RME-3044
ANNUAL REPORT FOR JULY 1, 1952 TO MARCH 31, 1953 By John W. Gruner Lynn Gardiner Deane K. Smith, Jr. April 1, 1953
[Site Issuance Date]
Division of Raw Materials, AEC
Information Service, Oak Ridge, Tennessee
Prop-en; iA
JOhn U T omHnson Ubrca r
M01.!ll Ststo College
RME-3044
ANNUAL REPORT FOR JULY l, l952 TO MARCH 3l, l953 PART I. PART II. PART III. PART IV. PART V. PART VI. FIELD WORK IN l952
URANIUM-BEARING CARBONACEOUS AND
ASPHALTIC MATERIAlS OF THE COLORADO PLATEAU
SYNTHESES OF URANIUM MINERAlS
TEE CHANGES FROM BED TO GRAY SHALES
AND SILTS IN URANIUM-BEARING AREAS
PRIMARY AND SECONDARY SOURCES OF
URANIUM IN TEE COLORADO PLATEAU SYNGENETIC VERSUS HYJlROTHERMAL HYPOTHESIS FOR THE ORIGIN OF TEE URANIUM DEPOSITS OF TEE COLORADO PLATEAU
by
John W. Gruner,. Lynn Gardiner, and Deane K. Smith, Jr. Parts I, II, and III.
John W. Gruner, Parts IV, V, and VI •
GEOLOGY AND MINERALOGY
The united States Atomic Energy Commission makes no representation or warranty as to the accuracy or completeness of the infor-mation herein and makes no recommendation
concerning it.
In the interest of economy, this report has been reproduced direct from copy as submitted to the
Technical Information Service.
Work performed under Contract No. AT-30-l-6lO.
ANNUAL BEFORT FOR JULY 1_, 1952 TO MARCH
J1,
122.:2.
Table of Contents
Abstract
5
Part I. , Field Work in 1952.
Introduction and Acknowledgments
6
White Canyon, Utah
6
San Rafael Swell, Utah
7
Flop-Over Area 8
Lucky Strike mine, Red's Canyon 12 Dirty Devil
#6
mine, Muddy River 12 Part II. Uranium-Bearing Carbonaceousand Asphaltic Materials of the
Colorado Plateau. 14
References 19
Part III. Syntheses of Uranium Minerals.
U-V Minerals 20 Becquerelite 20 Uranopilite 21 Johannite 22 Schoepite 22 Unknown 23 Table I 24 Table II 26
Part IV. The Changes from Red to Gray Shales and Silts in Uranium-Bearing Areas.
Introduction 28
Field and Laboratory Observations 28
Mineralogical Changes 31
Processes Responsible for Color
Changes 31
Inorganic Assimilation of Red Oxide 32 Processes Dependent on Organic
Compounds
33
Conclusions 34
RME-3044 -
4-Part
v.
Primary and Secondary Sources of Uranium in the Colorado Plateau.Introduction
36
Availability of Secondary Uranium
37
References 40
Part VI. Syngenetic Versus Hydrothermal Hypothesis for the Origin of the Uranium Deposits of the Colorado Plateau.
Introduction 41
Statement of the Question 41
Facts Supported by Observations 42 Distribution of Trace Elements 47 Some Chemical Considerations
50
Discussion and Conclusions
52
Table I
55
Table II
56
ABSTRACT
This report is divided into six parts, each inde-pendent of the other with its o~~ list of references. Since the main objective of the work is a study of the mineralogy of the deposits, their origin, and experiments which will support a plausible theory of the distribution of the ore and protore, these six divisions seem essential.
In Part I the results of the field work are discussed as far as conclusions have been reached with regard to certain features. For example, the Flop-Over at Temple Mountain is ciscussed in some detail. It does not seem appropriate at this place to write about things that need more field observations to support them. It would only add volume.
Part II contains an account of uranium-bearing car-bonaceous materials which fall into four categories as far as we know now. Part III deale with the syntheses of U-minerals: 1} carnotite, tyuyamunite, and rauvite in the presence of one of the reagents in solid form at room temperature and 100°0; 2} becquerelite from uranyl sulfate and Ca(HCOJ}2 solutions;
3}
uranopilite fromuranyl hydroxide and uranyl sulfate or H2S04; 4} johannite from uranyl and copper sulfates;
5}
schoepite from uranyl nitrate in the presence of copper nitrate. Numerous ex-periments are not yet completed.Part IV the subject of color changes from red to gray in shales and silts associated with uranium deposits is discussed. There are only two explanations: 1) the change is caused by reduction of hematite, or 2) the very small amounts of hematite which cause the red color enter the hydromicas with the result that the shales become gray. Part
V
is a discussion, hypothetical by its very nature, of how much uranium is in sedimentary rocks of the Plateau and how much of it could have been available for making the deposits. The balance is in favor of syngenetic con-tributions of uranium to the deposits. Part VI was written after attendance of the symposium at Grand Junction. It was felt that the various observations that lead either to a syngenetic or hydrothermal hypothesis of the origin of the ores should be collected in one paper.PART I. FIELD \WRK IN 1952 Introduction and Acknowledgments
The field work during parts of June, July, August, and September consisted largely of a study of deposits in the general area of 1'lhi te Canyon and of the San Rafael Swell with emphasis on U-Cu and U-asphaltite deposits respectively. Several days were spent in the ~1onument
Valley #2 mine and in the Lukachukai area. The newly discovered deposits between Laguna and Gallup, New 1-iexico, :l'lere examined. It is becoming more and more evident that
the problem of the origin and formation of the deposits of the Colorado Plateau is not divisible into definite types but must be considered as a whole. That does not mean that the deposits are all alike and originated under identical conditions. On the other hand, it would be a mistake to think of the sulfide type of having been formed by solutions auite different in character from those of the asphaltite type, or the latter being very different from the vanadium type, including the Salt 1iash fonnation deposits. The investigator, therefore, must be familiar with all kinds, though he may want to study one area more thoroughly than another. This principle has been follo~1ed
in our field work. We have had the full cooperation of all the geologists and mining men we met in the several areas and exchanged ideas freely. We were dependent on them for much information that we could not possibly have col-lected in the short time we had at our disposal compared with the time of their detailed areal geologic work. We relied largely on their knowledge of structures which they had carefully surveyed while we specialized in mineralogical details and in those features which we thought could give us a clue to the chemical processes which must have been operative. The best defined 11structural channel11 in the Moenkopi filled by Shinarump Conglomerate is of little value unless there existed the proper chemical conditions for the precipitation of uranium and unless uranium was present in the waters which used this channel. Finally, we want to·thank the officials and staff members of the Division of Exploration, Grand Junction Operations Office, for their efforts to help and make us comfortable in every way possible.
White Canyon, Utah
The work in White Canyon centered around the Happy Jack mine. All new workings (since 1951) are in uraninite-sulfide ores. Occasionally a veinlet filled with sulfates is seen which is a relatively late product of oxidation
of the present groundwater circulation. It is amazing how rapidly the transformation from uraninite and sul-fides to sulfates proceeds after a working is opened. In a span of about two months the U-sulfates uranopilite, zippeite, and johannite begin to appear. The same obser-vation has been made at the Prospector mine near Marys-vale, Utah, in which acidic conditions exist wherever pyrite is oxidizing. In the deposits of White and
adjoin-ing Red Canyon there is every evidence that given enough cover to keep out oxidizing conditions the ore minerals should be uranini te and sulfides as soc ia ted 'I'Ti th
carbon-ized wood. Whether the copper minerals in the process
o~ oxidation fona sulfates or carbonates depends largely on the amount of
so4=
radical as compared to calcite in the sandstones. Where carbonate is plentiful as, for e:xample in the Shinarump #2 mine, Seven Mile Canyon, north of Moab, Utah, oxidation is very shallow from the outcrop inward because the limey rocks were not only denser but they also neutralized the solutions rapidly.In this respect these deposita are quite different from the strictly carnotite tyuyamunite ones in which sulfate solutions play an unknown but subordinate role. At present we have little knowledge of whether these U-V
ores at one time were unoxidized or still are at depth where sufficiently covered. Geologists of the
u. s.
