WHAT IS REQUIRED FOR THE VIABILITY OF
METAL RECOVERY FROM MUNICIPAL
SOLID-WASTE INCINERATION FLY ASH? –
DESIGN AND ASSESSMENT OF A PROCESS
PLANT FOR COPPER EXTRACTION
Sten
Karlsson
1Patrik Carlsson
1Daniel Åberg
1Karin Karlfeldt Fedje
1Joakim Krook
2Britt-Marie Steenari
1 1Chalmers University of Technology, Sweden
2
Linköping University, Sweden
ABSTRACT
The incineration of municipal solid waste produces large amounts of fly ashes, today in Sweden around 200 000 tons/yr. The ashes normally contain a considerable amount of valuable and hazardous metals. To fulfil the environmental regulations, most of these fly ashes can be deposited only in specific sites. This handling costs, requires energy and leads to emissions in the transportation to the deposit site.
This work, taking departure in laboratory experiments for crucial steps, discusses possible chemical processing schemes and from this develops an overall design of a plant for the extraction and production of copper from the fly ash generated in a fluidized bed waste incineration plant. It also addresses the economic viability and environmental impact of the suggested processing in comparison to current handling, which involves transport to and disposal of the fly ash in Norway. The proposed process involves a leaching step, a solvent extraction process, a stripping step where the copper is transferred to an aqueous phase and finally electrolysis. By quantitative modelling and cost estimates of the processing steps of the proposed plant we identify the most important factors for the economics and environmental impact of the plant. In addition, we quantify the necessary recycling rates of the different process chemicals for achieving profitability. We conclude that a crucial factor is the recycling rate of the used organic solvent. Important parameters are also the handling costs and transportation needs of the rest products. For instance, a major benefit of the process is if treated fly ash can be reclassified such that it is allowed to be disposed into the own close-up hazardous waste landfill thus lowering the costs and environmental impacts. An extension of the process to include also the extraction of metals other than copper, for instance zinc, should be an interesting further development to consider.
KEYWORDS
1 INTRODUCTION
In Sweden, as in many other countries, generation of municipal solid waste (MSW) has continuously increased over time. At present, approximately 4.7 million tonnes of MSW, i.e. more than 500kg/capita, is annually generated in the country [1]. This is an increase of more than 100 kg/capita since 1995 [2]. Also within the EU-27, an increase in the amount of MSW has been observed during this period; from 474kg/capita in 1995 to 517kg/capita in 2006 [2]. In recent decades, Swedish waste management has gone through dramatic changes, partially due to new regulations emphasising material recovery and incineration [1-4]. An expansion of incineration capacity has played a central role for this development and approximately 50% of the MSW is currently energy recovered while less than 5% is land-filled.
Although incineration of waste offers several benefits compared to land-filling, e.g. substantial reduction in waste volume, recovery of energy and destruction of toxic organic compounds [5-7]; it also generates significant amounts of bottom ash and flue gas cleaning by-products (from now on termed fly ash). Such residues, especially the fly ash, are enriched in potentially hazardous substances and therefore have to be handled in ways limiting negative effects on the environment or human health [8]. Each year, Swedish waste incinerators generate about 700 000 tonnes of bottom ash and 200 000 tonnes of fly ash [1]. The bottom ash is either directly landfilled or used as construction material at waste deposits. For fly ash, on the other hand, the disposal routes are more complex and have also changed over time.
In the beginning of the 2000s, the fly ash was typically disposed of in Swedish landfills for hazardous waste. Then, however, the EC Directive on the landfill of waste was brought into legal force, making this disposal option more or less impossible [4]. This since the fly ash virtually always exceeds stated limit values for disposal at such deposits regarding leachability of, for instance, chlorides and potentially toxic metals. At present, there are only a few incinerators with permission to store their fly ash in domestic landfills or rock shelters. For most incinerators, however, there is not yet any developed domestic disposal route [9, 10]. Instead, the main part of the fly ash is transported to a company in Norway, where it is used to neutralize sulphuric acid residues from the paint industry. This procedure is classified as “recycling” and the generated “slurry” is disposed of in old mines at Langøya, Holmestrand, Norway. Although this disposal only renders slightly higher costs compared to the domestic disposal in hazardous landfills used before the Landfill Directive was implemented, it adds uncertainty since this option largely relies on one single actor with a monopoly status [11]. The costs for disposal could thus suddenly be increased or else this company might for some reason loose their permission to fill up old mines with fly ash, leaving Swedish incinerators without any developed alternative. Of course, the long-distance transportation of fly ash to Norway also adds environmental pressure to the disposal of Swedish fly ash.
