBBPA as a flame retardant include foam furniture,32 food packing,32 camping tents,42 low power electronics such as remote controls,32 higher power electronics including LCD televisions, electrical outlet coverings, and laptop PC components including the AC charger, keyboard, chassis, and power boards.43 It should be noted that the amount of TBBPA added to these products is usually quite low.2
Table 2. Compounds related to TBBPA and their commercial application.7,8,11,44,45 Derivative Reactive or Nonreactive Applications TBBPA (2,3-dibromopropyl ether) (TBBPA-DBPE)
Nonreactive Polyolefins and copolymers; found in building applications, textiles,
electronics TBBPA bis(allyl
ether)
(TBBPA-BAE)
Reactive Polystyrene foams
TBBPA bis(2-hydroxyethyl ether) (TBBPA-OHEE)
Nonreactive Engineering polymers, epoxy resins, electronic circuit boards
TBBPA brominated epoxy oligomers
Reactive Housings for business machinery and electronics
TBBPA carbonate oligomers
Nonreactive Engineering thermoplastics
TBBPA dimethyl ether
(TBBPA-DME)
Not applicable Not commercially used or available. Possible biological occurrence
TBBPA diacrylate (TBBPA-DA)
Unknown Automotive coatings, wire and cable coatings
TBBPA
bis(bromopropenyl
Not applicable Not commercially used or available. Elimination product from the
ether) (TBBPA-BBPE) hydrolysis of TBBPA-DBPE TBBPA bis(diglycidyl ether) (TBBPA-GE)
Unknown Epoxy resins
Tribromobisphenol-A (tri-BBPA)
Not applicable Commercial uses unknown, occurs as an impurity in the production of TBBPA
Dibromobisphenol-A (di-BBPA)
Not applicable Commercial uses unknown
Monobromobisphenol-A
(mono-BBPA)
Not applicable Commercial uses unknown
Bisphenol-A (BPA)
Nonreactive Stabilizer for plastics, monomer in the production of polycarbonate and epoxy resins, inhibitor of end oxidation in polyvinyl chloride
Once the TBBPA has made its way into the dust of the household or professional building, it then builds up wherever dust has a natural tendency to do so:
mattresses,46 pillows, blankets, and children’s stuffed animals; inside computers and
televisions, specifically on the cooling fans;43 and on
undeterred surfaces such as the floor.41 The dust can also be circulated throughout the Figure 3. The structures of the derivatives of TBBPA. Figure 2. The structures of the compounds related to TBBPA.
building via ventilation system of the building.21 Dust ingestion is the primary mode of exposure to humans of BFRs, especially dust in the home for those who are not exposed to TBBPA by occupation.32 However, the EFSA scientific opinion on TBBPA stated that ingestion of TBBPA through dust in the home, car, and classroom does not raise a health concern for children because none of their samples exceeded the limit of quantitation (LOQ) of 1 part per million (ppm), or 1 mg/kg, net weight.35
A substance flow analysis undertaken in Switzerland showed that WEEE at e-recycling plants accounts for the largest flow of BFRs, from consumption to waste management sites, as compared to other BFRs from other waste fractions at the recycling plant (such as automotive shredder residues and construction waste).4 This study found that roughly 66% of all TBBPA disposed of in the late 1990’s was disposed of through WEEE.4 WEEE fractions called small waste electric and electronic equipment (sWEEE) including small household appliances, office and communication appliances,
entertainment electronics, and small sized electric and electronic equipment were focused on for this study as they make up 90% of the sources of PBDEs and TBBPA in WEEE. By studying products disposed of at this facility in 2003, the study found that the highest concentrations detected were for TBBPA (1420 mg/kg plastic waste).4 This concentration was three times higher than even that of PBDEs observed at the facility. Only the plastic fraction of the sWEEE could be analyzed for BFRs, and the mean value concentration for TBBPA in this plastic fraction was 5.6 mg/kg plastic waste.4 Concentrations of
nonreactive TBBPA were 23 mg/kg for PC and TV housings, and 7.3 mg/kg for the rear covers of TV housings. These values were lower than estimates reported for plastics of monitor housings, but there was also an undefined amount of TBBPA found by the
study.4 Another study in a Chinese TV recycling center found that TV housings had the greatest concentration of TBBPA, 18.5 parts per thousand (ppth), followed by PCB, 205 ppm, but the levels in dust were found to be “relatively low.”23
After dust, the primary mode of exposure for all states of life except infancy is through dietary intake,35 especially ingestion of animal products,4 specifically meat.47 The mean concentrations in meat, eggs, and aquatic foods from a study in China were 263, 194, and 738 μg/kg lipid (or parts per trillion, ppt), respectively.47 An investigation was done on infant food in Japan to determine contamination levels of TBBPA, and while the levels for vegetable, potato, meat, and powdered milk for infants all displayed “minor contamination,” the levels of TBBPA were greater for the animal-based products than for the plant-based products.34 However, the plant-based foods were naturally lower in lipids than the animal-based products, and no high-lipid plant-based foods were studied, such as avocados, nuts, legumes, or soybean products. This same study also examined contamination from BPA – a known toxin and estrogenic possibly created from photolytic reduction of TBBPA35– was minor.34 TBBPA has also been found in an analysis of Styrofoam plates and containers,48 although the potential for leaching into food was not explored.