Geological Survey seem to have some evidence to this effect in the discovery of the mineral coffinite in the Morrison formation (Weeks, A. D., verbal communication at several symposia).San Rafael Swell, Utah
While the asphaltite deposita of the Shinarump also contain V in carnotite and tyuyamunite they do not resemble the Morrison type closely. They are best developed in
the San Rafael Swell and are definitely the primary ores in the sense that the yellow oxidation products are formed from them at a later date. These ores were studied thor-oughly in all mines open at present. The calix cores lying around on the surface at the Middle Workings of Temple Mountain were also examined closely though the ore portions had been removed before our arrival. As pointed out in other places, the ore extends as high as 30 feet above the :Moenkopi contact at Temple ~!ountain but rarely is directly in contact with the Moenkopi. In this respect Temple Mountain is an exception when compared with all other deposita which practically always are directly on the contact. There are no channels of ore at Temple
RME-3044 8
-Hountain. On the other hand, this locality is impregnated with oil which is also encountered in the \iingate sandstone about 300 feet above the Shinarump. This is not always evident because the rocks appear bleached on the surface but are light brovmish gray a few millimeters below the surface. The details concerning the mineralogy of the asphaltic and oily materials is given in Part II, P• 14. Flat Top Mesa, 3 miles west of Temple Mountain, is very similar to the latter but the ore horizons are thinner and at the Moenkopi contact.
Each of the two localities has associated with what, for want of a better name, may be called a collapse area. The one at the western edge of the Flat Top Mesa is more or less an equilateral triangle with sides about 300 feet long and one of its apices pointing into the center of the vlestern slope of the 1-fesa. The bedding of the Flat Top Nesa is nearly horizontal. The beds of the collapse area dip
zoo
to 30° westward. If the bleached rock (white only on surface) is Shinarump Conglomerate, the minimum down-ward displacement at the eastern apex is ZOO feet, and themaximum is about 300 feet at the western edge of the tri-angle. This small area is completely surrounded by brown Moenkopi formation which is almost flat-lying except for
the strata on the western side of the triangle which dip into the collapse at about 10° to 15°.
A smaller but very similar bleached collapse occurs half way between Flat Top Mesa and South Temple Wash. It is again somewhat triangular with its apex and minimum height of collapse southward. The beds of the collapsed sediments dip
Z0°
northward while the surrounding Moenkopi strata are flat-lying.Flop-Over Area. Few places in the lluranium country11 have been discussed more by geologists than the so-called Flop-Over between North and South Temple Mountains. The first detailed account of it was published by Frank Hess (19ZZ) and ever since it has been geologically investigated. The present writer interpreted the structure erroneously as a double landslide two years ago (l95Z, P• 17) because there is evidence that slides are superimposed on the real structure which is a collapse lying west of the ridge which forms the connection between the North and South Temple Mountains. The collapse on the western edge of the
Flop-Over has a vertical displacement of about ZOO feet.
East-ward it decreases and under the connecting ridge between the North and South 1:-lountains it seems to be no more than a minor sag. This statement is based on the fact that the Shinarump formation here can be traced through from
the north to the south. This structure, therefore, causes the beds in the wedge-shaped collapse area to dip about
zoo
westward. There is, hol>ever, no place where this can be seen.On the east side of the Flop-Over there is no evidence of a break, and the beds can be seen in normal continuity from north to south exceut for a few hundred feet v<here they are covered by large rock falls. The true nature of
the structure became evident when the Atomic Energy Commission drilled a vertical hole into the extreme western edge of the Flop-Over which here has been called the tongue because the projection of the bleached white rock into the yellowish brownish Moenkopi resembles such an appendage when seen from the air. The log as taken by the writer from the diamond drill cores is given below. As some portions of the core
had become slightly displaced during transport some discrepancies with earlier logging by other parties may exist.
Log of Diamond Drill Hole #Z-31 drilled on westernmost extension of 11tonguell of Flop-Over; depth in feet.
DePth
o.
0-16 16-17 17-19.519.5-Z0.5
20.5-39.0 39-41.0 4Z-43 43-50? Shinarump50-53?
53?-61? 61-62.56Z.5-64.5
64.5-7Z
7Z-77
77-78 78-8Z 8Z-84.5Light gray silty SS to sandy silt. Some count; a little zeuneri te.
Light gray SS, fine-grained.
Silt with some horizontal(?) bedding. Gray, fine
ss.
Gray, fine SS with brownish irregular layers (oil-bearing); dips Z0°-40°.
Silty SS with zeunerite.
SS, appears brecciated and contains some gray clayey material.
Gray silt; horizontal bedding above; becoming inclined gradually to 15° toward bottom.
Core may be out of place between 50-61. Badly brecciated SS + silt; like 4Z-43. Very similar to depth
Z0.5-39
above except for brecciated character.Coarse mottled 11salt and pepper11
ss.
Gray silty SS mixed with a little asphaltite which does not count.
Coarse 11salt and pepper11 SS with some clay galls.
Shaly rock + SS with much pyrite; dip may be
zoo.
Coarse SS +pyrite.
Mixed silty SS + SS; bedding obscure.
RME-3044
R 3tR
8 7 87-90 90-93 93-95 95-98 98-101 101-102 102-106 106-125 125-134 134-146 146-158 146-166.5 166.5-172 172-190 Hoenkoui 190-193 195-201 201-202 202-215 215-227 227-273·5 Stra ti~raphic 00-19. 19.5-53 or to 61 61-166.5 166.5-190 190-273·5 10-lHxed coarse + fine SS + fine pyrite.
Shaly + silty rock with brownish and red specks. Fine gray SS + silty SS + patches of pyrite. The same but slightly coarser; dip 5°-10°. Fine SS with some pyrite areas.
SS with almost black areas.
Asphaltite +asphaltic
ss;
high count.Fine SS with pyrite nodules; whitish except for pyrite areas.
Hedium SS ;;ith patches or nodules of coarse dark
ss;
some of the dark hasu;
pyrite nodulescommon. These may be surrounded by asphaltite. Conglomerate + SS with large dark areas; some asphaltite +much pyrite; ~ .9£.@.•
The same but less radioactivity.
Light gray SS + conglomerate +pyrite.
Conglomerate +coarse dark SS +pyrite patches. Almost white fine to medium SS; dip not over 10° at most.
Conglomerate, much like Shinarump; no red pebbles, but gray ones + pyrite.
Somewhat conglomeratic SS +pyrite. Light gray; sandy silt; dip
5°.
The same '1-<i th pyrite nodules.Silt + fine SS light gray; some dark pyrite bands, almost horizontal.
Medium gray, silty
ss.
Silty SS interstratified with silt; all light to medium gray. Some very thinly bedded; all contains disseminated pyrite.
Classification:
Doubtful as to its origin; it could be lvingate or Chinle.
Chinle. Shinarump.
Doubtful, perhaps Moenkopi but could be Shinarump.
Hoenkopi.