As a consequence, several Swedish initiatives have recently been conducted exploring possible options for storing the fly ash in domestic mines and dressing sand quarries [12,13]. Treatment of the fly ash as well as the bottom ash, e.g. through washing and leaching processes, in order to make it fulfil legal requirements for disposal at domestic hazardous landfills is another example of such initiatives [14-17]. A number of treatment methods have been suggested and an overview can be found in [18].
Apart from hazardous substances, however, waste incineration fly ash contains significant amounts of valuable metals, such as copper (typically 600-3200 mg/kg dry ash) and could also constitute a resource itself as, e.g., earth construction material [8,19]. The disposal of fly ash in old mines or hazardous landfills thus inherently lead to that limited natural resources are lost, cf. [20]. Quite recently, investigations of the applicability of hydro metallurgic methods to recover metals from MSWI ash have started [21-23]. Providing that the metal compounds present in the ash can be dissolved in, for example, an acid, either solvent extraction or electro chemical processes can be used to separate the metals into pure forms. Since solvent extraction is used in many other applications, a large number of extraction agents (extraction ligands) have been developed. Quite a few are available commercially, which can simplify the development of methods to recover metals from dissolved fly ash. In addition to the need for more research into the technical issues of metal recovery from fly ash, there is a lack of evaluations from a systems perspective. Such work is very important since it goes beyond the present focus on pollution concerns and also takes into account the related resource implications and points out the most environmentally and economically favourable paths to follow [24].
In this paper, we develop an overall design of a plant for extracting copper from the generated waste incineration fly ash from one combustion plant in Sweden. The economic and environmental feasibility of realizing this process is then evaluated by comparing to the present disposal route, involving transportation to Langøya in Norway for disposal in old mines. Emphasis is on identifying critical factors for economic and environmental performance such as efficiency of copper recovery and recycling rates of process chemicals.
2 METHOD
2.1 Process identification
Enhanced leaching of ash is widely used to release metal compounds from the ash. The probably most widespread leaching method is acid leaching [25-28]. However, due to the high alkalinity of ash large amounts of acid is needed to reach a sufficiently low pH. An alternative is to use other leaching agents, which form soluble complexes with metal ions at higher pH [29-30]. In addition leaching at slightly alkaline pH using NH4NO3 can be effective
for the release of Cu from ash [23]. The reason for the selective dissolution of copper is mainly that copper ions form soluble complexes with ammonia.
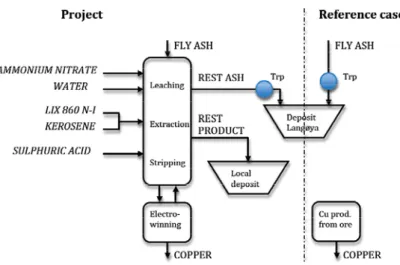
The proposed leaching-extraction method is given as a flow sheet in Figure 1. In the first
step, “Ash leaching”, the ash is leached using 3M NH4NO3. After filtration, the pH in the
leachate is adjusted to 2 using HNO3. In the second step, “Cu extraction”, the leachate is
mixed with an organic phase containing the extractant (LIX 860N-I). In the third step, “Cu stripping”, the loaded organic containing Cu is mixed with 2M H2SO4.The hydrogen ions
from the acid replace the Cu2+ in the extractant in the organic phase making the extractant available for re-use. In the fourth step, “Cu electrowinning”, high purity Cu metal is recovered through electrolysis and the spent electrolyte becomes available for another stripping cycle.
Figure 1. Flow sheet of the proposed recovery method developed for Cu from fly ash.