The Wikoff group calculated the lifetime average daily dose (LADD) as well as cancer-based margin of exposure (MOE) and margin of safety (MOS) for humans based on the findings of 20 different studies (Table 3).49 Their values were found using dose-response modeling conducted on cancer and non-cancer datasets using the US EPA’s Benchmark Dose Software v2.4 using dichotomous and continuous datasets.49 Reference
dose (RfD) values were derived for non-cancer values by adjusting each benchmark dose limit (BDLM) to a human equivalent dose (HED) level such that49
RfD = [HED/UFH x UFA x UFS x UFL X UFD] (1)
Where,
RfD = Reference dose (mg/kg/day)
HED = Human Equivalent Dose (mg/kg/day)
UFX = Uncertainty factor for (A) interspecies variation, (H) intraspecies variation, (S) subchronic-to-chronic extrapolation, (L) LOAEL-to-NOAEL (lowest observable adverse effect level to no observable adverse effect level) extrapolation, (D) database deficiencies (unitless)
The MOE is the quantitative measure between the dose associated with a small increase in adverse effect and level of exposure and the MOS, the exposure compared with dose associated with 10-6, 10-5, and 10-4 risk levels for cancer assessments.49 Table 3. Lifetime average daily dose (LADD) and
cancer-based margin of exposure (MOE) and margin of safety (MOS).49
Environmental Exposure
Given the places that this dust often accumulates, children (infants and toddlers especially) seem to be at an especially high risk of exposure to TBBPA because of their time spent on the floor.19,50 In fact, although dust exposure accounts for 34% of TBBPA exposure for adults, it accounts for 90% of the TBBPA exposure for toddlers.32 Children are exposed to more dust from their classrooms than adults are from their offices.20 The greatest incidence of exposure to children has been determined to be dust in the home and in cars, rather than at daycare centers or other microenvironments children may be
exposed to outside of the home, although the values for concentrations in daycares and those in the home are nearly indistinguishable.20 This makes sense, since most individuals are exposed to the greatest amount of TBBPA in dust in the home,32 and the median value for TBBPA in UK cars was found, by one study, to be 2 ng/g dust. It is important to know whether samples for vehicle studies are taken from the trunk or the cabin, however, because the printed circuit boards and padded seats in the cabin have been shown to have much greater concentrations of BFRs.18 A 2010 study found that the concentration of TBBPA found in dust at UK daycare centers exceeded significantly the concentrations of TBBPA in dust found in cars and homes.20 However, the total exposure for children in the UK from homes, daycares, classrooms, and cars was determined to be below the health-based limit value for the UK.20
Elevated levels of BFRs have been found in office buildings, probably because of the number of computers, fabrics, and foam padding furniture, which are potential
emission sources.16 A study of trends and mass balance of FRs in a new building showed that, prior to the building opening, concentrations of all BFRs monitored were low.21 In
eight months, TBBPA concentrations increased from 0.4 ± 0.1 ng/g dust and airborne vapor to 270 ± 250 ng/g.21 Only after nearly a year did the levels of TBBPA and other BFRs reach a steady-state mass balance. The long periods over which the levels of TBBPA continued to rise indicate that an equally long period of monitoring would be required to fully understand and see the effects on humans.21 The widespread distribution of BFRs in old and new office buildings suggests that workplace exposure may be more important than originally considered, even for those individuals not working directly to manufacture or destroy products with TBBPA.16
Occupational Exposure
Although dust in the home is the primary mode of exposure for most people, great consideration must be given to those whom are occupationally exposed, specifically people who work in factories producing or recycling consumer electronics and WEEE locations. These are the people who are in direct contact with TBBPA “from cradle to grave.” Even at compared environments with intact computers - such as offices, teaching halls, and daycares with computers on the premises – the levels of BFRs at production, repair, and disposal locations are orders of magnitude greater than any other
environment.24 Of these locations, the greatest amount of BFRs have been recorded at e-waste recycling facilities.3,24 These levels are reported in units of molecules, or parts per million, rather than by percent weight of air, because these numbers correspond to amounts that may be inhaled.24 The most overall exposure to TBBPA in these situations is via dust ingestion, dust inhalation, and dust dermal absorption.22 The incidences of dermal exposure likely occur mainly when using bare hands to work, and dust ingestion is likely from unintentional hand-to-mouth contact. The estimation of TBBPA exposure
via dust ingestion may be underestimated in instances of occupational exposure with significant TBBPA emission sources.22 Workers at these kinds of facilities have even tested positive for TBBPA in the blood,24 which is significant because TBBPA has been determined to be a site-specific contaminant rather than a ubiquitous environmental contaminant, like BPA or triclosan (TCS).3,12 These contaminants (BPA and TCS) have been detected in 90% and 75% of urine samples in the US.12 Even within a single production facility, exposure to TBBPA varies based on the area of a facility that a worker is in, but measures may be taken for these individuals to reduce their exposure, such as wearing gloves or a dust mask.22
PBDEs are still the dominant BFR in WEEE disposal locations,51 but increased usage of TBBPA in consumer products predisposed to obsolescence means that increased levels of TBBPA at these exposure sites will be likely. This increases any risks associated with TBBPA for those individuals working in WEEE disposal locations and the
environments surrounding those locations.
Biological Exposure
TBBPA, like PBDE, has a tendency to bioaccumulate.52 One of the main reasons PBDE was eventually banned was because of the high levels of the chemical that was being discovered in human breast milk,1 and now TBBPA is being discovered in breast milk worldwide.10,29-31 This means that not only are mothers being exposed, possibly through the routes discussed above involving consumer products and TBBPA in the workplace, but their babies are also being exposed to the chemical. Moreover, estimates of infant exposure to TBBPA exceed the exposure levels for both adults and toddlers, and this could possibly be attributed to exposure through breast milk.10 It has also been
suggested that TBBPA easily passes the blood-placenta barrier,34 and it is also concerning that TBBPA has been found in umbilical blood in French and Japanese studies.31,53
The estimated short half-life of TBBPA in human plasma suggests that
concentrations found in breast milk are likely to be indicative of current exposure, rather than past exposure.29 Detectable levels of TBBPA are rarely found in the breast milk of individuals whom are not occupationally exposed.29 Lower levels of TBBPA were detected in the breast milk of Boston mothers who reported using public transportation regularly, but no other significant associations for TBBPA exposure and breast milk levels of TBBPA were established for this group.10
HUMAN HEALTH RISKS
