R. Jimbo
Y. Xue
K. Mustafa
M. Andersson
A. Wennerberg
Photocatalytically induced
hydrophilicity influences bone
remodelling at longer healing
periods: a rabbit study
Authors’ affiliations:
M. Hayashi, R. Jimbo, A. Wennerberg,Department of Prosthodontics, Faculty of Odontology, Malmo¨ University, Malmo¨, Sweden
Y. Xue, K. Mustafa,Department of Clinical Dentistry, Center for Clinical Dental Research, Faculty of Medicine and Dentistry, University of Bergen, Bergen, Norway
M. Andersson,Department of Chemical and Biological Engineering, Applied Surface Chemistry, Chalmers University of technology, Gothenburg, Sweden
Corresponding author: Mariko Hayashi
Department of Prosthodontics, Faculty of Odontology
Malmo¨ University
Carlgustavsva¨gen 34 SE205 06 Malmo¨, Sweden Tel.: +46 40 665 8679
Fax: +46 40 665 8503
e-mail: mariko.hayashi@mah.se
Key words: gene expression, hydrophilicity, osseointegration, photocatalyst Abstract
Objectives: Previously, we have reported that photocatalytically active hydrophilicity of the anatase titanium dioxide (TiO2) nanoparticles coated onto commercially pure titanium discs
presented significantly improved hydrophilicity after ultraviolet irradiation. As hydrophilicity has shown enhancement of osseointegration, thein vivo responses were of great interest. The aim of this study was to evaluate whether or not the photo-activated hydrophilicity generated at the time of implant placement has an effect on the longer healing periods for osseointegration.
Materials and methods: Photocatatytically active nanostructured TiO2powder (Degussa P-25),
which consists of approximately 80% anatase and 20% rutile, was spin-coated onto commercially pure titanium discs and was heat-treated thereafter. These P25-coated discs were irradiated with ultraviolet (UV) light for the test (+UV) group, and non-irradiated discs were prepared for the control ( UV) group. Both groups of discs were placed in the rabbits’ tibiae. After 12 weeks of healing period, histological analysis and gene expression analysis using real-time RT-PCR were performed.
Results: From the histological analyses, there were no specific differences between UV and+UV groups. However, from the gene expression analysis, ALP, RUNX-2 and IL-10 were significantly upregulated for the+UV group compared with the UV group.
Conclusions: The biologically enhancing effect to photocatalytically activated surfaces remained even after 12 weeks of healing time in terms of genetic responses.
Surface hydrophilicity is an important factor
for the achievement of osseointegration
(Buser et al. 2004; Sawase et al. 2008; Sch-warz et al. 2009; Wennerberg & Albrektsson 2009; Rupp et al. 2010). There are many ways to obtain hydrophilic implant surfaces, such as sandblasted and acid-etched surfaces placed in liquid [modified sandblasted and
acid-etched (SLA)] (Buser et al. 2004),
plasma cleaning (Carlsson et al. 1989) and hydroxyapatite (HA) (Zhang et al. 2011). One way to achieve hydrophilicity is by using the TiO2 on the implant surface as a
catalyst to generate hydrophilicity under ultraviolet (UV) irradiation (Fujishima & Honda 1972; Wang et al. 1997; Jimbo et al. 2008, 2011a,b). Sawase et al. (2007) indi-cated that commercially available anodic oxidized implants possess photocatalytic properties, which is comprehensible as the TiO2 layer thickness created by the
oxida-tion can be as thick as 10lm (Jimbo et al. 2007). However, the commercially available
implants before and after UV irradiation did not present significant differences in bone-to-implant contact in a rabbit model after 4 weeks. It was speculated that this was due to the chemical composition of the TiO2, mainly being amorphous, and as it
has been suggested by Wang et al. (1997) that a certain crystalline structure, prefera-bly anatase or rutile, needs to be widely exposed to present strong photoreactivity (Wang et al. 1997). This was confirmed by Rupp et al. (2010) that the hydrophilicity of the anatase titanium dioxide (TiO2) surface
enhanced osseointegration (Rupp et al.