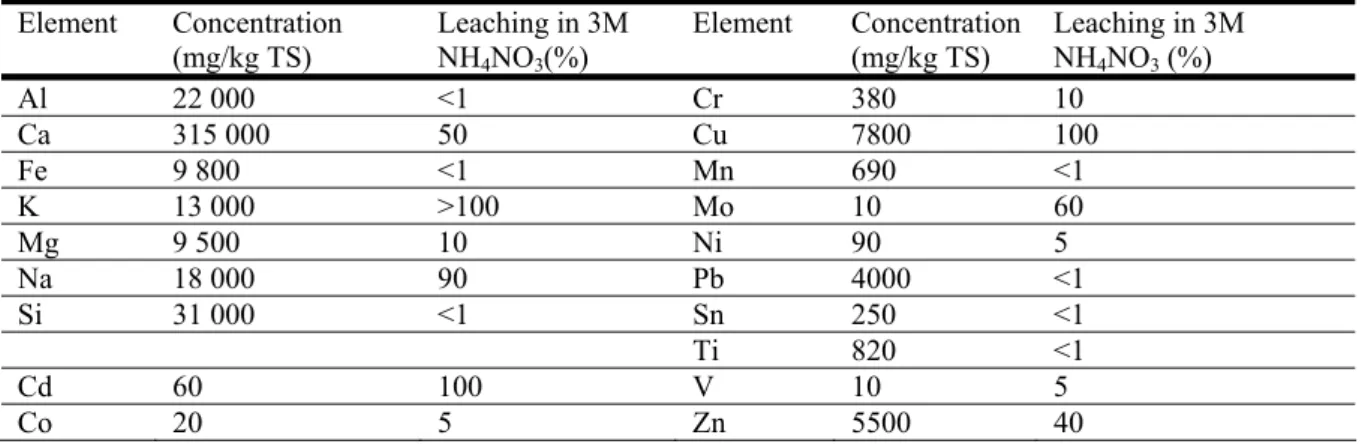
Table 1. The composition of selected elements in the fly ash and the shares leached in the 3M NH4NO3 leaching step used in the analysis.
Element Concentration (mg/kg TS) Leaching in 3M NH4NO3(%) Element Concentration (mg/kg TS) Leaching in 3M NH4NO3 (%) Al 22 000 <1 Cr 380 10 Ca 315 000 50 Cu 7800 100 Fe 9 800 <1 Mn 690 <1 K 13 000 >100 Mo 10 60 Mg 9 500 10 Ni 90 5 Na 18 000 90 Pb 4000 <1 Si 31 000 <1 Sn 250 <1 Ti 820 <1 Cd 60 100 V 10 5 Co 20 5 Zn 5500 40
In this work a fly ash from a bubbling fluidized bed (BFB) combustion plant mainly incinerating MSW and with a relatively high content of copper was studied, Table 1. The copper recovery process has been evaluated in laboratory tests carried out for a number of fly ash samples from different combustion units. It was found to give a copper yield of 50-95% of total Cu content in the ash, and 90-95% recovery of the dissolved copper [23]. Further optimization of the process is in progress.
We assess a project composed of a suggested process plant utilizing the identified copper recovery process. We assess the project in comparison to a reference case consisting of the current situation with transport of the fly ash for storage at Langøya, and production of the same amount of Cu from virgin ore, see Figure 2. The treatment capacity is 15 000 tons of fly ash per year containing 117 tons of copper. With some marginal for increased capacity, this corresponds to yearly output of the MSW plant generating the examined fly ash. The produced copper is sold to market price. The rest products are handled in the cheapest possible way, but in accordance with current legislation. In performed tests the rest ash has not fully fulfilled the legislation on leaching for disposal at a local landfill. In the base case, we therefore assume this part will still be sent to Langøya, Norway. The rest product from the extraction step is going to a nearby hazardous waste landfill site.
To estimate the operational costs for utilized major chemicals and energy as well as possible gains in energy use and CO2 emissions a modelling of the major materials and energy flows
and transports of the project and the reference case are performed, Figure 2. For a high recycling rate of chemicals in the process the transportation (tonkm) is dominated by the transport to the deposition at Langøya. For the transport and the production of each input the use of electricity, fuel and CO2 emissions are estimated. The data applied for the different
process flows in the assessment are given in Tables 2 and 3.