This core is entirely unoxidized and except for the top 20 feet did not undergo alteration caused by near surface condi-tions. All core except the upper 64 feet contains considerable pyrite. Some contains very much. This in itself explains the
It is, of course, impossible to tell whether the drill hole stayed in the collapsed mass or perchance entered the undisturbed Moenkopi at a depth of about 160 to 180 feet where the dip of the beds is low. It is extremely doubtful that a block of the Shinarump could have subsided without the same having happened to the underlying Hoenkopi.
The structure as interpreted. by the writer would be a triangular block elongated in E-\v direction pointing toward the east and ending somewhat beyond the cliffs of the Shinarump which, as mentioned above, can be seen to continue through from north to south with a sag of perhaps 20 to 40 feet. The west side of the triangle is about
400 feet long and must have dropped about 180 to 200 feet through the Hoenkopi which surrounds the block completely. The general dip of these undisturbed rocks is about
5°
to 10° s.E. 'l'lhile the 1-l:oenkopi near the western contact with the block has assumed dips toward it which reach
15°
to 20°, but only locally. In other words, this structure is in every respect similar to that on the ;;est edge of Flat Top Mesa but it is more elongated. It is so con-spicuous because the high cliffs on both sides of the block set it off. Also, the rock falls have added to the color contrast of white, brown and red. It was mentioned in previous papers by the writer that the white results from the removal of hydrocarbons from the surfaces of the sandstones, either Shinarump or Wingate. Much of the red is caused by .the oxide. tion of side ri tic sands tone of the Chinle and \iingate formations at the contact of the two. As all the formations contain at least some pyrite,
oxida-tion of it probably contributed to the contrasting colore. It is undoubtedly true that in the Flop-Over area fissures exist which pass from the Moenkopi to the Wingate but whether they are continuous and how far they are associ-a ted with the block which subsided is unkno;m. It is also unknown whether these fissures were responsible for the very small U-V deposits which occur near the bottom of the Wingate on both sides of the Flop-Over. The ore is also associated with plant remains in the Wingate, and it would seem strange that solutions coming from below would pick exactly this plant horizon in the Wingate to deposit their loads when they could have found other equally favorable beds on the way up at a lower level.
In this Flop-Over, particularly on the east side,
one finds locally a very peculiar stain in the White Wingate sandstone. It is a very light bluish green color. It does
RME-3044 - 12
-not react for Cu, Fe or Cr and probably is caused by
V.
It grades into a yellowish brown near the surface of the rocks. Since it is but a stain we have been unable so far to obtain a satisfactory chemical test.
From the foregoing description of the drilling, it is obvious that the asuhaltite deuosits were in existence before the collapse occurred. What caused these subsi-dences in the nature of a collapse is unknown and any attempted explanation is conjectural. The writer ••ould suggest that salt or gypsum plugs or masses were removed by leaching, if there is any reason to believe that salt beds existed in this part of the Plateau as they do fur-ther east in the Salt Valley Anticline. The leaching might cause solutions to rise through the overlying sedimentary rocks but they would be quite different from magmatic emanations. And as pointed out before (Gruner,
1951, P• 17)
there is nothing that suggests thermal l1aters, certainly the gypsum, asphaltites, oils, and other carbonaceous materials do not.Lucky Strike Mine, Red1s Canyon. This mine has been
considerably enlarged since we saw it last in
1951.
It has developed into an asphaltite ore body. Almost allzippeite and other secondary minerals have been removed. There are in places two conglomerate lenses or horizons, one directly on the Moenkopi which is the best ore and another separated by 1 to 4 feet of sandstone from the lower. The pebbles are quartz and chert. The latter
are full of fissures filled with asphaltite which is secon-dary. Judging by microfossils in similar pebbles they are Permian in age. Much of the ore is concentrated in irregu-lar asphaltite masses associated with trees. The ore values are extremely irregular and unpredictable, but, with the great thickness of sedime~ts overlying the deposit, nothing but asphaltite ore should be expected. Galena in almost microscopic cubes is scattered through the conglomerate. Cobalt sulfate (at one time bieberite before dehydration) forms a conspicuous efflorescence above the upper ore lenses at one of the portals.
Dirty Devil
££
Mine, Muddy River. This deposit is very similar to the Lucky Strike in mineralization, though5
to 10 miles distant from it. Its conglomerate lenses reach thicknesses of 10 feet. Trees are common. They have as usual much pyrite associated with them. Gray clay lenses and galls are very common. At the contact of the Moenkopi and Shinarump a large chert lens (at least5
x6
asphaltite and a little chalcopyrite in it. At first tbls was thought of some possible genetic significance, but these lenses have been found in other places* entirely
in the Moenkopi but near its contact. They are a brownish yellow to pinkish chert which is a replacement of original calcite lenses. No organisms have been found in them. They are geologically very unusual and perplexing where they are found directly at the l·ioenkopi contact as here. A large fault striking NW passes about
50
feet southwestof the deepest mine workings. At one time it was thought that it might have had some connection with the introduc-tion of the ore, but the ore gets poorer towara. it. The writer is practically certain that the ore is prefaulting in age.
It is not intended here to give a description of the various deposits visited, though this has been done for the mineral associations (Part III. Annual Report for July
1, 1951,
to June 30,1952;
see references in Part VI of the present report). Some have not been opened up sufficiently to form a picture of their primary setting. The accounts, however, that have been given in the pre-ceding pages should help in watching for criteria in ex-ploration and development. At present it appears that uranium might be found almost in any formation on the Plateau provided enough prospecting is done, but we know that this is not true. \ve have some criteria as, forexample, fossil '1-rood and discoloration of shales and silts. Can ;qe find any others as good as these?
*South Temple Wash, Pay Day Mine, and in several places of the Green Vein Mesa.
BME-3044 - 14
-PART II. URANIUM-BEARING CAF.BONACEOUS AND ASPHALTIC MATERIALS OF THE COLORADO PLATEAU
The present investigations on carbonaceous materials are confined to those in the Shinarump Conglomerate. The most sui ts.ble areas for study probably are in the San Rafael Swell, Utah. Practically all of the important U-bearing material in the Shinarump is probably within a stratigraphic distance of JO feet of the Moenkopi con-tact and is not found in the underlying l'!oenkopi forma-tion itself. J.!ost uranium is usually very close to the contact, though in the Temple Mountain area some may be as high as 20-30 feet above the contact.
Temple Nountain, the Flat Top Nesa (which is also called the Shinarump J.!esa), the Green Vein Hesa 10 miles further ~rest, and the Red Canyon (with the Lucky Strike mine) are best suited for study of U-bearing carbonaceous deposits. Still further west are the Dirty Devil Claims on the Muddy River which are of the same type as the Temple Mountain deposita. In the northern part of the San Rafael Swell are such claims as the Dexter group and the Lone Tree which are very similar.
In all places in the Swell more than one kind of car-bonaceous stuff, if hydrocarbons are included, may be observed. These are:
1) carbonaceous lignitic plant material in general.
2) aephaltite, or as it has also been called, thucholite, though this material does not contain thorium, for vihich thucholi te was named originally.
3) gileonite and similar hydrocarbons which are a kind of asphaltic material and used
to be named jet in the past. 4) liquid hydrocarbons.
The third and fourth are the only ones readily soluble in ordinary organic solvents.