2010). Thus, modifications to increase the surface area of a certain TiO2 crystalline
structure have been reported to be effective in increasing the initial cell attachment,
proliferation and early bone apposition
under UV irradiation (Jimbo et al. 2008; Sawase et al. 2008). Furthermore, Hirakawa et al. (2012) reported that the post-annealed plasma source ion implantation (PSII)
coat-Date:
Accepted 17 January 2013
To cite this article:
Hayashi M, Jimbo R, Xue Y, Mustafa K, Andersson M, Wennerberg A. Photocatalytically induced hydrophilicity influences bone remodelling at longer healing periods: a rabbit study.
Clin. Oral Impl. Res.25, 2014, 749–754
ing, which provided the implant surface dominantly an anatase crystalline structure,
presented significantly higher
bone-to-implant contact (BIC) of 42.7% after UV irradiation as compared to 28.4% for the commercial titanium implant without UV irradiation only after 2 weeks of healing time (Hirakawa et al. 2012). It can be emphasized here again that a broad expo-sure of a specific crystalline structure is one of the most important factors to pres-ent photoactivity sufficipres-ent enough to
posi-tively influence the initial biological
outcomes.
In our previous study, we focused on a
nanocrystalline form of TiO2 powder
(Degussa P25, Degussa GmbH, Germany), which has a controlled balance of anatase and rutile crystalline structures (Hayashi et al. 2012). As this material has been known to present strong photocatalytic properties with industrial materials (Karimi et al. 2010; Zhou
et al. 2010; Parussulo et al. 2011), we
explored the possibility of this material to be applicable as a biomaterial. The results showed that the surface coated with P25 onto commercially pure titanium discs presented significantly improved hydrophilicity after UV irradiation. Surprisingly, no enhancement of the initial cell response was confirmed at the time tested compared with the non-irradiated surface (Hayashi et al. 2012). We concluded that this might be due to the fact that the material surface was already extre-mely hydrophilic even before photoactivation,
due to the surface enlargement from the nanotopography. However, the unique chem-istry and nanotopography was of great interest for application as a biomaterial coating, and further testing in vivo was motivated by the obtained outcomes.
In animal studies investigating the
biologi-cal effect of photo-induced hydrophilic
implant surfaces, most of the studies focus only on the initial osseointegration (majority of the studies within 4 weeks) (Jimbo et al. 2007; Sawase et al. 2008; Hirakawa et al. 2012), and not much is known about the long-term aspects of osseointegration. As in general, it is believed that if the surface topo-graphical differences are noticeable only in the nanoscale, the long-term biological outcomes seem to be comparable as stated by Svanborg et al. (Svanborg et al. 2011). They showed that there seemed to have no signifi-cant differences between the SLA surfaces modified with HA as test and non-modified SLA surfaces as control after a long-term (9 weeks) observation. In addition, the photo-activated surface hydrophilicity is thought to be only active in the initial stages after implant placement, as reported by Sawase et al. to be approximately 48 h, and therefore will gradually lose its photocatalytic effects (Sawase et al. 2007). Thus, it was hypothe-sized that in longer time periods, the effects would fade away and the biological outcomes will not show any differences between photo induced hydrophilic and control surfaces. The objective of this study was to investigate
longer healing periods (12 weeks) of
P25-coated implants with and without UV irradiation placed in a rabbit model by using histological and gene expression approaches.
Material and methods
Surface preparationCommercially pure titanium discs with a 2.0-mm screw hole in the middle (cpTi, diameter 6 mm; thickness 1 mm, grade 4)
were used in this study. TiO2 powder,
which consists of 80% anatase and 20% rutile (particle size, approximately 20– 50 nm), was dispersed in 99.5% ethanol and prepared to 15 wt%. Thereafter, the discs
were spin-coated using the solution
(4000 rpm). To immobilize the TiO2powder
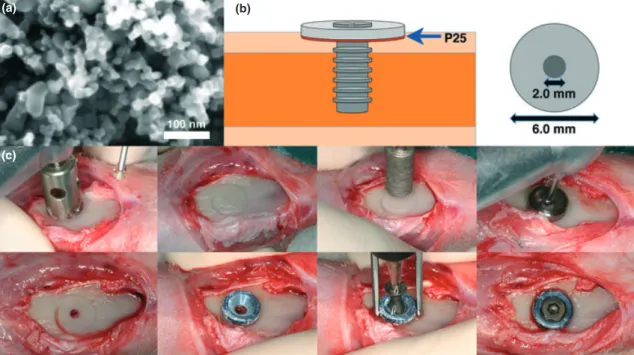
on to the titanium base as a thin coating, the coated discs were heat-treated in air at 500°C for 5 min, as shown in our previous reports (Hayashi et al. 2012). To confirm the surface morphology of the coated discs, scanning electron microscopy (SEM, LEO Ultra 55 FEG, Zeiss, Oberkochen, Germany) at an accelerating voltage of 6 kV was per-formed. A secondary electron in-lens detec-tor was used for visualization (Fig. 1). Three discs from each of the non-coated and coated discs were randomly selected for the observation, and the titanium base substrate was fully covered with nanoparticles.