Figure 2. The assessed project and its reference case. The inputs are marked in italics. For these five inputs, the three involved processes (Leach./Extr./Str., Electrow., and Cu prod.) and the two marked transport services, the energy and CO2 emissions are estimated.
Table 2. Applied data for the different process fluxes in the base case.
Flow Cost [SEK]a) Electricity [GJ] Fuel [GJ] CO
2 [tons]
Ammonium nitrate,1 ton 2142 b) 0,86 h) 15.3 h) 1.0 h)
Sulphuric acid, 1 ton 693 b) – - 1.1 i) - 0.07 j) LIX 860 N-I, 1 ton 70686 c) – 43.4 + 43.4k) 3.1+ 3.1k)
Kerosene, 1 m3 7300 d) – 35.3 + 5.1 l) 2.45+ 0.37 m)
Water, 1 m3 9.58 e) – – –
Electricity Sweden, 1 MWh 761f) – – 0.1n) Leach./Extr./Str., per ton Cu 4.5o) – 0.125n)
Electrowinning of Cu, 1 ton - 54193 g) 9p) – 0.25n)
Cu from ore, 1 ton 14.0q) 11.8q) 4.1q)
a) A conversion ratio of 1 US$ = 7.14 SEK and 1 € = 9.59 SEK is used; b) [31]; c) [32]; d) [33]; e) We use water rates from city of Göteborg [34]; f) Average electricity cost 2010 [35]; g) We
use the market copper price (27-months seller) for sales revenues of copper [36]; h) For production of used ammonia and nitric acid we use CPMdatabase, Chalmers [37]. Waste heat substitutes fossil fuel with the same CO2 intensity as the fuel used in the ammonium nitrate
production; i) The production in Europe of H2SO4 from the various sources is estimated to
give in average a net export of steam [37]; j) Assumed same CO2 intensity for heat
substitution as for ammonium nitrate production; k) No data for the production of LIX860 N-I has been available. It is an expensive hydrocarbon. We therefore use the same energy and CO2 density as for kerosene, but also assume a fuel and CO2 cost in the production equal to
the energy and CO2 content of the chemical itself; l) The energy content of kerosene is 35.3
GJ/m3 [38]. We assume the same fuel use share in kerosene production as for CO2 emission,
or ≈ 15 %; m) For the production of kerosene the same specific (ton/GJ) value as for diesel is used [37]. CO2 content in the fuel 71.5 kg CO2/GJ [38]; n) Average value for Swedish
electricity production is used [35]; o) Pumping costs in leaching, extraction and stripping can contribute considerably to the energy cost in large-scale mining industry [39]. We therefore assume here half of the electricity use in the electrowinning step per ton Cu at 100 % recovery; p) The value for zinc production in a corresponding process adjusted for the lower standard potential in Cu reduction is used [21]. This also corresponds to data (8 GJ/ton Cu) for large-scale Cu electrowinning in mining industry [39]; q) Average values for Chilean electrorefined (i.e., pyrometallurgical) copper cathodes in 2008. Also including indirect fuels (fuels for fuels and electricity) gives a total energy value of 30.9 GJ/ton [40].
Table 3. Applied costs, fuel use and CO2 emissions for transportation and deposition of fly ash and rest product in the base case.
Process Cost SEK/tona) Fuel CO
2
Truck transport to landfill, 310 km one way 384 b) 0.011 GJ/km 0.79 kg/kmc)
Disposal of fly ash and rest ash in Langøya 480 b) – –
Local disposal of the rest product 450 d) – –
a) A conversion ratio of 1 € = 9.59 SEK is used; b) Price for delivery of fly ash at Langøya
[41]; c)Average value of truck transportation with and without trailer [42] + for diesel production; d) The rest products is assumed to be deposited at an existing local hazardous waste deposit at negligible marginal cost, except for the Swedish waste deposit tax of 450 SEK/ton.
Table 4. Plant costs (investment / annuity), and operational costs in the base case.