The presence of liquid hydrocarbons under 4) con-fusee the question of origin in several respects. They have been in the Shinarump for geologic agee without having become appreciably polymerized. Being closely associated with radioactive material, they should have been polymerized if the ideas of Davidson and Bowie (1950) are to be accepted. The liquid hydrocarbons give off a strong odor and are easily distilled out of the rock. They do not contain any appreciable uranium, at least,
not any that can be found with the ordinary Geiger in-strument. The liauid hydrocarbons are not restricted to any particular-area in the Swell but are found in many including the ones already mentioned. In the Temple Hountain area they are found not only in the Shinarump Conglomerate but also locally in the Wingate Sandstone 300 to 400 feet above the Shinarump.
The liquid hydrocarbons stain and penetrate the sandstones along definite 'baxturally pervious bedding nlanes. The saturated sandstone has a dirty greyish brown to dark brown color and banding. If, as at the
Te~ple Mountain Flop-Over, for example, the rock is exposed to some oxidation, the hydrocarbons on the sur-face are removed and the rock assumes a whitish appear-ance vihich is a few millimeters thick and -vrhich has been
in the past mistaken for a sign for hydrothermal leaching by some geologists. Such whitish 11oxidized11 sandstone may contain small amounts of uranyl minerals, for example,
zeunerite and carnotite or tyuyamunite.
The hardened hydrocarbons mentioned under 3) which looll: like gilsonite or jet are commonly found in joints and fissures and are derived from the liauids and like
them contain no appreciable uranium. The consistency of this material differs widely, however. It looks
usually llpuren, that is, free of minerals, and is easily soluble in organic liquids.
The most puzzling substance is the asphaltite or thucholite mentioned under 2). It is unlike any of the other carbonaceous materials. It occurs almost free of impurities, that is, without any visible inclusions of sandstone or any other contamination by the sediments. From these purer masses it grades into areas in which it is present as fillings between the sand grains, that is, only interstitially, in very small amounts. The purer the asphaltite the lovrer is its specific gravity and the better conchoidal fracture it has. It is practically
opaque to transmitted light. In polished sections it may shorT a certain amount of anisotropism.
Chemcially it is a very complex substance. Some of the chemical data given here are those of Dr. Thos. 013rien of the School of Chemistry of the University of
Minnesota who works on this problem for the Atomic Energy Commission. He found~ for example, that not enough hydrogen is present for the material to be a hydrocarbon in the usual sense of the word. The excess
of carbon is great. Several per cent of vanadium oxide are present but not as Vz05• The highest grade material
RME-3044 16
-has several to seven or more per cent of U308• Spectro-graphic analyses show· many other elements. This asphal ti te is slightly soluble in chloroform, more in complex solvents in autoclaves at elevated temperatures. The asphaltite can be oxidized completely in concentrated nitric acid in about 24 hours after which the solution may be decanted and the clastic material by this ,method removed. Fror1 such solu-tions colloidal Si02 may be obtained. The writer determined the non-clastic Si02 in one of the 11pure11 masses as about 3 per cent. This Si02 may be chemically combined.
The asp hal ti te looks very interesting when vie'I'Jed in polished or thin sections under high power. Strange as it may seem, the asphaltic material replaces the grains of the sand leaving highly corroded remnants of quartz. The question has been raised whether the quartz grains could have been attacked and dissolved by some earlier inorganic solution and the asphaltite later deposited in the voids. There is no evidence of such an earlier nrocess. Under the microscope it looks as if the quartz grains had been corroded from the borders inward until all or most of the quartz had been consumed where the action was most intense. The action in the sandstone decreases outward from the almost pure asphaltite areas and the quartz grains show less and less corrosion till the asphaltite practically vanishes, leaving only sandstone. All shapes of such re-placements are found. Most of them follo•r more or less a particular sandstone or conglomerate lens. The asphaltic ore, that is, the cemented material, seems considerably tougher than the surrounding porous rock. The miners knovr this property very well, of course, and have mentioned it in every instance.
A puzzling feature is the occurrence of globules and balls of asphaltite ranging in size from pinheads to large marbles. These also are rich in uranium. Such spherical bodies are numerous in a few places and look as if they had been asphaltic pebbles originally that had rolled. into place where they are found now, an interpretation given
to them by Frank Hess (1922). It is, however, not impossible that the spherical bodies are replacements. This phase of the investigation is s·till in its early stages.
The uranium inthe asphaltite is present in at least two forms. One of these is uraninite~ This mineral is visible under high magnification in polished sections. The largest particles are about 10 microns in diameter; the smallest cannot be resolved with oil immersion. Pyrite of similar dimensions always is aBsociated with the uraninite. The arrangements of the particles suggest some structural or textural control (Rosenzweig, Gruner, and Gardiner, G.S.A., Boston, November 1952). Some resemble ellipsoidally outlined
bodies. There are also some fan-shaped outlines which suggest organic structures. In one section the uraninite narticles may follovr the boundaries of former auartz or other clastic grains. These uraninite particles do not account for all the uranium present. Preliminary tests by Dr. Thos. O'Brien indicate the presence of organic complexes containing uranium. For example, some speci-mens w·hen heated to about 200° in the absence of air o.eposi t a yellow sublimate on a cool surface ivhich is highly radioactive and contains uranium. This sublimate
can hardly be derived from uraninite. The volatility suggests an uranium compound which might be classified as an organo-metallic one.
The amount of uranium in asphaltite, of course, varies greatly and in places may be altogether absent
except for traces. Carbonized material which is definitely of plant origin because it shows cell structures carries little or no uranium if asphaltite is associated with it. If no asuhaltite is present, carbonized material may be quite rich. For example, we have a specimen from the Small Spot mine on the Calamity Mesa, Colorado, which by actual analysis contains 33;~ of U308• 'lie investigated this material by x-ray but could get no pattern except in one or two small areas where we found the mineral called coffinite by the United States Geological Survey. This is a new uranium silicate.
It is known, of course, that near the surface on oxidation asphaltite as well as the carbonized ;;ood dis-appear. In some cases the wood becomes silicified, as shown especially well as the Honument Valley No. 2 mine. The uranium in that case forms uranyl compounds, which with any vanadium also derived from the wood or asphaltite, may form carnotite or tyuyamunite or some of the lesser known uranium vanadates. In some cases it will go to torbernite, zeunerite or their meta minerals respectively. If the vanadium is in large excess, as is common, the
excess of vanadium has a tendency to be centrifugal in its behavior. We find, for example, at Monument No. 2 mine, Homtment Valley, that the concentrations of uranium are high in the centers of the "trees11 and decrease away from those places. Vanadium then is commonly found, if it is in great excess, in the very bottom of the Shinarumn and in the underlying DeChelly sandstone "lvhich it seems -to have replaced in part.
The writer believes that the separation of uranium and vanadium may also occur under other conditions. For example, vanadium salts are ~ soluble in reduced form,
RME-3044 18
-probably in the state of tetravalence. Under these condi-tions the uranium might be in the uranous state and little soluble. If reduction occured, let us say by HzS or
other compounds derived from the organic matter, the
uranium would be deposited as uraninite with the sulPhides of iron and copper while the vanadium, being soluble~ would be carried in solution to a new environment. Such behavior could account for the almost complete absence of vanadium in such sulphide-uranium deposits as the Happy Jack mine. Of course, as long as the vanadium and uranium were tied up organically there could be no separation under these conditions.
REFERENCES
Davidson,
c.
F., and Bowie,s.
H.u.
(1951), On thucholite and related hydrocarbon-uraninite complexes: Bul.Geol. Survey Gt. Britain, No.