The discs were divided into the following groups;
(a)
(c)
(b)
Fig. 1. (a) Scanning electron microscopy image of the P25-coated surface. The bar is 100 nm. (b) Schematic illustration of the disc model used in the study. (c) Photographs of the implantation procedure.
1. Control: P25-coated discs stored in a plas-tic box covered with aluminium foil to block any light source to avoid photo-activation ( UV).
2. Test: P25-coated discs irradiated with UV (wavelength 352 nm, 6 W) for 24 h (+UV).
Animals and implantation
Nine Swedish lop-eared rabbits (mean body weight 3.9 kg) were used in this study following the approval given by the Malm€o/ Lund Regional Animal Ethics Committee (approval no. M282-09). Before surgery, the hind legs of the rabbits were shaved and disinfected with 70% ethanol and 70% chlorhexidine. The animals were anaesthe-tized by intramuscular injection of a mixture of 0.15 ml/kg medetomidine (1 mg/ml Dorm-itor; Orion Pharma, Sollentuna, Sweden) and 0.35 ml/kg ketamine hydrochloride (50 mg/ ml Ketalar; Pfizer AB, Sollentuna, Sweden). Lidocaine hydrochloride (Xylocaine; Astra-Zeneca AB, S€odert€alje, Sweden) was
adminis-tered as the local anaesthetic at each
insertion site at a dose of 1 ml.
Two discs were placed in each rabbit: one test (+UV) disc and one control ( UV) disc werea seated on the left and right tibiae of the flattened proximal cortex, respectively, and thereafter securely stabilized with a screw (∅2.0 9 6 mm) (Fig. 1).
Post-operatively, buprenorphine hydrochlo-ride (0.5 ml Temgesic; Reckitt Benckiser, Slough, UK) was administered as an analgesic for 3 days.
After 12 weeks of healing time, the rabbits were euthanized with an overdose of sodium pentobarbital (60 mg/ml Apoteksbolaget AB, Stockholm, Sweden).
RNA extraction and real-time RT-PCR
The implants and the attaching cortical bone of six rabbits were carefully collected with the use of aΦ4.4-mm trephine bur (GC Den-tal, Tokyo, Japan); thereafter, the tissue spec-imens were placed in RNAlater solution (QIAGEN GmbH, Hilden, Germany) and stored at 80°C until analysis. Tissue sam-ples were homogenized with the TissueLy-serâinstrument (QIAGEN GmbH) and phase separated with QiaZolâ solution (QIAGEN GmbH). Total RNA was extracted from the separated aqueous phase with RNA Tissue Kit SII on the QuickGene extraction robot (Fujifilm Life Science, Tokyo, Japan) accord-ing to manufacturer’s instructions. Duraccord-ing extraction, all samples were DNase-treated with Rnase-free DNase set (QIAGEN GmbH) to reduce gDNA contamination. All RNA
samples were analysed for RNA quantity with NanoDrop ND-1000 spectrophotometer (ThermoScientific NanoDrop Technologies, Wilmington, DE, USA).
RNA samples were normalized to 50 ng/ll, and then they were reverse-transcribed in sin-gle 20-ll reactions, 5-ll RT Mix and 15-ll samples. All RT was performed using iScript cDNA Synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA) to generate cDNA for
relative quantification on mRNA. After
reverse transcription, cDNA samples were stored in -20°C.
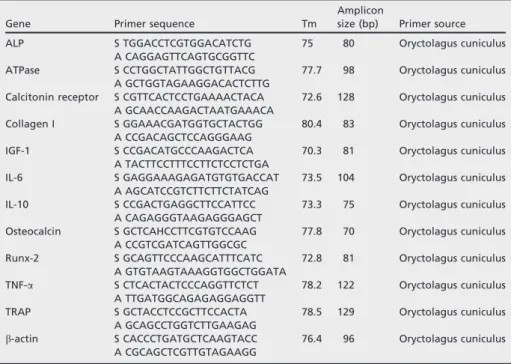
Real-time quantitative reverse transcription (RT-PCR) was performed in 20-ll reaction in triplicate for each sample, with custom-designed primers (Table 1) of SYBRâ green detection (PrimerDesign Ltd, Southampton,
UK) on a 96-well StepOnePlusTM system
(Applied Biosystems, Foster City, CA, USA).