Investment 10.8 MSEK / 1.26 MSEK/yr
Initial cost for chemicals 0.92 MSEK / 0.11 MSEK/yr
Residual value - 1.0 MSEK / -0.02 MSEK/yr
Operational and maintenance costs 0.75 MSEK/yr
The process plant costs are given in Table 4. The plant cost consists of investment costs and cost for start-up chemicals assuming a turnover time for the processing batch of 6 hours. The investment costs is estimated by taking an average specific value for two existing hydrometallurgical plants producing yearly 40 000 and 2 100 tons, respectively, of Cu from ore [43], while applying a cost-capacity scale factor of 0.7 [44]. The operation of the plant is assumed handled by a personnel of one manyear/yr [45]. The project is evaluated by calculating the net present value, with an applied lifetime of 20 yrs and interest rate of 10%.
3 RESULTS
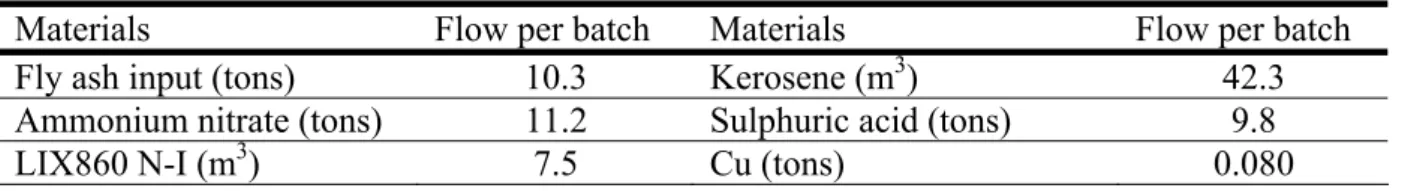
The involved materials turnover for each batch is shown in Table 5. The amounts of chemicals involved are in the order of the treated ash or larger (kerosene), and very large in comparison to the copper. The net present value (NPV) of the project is shown in Figure 3. For the base assumptions, the NPV is maximally around 44 million SEK. For a base case assumption of 95 % Cu overall yield, the sales of copper gives a revenue of 49 millions while 15 millions are due to the saved transport and deposition costs, Figure 3b. The income from copper sales is lowered with roughly 4 millions for each 10 % decrease in yield (or copper price) but the NPV is still positive at no copper yield showing that the savings of transport and dumping cost are paying for the project alone. The local dumping of the rest product is now assumed to be free of charge except for the waste deposit tax. Assuming a handling cost equal to dumping at Langøya would almost eradicate the cost benefits from avoiding the transport to Langøya. However, the energy and CO2 gains, discussed below, would prevail. Table 5. The materials turnover in one batch (6 hours operation).
Materials Flow per batch Materials Flow per batch
Fly ash input (tons) 10.3 Kerosene (m3) 42.3
Ammonium nitrate (tons) 11.2 Sulphuric acid (tons) 9.8
LIX860 N-I (m3) 7.5 Cu (tons) 0.080
On the other hand, because only a few species, mainly Ba, do not fulfil leaching limits in our tests, a further development of the leaching process and/or rest ash handling may make possible a local disposal also of the rest ash, and consequently avoidance of all the transport to Langøya, saving money, energy and CO2. A three times higher investment cost, as
suggested for a zinc extraction plant with similar metal recovery, but, due to higher metal concentration in that case, almost a factor of ten less treated fly ash [21], lowers the NPV by 20 million SEK. Such an increase in plant investment cost may still give a positive NPV, though. The NPV is sensitive to specifically the recycling rate of the kerosene, Figure 3a. At
the project non-profitable. For the other chemicals, and especially sulphuric acid, such a high recycling rate is not crucial for the profitability.