TBBPA is readily absorbed up to 90% in the gastrointestinal tract, rapidly metabolized by the liver, and excreted mainly through bile acid.54 Tribromobisphenol-A is formed, theoretically, by TBBPA reduction by intestinal microflora. TBBPA can also be metabolized primarily to TBBPA-glucuronide and TBBPA-sulfate, but
tribromobisphenol-A-glucuronide has also been found as a metabolite from oral
administration of TBBPA.55 When used as a flame retardant, TBBPA contains <0.01 to 0.05 ppb PBDDs and <0.01 to 0.02 PBDFs.11 Literature on which dioxins and furans are present in TBBPA was unavailable and warrants research given the wide range of toxicity for these industrial byproducts, especially considering the discontinued use of PBDEs partially for their dioxin-like characteristics at high temperatures.6
Detectable levels of TBBPA have been found in blood samples across the globe at comparable levels (<1 to 8.4 ng/L serum for computer technicians) despite Asia being the largest consumer of BFRs and TBBPA (although levels in Asia were slightly higher than the global average).47 Blood levels of TBBPA are typically lower than what is
representative of exposure because of the absorption in the gastrointestinal tract.55 A 2008 study of TBBPA in human adipose tissue found that levels of TBBPA in samples from New York City was comparable to or lower than levels reported in breast milk, plasma, and adipose tissue samples in Europe and Mexico.48 The possible toxic effects of TBBPA are potentially mitigated by the experimentally estimated human half-life of 2.2 days.19 TBBPA contains a phenol group, making it easily conjugated and excreted, which may account for the short half-life.32 This also means that detection levels for TBBPA in humans is typically low and representative of current, rather than past, exposure.29,32 It is important to note that the estimated half-life in human plasma and adipose tissue is longer (6.6 – 76.7 days) if 14C-labelled TBBPA is used and all the metabolites are included.48
In studies that explored the levels of TBBPA, PBDEs, and HBCDs in humans, there was a lack of correlation between the concentrations of the three BFRs in the human body, which indicates differences in pharmacokinetics.48
Immunotoxicity
Studies have shown that TBBPA is cytotoxic and can interfere with cell signaling pathways, and it is also immunotoxic in vitro and can inhibit T-cell activation.32 Human exposure to a minimal amount of TBBPA over a short period time has resulted in
function.37 This persisted even after the TBBPA was removed from the system37 and suggests that the exposure dosage is not as damaging to the cells as exposure duration or the cumulative effects of multiple exposures.56 Such alterations to NK cells could result in viral infection or induce a predisposition to the development of certain tumors.37
TBBPA has also shown cytotoxicity for TM4 Sertoli cells essential to sperm development by causing an increase in intracellular [Ca2+] levels (denoted [Ca2+]i) , similar to BPA.57 This then initiated cell death through a mechanism due, hypothetically, to Ca2+-induced mitochondrial depolarization. Acute effects of TBBPA on these cells was found to occur at μM levels, 100-fold greater than the greatest level reported in humans.57
Neurotoxicity
The neurotoxic abilities of TBBPA have been classified as low compared to other BFRs, however there are several conflicting in vitro and in vivo studies that show adverse effects of TBBPA on the nervous system.58 Disturbances in intracellular Ca2+
homeostasis in vitro has been observed due to TBBPA exposure, specifically increases in basal [Ca2+]i was observed in dopaminergic pheochromocytoma (PC12) cells.58 This is a cell line derived from rat adrenal medulla, and is often used for researching brain
diseases. Prolonged increases in [Ca2+]i can potentially affect gene expression, protein phosphorylation, neurotransmission, and caspase-mediated apoptosis.58 TBBPA is also an antagonist for human α4 2nicotinic acetylcholine receptors, giving it a “low potency” neurotoxic potency designation (lowest observable effect concentration between 1 μM and 10 μM) by Hendricks, et al. in β014.58
TBBPA is also toxic to primary cultured cerebellar granule neurons.59 Of four derivatives analyzed for neurotoxicity
(TBBPA-DBPE, TBBPA-DAE, TBBPA-OHEE, TBBPA-GE), only TBBPA-DAE was determined to induce high neurotoxicity. The overall structure of TBBPA contributes to its toxicity, however the unique branching on TBBPA-DAE (Figure 2.1) may make this compound particularly toxic.59 Potential concerns for neurotoxicity of TBBPA include effects on locomotor activity, memory, and learning, especially when exposure occurs during the perinatal stage.60 Many studies have been conducted to assess these concerns, but the results are conflicting and inconclusive owing to differences in test methodology, reproducibility, and difficulties in interpreting animal studies for human health assessments.60
Endocrine Disruption
Endocrine disruption compounds are compounds or mixtures that “alter the function(s) of the endocrine system and consequently causes adverse health effects in an intact organism, or its progeny, or (sub)population.”61
These health effects can include infertility, disrupted thyroid function, obesity, type 1 diabetes, cancer, immune dysfunction, as well as cognitive and behavioral disorders.61 TBBPA has been identified as an endocrine disruptor because of its structural similarity to 17 -estradiol (Figure 4) and thyroxine (Figure 5).20 TBBPA presents an estrogen antagonist response which lowers the induced response of 17-estradiol by 24%.61 For this reason, the UK Committee on Toxicity recommended a total daily intake (TDI) of 1 mg/kg bw/day in 2004.20,49 TBBPA also has a potential
interaction in the body similar to that of BPA (Figure 3.4), a well-known debrominated version of TBBPA that exhibits estrogenic effects.62 Other brominated bisphenols
Figure 4. The structure of 17 -estradiol.
possibly formed from the physical or biological degradation of TBBPA including mono-, di-, and tri-BBPA63 have been determined to have estrogenic potencies with
concentrations on the range of 10 μM for the mono- and di-BBPA and 2 μM for the tri-BBPA.64 Thus, even if TBBPA has little to no
estrogenic properties, the debrominated products that can form during combustion (as in at a WEEE location) or as metabolic byproducts are capable of endocrine disruption.
Thyroid Dysfunction
One of the main human health concerns of all BFRs is disruption of thyroid hormone homeostasis because even minor alterations in maternal and fetal thyroid levels can cause neurological disorders and birth defects.32 Disruption of thyroid homeostasis may be the main toxic effect of TBBPA because it has the potential to compete with T4 (thyroxine), a circulating thyroid hormone, for binding to a key serum transport protein transthyretin (TTR).65 TBBPA is structurally similar to thyroxine (Figure 5) and has shown thyroid hormone agonism and antagonism in laboratory animals.32 TBBPA has shown 10 times more binding affinity to T4 than TTR.65 In a study on male Sprague-Dawley rats, serum total T4 concentration dramatically decreased in a 250 and 500 mg/kg dose of TBBPA.54 For zebrafish, and other marine life, TBBPA is particularly toxic to the thyroid even in nominal concentrations.36 In humans, changes in T4 production during development are associated with developmental delay, low body mass, brain
development abnormalities, and neurobehavioral developmental disorders.66 It is worth noting, however, that thyroid abnormalities due to decreased T4 were only seen in rats
Figure 5. The structure of thyroxine.
