Final Thesis
Feto-Maternal
Communication in Broiler Chickens
(Gallus gallus domesticus)
Albin Gräns
Rapporttyp Report category Licentiatavhandling x Examensarbete C-uppsats D-uppsats Övrig rapport _______________ Språk Language Svenska/Swedish x Engelska/English ________________ Titel Title
Feto-Maternal Communication in Broiler Chickens (Gallus gallus domesticus)
Författare
Author Albin Gräns
Sammanfattning
Abstract
Bird incubation is a natural phenomenon that balances the needs of the parents for nourishment with the needs of the fetus for heat provision and protection. In this context, any means of communication between the fetus and the parents would have an adaptive value. The aim of the study was to investigate whether putative means of feto-maternal communication would correlate to physiological changes caused by environmental alterations. Oxygen consumption was used to measure fetal well being and six independent variables associated with fetal vocalizations and fetal movements were used to evaluate their potential for communicating the fetus statu quo. Broiler fetuses (Gallus gallus domesticus) of three developmental stages (day 18, internally pipped and externally pipped) were challenged by a stepwise reduction in ambient temperature down to 30ºC. A linear drop in oxygen consumption in response to lowered temperatures was found in all three developmental stages indicating that the fetus was affected by the temperature changes. No differences correlating with temperature variations were found in any of the variables associated with fetal vocalization. Fetal vocalizations are consequently not used to communicate the thermal status of the fetus. Movement occurrence, movement intensity and ventilation frequency, however, followed a “maximum peak” trend, with a highest response at the third temperature interval (35.0-35.5ºC). Considering that the lower limit of optimal development is between 35-36 C, the results suggest that fetal movements can be of potential use to the incubating parent to assess the well-being of the fetus.
ISBN
__________________________________________________ ISRN
__________________________________________________
Serietitel och serienummer ISSN
Title of series, numbering LiTH-IFM-Ex—06/1632—SE
Supervisor:
Assoc. Prof. Jordi Altimiras
Datum Date 2006-05-24
URL för elektronisk version
Avdelning, Institution Division, Department
1. Abstract ...1
2. Introduction ...1
3. Material and Methods...3
3.1 Temperature control...4
3.2 Oxygen consumption (VO2) ...5
3.3 Sound recordings ...5 3.4 Force recordings ...6 3.5 Data analysis ...6 3.5.1 VO2 analysis ...6 3.5.2 Sound analysis...6 3.5.3 Force analysis...8 3.6 Statistical analysis...9 4. Results ...9
4.1 Oxygen consumption (VO2) ...9
4.2 Fetal vocalization...10
4.3 Fetal movement...10
4.4 Ventilation frequency ...11
5. Discussion ...11
5.1 Oxygen consumption (VO2) ...11
5.2 Vocalization activity...12
5.3 Fetal movement and ventilation frequency ...13
5.4 Consequences of temperature variations to fetal well-being...15
6. Conclusion...17
7. Acknowledgements ...18
8. References ...19
1. Abstract
Bird incubation is a natural phenomenon that balances the needs of the parents for nourishment with the needs of the fetus for heat provision and protection. In this context, any means of communication between the fetus and the
parents would have an adaptive value. The aim of the study was to investigate whether putative means of feto-maternal communication would correlate to physiological changes caused by environmental alterations. Oxygen
consumption was used to measure fetal well being and six independent
variables associated with fetal vocalizations and fetal movements were used to evaluate their potential for communicating the fetus statu quo. Broiler fetuses (Gallus gallus domesticus) of three developmental stages (day 18, internally pipped and externally pipped) were challenged by a stepwise reduction in ambient temperature down to 30ºC. A linear drop in oxygen consumption in response to lowered temperatures was found in all three developmental stages indicating that the fetus was affected by the temperature changes. No
differences correlating with temperature variations were found in any of the variables associated with fetal vocalization. Fetal vocalizations are
consequently not used to communicate the thermal status of the fetus. Movement occurrence, movement intensity and ventilation frequency, however, followed a “maximum peak” trend, with a highest response at the third temperature interval (35.0-35.5ºC). Considering that the lower limit of optimal development is between 35-36ºC, the results suggest that fetal
movements can be of potential use to the incubating parent to assess the well-being of the fetus.
Keywords: Feto-maternal communication, prenatal, oxygen consumption, fetal
vocalization, fetal movement, development.
2. Introduction
During the early evolution of birds, development of a higher body temperature might have, in combination with egg-parent contact incubation, increased the egg temperature and thereby led to a higher hatching success (Lundy 1969). Higher egg temperatures reduce the time of incubation (Hepp et al. 2006) and consequently the risk of predation. Selection pressure probably strengthened and further improved the contact incubation behaviours due to its
advantageous control of the developmental environment.
parents (Tazawa & Rahn 1987). During natural incubation, the incubating parent must find a balance between their own need for energy with the thermal and protective needs of the fetuses (Conway & Martin 2000). Consequently, any way for the incubating parent to know the status of the fetus would have an adaptive value. Late in incubation, feto-parental communication could proceed via vocalizations, movements or even chemical signaling but these mechanisms have not been extensively studied. Alternatively, parental attendance could still occur in the absence of fetal communication by
temperature sensitive mechanisms arising from the brood patch. The present study only focused on suggested means of feto-maternal communication and if measured responses were correlated to physiological changes caused by
environmental alternations.
Once the physiological effects that temperature has on the fetuses are known, any behavioural responses correlated with these effects become interesting.
The ontogeny of both prenatal vocalizations and movements has been thoroughly described and both parameters can be measured using
non-invasive methods (Kuo 1932a). For a full characterization of fetal movements and fetal vocalizations all possible aspects dealing with these activities need to be taken into consideration.
The frequency of sounds can be separated into different kinds of sounds as well as the duration of the sounds (Collias 1952). Vocalizations are
important for communication between hatchling and parent in several different aspects, including as a mean to communicate the need of heating assistance (Collias 1952, 1987). Studies on temperature related vocalizations prior to hatching have yielded contradictory results (Bugden & Evans 1999).
Gallus gallus domesticus (Broiler chickens) of three developmental
stages were chosen as test subjects. It is a domesticated breed selected for fast growth and known to, in adult life, have problems when coping with extreme temperatures (Yahav et al. 2004).
The focus of the VO2 part of the study was to measure how broiler
chicken embryos are affected by decreasing temperatures, indicated by VO2.
Thermal conditions have an important effect on developing fetuses. Oxygen consumption (VO2) is strongly correlated with body temperature (Tazawa et
al. 1988) so declining VO2 indicates that the fetus is affected by the decreasing
temperatures. Whether or not bird fetuses show a feeble increase in metabolic rate when temperatures are decreased, as a weak thermoregulation response, has been a topic of debate (Tazawa et al. 1988).
The prediction was that VO2 would show a feeble increasing response
to moderate cooling and then decrease linearly as a response to continuously decreased temperatures. It was also predicted that a maturation of the
metabolic response would be seen as time of hatching approached.
The focus on the behavioural part of the study was to test whether or not fetal vocalizations and fetal movements in any way respond to decreased
temperatures in broiler chicken fetuses.
The prediction was that all variables associated with fetal vocalization and fetal movements would increase as the temperature dropped and that the responses would increase as time of hatching approached.
3. Material and Methods
Experiments were conducted on fertile eggs from broiler chickens (Gallus
gallus domesticus) of the strain Ross 308 obtained from a local hatchery
(Kläckeribolaget, Väderstad, Sweden). Prior to incubation the eggs were stored at a constant temperature of 15ºC and automatically turned once every 3 hours (Hova-bator, Invansys, USA).Fetal development was monitored by candling the eggs periodically (every 7 days) and removing non-fertile and dead eggs. Masses of the eggs and fetuses are presented in table 1.
On day 0 the eggs were weighed to the nearest hundredth of a gram (BP 221S, Sartorius AG, Germany) and placed in a still air incubator (25 HS, Masalles Comercial, Spain) set at 37.8ºC and 45% RH. The eggs were
automatically turned once every hour until day 19 when they were placed in a hatching tray in the bottom of the incubator.