3,
P• 1-19.Hess, Frank 1. (1922), Uranium-bearing asphaltite sedi-ments of Utah: Engineering and Mining Journal,
RME-3044 - 20
-PART III. SYNTHESES OF URANIUM MINERALS U-V Minerals
Since the publication of 11New Data of Syntheses of
Uranium Minerals n (Part I. Annual Report for July 1,
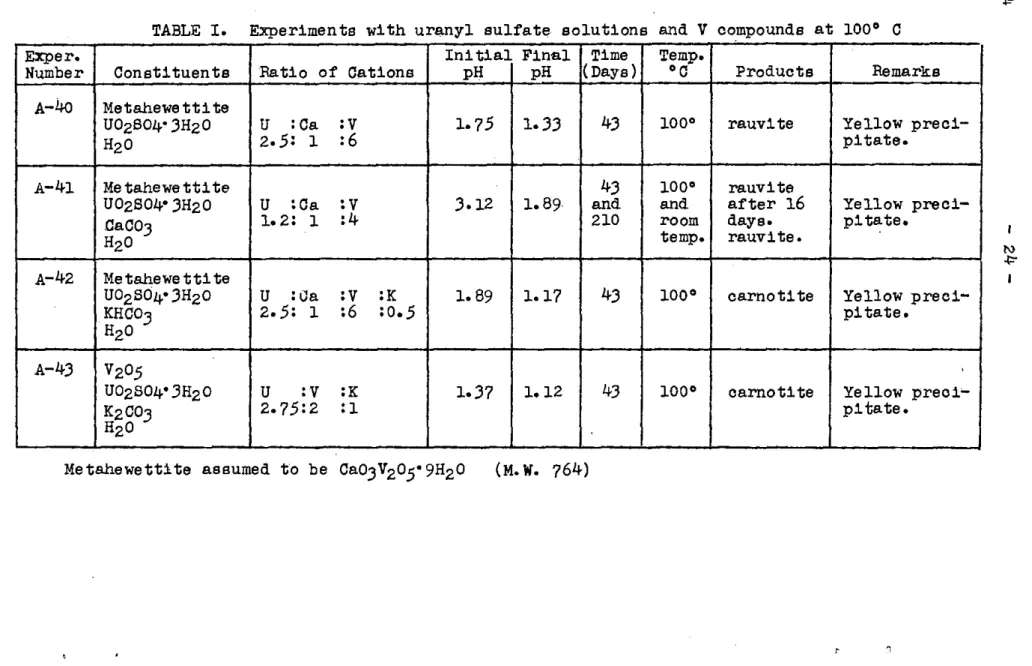
1951 to June 30, 1952, A.E.C., RM0-983) a considerable number of new experiments have been set up. Some of them are completed, others are still running. One series deals with the reactions and products that re-sult when uranyl sulfate solutions* come into contact with chemically pure vanadium oxide, V205, or minerals like metahewettite (Ca0•3V205•9H20) which is found as fairly good crystalline natural material. With the appropriate additions of [+ or ca++ it was expected that carnotite or tyuyamunite would result. As may be seen in Tables I and II this happened in some ex-periments but not in others. Only those experiments of this series are listed which produced compounds corresponding to natural ones. Some resulted in un-known crystalline materials.
After the experiments had been prepared, they were occasionally shaken and their pH's and x-ray patterns determined. These intermediate determination$ are not
recor~ed in the tables. Experiments of Table I were
put on a steambath to speed up the reactions. The others were kept at room temperature, at which reaction is
naturally very slow and still incomple"te in some. In all the experiments the pH1s decreased fairly rapidly. This was caused by the withdrawal of cations from the solutions to make insoluble compounds. The only excep-tion was Exp. A-49 in which, as was expected, an in-soluble precipitate formed at once. Even after 325 days no crystalline substance had separated, though the
solu-tion was less basic than at the start and all the brown V205 had disappeared. The conclusion that one may draw is that in a basic environment a reaction between U and
V
is so slow that it is beyond the range of experimenta-tion. It is of considerable interest that rauvite or uvanite are intermediate products in those experiments in which carnotite or tyuyamunite are the end products. This is contrary to the belief of some investigators that rauvite is a decomposition product of tyuyamunite.Becquerelite, 2U03•3H20
Exp. J-229 and J-230 were duplicates. Solutions of 0.01 M uranyl sulfate (pH 2.9) were treated with saturated *Solutions from 0.1 to .3 molar.
Ca(HCOJ) 2 solution which was added dropt1ise for an hour a day. After about a week a precipitate began to form at a pH of
5.0.
In the next 90 minutes about 90 more drops of Ca(HCOJ)2 were added. The pH was5.6.
After standing J weeks and 7 weeks respectively, samples were withdrawn and x-rayed. They were typical becquerelite. More of this type of experiments are in progress to findout the influence of concentration on the products. It is quite certain that the earliest precipitate, which was withdrawn within a day after it had formed, is prac-tically amorphous. An important point we would like to establish is the exact pH at which precipitation begins. This caru1ot be done unless the pH is changed very slowly, which results in a large volume of Ca(HCOJ)2 solution that must be added.
Uranopilite, (U02)6S04(0H)lo
This mineral and zippeite (2UOJ•S03•nH20) commonly are early products of oxidation on uranlnite in places where pyrite is also plentiful. It is found at Marysvale, Utah, and at the Happy Jack mine, White Canyon, Utah,
where it forms very rapidly after exposure of the workings to air. It would seem that there would be no difficulty in synthesizing it by simple hydrolysis of uranyl sulfate. There are some difficulties involved, however, as numerous experiments have shown. Some qualitative experiments
have given the mineral but the reactions did not go to completion except in one case.
Exp.
J-258.
Some U02(0H)2 was spread on.a large glass slide and a few drops of 0.1 N H2S04 were put in contact with U hydroxide. Another glass slide was used as cover. In this way it was possible to examine any changes under the polarizing microscope. The whole assem-bly was put under a bell jar with a dish of water to keep the humidity at value corresponding to the temperature of the laboratory. After 10 weeks three greenish yellow circular aggregates of crystals had formed among the re-maining hydroxide which were highly fluorescent. The pH of the acid had increased from about 1 to J (hydrion paper). The x:-ray pattern of this new growth is.'that of uranopilite. The optics also check fairly well with this mineral. Another experiment,J-257,
in which the acid was about5
N resulted in the conversion to ordinary U02S04 which dissolved on the glass plate.Exp.
J-256.
A small amount of UOz(OH)z, which is anRME-3044 22
-artificial compound, is lvetted with dilute H2S04 (1:,3). The sample is left to dry. After this, it is kept just wet with distilled v•ater. All of the hydroxide had been converted to uranopilite in 4 weeks as checked by x-rays and optically. Also, uranopilite is highly greenish yellow fluorescent, lthile the hydroxide has a very dull fluorescence. If too much acid is used, it is converted to the soluble uranyl sulfate. No zippeite was obtained as an intermediate product in these experiments.
Exp. A-76 and J-250 were quantitative. To 2g. of U02(0H)2 enough uranyl sulfate solution (0.20 M) was added to make the ratio u:so4 = 6:1. The amount of
solu-tio~ was 6.6 cc. After 4 days the pH ~~s 3·9· On fre-quent stirring, two different yellow colors could be seen after about a month. As both remained, a sample was
x-rayed. It showed uranopilite and the original hydroxide in perhaps equal amounts; pH = .3·9· For the next .3 weeks about .30 cc of water was kept on the material and a few drops of H2S04 -vrere added to keep the pH at ,3. 7· The former high fluorescence disappeared gradually and an x-ray showed that all of the sample had gone back to the hydroxide. Apparently the concentration of solution to solid is more important in this case than the pH. The solutions are no~; gradually evaporating and someuhere a point should be reached where uranopilite will appear again. This will be reported at some later date.