a) b)
Figure 3.a) Net present value of the project (MSEK) for copper yield and recycling rates for the different chemicals, respectively, varied between 100 and 80 %. (Note: Losses for LIX 860 N-I is a given at 3 kg/kg Cu.); b) Contribution of different factors to the net present value (MSEK) (Cu yield is held at 95 % and recycling rates at 99.9%)
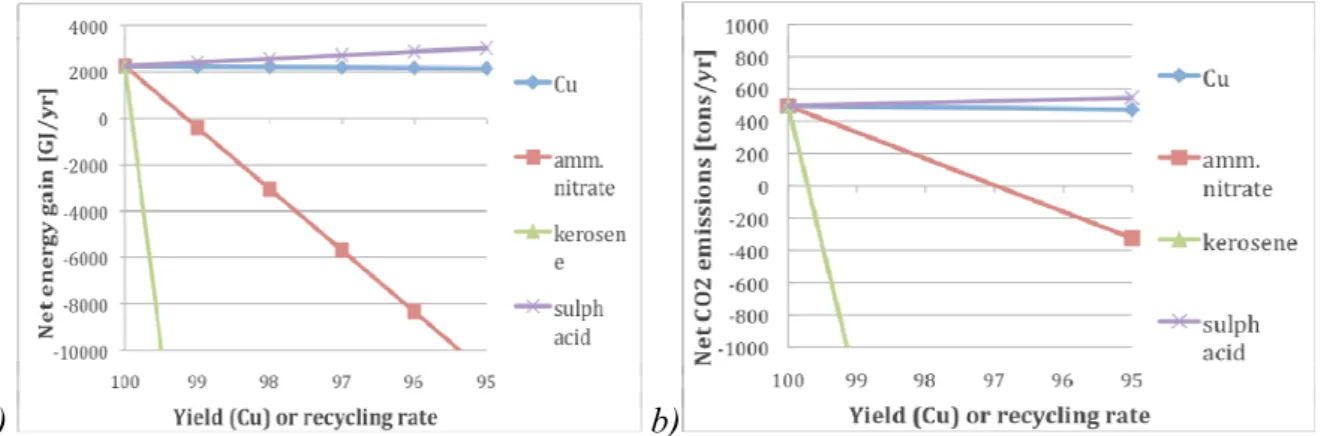
Figure 4 depicts, relative to the reference case, the project’s net energy gain and net CO2
emissions, respectively. Figure 5 shows how the different inputs and processes contribute to the energy costs and CO2 emissions, respectively, at an assumed loss rate of chemicals of 1‰
and a 95% recovery yield of copper. Both for energy and CO2 the recycling rate of process
chemicals is even more critical than for the cost, with energy turnover the most critical of the two.
a) b)
Figure 4. a) The project’s a) net energy gain (GJ/yr), and b) the net CO2 gain (tons/yr) for the project (incl. of initial set up of chemicals) for copper yield and recycling rates for the different chemicals, respectively, varied between 100 and 95 %. (Note: Losses for LIX 860 N-I is a given at 3 kg/kg Cu.)
a) b)
Figure 5. Processes contributing to the project’s a) net energy cost (GJ/yr), and b) net CO2 emission (tons/yr). The process chemicals are shown for a case of 0.1 % loss (recycling rate 99.9%) of the yearly process through-flow and 95% Cu yield. For kerosene the emission also is divided into the contributions from the solvent itself (bottom) and its production, respectively. (Note: Losses for LIX 860 N-I is a given at 3 kg/kg Cu.)
For the project to have a positive energy balance compared to the reference case the loss rate of kerosene, the most critical flow, has to be less than around 1‰, Figure 5a. For kerosene, the energy loss as well as the CO2 emissions are dependent on how the losses are handled; can
the energy in the solvent substitute for another CO2-emitting fossil energy use, then the
balance can be considerably more favourable. The energy and CO2 balances are relatively
insensitive to copper yield and losses of sulphuric acid.