Fetuses at three different developmental stages were used in the
experiments: 18 days after incubation started (18d), internally pipped (IP) and externally pipped (EP). IP and EP are considered better markers than fetal age because the time of hatching can vary considerably (Webb 1987) and
consequently the physiological status of the fetus can be better judged by the pipping state. A second reason for using IP and EP to define the fetus
developmental stages instead of incubation age is that IP and EP are crucial events for fetal maturation and onset of lung breathing (Tazawa et al. 1988). To determine whether or not an egg was internally pipped a 1 cm2 piece of the shell above the air cell was removed under sterile conditions and replaced by a microscopy cover glass that was subsequently glued (Super epoxy 2
component glue, Loctite, Sweden) to the shell. The operation was conducted on day 16 to minimize interference to the air cell gas composition which could
onset of internal pipping the egg was moved into a custom-made experimental incubator set at the same conditions as the commercial incubator.
The experimental measurements included four parameters divided into several tested variables dealing with their amplitude and frequency. The four parameters where: VO2, ventilation frequency, fetal vocalization and fetal
movement.
3.1 Temperature control
All variables were recorded simultaneously under five different temperature settings labeled T1-T5 for convenience. A manual ventilation fan (Ø80 mm) was installed in a circular hole located in the door of the experimental
incubator to enhance temperature control.
K-type thermocouples were fitted to the lid of the chambers to measure inside temperatures. A custom made data acquisition software program (Lab View; National Instruments, USA) was used to record the temperature.
Each trial took 150 min and alternations of the incubation temperature was conducted every 30 min. Since the temperature settings on the incubator did not represent the temperature inside the experimental chambers a custom made test schedule (see table 2) was needed to obtain the wanted temperature intervals, ranging from 40 to 30°C. The experiment started 1 hour after the eggs were moved to the experimental incubator to give them time to settle after handling and manipulation. Each egg was used once and the fetus was afterward euthanized with an injection of 0.5 ml pentobarbital 100 mg/ml (Pentorbarbitalum 100g, ethanolum 290g, Apoteket, Sweden).
In the experimental incubator the eggs were placed in an airtight
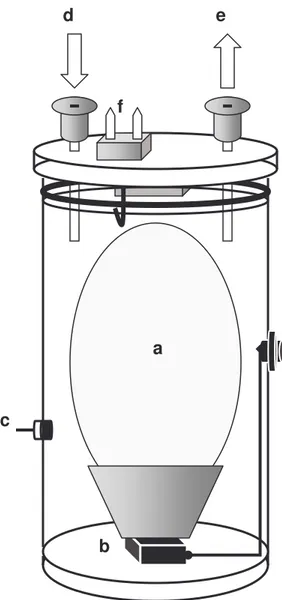
plexiglas chamber (inner dimensions: height 95 mm, Ø50 mm) built to fit the size of broiler eggs (see fig 1 for a detailed description).
To account for possible discrepancies between chamber temperature and egg temperature due to the thermal inertia of the egg, a separate
temperature test was conducted. A T-type thermocouple was, on day 15,
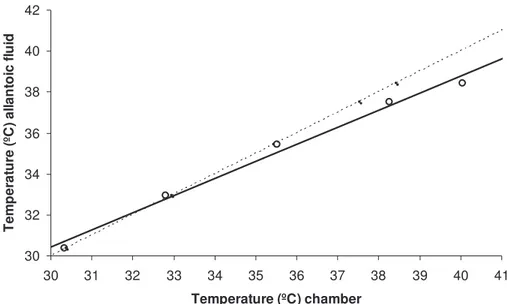
inserted 1.5 cm into the egg under sterile conditions. Inspection of the eggs, on the day of hatching, revealed that the tip of the thermocouples was located in the allantoic fluid. The eggs were treated identical to the eggs used in the experiments. The results were then compared with the chamber temperatures (see fig 2). The procedure was noninvasive and all six fetuses used in the temperature difference test developed normally.
3.2 Oxygen consumption (VO2)
Oxygen consumption was measured by open respirometry in a push-mode configuration. Two chambers were used for the tests: one containing a nonfertilized egg, which was used for baselining, and one containing the experimental egg. The chambers were equipped with two sets of tubing (Tygon R3603 3.2 X 4.8 mm) leading air in and out with a controlled flow of 50 ml min-1 (FOX II Analyzer, Sable systems International, USA). In the lid of the chambers, small fans (Ø25 mm) were installed to increase air mixing. Air flow was switched between the two chambers every 15 min using a
multiplexer (TR-RM8 Respirometer Multiplexer, Sable systems International, USA). The chamber used for sampling was flushed using a second pump.
The system was setup as shown in figure 3. The push mode was used since it is the setup least sensitive to leaks (Mortola & Labbe 2005). In such a system the oxygen concentration ([O2]) of the air is measured after being
pushed through the chamber containing the oxygen consuming sample and compared with air pushed through a baseline chamber (Mortola & Labbe 2005). In our test a Fox II Analyzer was used to sample the [O2] of the air.
Before the air reached the Fox II Analyzer it was desiccated by being pushed through a tube filled with drierite (Anhydrous calcium sulfate, W.A.
Hammond drierite company Ltd, USA). The Multiplexer and the FOX II Analyzer were connected to a PC-computer (Dell Optiplex GXa). The data collected was analyzed using a custom made data acquisition program (Lab View; National Instruments, USA). VO2 was measured on all three
developmental stages.
To make sure each sample was independent and that the chamber was completely washed out between sampling periods a wash-out test was
conducted (see figure 4). The chamber was flushed with nitrogen until oxygen concentration [O2] read zero, whereafter it was flushed with air from inside the
experimental incubator ([O2] 20%) at 50 ml min-1. The test was stopped when
[O2] was stable. It took 6 min 20 sec to get a 95% flush out. Based on the
results of the wash-out test each chamber was sampled for 15 min at each temperature interval but only the last 5 min were used for VO2 data analysis to
ensure independency between the samples.
3.3 Sound recordings
A condenser microphone (Ø=4.5mm, Veco Vansonic, PVM-6027) was
recorded for 15 min at a sampling rate of 11025 Hz with an 8 bit resolution during each of the temperature intervals. A custom made data acquisition software program (Lab View; National Instruments, USA) was used to record the sounds. From the sound recordings, the variables associated with fetal vocalizations were extracted. Sound recordings were measured on IP and EP fetuses but not on 18d fetuses since vocalizations are only possible after internal pipping (Bugden & Evans 1999).
3.4 Force recordings
The egg was placed on a force sensor (Micro switch, FSL05N2C, force range 1500 g and sensitivity 0.02 - 0.28mVgram-1, Honeywell, Canada). To amplify
the force signal from the force sensor a Grass RPS 107 pre-amplifier and a Grass polygraph D.C. driver amplifier was used. The amplifier was connected to a commercial data acquisition system (ML865 PowerLab 4/25T Data
acquisition System; ADinstruments, USA) running the software program Chart5 for Windows in a PC-computer (Dell Optiplex 17OL). From the force recordings the variables associated with fetal movements and ventilation frequency were extracted. Force recordings were measured on 18d and IP fetuses but not on EP fetuses due to technical problems. The ventilation frequency was only obtained in IP fetuses since fetuses do not ventilate prior to pipping (Kuo 1937).
3.5 Data analysis
3.5.1 VO2 analysis
[O2]was collected every 10 sec during the last 5 min of each 15 min trial. The
value for VO2 was then calculated as the average of all the readings during
this period using formula 1.
VO2 = [O2] * flow (1)
[O2] = the measured [O2] in the trial chamber subtracted from the [O2] in the
baseline chamber.