Johannite, (Cu, Fe)o·uo.3•so.3•4H2o
EXP. J-261A and J-261B. To 10 cc of 0.20 M uranyl sulfate-solution+las added 0.5g. of CuS04•5H20• This gave a ratio U02 :cu
=
1:1. A piece of an aragonite crystal was added to A and a cleavage piece of iceland spar to B. The underlying thought v1as that aragonite might react more rapidly than calcite; pH at start 1.9. The reaction was very slow but gradually greenish hair-like crystals began to form. They were in spherical aggre-gates about 1-2 mm. in diameter. Also, thin hairs of gypsum developed. The pH at this stage was .3·9· After .35 days the green crystals were x-rayed and examined optically. They are johannite; pH= 4.05 for A and,3.65
for B.Schoepite, 4UOJ"9H2o
Exp. J-264A and J-264B were set up to find out whe·ther U and Cu would combine in the absence of so4=. To
5
cc of 0.20 M uranyl nitrate solutions were added 0.24g.ot
Cu(N03)2•3H20 dissolved in 10 co H20; pH of solutions 2.95. To experiment A was added a cleavage piece of iceland spar, to B a fragment of aragonite, After some time aggregates of rounded grains from 0.1 to 0.3 mm. in diameter formed in both solutions. These were yellow in color and non-fluor-escent. After a month they were x-rayed and proved to to achoepite; pH= 5.20 for A and 4.64 for B. This shows that Cu does not enter into hydroxides of u. The absence of fluorescence suggests, however, that traces might be present. Blue green crystals of a copper compound formed gradually after the achoepite had started to precipitate.
Unknown
Two exoeriments J-263A and J-263B contained solutions of uranyl sulfate and cupric nitrate; 10 co 0.20 H sulfate and 0.24 g. of Cu(N03)2•3H2o which gave a ratio uo2++:cu = 1:1; pH = 2.2. Iceland spar and aragonite respectively were added. After a week pistachio green moss-like aggre-gates began to form. These were x-rayed after 4 weeks and gave a good but unknown pattern; pH for both 4.65. A
number of attempts ~rere made to precipitate Pb-U-phosphates but they were failures because the Pb-phosphates appear to be more insoluble than the double salta. Therefore, these experiments will not be described.
All described experiments show clearly, as also dis-cussed in Part VI, that these reactions take place easily in acidic solutions and that the minerals are stable in some cases even at low pH1 s. This, of cottrse, does not
prove that they could not form in a sometvhat basic environ-ment, but since U is practically insoluble in neutral or slightly basic solutions, the solutions could not carry more than infinitesimally small amounts of the metal. As natural carbonates of U are extremely scarce, for ex-ample, such minerals as voglite and liebigite, it must be concluded that they originate under very unusual conditions which could be basic ones.
I
)~TABLE I. Experiments with uranyl sulfate. solutions and V compounds at 100° C
Exper. Initial Final Time Temp.
Number Constituents Ratio of Cations pH pH (Days)
•c
Products Remarks A-40 MetahewettiteU02804• 3H20
u
:ca:v
1. 7.5 1·33 43 100° rauvite Yellowpreci-H2o 2 • .5: 1 :6 pi tate.
A-41 Me tahewe tti te 43 100° rauvite
U02804• 3H20
u
!Ca:v
3-12 1.89 and and after 16 Yellow preci-CaC03 1.2: 1 :4 210 room days. pi tate.H2o temp. rauvi te.
A-42 Metahewettite
uo
2s04• 3Hz0u
:ca:v
:K 1. 89 1.17 43 1oo• carnotite Yellowpreci-KHC03
z •
.5: 1 :6 : o • .5 pi tate.H2o
A-43
v
2o.5 'UOzS04• 3Hz0
u
:v
:K 1-37 1.12 43 100° carnotite Yellowpreci'-K2C03 2-7.5:2
a
pi tate.H2o
Me tahewett1te assumed to be Ca03
v
2o.5• 9H2 o (M.w. 764)'
~---·~~--~~---~~---Number Constituents Ratio of Cations pH pH (Days) A-44 V205 U02S04• 3H20 u :v :K lo 60 lol3 43 KHC03 5 :4 :1 H20 A-75 v2o5
uo2s04• 3H20 u :v
:ca
3.20 lo8? 17CaC03 2.?5:2 :1
H20
Metahewettite assumed to be Ca03V205•9H20 (M. W. 764)
I Products 100° oamoti te 100° tyuyamunite Remarks Yellow preoi-pi tate. Yellow preoi-pi tate. 1\) \J\ I
','
~---~---~---
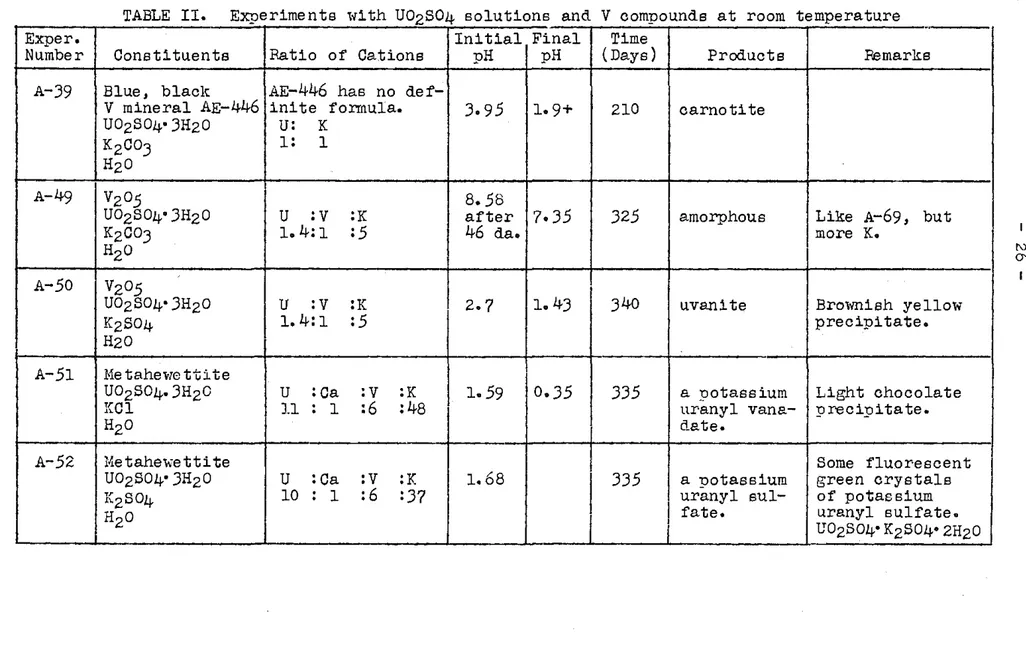
-TABLE II. Exueriments with UOzS04 solutions and V comnounds at room temperature
-Exper. Initial Final Time
Number Constituents Ratio of Cations pH pH (Days) Products Remarks A-39 Blue, blaclc AE-446 has no
def-V mineral AE-446 inite fonnula. 3. 95 lo 9+ 210 carnotite U02S04•3Hz0 u: K
K2co3 1: 1
H2
o
A-49
Vzo5
8.58UOzS04• 3Hz0 u
:v
:K after 7o35 325 amorphous Like A-69, butKzC03 1. 4:1 :5 46 da. more K,
HzO A-50
Vz05
UOzS04•3HzO u
:v
:K 2.7 1. 43 340 uvanite Bro1-mish yellowK2S04 1. 4:1 :5 precipitate.