when a non-biologically relevant dose of TBBPA was applied and these abnormalities were seen only in rats, not mice, and may be specific to rats alone.66
In addition, TBBPA is also an obesogen candidate, meaning that in humans it can disrupt the “normal developmental and homeostatic controls over adipogenesis and energy balance.”67
TBBPA is such a xenobiotic, as well as some of its derivatives,68 because of its potential for binding to the thyroid hormone receptor and a group of receptor proteins that function as transcription factors regulating the expression of genes, peroxisome proliferator-activated receptors (PPAR), specifically PPAR .9 Both of these are nuclear receptors which are directly implicated in metabolic control in the periphery and hypothalamus.9 The derivative BPA works in direct conjunction with insulin to change the insulin dependent kinase pathways and enhance glucose uptake, and positive correlations for measureable serum BPA levels and obesity, as well as polycystic ovarian syndrome, have been observed in human females.68
Carcinogenicity
Both PBDEs and TBBPA have dioxin-like characteristics at high temperatures6,11 that could potentially pose a cancer risk to individuals exposed to them, and begs the question as to whether they are also are carcinogenic as environmental contaminants without exposure to such high temperatures. Of primary concern is the increased
incidence of uterine tumors corresponding with exposure to TBBPA. There have been no studies to evaluate the carcinogenicity of TBBPA, although studies by the National Toxicology Program (NTP) have shown that BFRs, in general, have the potential to be multispecies carcinogens.66 The NTP conducted a two-year study where rats were given a maximum of 1,000 mg/kg TBBPA orally 5 days a week and found grossly increased
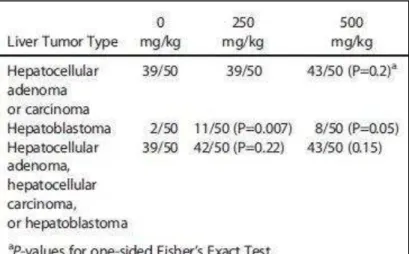
incidences of uterine tumors and adenocarcinomas as well as some increased incidence of tumors in rat testes and also in the liver, large intestine, and the vascular system of male mice.66 A summary of the liver tumors observed in the male mice can be seen in Table 4.
The modes of action relating TBBPA exposure to uterine tumors is unclear, but it was hypothesized by Lai et al. that exposure may promote pre-existing Tp53 mutations in the uterus.69 The Tp53 tumor suppression gene in both humans and rats is responsible for cell cycle maintenance and genomic stability.69 The proposed mode of action of suppressing this suppression gene by Lai et al. was thus: TBBPA competitively inhibits estrogen conjugation, which leads to increased serum estrogen and an increase in hormone-responsive genes that promote cell proliferation. This increased cell proliferation could then promote the Tp53 mutations in the uterus.69 To validate this hypothesis, however, more studies are needed. Also, as with the thyroid studies, these findings may still only apply to rats and not be relevant for human exposure assessments.69
Table 4. Summary of liver tumors observed in male mice.66
IMPACT ON ECOSYSTEMS
Transport and Persistence
The bioaccumulative potential for TBBPA derivatives is considered low because of their molecular weight, but their overall persistence is estimated to be high because of particle association.8 TBBPA also has a high Kow value (logKow = 6.53) in spite of its relatively high aqueous solubility, Sw (3.19 × 10-7) (Table 1).
log � = log �� − . (2)
As Kow increases, so does the bioaccumulation factor, or KB, and as the bioaccumulation
increases, so does the potential for biomagnification in higher levels of the food chain.70 The investigated transport mechanisms for TBBPA derivatives in the environment also increase the likelihood of becoming global pollutants.8 Plastic debris is a common source for toxic chemicals in the ocean. Moreover, there is a synergistic effect between plastic debris and persistent, bioaccumulative, and toxic chemicals as plastic concentrates and transfers these chemicals from the ocean into the marine food web.71 Plastic debris is also able to float, making it able to transport toxins over greater distances, however they also have the potential to sink and remain on the ocean floor. Plastics are generally also not biodegradable so they have a greater tendency to persist than other forms of oceanic debris.71 This, of course, is not the only means by which TBBPA is transmitted in an abiotic manner to water, and other means will be discussed later. Other abiotic and biotic transmission modes are also of concern.
The Voluntary Emission Control Action Programme conducted a survey on TBBPA emission for the European TBBPA manufacturing industry for 2008-2010 and
reported that emission to land (5.8 × 104 to 2.6 × 105μg/kg product) was much greater than emission to air and water (100 to 1.4 × 104μg/kg product).22 Despite this,
manufacturing of electronics and electrical equipment has been reported as a source of BFR pollution for aquatic ecosystems.63 TBBPA concentrations in landfill leachates from industrial waste sites have been reported to be as high as 620 ppt. Concentrations of TBBPA have also been observed as higher in sediment downstream from a plastic manufacturing facility (270 ppb) than concentrations in sediment upstream from the facility (34 ppb).63
In countries such as China, the environmental exposure to TBBPA and other BFRs from e-waste can be greater than estimated or measured for other regions because of the high incidence of illegal electronics dismantling sites that practice crude and illegal methods of disposing of e-waste.51 These methods not only seriously contaminate the immediate area, but also further regions because of river runoff, contaminated fish, and air or dust transport.51 A survey of TBBPA transport and persistence conducted in the Pearl River Delta of China revealed that the highest concentrations in river sediment occurred in rivers that ran near e-waste recycling facilities.25 This same survey indicated that the concentration of TBBPA in river sediment increased with increasing population density, which implied that domestic sources of TBBPA were strongly influential, although still not as influential as industrial and commercial sources.25 Another 2010 survey in Hunan, China, found that sediment and soil samples collected near an
electronics manufacturing plant had higher concentrations of TBBPA and its derivatives (TBBPA-AE, TBBPA-BAE, TBBPA-DBPE) upstream and nearer to the facility than the concentrations downstream.72 The concentration of TBBPA-DBPE (Figure 2.4) was not
determined, either due to the sensitivity of the instrumentation employed, or because this compound is susceptible to hydrolysis in the same range as DDT and its elimination product, TBBPA-BBPE (Figure 2.9), may be a more prevalent compound in sediments but was not analyzed by this group.73 Of these, TBBPA was the lowest concentration overall and had the greatest exponential declining trend as distance from the facility increased. Earthworms and rice hulls were also analyzed, and the decline of
concentrations of TBBPA and derivatives detected was consistent with the decline of concentrations in the soil in relation to distance from the facility.72 Biota, then, has a proportional relationship to environmental exposure, to some degree.