3.5.2 Sound analysis
Analysis of the sound recordings was conducted using Adobe Audition 1.0. For a schematic description of the sound analysis procedure see figure 5. The sound data file was a mixture of background noise from the incubator and vocalizations from the fetuses. To reduce the noise and other unwanted
disturbances, the sound file had to be extensively processed. A noise reduction profile was constructed from a vocalization-free section of the data. The noise profile was used to remove most of the background noise (see 1. figure 5). A band pass filter fitted to the hearing bandwidth of G. gallus (Saunders & Salvi 1993) removed all other sounds from unwanted frequencies (see 2. figure 5). The amplitude (dB) of the least detectable peeping sound, after the data had gone through the noise reduction and the band pass filter, was used as the minimum threshold, when defining a sound. The sound files consist of hundreds of readings which exceed the value chosen as a minimal threshold (see 3. figure 5). With the minimal thresholds defined the program
automatically found and marked any event in the data exceeding the threshold for more than 1ms (see 4. figure 5). For a full description of the recorded sounds, readings exceeding the minimal threshold were divided into bouts. The bout-method was based on the work of (Tolkamp & Kyriazakis 1999). A sound period was the period during which the sound readings were higher than the minimum threshold. The episode used to find suitable bout-definitions was the time between the starts of these sound periods. The chosen definitions were the times containing least episodes and would thereby give the minimal amount errors (errors = episodes with the same length as the breakpoints and thereby impossible to define). Two thresholds were chosen, one short (a sound pulse) which defined a peep and a longer one (sequence of shorter peeps), which defined a phrase. All silence periods longer than the threshold defining a phrase point out the start of a new phrase. Figure 6 shows an example of what a treated sound sequence looked like and how it would be analyzed.
The average sound intensity for all readings between the minimum and maximum values was normalized relative to the fetuses’ specific settings and calculated by using formula 2. The more common RMS (Root Mean Square) method to measure signal-strength could not be used because the distance between the microphone and the sound source was not fixed as the fetus (egg) moved during recordings.
intensity = readings
(
(x – min)/(max –min))
/number of readings (2)x = (point value) ^2 *1000 (using all points greater than the threshold) min = lower threshold ^2 *1000
Mean phrase duration was calculated by dividing the time left after removing all silence periods (see 5. figure 5) by the number of phrases in that interval.
3.5.3 Force analysis
The software program Chart5 for Windows was used to record the force traces and process the data.
The data contained noise (unwanted movements) from the experimental incubator. To improve the signal-to-noise ratio and find the movements
originating from the fetuses the data signal was filtered and intensified (for a schematic description of all movement analyses see figure 7). A high pass filter was used to remove all unwanted movements and remove baseline drifting (see 1. figure 7). All remaining values were than squared and
amplified (see 2. figure 7). A lower threshold was set individually for each fetus based on their individual movements and all data exceeding this
threshold was marked using a feature in the program (see 3. in figure 7). Time between the marks and peak value of the markings was then used to calculate the movement occurrence (markings) and event intensity.
Fetal movement was recorded for 15 min at a sampling rate of 1000 samples per second during each of the temperature intervals. From each of the 15 min interval three different variables of movements were analyzed: movement occurrence per 15 min, event intensity and ventilation frequency.
The maximal intensity of movement events of each egg was normalized relative to the fetuses’ specific settings and calculated using formula 3.
intensity = readings
(
(x – min)/(max –min))
/number of readings (3)x = local maximum value of marked event.
min = lower threshold = ( max – min)/5))*1.5 (during 5 sec intensity
ranges in low activity episodes of the first 15 sec of each temperature interval) max = highest recorded value for a specific egg, with the only exception that values more than 50 times higher than the threshold value were only counted as events and not as highest value.
The last 5 min of each temperature interval (same as for VO2) was used
to acquire the fetal ventilation frequency. The ventilation frequency was obtained by transforming the peak value obtained form the spectrum view from Hz to min-1 (see 4. figure 7).
3.6 Statistical analysis
All statistical analysis were conducted in SPSS 12.0.1
VO2 differences covariate with temperature sensitivity were tested
between the three developmental stages using one-way ANOVA with Bonferroni’s post hoc test.
To test for significant differences between fetal developmental stages in all variables measured (namely phrase occurence, peeps per phrase, average sound intensity in a phrase, phrase duration, movement occurence and
maximal intensity of the movement events) a repeated measures ANOVA was used with the tested variable as dependent repeated factor and developmental stage as between subject’s factor. The different temperature intervals were used as the repetitions.
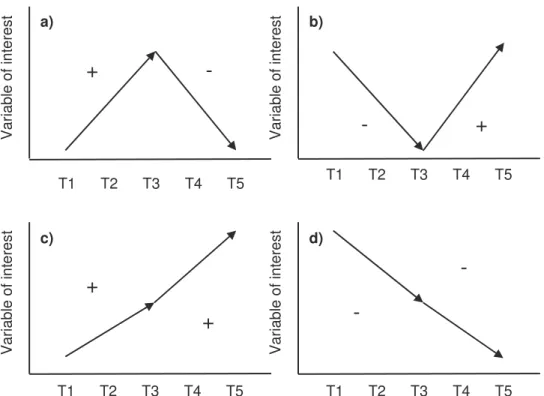
Due to the limitations of repeated measures ANOVA and the fact that measurements need to be independent and random (which is impossible when measuring gradual cooling) a different test was used to find differences and trends between temperatures. To find overrepresented temperature trends within the fetal activity variable a trend test was used. Data from all fetuses from all developmental stages (all vocalization and movement variables except peep per phrase, which did not contain enough data) was split into two subsets: subset 1 – data from T1, T2 and T3 and subset 2 – data from T3, T4 and T5. A linear slope was calculated for each of the two subsets and the signs of those lines were combined to define a trend. The four possible trends are shown in figure 8. Tend a) and b) in figure 8 indicates the occurrence of temperature threshold and can be used as indicators for stress or comfort. Trend c) and d) occurs when the response is correlated to the strength of the exposure. A chi-square distribution analysis was used to evaluate the
frequency of the different trends. A 1:4 distribution was assumed if all trends were equally likely to occur. To compensate for the fact that we only have two categories and thereby only one degree of freedom the Yates correction was used (Sokal & Rohlf 1995).
4. Results
All data is presented as Mean (SD) (Curran-Everett & Benos 2004).
4.1 Oxygen consumption (VO2)
Figure 9 shows the VO2 responses to gradual cooling on fetuses of three
All the three developmental stages showed a decline in VO2 as
temperature decreased. The difference between T1 and T5 was more than 60% for all developmental stages, 18d 62.22 %, IP, 61.64 % and EP 62.61 %.
There was a significant difference in the temperature sensitivity between 18d and IP (p = 0.008) and 18d and EP (p = 0.000) (see fig. 10). The lowest metabolic temperature sensitivity occurred in 18d 0.017 ml min-1 °C-1 (0.001) and the highest in EP 0.026 ml min-1 °C-1 (0.004).
4.2 Fetal vocalization
Figure 11 shows the histograms of IP and EP used to divide sounds between phrases and peeps in the vocalization analysis. The times chosen were 0.15 s and 0.8 s and gave the following definitions: a peep is an episode shorter than 0.15 s, a phrase is an episode shorter than 0.8 s. After a silent period longer than 0.8 s a new phrase started.
Figure 12 shows all vocalization variables (namely phrase occurrence, peeps per phrase, average sound intensity in a phrase and phrase duration) comparing the two tested developmental stages during the five temperature intervals. The lowest phrase occurrence was found on IP fetuses with the lowest interval average being 2 phrases during the 15 min period, 1.3 peeps per phrase and phrase duration of merely 0.024 s. The highest phrase
occurrence was found on EP fetuses with the highest interval average of 8 phrases during the 15 min period, 2.9 peeps per phrase and phrase duration of 0.11 s. The average sound intensity was highest on IP fetuses during T3 [3.05 a.u. (3.41)] and lowest on EP fetuses during T4 [0.49 a.u. (0.50)].