4 CONCLUSIONS
We have performed an investigation of the requirements for a viable recovery of copper from municipal solid-waste incineration fly ash from the point of view of the net present value, the energy use and the CO2 emissions. It is a first preliminary assessment that has relied on a
process tested on a laboratory scale and applied to a specified ash from a fluidized bed boiler. We have in the base case applied a Cu overall yield of 95%, somewhat higher then achieved in the laboratory, 90% at best, but the process should be possible to further optimize. The suggested plant has been compared to the current alternative of copper production from ore and continued dumping of fly ash abroad involving a considerable amount of truck transport. At current or higher copper market prices, the sales of copper dominate the contribution to the net present value but also avoided transportation costs are significant. Due to the large turnover of chemicals in the suggested process, high recycling rates are required. The most stringent requirements come from the energy turnover; to be positive a loss rate of 1‰ or less is required for the process chemical kerosene. Regarding net CO2 emissions, the recycling
rates of process chemicals are less important due to considerable amounts of avoided emissions from transport of fly ash and virgin Cu production. However, in order to have a positive economic net present value, the recycling rate of kerosene needs to be around 99 %. An extension of the process to include also the extraction of metals other than copper, for instance zinc, should be an interesting further development to consider.
ACKNOWLEDGMENT
This project has received funding from the Swedish Research Council Formas, which is gratefully acknowledged.
REFERENCES
[1] The Swedish Waste Association, 2009. Svensk avfallshantering 2008. Malmö, Sweden. (in Swedish)
[2] Eurostat, 2009. Key figures on Europe, 2009 edition, Luxembourg, Louxembourg, Office for Orficial Publications of the European Communities.
[3] SFS Miljödepartementet, 1998. Miljöbalk (Environmental laws) 1998:808, updated to SFS 2007:661, Swedish Ministry of the Environment, Stockholm, 1998. (in Swedish) [4] Council Directive 99/31/EC. Directive 99/31/EC of the European Parliament and of
the Council on the Landfill of Waste.
[5] Björklund, A., Finnveden, G., 2005. Recycling revisited – Life cycle comparisons of global warming impact and total energy use of waste management strategies.
Resources, Conservation and Recycling 44, 309-317.
[6] Cheremisinoff, N.P., 2003. Handbook of solid waste management and waste minimization technologies, Elsevier Science.
[7] Sabbas, T. et al., 2003. Management of municipal solid waste incineration residues,
Waste Management 23, p. 61-88.
[8] Chandler A.J. et al.,1997. Municipal solid waste incineration residues, Elsevier: Amsterdam; New York.
[9] Engfeldt, C., 2007. Aska från energiproduktion – producerad och använd mängd aska i Sverige 2006. Svenska Energiaskor AB (in Swedish).
[10] SEPA, 2010. Nyhetsbrev nr 06/07–3 April. The Swedish Environmental Protection Agency, Stockholm, Sweden (in Swedish).
[11] Taberman, S-O., Tekniska Verken i Linköping AB. +46 13 208122. Personal communication.
[12] The Swedish Waste Association, 2009. Deponering eller utfyllnad av bergrum med RGR. Report F2009:05, Malmö, Sweden (in Swedish).
[13] The Swedish Waste Association, 2009. Möjligheter att använda rökgasreningsrester vid efterbehandlingen av deponier med sulfidhaltigt gruvavfall – rapport över laboratorieförsök. Report F2009:06, Malmö, Sweden (in Swedish).
[14] Wilewska – Bien, M., Lundberg, M., Steenari, B-M., Theliander, H., 2007. Treatment process for MSW combustion fly ash,laboratory and pilot plant experiments. Waste
Management 27, 1213-1224.
[15] Steenari, B.-M., Zhao, D., 2010. Washing of fly ash from combustion of municipal solid waste using water as leachant, Waste Refinery Report Project WR-17.
[16] Ecke, H., 2001. Carbonation for fixation of metals in municipal solid waste incineration (MSWI) fly ash. Doctoral thesis, Avfallsteknik, Luleå University of Technology, 2001:33.
[17] Steketee, J., Timmerije M., van Aalten, J., 2009. Accelerated carbonation and washing of MSWI bottom ash: from laboratory to full scale. Presentation at Wascon 2009, Lyon.
[18] Bjurström, H., Steenari, B.-M., 2003. ”Våt rening av askor, metodöversikt” (Wet treatment of ashes - a survey of methods), Värmeforsk, Miljöriktig användning av askor, Rapport Q4-129, ISSN 0282-3772, juli 2003. (in Swedish)
[19] Ferreira, D., Ribeiro, A., Ottosen, L., 2003. Possible applications for municipal solid waste fly ash. Journal of Hazardous Materials B96, 201-216
[20] Ayres, R.U., 1997. Metal recycling: Economic and environmental implications.