In a similar study, TBBPA and its related compounds were determined
simultaneously using a novel analytical method for sewage sludge and sediment from Spain’s Ebro River basin.26
TBBPA was detected in six samples with values ranging from 287 ppt to 1.3 ppb. These values were higher than the previous literature values for TBBPA and may be owing to a low repeatability for the method.26 Mono-, di-, and tribromobisphenol-A were found between non-detectable limits and 561 ppt, 660 ppt, and 637 ppt, respectively. BPA was also found in concentrations from 6.6 to 26 ppt.26
Another source of TBBPA to the aquatic environment is final effluence from sewage treatment works (STW). TBBPA, and other BFRs, were present in UK samples of STW influents, but TBBPA specifically was present in particulate fractions (<0.4 to 29 μg/kg dry weight) and in the dissolved phase (up to 24 ng/L).28
These were higher than average influent concentrations in Canadian municipal wastewater treatment plants (5.27 ng/L).45 Maximum concentrations of TBBPA were found in secondary treated and dewatered sludge samples from Cork, Ireland (192 ppb), which were comparable to
concentrations reported from Swedish sewage sludge.28 Influent tri-BBPA concentration ranges measured in Canada were 1.0-2.2 ng/L, and these seem to be the only available values measured for tri-BBPA concentrations in wastewater influent.45 Since there are few, if any, commercial sources of tri-BBPA, environmental sources are minimal. The Canadian findings, then, suggest that TBBPA can degrade prior to water treatment in STW.
TBBPA has been tested for degradation by activated sludge, but even partial biodegradation into BPA was not detected.27 However, TBBPA has been found to degrade slowly to BPA in anaerobic soil.73 Soils in most environments are usually aerobic, but during flooding anaerobic conditions can develop. Under anaerobic conditions, TBBPA may be bacterially O-methylated to mono- and dimethyl ethers.74 One study used microcosms from Kearny Marsh, New Jersey and Kymijoki River, Finland spiked with a TBBPA concentration of 10 μM.75
After 80 days over 50% of the original TBBPA had
biotransformed to TBBPA mono- and di-methyl ether from the Kearny Marsh, and after 60 days approximately 10% of the TBBPA from the Kymijoki River had biotransformed.75
TBBPA-dimethyl ether (TBBPA-DME) is more lipophilic than TBBPA, which increases its potential for bioconcentration.75
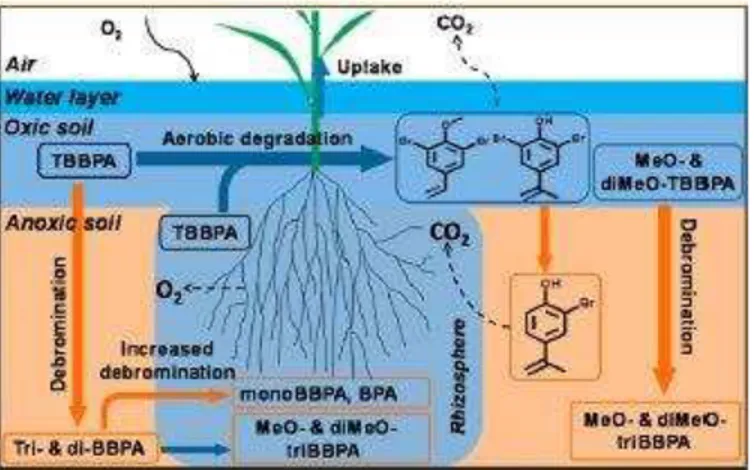
Figure 6. Plants submerged in soil with aerobic-anaerobic interface.75
Flooded soils can have adjacent aerobic-anaerobic layers, as in rice paddies where TBBPA has already been detected in China.74 In flooded soils with no plants, lower-brominated BPAs and O-methylation products of BBPAs were detected, indicating simultaneous aerobic and anaerobic transformation of TBBPA.74 Further debromination products of di-BBPA in this soil and the decrease in tri- and di-BBPA suggests that debromination under these conditions is slow (although faster than previously reported in purely aerobic or anaerobic soil) (Figure 6).74
Environmental Fate and Toxicity
The likelihood of TBBPA to be associated with suspended particles in water and buried in sediment is owed to the low water solubility and high log Kow. At a neutral pH,
TBBPA has a low solubility, but at higher pH levels (as in arid regions) the solubility increases. This increases soil mobility and potential for groundwater contamination considerably.76 TBBPA has been found in abiotic matrices including air, soil, sediment, and sewage sludge77 and may undergo abiotic degradation. The calculated half-life by UV radiation was the following for each season: 6.6 days in summer, 25.9 days in autumn, 80.7 days in winter, and 10.2 days in spring.40
Persistence and Toxicity in the Marine Environment
In water, debrominated TBBPA derivatives (tri-, di-, and monobromobisphenol-A) are generated from either photochemical degradation or plastic decomposition.63 The fate of TBBPA and its derivatives is highly dependent on the nature of each compound. TBBPA-DBPE is susceptible to hydrolysis at the same level as DDT and creates an elimination product, TBBPA-BBPE, more often found in sediment than TBBPA-DBPE,
much in the same way that DDE is found in sediment compared to DDT.8 TBBPA-OHEE and TBBPA-BAE are both theoretically resistant to environmental degradation, though it seems there is a lack of information on these compounds in the environment. Most of the derivatives of TBBPA are hydrophobic, so transport is likely to occur from adsorption to solids. TBBPA-DBPE and TBBPA-BAE have a low volatility and are likely to be transferred in the atmosphere by particle transport.8 These last two derivatives are larger and have a lower volatility than most potential Arctic contaminants, but are included on such a list to continue monitoring and investigating their usage as additive BFRs.8
The Wang group at the University of Chinese Academy of Sciences demonstrated in 2015 that the photolysis of TBBPA in aqueous solutions occurs under a simulated solar light irradiation in air, and an N2 atmosphere at only a slightly slower rate.78 They found that dissolved oxygen is not required for the photolysis of TBBPA, which means that TBBPA can undergo direct photolysis independent of O2 oxidation.78 TBBPA can undergo at least three different pathways for photodegradation, each of which result in different degradation products with yet unknown effects on the environment.78
Despite having no current regulation for human exposure and being regarded as having little to no toxicity, TBBPA is highly toxic to marine life.63,65 TBBPA is on the Convention for the Protection of the Marine Environment of the North-East Atlantic (OSPAR) list of chemical under priority action for their Hazardous Substances Strategy.47 The toxicity of TBBPA and BPA have been studied and found to be significantly lower than the toxicity of mono-, di-, and tribromobisphenol-A.63 This study found that TBBPA and BPA were also less toxic than the mono-, di-, and tri-brominated derivatives on the
cell viability of trout hepatocytes; this does not imply nontoxicity, however.63 The toxicity of the derivatives appeared to increase with greater bromine count, between the mono-, di-, and tri-brominated compounds. BPA was found to be more toxic to micro-algae compared to TBBPA, but this is a finding inconsistent with previous observations.63
In marine sediment, TBBPA can degrade to, and persist as, BPA.71 Disruption of the thyroid system in frogs exposed to tri- and dibromobisphenol-A has been observed.63 For zebrafish, and other marine life, TBBPA was particularly toxic to the thyroid even in nominal concentrations comparable to measured water concentrations.36 Toxic effects to marine life include edema, hemorrhaging, decreased heart rate, and tail malformation. Tail and trunk malformation from TBBPA could be a result of alteration of proper matrix metalloproteinase expression and activity.79 A 2008 study of sharks and dolphins in the USA found TBBPA in all samples analyzed, at concentrations ranging from 0.056 to 8.48 ng/g lipid weight for bottlenose dolphins, 0.035 to 35.6 ng/g lipid weight for bull sharks, and 0.495 to 1.43 ng/g lipid weight for Atlantic sharpnose sharks.48 This information could be useful in assessing appropriate safety levels of TBBPA in the environment.