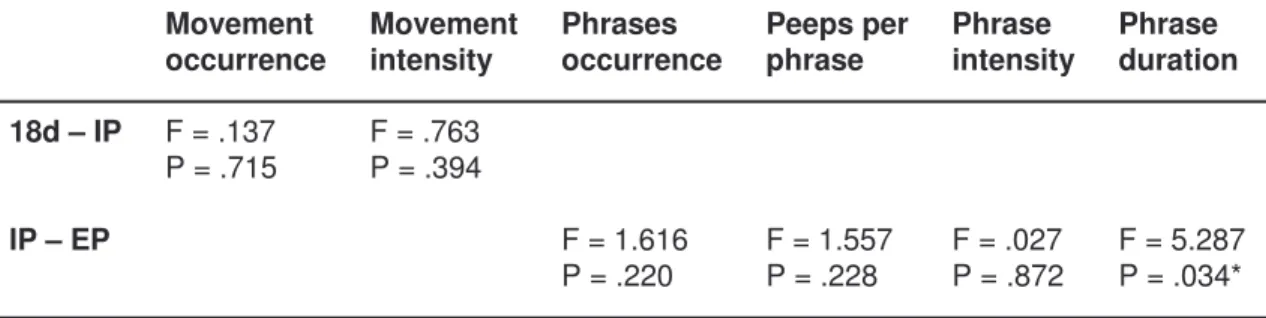
Table 3 shows the results from the repeated measures ANOVA on all variables testing for differences between developmental stages. Significantly longer phrase duration was found for EP fetuses compared with IP fetuses (F = 5.287 and P = .034)
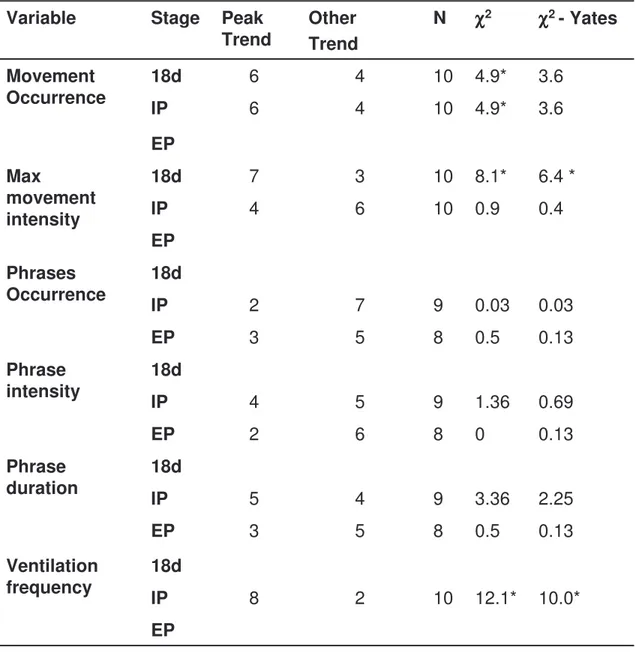
As shown in table 4, neither of the vocalization variables showed a significantly higher number of fetuses showing a maximum peak trend than expected if the four trends were evenly distributed.
4.3 Fetal movement
Figure 13 shows movement occurrence and maximal intensity of the movement events of 18d and IP during T1, T2, T3, T4 and T5. Movement occurrence was highest at T3 with 3.29 events min-1 (2.21) for IP and lowest
The maximal intensity of the movement events was highest on 18d during T3, 10.05 a.u. (4.99) and lowest on IP fetuses during T5, 3.11 a.u. (2.06).
Table 3 shows the results from the repeated measures ANOVA on all variables testing for differences between developmental stages. No significant differences between developmental stages and any of the fetal movement variables were found.
As shown in table 4 there was a significantly higher number of fetuses showing a maximum peak trend on ventilation frequency at IP (p<0.05) and movement event intensity at 18d (p<0.05) than expected if trends were evenly distributed among the fetuses. Prior to using Yates correction, which has been criticized for overcompensating (Sokal & Rohlf 1995), the frequency of
movement at both 18d and IP also showed a significantly higher amount of maximum peak trends (p<0.05).
4.4 Ventilation frequency
Different breathing frequencies of IP fetuses during the tested temperature intervals are shown in figure 14. The average breathing frequency went up from 67 breaths min-1 during T1 temperature (40.1ºC ) to a maximum of 77 breaths min-1 during T3 (35.5ºC) and then down, as the temperature dropped, to 58 breaths min-1 at T5 (30.3ºC).
As shown in table 4 there was a significantly higher number of fetuses showing an activity peak trend on ventilation frequency (p<0.05).
5. Discussion
5.1 Oxygen consumption (VO2)
The two alternative outcomes of decreasing temperatures on the
metabolic rate is either a conforming response or a regulated response (Webb 1987, Mortola & Labbe 2005). The conforming response is an inactive
response where a lower ambient temperature leads to a lower body temperature which in turn leads to a lower metabolic rate. A conforming
response is expected for an ectothermic animal (Tazawa et al. 1988, Tazawa et al. 1989a, Tazawa et al. 1989b, Mortola & Labbe 2005). The regulated
response is an active way to keep body temperature constant, above or below ambient temperature. Regulated responses can be both behavioural and physiological.
During normal incubation temperature (37.8ºC) the measured VO2
levels agreed well with the levels reported in previous studies (Tazawa et al. 1988, Tazawa et al. 1989c, Mortola & Labbe 2005). All tested developmental stages showed a linear decrease in metabolic rate as the temperature went down. Significantly higher temperature sensitivity was seen in fetuses closer to hatching than in younger fetuses (see fig 10). Although significant, the differences were small between the developmental stages for both temperature sensitivity and VO2 changes in percent. Thereby the results challenge all
studies suggesting a prenatal increase in metabolic rate as a response to coolingas hatching is approached (Freeman 1964, Decuypere et al. 1979, Tazawa et al. 1988, Whittow & Tazawa 1991, Mortola & Labbe 2005). The results also challenge studies suggesting that there is no difference (Romijn & Lockhorst 1995), since significant differences did occur. If a maturing
thermoregulation system occurs already in prenatal life the responses seen in the results, with higher temperature sensitivity closer to hatching, is the opposite of what would be expected.
The predicted feeble increase in VO2 when the temperature was
moderately decreased did not occur in any of the developmental stages. Consequently, broiler fetuses do not show any signs of thermoregulation through an increase in VO2. The results are relevant because it means that the
parents need to be present during the entire incubation period. After hatching, chicks are known to thermoregulate, and the
thermoregulatory system matures rapidly after hatching (Abraham & Evans 1999b, Bugden & Evans 1999, Shinder et al. 2002). Still, the fetus can not sustain its own body temperature even when being externally pipped, just before the time of hatching.
The reason for the differences in temperature sensitivity for different developmental ages remains unclear. Further studies are needed to explain this unexpected phenomenon.
5.2 Vocalization activity
Vocalization from the fetus can be important in two different aspects. It is either important as a self stimulus or as a stimulus triggering responses from the incubating parent or other conspecific (Rumpf & Nichelmann 1993, Brua 1996).
Numerous studies have outlined the importance of fetal vocalizations as a response to temperature variations dealing with various species of bird’s e.g.
nigricollis (Eared Grebes) (Brua et al. 1996) Larus delawarensis (Ring-Billed
Gulls) (Evans et al. 1994) and Pelecanus erythrorhynchos (American White Pelican) (Evans 1989, Abraham & Evans 1999a). The prediction was that vocalizations in broiler fetuses would increase as the temperature dropped.
The results show no significant effect of decreased temperatures on fetal vocalization activity. These results agree well with several earlier studies (Dawes 1981, Bugden & Evans 1999). Rumpf & Nichelmann (1992) saw no evidence of feto-maternal communication in Cairina moschata (Muscovy Ducks). However, a second study conducted by the same research team showed that fetuses of the same species of ducks influenced each other to vocalize more (Rumpf & Nichelmann 1993). Consequently, it seems like vocalization activity in C. moschata is important as a sensory stimulation rather than as a means to communicate thermal needs (Rumpf & Nichelmann 1992, Sleigh & Lickliter 1996).
A significant rise in the vocalization amplitude between IP and EP as shown in the results partly agrees with the results of Bugden & Evans (1999) who found trends of increased vocal activity as hatching approached.
One suggested explanation to the different results in studies dealing with the role of vocalizations as a mean to communicate heating assistance is the hatchling maturity types (Matsunaga et al. 1989, Kuroda et al. 1990). The argument is that fetal-vocalization is only of importance in precocial species, since they are further developed when hatching (Matsunaga et al. 1989, Kuroda et al. 1990). This is not a convincing argument since species of different hatchling maturity types are represented in the studies earlier mentioned. Of the tested species, chickens, ducks and grebes are considered precocial, gulls are semi-precocial and budgerigars and pelicans are altricial (Nice 1962). No obvious trends can be found between classifications and presence or absence of vocalization responses to cooling.