Resources, Conservation and Recycling 21, 145–173.
[21] Schlumberger, S., Schuster, M., Ringmann, S., Koralewska, R., 2007. Recovery of high purity zinc from filter ash produced during the thermal treatment of waste and inerting of residual materials. Waste management and research 25, 547-555.
[22] Karlfeldt Fedje, K., Ekberg, C., Skarnemark, G., Steenari, B.-M., 2010. Removal of hazardous metals from MSW fly ash - An evaluation of ash leaching methods. Journal
of Hazardous Materials 173, 310-317
[23] Karlfeldt, K., 2010. Metals in MSWI fly ash – problems or opportunities?, PhD Thesis, Department of Chemical and Biological Engineering, Chalmers University of Technology. ISBN 978-91-7385-386-6.
[24] Roth, L., Eklund, M., 2003. Environmental evaluation of reuse of by-products as road construction materials in Sweden. Waste Management 23, 107–116.
[25] van der Sloot, H.A., Kosson, D.S., Hjelmas, O., 2001. Characteristics, treatment and utilization of residues from municipal solid waste incineration, Waste Management 21, 753-765.
[26] Brunori, C., Balzamo, S., Morabito, R., 1999. Comparison between different leaching/extraction tests for the evaluation of metal release from fly ash. International
Journal of Environmental Analytical Chemistry 75, 19-31.
[27] Ariese, F., Swart, K., Morabito, R., Brunori, C., Balzamo, S., Slobodnik, J., et al., 2001. Leaching studies of inorganic and organic compounds from fly ash.
International Journal of Environmental Analytical Chemistry 82, 751-770.
[28] Liu, F., Liu, J., Yu, Q., Jin, Y, Nie, Y., 2005. Leaching Characteristics of Heavy Metals in Municipal Solid Waste Incinerator Fly Ash. Journal of Environmental
Science and Health Part A 40, 1975 – 1985.
[29] van Gerven, T., Cooreman, H., Imbrechts, K., Hindrix, K., Vandecasteele, C., 2007. Extraction of heavy metals from municipal solid waste incinerator (MSWI) bottom ash with organic solutions. Journal of Hazardous Materials 140, 376-381.
[30] Olsson, S., v. Schaik, J.W.J., Gustafsson, J.P., Kleja, D.B., canHees, P.A.W., 2007. Copper(II) binding to dissolved organic matter fractions in municipal solid waste incinerator bottom ash leachate. Environmental Science & Technology 41, 4286-4291. [31] Caterina Camerani, Eka Chemicals AB. Personal communication, May 3, 2010. [32] Simon Frauchiger, European sales manager, Cognis GmbH. Personal communication,
February 4, 2010.
[33] Kundservice, AB Svenska Shell. Personal communication, April 16, 2010. [34] http://www.goteborg.se/wps/portal
[35] Kanlén Fredrik, Swedish Statistics. Personal communication, April 23, 2010. [36] LME, http://www.lme.com/copper.asp, assessed Sept 24, 2010.
[37] CPMdatabase, Chalmers. http://www.cpm.chalmers.se/CPMDatabase/. [38]
http://www.naturvardsverket.se/sv/Klimat-i-forandring/Minska-utslappen/Verktygslada-for-kommuner-och-foretag/ [39]
http://www.copper.org/publications/newsletters/innovations/2001/08/hydrometallurgy. html
[40] Hunt, S., 2009. Energy consumption and greenhouse gas emissions in the Chilean copper mining industry, Chilean Copper Commission Research and Policy Planning Department, Chile.
[41] Morten Hallan, Noah, Production manager. Personal communication, April 14, 2010. [42] SMHI och IVL Svenska Miljöinstitutet AB med underkonsulter, Handbok för
vägtrafikens luftföroreningar, ISSN: 1401-9612.
[43] http://www.allbusiness.com/manufacturing/primary-metal-mfg-nonferrous/7249913-1.html
[44] Green, D.W., Perry, R.H., 2008. Perry's Chemical Engineers' Handbook (8th Edition). McGraw-Hill.