Toxicity in Mammals and Birds
Given that household dust is a sink for TBBPA and the sensitivity of many
animals to chemical exposure tends to be lower than that of humans, consideration should be given to the non-human mammals and birds that are kin, however distant, to common pets and companion animals.
TBBPA was measured in the muscles of six different bird species and the
e-waste recycling region in South China. TBBPA was detected in the muscle tissues of all the birds, ranging from 9.0 to 1482 ng/g lipid weight. The grain, plant leaves, and soil samples ranged from 2.9 to 780 ng/g dry weight TBBPA, which were consistent with TBBPA levels in sediment near an electronics manufacturing facility in the Pearl River Delta.80,81 This indicates that e-waste production and recycling is an important source of environmental TBBPA.
Herring gull egg samples were taken from the St. Lawrence River and Great Lakes Basin in Canada for a 2010 study, as herring gulls are commonly monitored for bioaccumulative contaminants in that watershed ecosystem.82 In this study, 83% of the eggs were contaminated with TBBPA-AE and 67% contained TBBPA-DBPE. Although the concentrations found in the eggs were rather low (not detectable to 0.56 ng/g wet weight) this is another indication of the bioaccumulative properties of TBBPA and its derivatives.82
Toxic Effects on Plants
Plants are already known to readily uptake BPA through their roots from water and metabolize it into glycosidic compounds, thereby making these byproducts more estrogenic.38 Plants are most susceptible to toxicity during the germination stage, and chickpea plants specifically were found to be inhibited from germinating by the presence of TBBPA and BPA at concentrations of 100 mg/L. TBBPA and BPA were also shown to create oxidative stress in roots by inducing reactive oxygen species, although the mechanism remains unclear.38 Oxidative stress is a condition when the defenses of an organism can no longer rid themselves of radicals and other active oxygen species, which
leads to reduction of growth and productivity, and ultimately death.65 Oxidative stress in chickpea roots because of BPA and TBBPA was shown to positively correlate with destruction of proteins in the plant.38 In wheat leaves, TBBPA induced oxidative stress is indicated by the formation of malondialdehyde (MDA). The greatest levels of MDA were detected after the wheat leaves were exposed to 5000 mg/kg TBBPA for seven days, as would be expected since this was the greatest level of TBBPA used.65
Translocation and accumulation of TBBPA was studied in cabbage and radish plants and determined that the roots of the leafy cabbage contained three times as much TBBPA as the radish root.83 However, the cabbage showed a reduced tendency towards translocation and accumulation in the shoots of the plant. The sorption of TBBPA to the soil matrix was the most important factor in determining the short-term fate of TBBPA in soil.83 There was no interspecific competition between plants that affected TBBPA uptake, and the most important factors to determine the fate plants exposed to TBBPA appears to only be the properties of TBBPA. However, it was noted that the presence of plants seemed to enhance the bioavailability of TBBPA in soil,83 which could be cause for concern regarding human and feedstock exposure, especially if plants are grown in soil contaminated with TBBPA from TBBPA-containing dust and water, or especially if they are fertilized by waste-based fertilizers containing TBBPA.84
Sun et al. found that rice and reed seedlings were able to grow in TBBPA contaminated soil (5 mg/kg soil) (Figure 6).74 Plant-mediated stimulation was found to alter the amount of metabolites in the soil, as well as the time course of their occurrence, compared to unplanted soil.74 The presence of reed and rice plants stimulated anaerobic reduction that that mono-BBPA and BPA were detected in these soils, and the reed plants
more strongly stimulated TBBPA transformation than the rice plants. The rice and reed seedlings both showed high accumulation of TBBPA and its metabolites from the soil.74 These findings are compatible with the studies of cabbage and radishes, and the
researchers suggest that the species-specific accumulation of TBBPA reflects the microbial community and its activity in the soil.74
TBBPA needs to continue to be monitored as an emerging persistent organic pollutant as there is clear evidence of toxicity to the flora and fauna of the global
environment, as well as to the humans that inhabit the planet and continue to use TBBPA. In doing so, it would be foolish to conduct research in a way that served only to further damage the environment. By applying green chemistry techniques, researchers can study TBBPA in an environmentally and fiscally responsible way that poses little threat of hazard exposure to the scientists and their laboratories.