The predicted outcome, an increase in fetal vocalizations as the
temperature dropped, did not occur. Neither could any differences, correlated with temperatures, be seen between the developmental stages. From the results it is clear that the major importance of fetal vocalization is not as a means to communicate its thermal status. The role that vocalization duration has in the hatching process remains unclear and needs to be studied further.
5.3 Fetal movement and ventilation frequency
importance for the parent to know the thermal status of the fetus makes it likely that the incubating parent can detect movements within the eggs and thereby determine the physiological status of the fetuses. Even so, several occasions of birds incubating dead eggs have been observed (Afik & Ward 1989, Conway & Martin 2000). It has even been suggested that keeping dead eggs could be advantageous (Afik & Ward 1989). A dead egg in the nest will reduce heating and cooling rates of the entire nest and thereby work as a heat buffer for the other eggs (Afik & Ward 1989). Even if this is the case,
knowledge of the status of the fetus is of great importance, especially for birds incubating one or very few eggs.
The first possible response from the movement tests is a one way trend. An increasing or decreasing slope, illustrated in figure 11 as trend c) and d).
The second possible response is a maximum or minimum activity peak. These responses are illustrated as trends a) and b) in figure 11 and indicate that temperatures important for development were reached or exceeded. These four responses (a-d in figure 11) can all indicate comfort or distress of the fetus, depending on when they occur.
The results from our study showed a significantly higher number of fetuses showing the maximum activity trend on ventilation frequency at IP, movement occurrence at IP and both movement occurrence and maximal intensity of the movements at 18d. These results indicate an importance of the temperature associated with this maximum peak. That a high ventilation frequency and a high movement activity in an egg would indicate comfort is challenged by the low temperature at the peak (35.5 – 36.0°C from figure 9). If high movement activity was a sign of comfort the peak would be expected in temperature interval T2 which is the interval including optimal incubation temperatures (Webb 1987, Conway & Martin 2000). This was not the case.
The most likely explanation of high activity would be an indication of distress. It implies that temperatures around T3 are important for fetal
development. Several studies dealing with the effects of ambient temperatures on avian incubation suggest three important thresholds: physiological zero temperature, lower limit of optimal development and upper lethal temperature (Lundy 1969, Webb 1987, Conway & Martin 2000, Yahav et al. 2004). In chickens the physiological zero temperature is 26.0°C, the lower limit of optimal development is 36.0°C and the upper lethal temperature is 40.5°C (Lundy 1969). These are the temperature thresholds were behavioural responses can be expected.
The common temperature of naturally incubated avian fetuses is between 30°C and 40°C (Webb 1987). Consequently, most birds will be exposed the lower limit of optimal development during natural incubation and a response evolving at this threshold seems possible. If this is an all or nothing response the expected temperature to trigger this would then be just below 36.0°C. The significantly higher occurrence of maximum peak trend in our study occurred at 35.24°C for 18d and 35.53°C for IP which are both just below the suggested temperature for the lower limit of optimal development. Tazawa et al. (1989a) and Nichelmann et al. (1998) suggest, in their studies on the temperature coefficient Q10, that an important threshold occurs when the fetus goes under a Q10 of 2.0, and that this occurs for 18d fetuses around 36°C. Thereby, these studies support the theory of increased fetal movement indicating that a critical temperature threshold has been reached, and that this threshold occurs at a temperature close to 36°C.
The strongest trend responses were found in ventilation frequency of IP fetuses and at maximum event intensity in fetuses 18d and a weaker trend response in movement occurrence in 18d and IP. The weaker response in movement occurrence is likely to be due to the high activity in the egg involved with internal pipping (Kuo 1932b, 1937).
As predicted, movement intensity showed a significant increase, but only for 18d and not for IP fetuses. Again the result is most likely due to the large movements involved with internal pipping (Kuo 1932b, 1937).
The predicted outcome was an increase in fetal movements as the
temperature dropped. The observed response was a significant increase in both amplitude and frequency until temperatures around 36°C, followed by a
decline as the temperature continued to fall.
Fetal movement is correlated with temperature variations and consequently may be a means of feto-maternal communication.
Two further studies to conclude the suggested role of fetal movement as a means of feto-maternal communication would be I) repeating the same study on fetuses from the egg-burrowing family Megapodiidea where fetal-
movements can not play a role in feto-maternal communication and II), a study focusing on the parental behavioural responses to manually controlled fetal movements.
5.4 Consequences of temperature variations to fetal well-being
relatives (Rhen & Lang 1995, Shine et al. 1997). It has even been shown that the influence of incubation temperatures can over-ride genetically
predetermined properties as sex (Shine et al. 2002). It has also been
discovered that incubation temperatures influences phenotypic plasticity in birds (Göth & Booth 2005, Hepp et al. 2006). Recent research shows that incubation temperatures can affect the sex ratio, also in birds (Göth & Booth 2005). Here it seems to be due to different survival rates between the sexes, rather than an over-ride of genetics, but it still points out a new importance of incubation temperatures in birds.
Broilers have problems to cope with extreme external environments (Arjona et al. 1990, Yahav et al. 2004). Fetuses that have been exposed to temperature variations cope better to extreme environments later in life
(Arjona et al. 1990, Holland et al. 1997, Janke et al. 2002, Shinder et al. 2002, Moraes et al. 2003, 2004, Nichelmann 2004, Yahav et al. 2004). This
phenomenon is explained by the positive effects that thermal manipulation has on the development and efficiency of the thermoregulatory system (Arjona et al. 1990, Janke et al. 2002, Yahav et al. 2004). For thousands of generations broiler eggs have been hatched in incubators with a steady temperature optimal for fetal development. These unnatural conditions seem to have lead to a successive deterioration of the efficiency of their thermoregulatory system.
The effect of this on our study is that exposure to temperature gradients during prenatal development might influence the ability to respond to
temperature changes by adjusting the metabolic rate. The variations in pre-test environmental conditions between the studies would then produce biases in the test results and could partly explain the variation in results between different studies (Tazawa et al. 1988, Tazawa et al. 1989a, Tazawa et al. 1989b, Romijn & Lockhorst 1995, Mortola & Labbe 2005). Also, the results of studies on vocal responses to temperature gradients can be biased because of the same reason. It is obvious that studies conducted on eggs either in, or collected from, their natural environment show significant vocal responses to cooling: M. undulates (Berlin & Clark 1998), P. nigricollis (Brua et al. 1996)
L. delawarensis (Evans et al. 1994) and P. erythrorhynchos (Evans 1989,
Abraham & Evans 1999a). These studies were all conducted on eggs
incubated in a natural environment until latest a few days prior to hatching. Studies on ducks (Rumpf & Nichelmann 1992) and chickens (Bugden & Evans 1999, present study) show no occurrence of vocal responses to different
temperatures and are conducted on eggs incubated in incubators and have consequently never been exposed to any larger temperature variations.
To learn more about the effects that temperature variations have on the development and hatchability of chickens, several pilot studies were
conducted for future research. In one of the pilot studies, conducted on Gallus
gallus (red jungle fowls), temperature loggers were placed in the air cell of
eight eggs distributed in two nests and incubated between days 17 to 20 of incubation. All eight eggs hatched within the normal time even though incubation temperatures varied both between the eggs and the nests. The lowest average temperature measured in an egg was 32.2°C and the highest was 37.6°C. The eggs had a combined temperature difference of 22.9°C and still no defective effects could be seen. In a second unrelated pilot study eight broiler eggs incubated in large scale incubators (37.8°C) for 18 days were placed in room temperature (>20°C) for 17 h and were then returned to a incubator. As in the previous pilot study, all eight chicks hatched. These studies show that temperature fluctuating during the last days of incubation is not lethal for the fetuses.