H20
A-51 Ke tahe~1e tti te
UOzS04.3HzO u :ca
:v
:K 1.59 0.35 335 a uotassium Light chocolateKCl l l : 1 :6 :48 uranyl vana- precipitate.
HzO ctate.
A-52 Hetahel\ettite Some fluorescent
UOzS04•3H20 u :ca
:v
:K 1.68I
335 a uotassium green crystals
KzS04 10 : 1 :6 :37 uranyl sul- of potassium
H2
o
fate. U02S04•KzS04•2HzO uranyl sulfate.--A-66 Ne tahel-ie tti te
U02S04• 3H2o
u
:ca :v 1.75 1.10H2o 2. 5: l :6
A-67 14etahewetti te
U02S04• 3H20
u
:ca :v 3.12 1.55CaC03 1.2: l :4
H20
A-68 ~!e tahewetti te
U02S04•3H2o
u
:ca :v :K l. 89 1.05 KHC03 2.5: l :6: o.
5 H20 A-69 v2o5 U02S04•3H2ou
:v :K l· 37 0.90 K2co3 2. 8:2 :1 H2o A-70 v2o5 U02S04•3H20u
:v :K 1.60 0.9 KHC03 5 :4 :1 H2o 290 rauvite 300 rauvite .. 290 rauvite + carnotite uvanite + 310 carnotite(?) + unknown 290 uvanite Yellow preci-ni tate. - ' Golden yellow precipitate. Yellow preci-pi tate. Yellow preci-pi tate. Golden ye llo1·1 precipitate. !\) """' IRME-3044 28
-PART IV. THE CHANGES FROM RED TO GRAY S!W..ES AND SILTS IN URANIUM-BEARING AREAS
Introduction
The relationship to one another of certain red and gray shales of the Colorado Plateau is a problem closely tied up with the distribution of uranium in this region
(Fischer, R. p., and Blackman, D., 1949; Fischer, R. P., '1
1950). For this reason in particular, it has been studied lately to a considerable extent. A paper by A. D. Weeks
(1951) deals with the red and gray clays in the ore-bearing sandstones of the Morrison formation of Western Colorado. The study may be extended geographically to include parts of Utah, Arizona, and New Mexico as well as arid regions in other continents. StratigraPhically it reaches from the Upper Paleozoic to the bottom of the Cretaceous. In the present investigation we are chiefly concerned with the Triassic rocks though there is some mention of others.
The upper few feet of the Moenkopi formation at the contact of the Shinarump Conglomerate deserve our attention because here a change of the sediments from red to gray is very common, almost the rule, in uranium areas. The Shina-rump itself, while made up largely of conglomerate and coarse sandstone, contains also numerous lenses, blebs, balls, and other shapes of clay or shale which are greenish gray where uranium and fossil plants are found. The over-lying Chinle formation does not have a sharp contact with the Shinarump but grades into it. In places it may be difficult to find the Shinarump Conglomerate because it is very thin. The Chinle has red and gray silt and shale beds or mottled red and gray strata. In uranium areas the Chinle shales just above the ore bodies are usually gray. As this gray color is much more widespread than the immedi-ate ore areas according to E.
v.
Reinhardt (1952, p. 12), it is not certain that any change from red to gray or to grayish green is tied up with the immediate presence of uranium. It is a fact, however, that in many places a conspicuous color change has taken place which is not for-tuitous but in some way connected with the solutions which brought the uranium.Field and Laboratory Observations
Anyone familiar with the sedimentary formations of the Colorado Plateau knows that the removal of red colora-tion is a phenomenon not solely associated with areas of
uranium-concentrations (Houlton, 1926, and Van Houten, 1948),
though it has been studied more in these places. Where, for example, white bands and lenses 'l'lith blunt and rounded terminations occur in reddish sandstone of the Entrada, it may be seen under the microscope that the red hematite is in such minute amounts that it is almost impossible to observe it in thin sections. In other words, a thin section from medium-grained reddish sandstone is apparently identical to one of adjacent white rock. The little bit of hematite which can be seen is usually in extremely small dusty aggregates around and between the quartz grains and the micaceous cement. It can hardly be more than a small fraction of one per cent by volume. Such widespread, large-scale discoloration is usually ascribed either to primary deposition or diagenetic processes having followed soon after (MacCarthy, 1926, p. 35; Van Houten, 1948, P• 2122).
A more difficult case to explain is the bleaching of sandstones along almost vertical fissures which cut across nearly flat-lying beds for distances of hundreds of feet. The width of discoloration may be as much as
50
feet but varies considerably. The best example knom1 to the writer is just south of the highway about 3 miles northwest of the Happy Jack mine in White Canyon, Utah. We know that something like a solution must have moved along such fis-sures but why the contacts should be sharp in the porous rock and what had the power to dispose of the hematitic duet, even though there was not very much, is not clear. l.foulton (1926, P• 306) gives some similar large scale examples outside of the Colorado Plateau in Montana.It has been shown by Keller (1929) that the following reactions may occur: Hematite is reduced by H2S• The resulting ferrous iron may then be removed by waters high in carbon dioxide in the form of a soluble iron bicarbonate. The equations could be written:
Fe203
+
3H2S----+
FeS+
FeS2+
3H20 FeS+
H2C03 ----~ FeC03+
H2S1'
FeC03+
H2C03 ----~ Fe(Hf03)2soluble in water in absence of air.
These reactions locally are of importance, as organic matter does generate sulfide ions in considerable amounts and C02 is also found under these conditions. It is doubt-ful, however, that these charged solutions could have diffused
RME-3044
30
-through dense shales to any great extent. After all, solutions or gases travel the road of least resistance, which is, if possible, through sandstones and around shale lenses. Any changes, however, from red to gray at contacts or in fissures of shales could be explained by such reactions. Proof for the movement of some kind of solution and diffusion is seen, for example, at the Frey #4 mine in Frey Canyon, Utah. Here many small fissures may be observed in the silty shale which forma the top of the Moenkopi and bottom of the 11ore channel"· The shale is reddish brown except for a few millimeters on both sides of fissures or joints where the color is light gray. The contacts are sharp. The discoloration along the fissures does not reach down into the Moenkopi more than perhaps a foot. A little metatorbernite was
found in these cracks.
As already mentioned there are vast masses of Chinle formation in the areas under consideration which consist of alternating bro~mish red, gray and greenish gray beds of silts and shale. But there are also thick beds in
~rhich a mottling of red and gray occurs. In some places the gray predominates and surrounds irregular bleb- to boulder-like masses of red. In others the color scheme is reversed. Except for color there is no other physical distinction diacernable in the field. This mottling has been observed in drill holes and mine drifts. Therefore, it is not just an expression of weathering and oxidation. Many geologists may agree with the author that these
rook masses are so vast in scale that diagenetic processes more likely than not produced this variegated color pattern.
The color changes from red to gray in the Shinarump and top of the 1-l:oenkopi, however, had a different geolo-gical history, though chemically and mineralogeolo-gically they are closely related. The change from red to gray in the Moenkopi at the contact with the Shinarump is definitely
later than Moenkopi. Since it parallels the channels which the Shinarump out into the Moenkopi it cannot be any older. Most geologists, including the writer,
associ-ate the color change with the solutions that were instrumental in concentrating U deposits. As these solutions, probably sulfate ones which may also have carried colloids of organic origin, were more widespread than the areas of present vrork-able uranium concentrations, the bleaching of the Moenkopi contact reaches beyond these limits.