GREEN CHEMISTRY TECHNIQUES
The Twelve Principles of Green Chemistry
The twelve principles of green chemistry were outlined by Paul Anastas and John C. Warner in their 1998 book, Green Chemistry: Theory and Practice. These principles had been in use in the environmentally-minded parts of the scientific community since approximately the era of Rachel Carson and Silent Spring, and are practiced today to maintain thoughtful, sustainable practices in academic and commercial labs, as well as in the chemical industry. When considering an emerging chemical such as TBBPA and its derivatives, it makes sense to reduce the environmental impact of the associated research
and to attempt to follow these principles to avoid further exposure and contamination. Data from the Environmental Protection Agency’s annual Toxics Release Inventory Analysis from 2013 shows that the amount of chemicals being released to land, water, and air has decreased 7% in the last decade. This can be attributed largely to the
implementation of green chemistry principles in the chemical production industry in the last two decades.85
Table 5. The Twelve Principles of Green Chemistry and Their Application to TBBPA86
Principle Description Relating to
TBBPA Prevention It is better to prevent waste than to treat or clean up
waste after it has been created.
Yes
Atom Economy Synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product.
No
Less hazardous chemical
synthesis
Wherever practicable, synthetic methods should be designed to use and generate substances that possess little or no toxicity to human health and the
environment.
No
Designing safer chemicals
Chemical products should be designed to affect their desired function while minimizing their toxicity.
No
Safer solvents and auxiliaries
The use of auxiliary substances (e.g., solvents, separation agents, etc.) should be made unnecessary wherever possible and innocuous when used.
Yes
Design for energy efficiency
Energy requirements of chemical processes should be recognized for their environmental and economic impacts and should be minimized. If possible, synthetic methods should be conducted at ambient temperature and pressure.
Yes
Use of renewable feedstocks
A raw material or feedstock should be renewable rather than depleting whenever technically and economically practicable.
No
derivatives protection/deprotection, temporary modification of physical/chemical processes) should be minimized or avoided if possible, because such steps require additional reagents and can generate waste.
Catalysis Catalytic reagents (as selective as possible) are superior to stoichiometric reagents.
No
Design for degradation
Chemical products should be designed so that at the end of their function they break down into innocuous degradation products and do not persist in the
environment. No Real-time analysis for pollution prevention
Analytical methodologies need to be further developed to allow for real-time, in-process monitoring and control prior to the formation of hazardous substances. No Inherently safer chemistry for accident prevention
Substances and the form of a substance used in a chemical process should be chosen to minimize the potential for chemical accidents, including releases, explosions, and fires.
Yes
Techniques for the Analysis of TBBPA
The most common matrices for the analysis of TBBPA are dust,2,12,14,15,17-20,46,87-97 soil and sediment,25,26,73,74,81,83,84,98-103 sewage and wastewater,26,28,45,104 plastic consumer products,42,43,105-107 human breast milk,10,29-31 computer circuit boards,22,43,108 and
foodstuffs or animals for human consumption and its packaging.28,34,100,109-117 Although the number of studies on food and food packaging are increasing as concern for exposure to TBBPA increases, the phenolic character of the molecule makes it very difficult to analyze in complex matrices such as food.
To prepare samples for analysis, the TBBPA must often be extracted. In the analysis of sediment, soil, and sludge, any sulfur present must also be removed to prevent
the effects of sulfur on pH.77,118 This is often done by copper powder or gel permeation chromatography to Extraction can be accomplished by Soxhlet,8,24,25,28,30,80,118,119
accelerated solvent extraction,10,29,120 solid-phase extraction,7,59,82,121 and pressurized liquid extraction.104,122 The main extraction technique for TBBPA that will be discussed in greater depth later is dispersive liquid-liquid microextraction (DLLME).92,123-125 This is a technique successfully employed for other emerging environmental contaminants92 and has been used in food analysis.123 It involves using several organic water-miscible
dispersive solvents (methanol, acetone, tetrahydrofuran, etc.) but will help disperse the water-immiscible extraction solvent (toluene, chlorobenzene, chloroform, etc.) into the aqueous sample so that the turbulence produces fine droplets in the aqueous layer.123 These droplets have large interstitial area and the equilibrium is reached rapidly so that extraction is nearly instantaneous, and the equilibrium is often assisted through the use of ultrasound to aid in the dispersion. Techniques utilizing this method include ultrasound-assisted emulsification-microextraction.126,127 DLLME is a rapid, efficient technique compared to solid-phase or liquid-liquid extraction.92 Optimal extraction and dispersive solvents for TBBPA quantitation has been studied by other students completing research here at UCCS.92,128
Analysis of TBBPA is often done by gas chromatography-mass spectrometry (GC/MS), but because TBBPA is a polar compound it is necessary to acidify and derivatize the samples before injection to improve both peak shape and analytical sensitivity. This is not necessary with high performance liquid-chromatography mass spectrometry (LC/MS), which is well-suited to analyze the polar, phenolic character of TBBPA. Liquid chromatography tandem MS (LC/MS/MS) methods have been developed
to optimize analysis without intense sample preparation.7,55,77 Analysis done with electrospray ionization in negative mode with a 1:1 (v/v) methanol/water and 2 mM ammonium acetate solution for the first mobile phase and a second, 100% methanol, mobile phase has been shown to be particularly effective.10,19 TBBPA can also be analyzed in biological contexts using assays.9,129,130
Previous work analyzing TBBPA in infant food has been done only in Japan by two groups. One study by the Nakao group was conducted by ultrasonic wave assisted gas chromatography with high resolution mass spectrometry (HRMS),34 the other by GC-HRMS without ultrasonic wave assistance.110 They analyzed TBBPA and BPA
concurrently, using 13C-labeled TBBPA and D-labelled BPA to spike samples, which were defatted by liquid-liquid partitioning with ethyl acetate and extracted using
methanol.34 The samples of infant food collected for this study were vegetables, potatoes, meats, and powdered milks purchased from grocery stores and a baby product store in Japan.34 This group found TBBPA on the range of 0.037-0.71 ng/g in vegetables, 2.2-3.9 ng/g in meats, and 3.3-3.8 ng/g in powdered milks.34 Further study by this same group in 2013 showed that infant foods composed of vegetables, fungi, and/or fruit were low in TBBPA concentration (> 1 ng/g) compared to foods composed of meats (2-4 ng/g).111
The Murata group analyzed TBBPA and other environmental contaminants in food, but needed to analyze for TBBPA separately. They used a 13C-TBBPA labeled internal standard to spike samples, which were then defatted by liquid-liquid partitioning with hexanes and extracted multiple times with methanol.110 This group used a market basket method to obtain food samples in Fukuoka, Japan between the years 2002-2005.110 Given the values of TBBPA found in these items, the estimated dietary intakes of
TBBPA for an adult was determined to be 1.1 ng/kg bw/day.110 Both groups found values for TBBPA contamination that fall far below the TDI established in the UK of 1 mg/kg bw/day.20
The phenolic character of TBBPA makes it particularly challenging to manipulate into the water or lipid phase of fatty foods, especially animal fat. Typically, the solutions containing phenols in water are acidified to a pH of at least 2131 and then extraction can be completed, using a solvent such as methanol or methylene chloride.132 Unfortunately, the acidification step can introduce errors or losses to the experiment or the instrumental determination.133
Supporting Sustainable Research
Given that TBBPA is found at trace levels, even in “high concentration” sinks like dust, research and analysis lends itself to sustainable chemistry techniques because only small amounts of both sample and solvent are required. Often, this is achieved on a milliliter scale appropriate for LC or GC.2,26 Analysis can also be done on a microliter scale utilizing techniques such as DLLME, which is considered especially useful for analyzing emerging environmental contaminants.92 Techniques that use solvents on these scales reduce solvent waste and researcher exposure to solvents and support a sustainable ethic. Small-scale solvent use also reduces the potential for chemical accidents like dermal exposure or chemical fires.