In a third pilot study 16 fertile developing eggs were on day 18 divided into two incubators. Eight eggs were incubated in 30°C and eight in 37.8°C. The results were 100% mortality for the fetuses incubated at 30°C and 100% hatchability on the fetuses incubated at 37.8°C. These preliminary results show that the extended periods of low incubation temperatures is lethal for fetuses during the last days of incubation and this agrees well with results reported in previous studies (Lundy 1969, Webb 1987, Conway & Martin 2000)
Exposure to different temperatures is important as stimulus for activations and maturation of physiological mechanism. The role these
exposures have on the thermoregulation, both physiological and behavioural, remains unknown.Studies dealing with how different temperature exposures affects the fetus abilities to, physiologically and behaviourally, respond to temperature variations, might help to explain the contradictory results published in this field of research.
6. Conclusion
The main results of the study indicate that all developmental stages showed a linear decrease in metabolic rate in response to lowered temperatures without indication of compensatory thermoregulation as temperatures decreased. The
18d. At the same time, EP fetuses vocalized with significantly longer phrase duration than IP fetuses but none of the vocalization variables correlated with temperature. Fetal movements, on the other hand, responded to the
temperature challenge following a maximum activity trend. Ventilation frequency at IP, movement occurrence at IP and both movement occurrence and average maximal intensity of the movement events at 18d peaked at the third test temperature interval between 35-35.5ºC.
7. Acknowledgements
I want to thank my supervisor Associate ProfessorJordi Altimiras, for all the help guidance and critical reading of the manuscript. I also like to
acknowledge Kläckeribolaget in Väderstad for supplying me with broiler eggs, ELFA forskningsstiftelse for the grant 2005, Ingevald Abrahamsson for all his assistance with building and developing the experimental equipments, Christer Blomqvist for helping me with the acoustic analysis, Alf Blomqvist for all his help at Götala research station and Petronella Steen for critical reading of the manuscript.
8. References
Abraham CL & Evans RM (1999a) Metabolic costs of heat solicitation calls in relation to thermal need in embryos of American white pelicans.
Animal Behaviour 57, 967-975.
Abraham CL & Evans RM (1999b) The development of endothermy in american white pelicans. The Condor 101, 832-841.
Afik D & Ward D (1989) Incubation of Dead Eggs. Auk 106, 726-728. Arjona AA, Denbow DM & Weaver LDJ (1990) Neonatally-induced
Thermortolerance: Physiological responses. Comp Biochem Physiol 95A, 393-399.
Berlin KE & Clark AB (1998) Embryonic calls as care-soliciting signals in budgerigars, Melopsittacus undulatus. Ethology 104, 531-544.
Brua RB (1996) Impact of embryonic vocalizations on the incubation behaviour of eared grebes. Behaviour 133, 145-160.
Brua RB, Nuechterlein GL & Buitron D (1996) Vocal response of eared grebe embryos to egg cooling and egg turning. The Auk 113, 525-533.
Bugden SC & Evans RM (1999) The development of a vocal
thermoregulatory response to temperature in embryos of the domestic chicken. Wilson Bulletin 111, 188-194.
Collias NE (1952) The development of social behaviours in birds. Auk 69, 127-159.
Collias NE (1987) The vocal repertoire of the Red Junglefowl: a spectrograpic classification and the code of communication. The Condor 89, 510-524. Conway CJ & Martin TE (2000) Effects of ambient temperature on avian
incubation behavior. Behavioral Ecology 11, 178-188.
Curran-Everett D & Benos DJ (2004) Guidelines for reporting statistics in journals published by the Americal Physiological Society. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology 287, R247-R249.
Dawes CM (1981) The effects of cooling the egg on the respiratory
movements of the hatching fowl (Gallus domesticus) with a note on vocalization. Comp Biochem Physiol 68A, 399-404.
Decuypere E, Nouwn EJ, Kühn ER, Geers R & Michels H (1979) Iodohormones in the serum of chick embryos and post-hatching
chickens as influenced by incubation temperature. Relationship with the hatching process and thermogenesis. Ann. Biol. Anim. Biochem.
Biophys 39, 255-264.
Evans RM (1989) Egg temperatures and parental behavior during the
transition from incubation to brooding in the american white pelican. Auk 106, 26-33.
Evans RM, Whitaker A & Wiebe MO (1994) Development of Vocal
Regulation of Temperature by Embryos in Pipped Eggs of Ring-Billed Gulls. Auk 111, 596-604.
Freeman BM (1964) The Emergence of the Homeothermic-Metabolic
Response in the Fowl (Gallus Domesticus). Comp Biochem Physiol 13, 413-22.
Göth A & Booth DT (2005) Temperature-dependent sex ratio in a bird. Biology Letters 1, 31-33.
Hepp GR, Kennamer RA & Johnson MH (2006) Maternal effects in Wood Ducks: incubation temperature influences incubation period and neonate phenotype. Functional Ecology 20, 307-314.
Holland GB, Nichelmann M & Höchel J (1997) Development in heat loss mechanisms in avian embryos. Verh.Dtsch.Zool.Ges. 90, 105. Janke O, Tzschentke B, Hochel J & Nichelmann M (2002) Metabolic
responses of chicken and muscovy duck embryos to high incubation temperatures. Comp Biochem Physiol A Mol Integr Physiol 131, 741-50.
Kuo ZY (1932a) Ontogeny of embryonic behavior in aves. I. The chronology and general nature of the behavior of the chick embryo. Journal of Experimental Zoology 61, 395-430.
Kuo ZY (1932b) Ontogeny of embryonic behavior in aves. I. The mechanical factors in various stages leading to hatching. Journal of Experimental Zoology 62, 453-487.
Kuo ZY (1937) Ontogeny of embryonic behavior in aves. XI. Respiration in the chick embryo. Journal of Comparative Psychology 24, 49-58. Kuroda O, Matsunaga C, Whittow GC & Tazawa H (1990) Comparative
metabolic responses to prolonged cooling in precocial duck (Anas
domestica) and altricial pigeon (Columba domestica) embryos. Comp
Biochem Physiol A 95, 407-10.
Lundy H (1969) A review of the effects of temperature, humidity, turning and gaseous environment in the incubator on the hatchbility of the hen's egg. in T.C. Carter and B.M. Freeman, eds. The Fertility and
Hatchability of the Hen’s Egg. Oliver & Boyd, Edinburgh, 143-176. Matsunaga C, Mathiu PM, Whittow GC & Tazawa H (1989) Oxygen
consumption of brown noddy (Anous stolidus) embryos in a
quasiequilibrium state at lowered ambient temperatures. Comp Biochem Physiol A 93, 707-10.
Moraes VMB, Malheiros RD, Bruggeman V, Collin A, Tona K, Van As P, Onagbesan OM, Buyse J, Decuypere E & Macari M (2003) Effect of thermal conditioning during embryonic development on aspects of physiological responses of broilers to heat stress. Journal of Thermal Biology 28, 133-140.
Moraes VMB, Malheiros RD, Bruggeman V, Collin A, Tona K, Van As P, Onagbesan OM, Buyse J, Decuypere E & Macari M (2004) The effect of timing of thermal conditioning during incubation on embryo
physiological parameters and its relationship to thermotolerance in adult broiler chickens. Journal of Thermal Biology 29, 55-61.
Mortola JP & Labbe K (2005) Oxygen consumption of the chicken embryo: interaction between temperature and oxygenation. Respir Physiol Neurobiol 146, 97-106.
Nichelmann M (2004) Perinatal epigenetic temperature adaptation in avian species: comparison of turkey and Muscovy duck. Journal of Thermal Biology 29, 613-619.
Nichelmann M, Burmeister A, Janke O, Hochel J & Tzschentke B (1998) Avian embryonic thermoregulation: Role of Q(10) in interpretation of endothermic reactions. Journal of Thermal Biology 23, 369-376. Rhen T & Lang JW (1995) Phenotypic Plasticity for Growth in teh Common
Snapping Turtle: Effects of Incubation Temperature, Clutch, and Their Interaction. The American Naturalist 146, 726-747.
Romijn C & Lockhorst W (1995) Chemical heat regulation in the chick embryo. Poultry Science 34, 649-654.