It is probable that in the Shinarump Conglomerate some of the gray lenses and irregular bodies of shale and shaly silt so prominent in and near plant fossils and
I
uranium concentrations were changed from red by the se~e
kinds of sulfate solutions. Since the shales of the Shinarump were associated with plant material from the
~ of deposition, a portion of them, however, may have been gray from the start. Otherwise, in many respects these changes from red to gray should be identical in origin to those in the l~orrison mineralogically described by \'leeks (1951).
Mineralogical Changes
As was shown by Weeks (1951) for the shales of the Morrison, the compositional changes are not very signifi-cant. There is no noteworthy increase in ferrous iron in going from red to gray. In both shales FeO averages below one per centf There is, however, more Fe203 in the red rocks than in the gray, on an average by a factor of 2. The average of 8 red shales (Weeks, P• 13) is
Fe203 = 3-39 and of the gray ones Fe203 = 1.67 per cent. There are no other chemical differences that point to any particularly significant chemical reactions. 1Uneralogically all the shales under consideration, whether Triassic or
Jurassic, consist largely of hydromicas, quartz, and a little carbonate. These hydromicas contain considerable amounts of Fe203 in their structures. The montmorillonite clay group is present, but its contribution may be small, albeit this is hard to ascertain. Hematite is chiefly a coloring agent and very rarely appears as faint lines in x-ray diagrams. Pyrite is observ::ed only in those gray shales which are associated with plant remains.
Processes Responsible for Color Changes
As there is nowhere any evidence that the gray shales might have been changed to red unless it were by weathering where they contained considerable iron carbonate or iron sulfide, the change ~ have proceeded from red to gray. It is believed by the majority of observers (Moulton{ 1926; Keller, 1929; Van Houten, 1948, P• 2120; ·weeks, 19511 that the change is caused by the reduction of the ferric iron of hematite to ferrous compounds, though the amount of such change would not have had to affect more than ~ fraction of the total iron present.
This would probably be acceptable provided one over-looked those minerals of ferric iron which are not red. *Van Houten (1948, p. 2093) shows this also for other
RME-3044 32
-These have been listed, for example, by MacCarthy (1926,
P• 19). Glauconite, a hydromica structurally nearly
the same as illite, which makes un the bulk of the shales is not rea-though largely ferric.- Illite itself contains considerable Fe203, and nontronite, a member of the mont-morillonite group, is all ferric and dull greenish yellow as a rule. Another mineral commonly found in the shale areas is jarosite, K Fe3(S04) 2 (0H)6, which is ferric but yellow in colors. It is associated with other sulfates like gypsum which are plentiful in the rocks under dis-cussion.
The only inorganic reducing material we might have in this environment is some ferrous iron contained in pyrite or marcasite. But it is perfectly obvious that
it can reduce hematite only by being itself oxidized, which is thermodynamically impossible. Reduction by organic pro-cesses, however, is not excluded and is one of two proposed below to explain the change from red to gray. In all pro-cesses, diffusion, which is slow, would have had to take over the role of flow of groundwater, as the shales are too dense for flow. Base exchange reactions would also have been important, as the shales are largely layer struc-tures of silicates.
Inorganic assimilation of red oxide. In the first process or mechanism proposed, only inorganic constituents would take part, and £Q reduction of the ferric iron would occur. Instead, the finely divided hematite would be dis-solved and the ferric ions incorporated into the silicates by base exchange or filling any vacant structural positions in the hydromica. A necessary assumption in this process is that sulfate or other highly ionized waters were the medium in rlhich the reaction could take place. The grain boundaries in the shales offer enormous surfaces to inter-stitial solutions for specific ionic exchange reactions. Any so-called 11primary11 clay or mud colored red by hematite would have been in a metastable eauilibrium if it had come
in contact with such solutions. The latter would have
be-come acidic because of adsorption of cations like those of i~
potassium by the clays and would have slowly dissolved the
l
otherwise almost insoluble red oxide. The ferric ions weremade a part of the newly forming mica layers as fast as they became available. The reaction proceeded toward the mica product because the hydromica is almost insoluble in acids and there was a large excess of those constituents necessary to shift the equilibrium in the direction of illite. Where the shales are still red today there may be t1-1o or more reasons for this fact: First, and most important, there were insuffi-cient amounts of sulfate solutions, and second, there was
more red oxide than could be assimilated in the hydromica structures.
We have to admit that we know practically nothing about chemical reactions in an environment as outlined. We have some data by Gruner on how to make mica hydro-thermally in acid solutions which~e partly responsible for the ideas outlined above. Another motivation for the author was provided by the still unsolved problem of the fonnation of glauconite, for it is similar in these re-spects, that a ferric mica structure forms in an environ-ment of mud and potassium ions derived from the salts of the sea. Though the all important time factor is not
re-~roducible, some experiments have been started on possible substitution reactions which would lead to the elimination of the red oxides in clays. The first results are encour-aging.
Processes denendent on organic compounds. Such pro-cesses can be of two kindB; 1) those involving bacterial activity and 2) those in which reduction is brought about by simple oxidation of carbon and hydrogen usually at an elevated temperature.
It is difficult to imagine that bacterial life could exist in dense clays and shales but in the decay of plant matter so abundant in the Shinarump, for example, it must have played an important role in generating gases like HzS which could have ·reduced ferric iron as pointed out above. No one doubts that red soils can be bleached by
the action of so-called humic and other organic acids but it is not known whether this could happen in the ab-sence of micro-organisms. As these organic solutions are usually colloidal in nature, it is difficult to see how they could penetrate the shales to any depth and pro-duce such a uniformly gray rock unless it were done by gases which can more easily diffuse through dense rocks. But there still remains the question why the gases would not flow around the shale lenses in looking for an outlet in the highly porous sandstones, especially since plant decay took place largely at the contact with porous rocks and not in the shales.
The second possibility of reduction of ferric iron without the aid of bacteria is so improbable at tempera-tures below 200°0 that it does not need consideration.
Anyone working in the San Rafael Swell of Central Utah is impressed by the large amounts of hydrocarbons in the
RME-3044
-
34-Shinarump and higher formations. Some liquid oils impregnate large volumes of sandstone and it is not un-usual to find them, for example, in fossil logs which are also rich in uranium. They intimately surround the shale masses but do not seem to penetrate any of them. It, therefore, is not possible to make them directly responsible for any kind of reducing action
that might have occurred.
Cone lusions
While it is well known that certain Triassic and Jurassic sediments underwent a change in color from red to gray, the mechanisms and reasons for this change are little understood because they are not the same in all instances. Where the change is on a regional, vast scale in silt and shales as in the Chinle, it is not possible to make local conditions responsible. They must be regional, that is, connected with widespread primary or diagenetic environment. On the other hand, some.of the changes are more localized and are associated with areas containing uranium concentrations in portions of the formations. In these areas which are unusually high in sulfates of Ca, Al, K, and Fe+++ (jarosite) it is probable that the ground~;ater solutions were high in sulfate ions. We may also attribute, for the moment, the movement of uranium to such waters, though its im-mediate precipitation was caused by organic matter.
It is here suggested that diffused sulfate waters in the red shales and silts acted as a medium in trans-ferring the small amounts of red hematitic iron into the crystal structures of the abundantly present hydromicas which usually are high in ferric iron though their color is
n£1
red but gray or greenish gray. This would be the mechanism by which red shales became gray. It does notinvolve any reduction of iron and, therefore, eliminates the almost insurmountable difficulty of reducing ferric oxide in very dense rocks. If this cannot be the mechanism, then reduction by organic agents directly or indirectly seems to be the only other means that nature would have to bring about the change.