The work done in this research is sustainable and “green” for following small-scale solvent techniques. DLLME is a green micro-small-scale analytical method that was implemented for this work, and so the samples were analyzed using GC/MS and LC/MS
utilizing solvent volumes on the order of microliters. Previous work extracting TBBPA from food using other liquid-liquid extraction methods require the use of solvents such as chloroform, tetrachloroethylene, carbon tetrachloride, and other known carcinogens and mutagens,123 however the solvents used with DLLME are common solvents that do not create additional financial burden for purchasing them (methanol, acetonitrile,
dichloromethane, etc.) and do not exacerbate waste disposal.
Statement of Hypotheses
The goals of this research was to determine if there exists a statistically significant difference in the levels of TBBPA in different types of baby food packaged in the same kinds of containers, namely comparing animal product-based (dairy, meats, etc.) foods to plant-based (no dairy or meat) foods. It is also necessary to determine if there is a
statistically significant difference between the levels of TBBPA in the same kind of food before and after microwaving. As there is currently no set TDI for TBBPA in the United States, the concentrations of TBBPA found in the foods will be compared to current literature values and the TDI of TBBPA for European countries. The current hypothesis is that there will be a higher concentration of TBBPA in animal product-based baby foods (because of the high lipid content) compared to plant-based foods (with a lower lipid content and less bioaccumulation). Also, it is hypothesized that there will be a significant difference in the concentration between microwaved and non-microwaved samples, and that the lipid-rich food will exhibit a higher increase in concentration of TBBPA
compared to the concentration increase that less lipid-rich foods exhibit after microwave treatment. TBBPA has previously been found in food packaging, especially plastic food
packaging, and so this part of the research will examine the TBBPA which leaches from the food packaging of foods in glass and plastic containers upon microwave heating.
CHAPTER 2
II. EXPERIMENTAL PROCEDURES
INSTRUMENTATION AND TECHNIQUES
In accordance with both previous literature and prior work done at the University of Colorado Colorado Springs with TBBPA, chromatography coupled to a mass
spectrometry detector was utilized for the analysis of TBBPA.128 Both gas chromatography and liquid chromatography were employed.
Chemicals and Reagents
A 13C-TBBPA internal standard (99% purity, 50 μg/mL in methanol, Cambridge Isotope Laboratories, Andover, MA) was used for analysis on both GC/MS and LC/MS. A 13C-TBBPA internal standard (99% purity, 25 µg/mL in methanol, Cambridge Isotope Laboratories, Andover, MA) was also used. For all LC/MS studies, the same LC/MS grade methanol (Fisher Scientific, Fair Lawn, NJ) was used, including in mobile phase solvents. The concentrated hydrochloric acid, toluene, 50% sodium hydroxide solution, HP-LC grade acetonitrile, ethyl acetate, and hexanes were also from Fisher Scientific (Fair Lawn, NJ). Dichloromethane (Macron, Center Valley, PA) and acetic anhydride (Acros, NJ) were used for GC/MS calibration curve samples. Chlorobenzene (Acros, NJ) was used for microwave-assisted extraction. The 18MΩ deionized water was produced using a Bamstead E-pure filtration system (Thermo Scientific, Asheville, NC). For solid phase extraction techniques, a sodium phosphate buffer was prepared using monobasic sodium phosphate monohydrate (J.T. Baker Chemical Co, NJ), and an ammonium acetate
buffer was prepared using ammonium acetate (Mallinckrodt Inc, KY). To wash the columns, a 1 M acetic acid solution was prepared using glacial acetic acid (Fisher Scientific, Fair Lawn, NJ). Ammonium hydroxide (Sigma-Aldrich, St. Louis, MO) was added to mobile solvents A and B over the range of 0.25-0.0025%. Stock TBBPA solutions were prepared in methanol (Sigma-Aldrich, St. Louis, MO).
Mass Spectrometry
Mass spectrometry (MS) is a powerful analytical tool based on the fragmentation of ions. With electron ionization (EI), “fingerprint” data can be obtained by complete fragmentation of the ions. This sort of data can be used to deduce the structural data for a molecule, but does not allow for the deduction of molecular mass. In other, softer,
ionization methods, a molecular ion signal and few fragment ions are obtained, which can be used to obtain the molecular mass data for a molecule. To obtain structural data using other ionization methods than EI, which may provide higher sensitivity or ion energy, tandem mass spectrometry can be employed (often abbreviated as MS/MS). Another benefit of MS is that, depending on the analyte, different mass analyzers and detectors can be coupled with the ionization source for optimized analysis.134 For analysis of TBBPA by GC/MS, samples must be acidified and derivatized. This is not necessary for analysis by LC/MS.7 Derivatization can potentially produce errors or analyte loss, so this could be a major disadvantage for GC/MS. Another advantage of LC/MS over GC/MS for TBBPA analysis is that 13C-labeled TBBPA can be used as a surrogate standard, which improves the data obtained by compensating for matrix-effects that disturb ion intensity.7