Rumpf M & Nichelmann M (1992) On Ontogeny of Embryo-Maternal Acoustic Communication in the Muscovy Duck (Cairina moschata). Zoologische Jahrbucher-Abteilung Fur Allgemeine Zoologie Und Physiologie Der Tiere 96, 379-394.
Rumpf M & Nichelmann M (1993) Development of prenatal acoustic interaction in the muscovy duck (Cairina moschata). British Poultry Science 34, 287-296.
Saunders JC & Salvi RJ (1993) Psychoacoustics on normal adult chickens: thresholds and temporal integration. J Acoust Soc Am 94, 83-90. Shinder D, Luger D, Rusal M, Rzepakovsky V, Bresler V & Yahav S (2002)
Early age cold conditioning in broiler chickens (Gallus domesticus): thermotolerance and growth responses. Journal of Thermal Biology 27, 517-523.
Shine R, Elphick MC & Harlow P (1997) The influence of Natural Incubation Environments on teh Phenotypic Traits of Hatchling Lizards. Ecology 78, 2559-2568.
Shine R, Elphick MC & Donnellan S (2002) Co-occurrence of multiple, supposedly incompatible modes of sex determination in lizard population. Ecology Letters 2002, 485-489.
Sleigh JM & Lickliter R (1996) Type and amount of prenatal stimulation alters perceptual responsiveness in bobwhite quail chicks. Infant Behavior and Development 19, 325-338.
Sokal RR & Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. New York: W.H.Freeman.
Starrs AP, Orgeig S, Daniels CB, Davies M & V. LO (2001) Antioxidant enzymes in the developing lungs of egg-laying and metamorphosing vertebrates. Journal of Experimental Biology 204, 3973–3981.
Tazawa H & Rahn H (1987) Temperature and metabolism of chick embryos and hatchlings after prolonged cooling. J Exp Zool Suppl 1, 105-9. Tazawa H, Wakayama H, Turner JS & Paganelli CV (1988) Metabolic
compensation for gradual cooling in developing chick embryos. Comparative Biochemistry and Physiology 89A, 125-129. Tazawa H, Okuda A, Nakazawa S & Whittow GC (1989a) Metabolic
responses of chicken embryos to graded, prolonged alterations in ambient temperature. Comparative Biochemistry and Physiology 92A, 613-617.
Tazawa H, Whittow GC, Turner JS & Paganelli CV (1989b) Metabolic responses to gradual cooling in chicken eggs treated with thiourea and oxygen. Comparative Biochemistry and Physiology 92A, 619-622. Tazawa H, Okuda A, Nakazawa S & Whittow GC (1989c) Metabolic
Responses of Chicken Embryos to Graded, Prolonged Alterations in Ambient-Temperature. Comparative Biochemistry and Physiology A-Physiology 92, 613-617.
Tolkamp BJ & Kyriazakis I (1999) To split behaviour into bouts, log-transform the intervals. Animal Behaviour 57, 807-817.
Webb DR (1987) Thermal tolerance of avian embryos: a review. The Condor 89, 874-898.
Yahav S, Sasson Rath R & Shinder D (2004) The effect of thermal manipulations during embryogenesis of broiler chicks (Gallus
domesticus) on hatchability, body weight and thermoregulation after
41.64 (40.95 – 42.34) 66.81 (61.22 – 76.56) 10 EP 39.91 (35.99 – 43.76) 66.65 (59.55 – 73.10) 10 IP 33.66 (30.10 – 36-67) 67.03 (62.38 – 72.41) 10 18d Fetal mass trial day (g) Egg mass (g) No. eggs Stage
Table 1. The egg mass (g) prior to incubation and the fetal mass (without yolk sac) after trials. Fetal mass of the EP eggs was obtained for only two eggs. Values shown as mean (min-max).
on on on 1 25.8 135-150 off off on 2 25.8 120-135 T5 on on on 1 28.8 105-120 off off on 2 28.8 90-105 T4 on on on 1 31.8 75-90 off off on 2 31.8 60-75 T3 on on on 1 34.8 45-60 off off on 2 34.8 30-45 T2 on on on 1 37.8 15-30 off off on 2 37.8 0-15 T1 Sound Force [O2] Chamber Temp (°C) Time (min) Interval
9. Tables and Figures
Table 2. Run schedule for trials in experimental incubator. The temperatures are the controller settings for the experimental incubator. The on or off option refers to whether the variable was recorded or not. Channel 1 contained the tested fetus and channel 2 the unfertile egg used as baseline.
F = 5.287 P = .034* F = .027 P = .872 F = 1.557 P = .228 F = 1.616 P = .220 IP – EP F = .763 P = .394 F = .137 P = .715 18d – IP Phrase duration Phrase intensity Peeps per phrase Phrases occurrence Movement intensity Movement occurrence
Table 3. Statistical results of the repeated measures ANOVA over the
temperature interval T1-T5 (repeated factor) at different developmental stage (main factor), (N=10, df=1). * indicates a significant difference between the tested stages (P<0.05).
8 3 5 2 4 3 2 4 7 6 6 Peak Trend 2 5 4 6 5 5 7 6 3 4 4 Other Trend EP IP 18d EP IP 18d EP IP 18d EP IP 18d EP IP 18d EP IP 18d Stage 10.0* 12.1* 10 Ventilation frequency 0.13 0.5 8 2.25 3.36 9 Phrase duration 0.13 0 8 0.69 1.36 9 Phrase intensity 0.13 0.5 8 0.03 0.03 9 Phrases Occurrence 0.4 0.9 10 6.4 * 8.1* 10 Max movement intensity 3.6 4.9* 10 3.6 4.9* 10 Movement Occurrence χχχχ2 - Yates χχχχ2 N Variable
Table 4. Distribution of the “maximum peak” trend in comparison with other trends (see Figure 8). A 1:4 ratio is expected if the distribution is random. The
χ2value of the observed-expected comparison before and after the application
of Yates correction is displayed in the last two columns (critical values 0.1 = 2.71 and 0.05 = 3.84 when df = 1 ). * indicates a significantly higher number of “maximum peak” trends.
Figure 1. Experimental chamber. Egg (a) placed with the air cell facing up in a light plastic egg cup glued to a force transducer (b) measuring fetal-movements and ventilation frequency. Microphone (c) fitted to the wall of the chamber measuring vocalizations. Two tubing connectors used to bring air in (d) and leading it out (e). Thermo couple fitted to the lid measuring the chamber temperature (f). a b c d f e
Figure 2. The correlation between chamber temperature and temperature of the allantoic fluid (N=6). The dotted line indicates line of equality. 30 32 34 36 38 40 42 30 31 32 33 34 35 36 37 38 39 40 41 Temperature (ºC) chamber Te m p er at ur e (º C ) a lla nt o ic fl u id Pump in out N- valve in out Multiplex. in out Chamber in out Analyzer in out Flowmet. in out Air from incubator
Included in FOX II Analyser
Figure 4. Wash-out test. The chamber was flushed with nitrogen until
oxygen concentration [O2] read zero. Thereafter the chamber was
flushed with air from inside the experimental incubator ([O2] = 20%)
at a flow of 50 ml min-1.The test was stopped when [O
2] was stable. 95% 0 4 8 12 16 20 0 100 200 300 400 500 Time (s) [O2 ] ( % )
Sound signal (15 min)
Noise reduction profile: FFT size : 512 p
Reduce by: 40dB
Precision Factor: 7 Smoothing amount: 1 Spectral Decay rate: 65% Sound signal free
from background noise (15 min)
Band pass filter: High cut of 5000 Hz Low cut of 2000 Hz Sound signal free from
background noise and filtered (15 min)
Thresholds:
IP: -42 db
EP: -36 db
For more than 1 ms Sound signal free from
background noise and filtered with marked events (15 min)
Sound signal free from background noise and filtered with marked events and without silence (<15 min) Export event list
to Excel for event analysis
Export data to Excel for total sound intensity and phrase duration analysis In te ns ity (d B ) Time (s) 0 1 2 3 4 0 1 2 3 4 0 1 2 3 4 In te ns ity (d B ) In te ns ity (d B ) Time (s) Time (s)
S ou nd in te ns ity
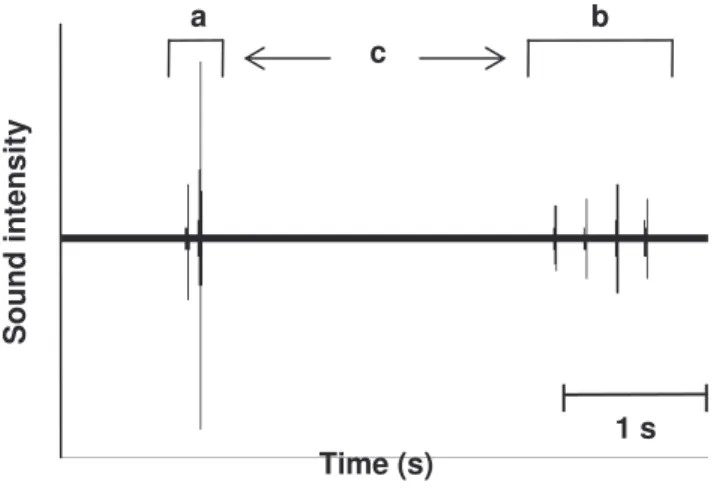
Figure 6. Section of a sound signal with two phrases, a and b, containing 2 and 4 peeps respectively. The time of silence between the two phrases, here labeled c, consequently must exceed the time used to define a phrase or else both clusters of peeps would be within the same phrase.
a b
1 s Time (s)
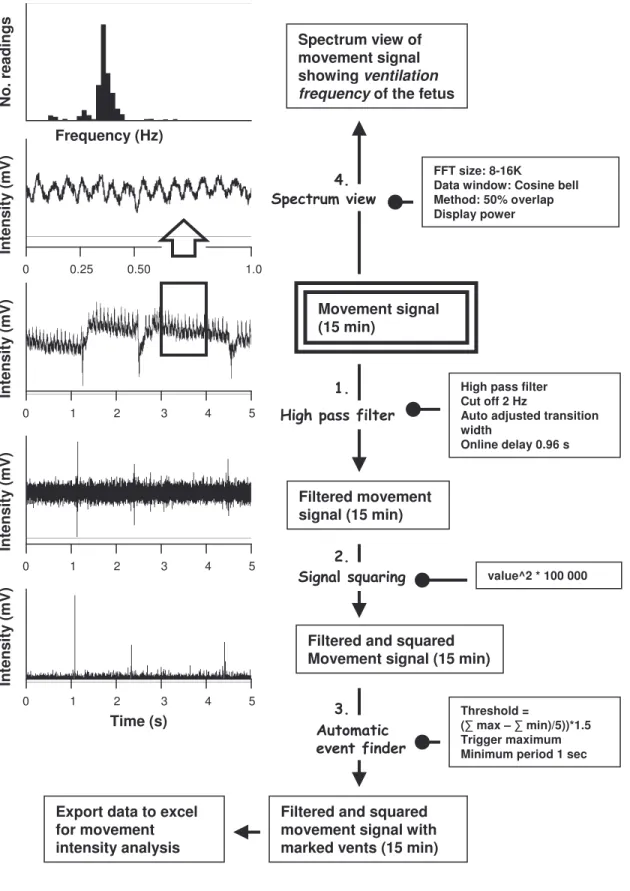
Movement signal (15 min)
FFT size: 8-16K
Data window: Cosine bell Method: 50% overlap Display power Spectrum view of
movement signal showing ventilation frequency of the fetus
Filtered movement signal (15 min)
High pass filter Cut off 2 Hz
Auto adjusted transition width
Online delay 0.96 s
value^2 * 100 000
Filtered and squared Movement signal (15 min) Time (s) In te ns ity (m V ) N o. r ea di ng s Frequency (Hz) Threshold = ( max – min)/5))*1.5 Trigger maximum Minimum period 1 sec
Filtered and squared movement signal with marked vents (15 min) Export data to excel
for movement intensity analysis 0 1 2 3 4 5 0 1 2 3 4 5 0 1 2 3 4 5 0 0.25 0.50 1.0 In te ns ity (m V ) In te ns ity (m V ) In te ns ity (m V )
+ + + + -T1 T2 T3 T4 T5 T1 T2 T3 T4 T5 T1 T2 T3 T4 T5 T1 T2 T3 T4 T5 a) b) d) c) V ar ia bl e of in te re st V ar ia bl e of in te re st V ar ia bl e of in te re st V ar ia bl e of in te re st
Figure 8. From the combination of the signs calculated from two lines there are four possible outcomes: a) +/- = ”maximum peak” trend, b) -/+ = ”minimum peak” trend, c) +/+ = ”rising” trend and d) -/- = ”falling” trend.
0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 29 30 31 32 33 34 35 36 37 38 39 40 41 Temperature (ºC) V O2 (m l m in -1 )
Figure 9. Oxygen consumption (VO2) at different ambient temperature
intervals (T1-T5) in chicken fetuses of three different developmental stages: 18 days old fetuses ( 18d), Internally pipped fetuses ( IP) and externally pipped fetuses ( EP). Data as means (SD) N=10.
T1 T2 T3 T4 T5 0 0.01 0.02 0.03 0.04
Figure 10. The temperature sensitivity of 18d in grey, IP in white
and EP in black. indicates a significant difference (P < 0.05), in
temperature sensitivity compared with 18d. Data as means (SD) N=10. Te m pe ra tu re s en si tiv ity (m l m in -1ºC -1)
0 2 4 6 8 10 0 0.2 0.4 0.6 0.8 1 3 5 9 Time (s) Time (s) 0 2 4 6 8 10 0 0.2 0.4 0.6 0.8 1 3 5 9 % % %
Fig 11. Frequency distribution of the time duration between sound events in fetuses of two different developmental stages (IP-top panel and EP-bottom panel). The natural gaps at 0.15 s and 0.8 s were used to minimize the error when defining peeps (>0.15 s and <0.8 s) and phrases (>0.8 s) in the sound analysis.
0
10
20
30
T1
T2
T3
T4
T5
0 1 2 3 4 5 T1 T2 T3 T4 T50
2
4
6
8
T1 T2 T3 T4 T5
0
0.1
0.2
0.3
0.4
T1
T2
T3
T4
T5
0 10 20 30 0 2 6 8 4 0.2 0.3 0.1 0 T1 T2 T3 T4 T5 T1 T2 T3 T4 T5 T1 T2 T3 T4 T5 T1 T2 T3 T4 T5 0.4 2 3 1 0 4 5Figure 12. Sound-related variables: phrase occurrence (phrases in 15 min), peeps per phrase, average phrase intensity and phrase duration at two developmental ages (IP – open bars, EP – closed bars) and at the five temperature intervals (T1-T5). Data as mean (SD) N=10. indicates a significant difference between the two developmental stages (P < 0.05).
P hr as e O cc ur re nc e P ee ps ph ra se -1 P hr as e du ra tio n (s ) P hr as e In te ns ity (a .u .)
0 2 4 6 T1 T2 T3 T4 T5
T1
T2
T3
T4
T5
50 60 70 80 90 100 T1 T2 T3 T4 T5 T1 T2 T3 T4 T5 0 T1 T2 T3 T4 T5 5 15 10 20 0 2 4 6Figure 13. Movement-related variables: movement occurrence (min-1) and maximal movement intensity at two developmental ages (18d – shaded bars, IP – open bars) during temperature intervals T1-T5. Data as mean (SD) N=10.
M ax M ov em en ti nt en si ty (a .u .) M ov em en tO cc ur re nc e (m in -1)
Figure 14. Ventilation frequency of IP fetuses at five temperature intervals T1-T5. Data as mean (SD) N=10.
V en til at io n fr eq ue nc y (m in -1)
![Figure 4. Wash-out test. The chamber was flushed with nitrogen until oxygen concentration [O 2 ] read zero](https://thumb-eu.123doks.com/thumbv2/5dokorg/5516142.143826/33.918.202.722.237.476/figure-wash-test-chamber-flushed-nitrogen-oxygen-concentration.webp)