The comparative Ct or △△Ct method was
applied, and b-actin was served as house-keeping gene (Schmittgen & Livak 2008). The control group was used as reference and normalized with test group in the calcula-tions.
Cut and ground sectioning
To observe the bone-to-implant interactions, tibiae from three rabbits were subjected to histological processing (Johansson et al. 2011). In brief, the samples were first placed
in a series of ethanol for dehydration
(70–100%), thereafter, a series of resin for infiltration (30–100%). The samples were
finally embedded in light-curing resin (Tech-novit 7200 VLC; Heraeus Kulzer Wehrheim, Germany). Cut and ground sections were pre-pared from each resin blocks by using Exakt sawing and grinding equipment (Donath 1987). The sections were ground to a final
thickness of approximately 20lm and
stained with toluidine blue and pyronin G. Histological analysis
To observe whether biological reactions (both negative and positive) exist between the con-trol ( UV) and the test (+UV) discs, the histo-logical sections were observed under a light microscope (Eclipse ME600; Nikon, Japan), and bone-to-implant contact (BIC) along the entire implant for both groups was quantified by an image analysis software (Image J v. 1.43u; National Institutes of Health) at9 10 objective magnification (Jimbo et al. 2011a).
Statistical analysis
For the gene expression analysis, Student’s paired t-test was used to compare the two groups. P values less than 0.05 were consid-ered significant.
Results
Real-time RT-PCRThe gene expression analysis presented that at 12 weeks, the test (+UV) group presented statistically significant higher values of alkaline phosphatase (ALP), runt-related Table 1. Primer sequences used for real-time RT-PCR
Gene Primer sequence Tm
Amplicon
size (bp) Primer source
ALP S TGGACCTCGTGGACATCTG A CAGGAGTTCAGTGCGGTTC 75 80 Oryctolagus cuniculus ATPase S CCTGGCTATTGGCTGTTACG A GCTGGTAGAAGGACACTCTTG 77.7 98 Oryctolagus cuniculus Calcitonin receptor S CGTTCACTCCTGAAAACTACA
A GCAACCAAGACTAATGAAACA 72.6 128 Oryctolagus cuniculus Collagen I S GGAAACGATGGTGCTACTGG A CCGACAGCTCCAGGGAAG 80.4 83 Oryctolagus cuniculus IGF-1 S CCGACATGCCCAAGACTCA A TACTTCCTTTCCTTCTCCTCTGA 70.3 81 Oryctolagus cuniculus IL-6 S GAGGAAAGAGATGTGTGACCAT A AGCATCCGTCTTCTTCTATCAG 73.5 104 Oryctolagus cuniculus IL-10 S CCGACTGAGGCTTCCATTCC A CAGAGGGTAAGAGGGAGCT 73.3 75 Oryctolagus cuniculus Osteocalcin S GCTCAHCCTTCGTGTCCAAG A CCGTCGATCAGTTGGCGC 77.8 70 Oryctolagus cuniculus Runx-2 S GCAGTTCCCAAGCATTTCATC A GTGTAAGTAAAGGTGGCTGGATA 72.8 81 Oryctolagus cuniculus TNF-a S CTCACTACTCCCAGGTTCTCT A TTGATGGCAGAGAGGAGGTT 78.2 122 Oryctolagus cuniculus TRAP S GCTACCTCCGCTTCCACTA A GCAGCCTGGTCTTGAAGAG 78.5 129 Oryctolagus cuniculus b-actin S CACCCTGATGCTCAAGTACC A CGCAGCTCGTTGTAGAAGG 76.4 96 Oryctolagus cuniculus
transcription factor 2 (RUNX-2) and interleu-kin-10 (IL-10), compared with the control ( UV) group (ALP, 0.9800-fold, P= 0.0229; RUNX-2, 2.473-fold, P= 0.0447; IL-10, 1.235-fold, P= 0.0479) (Fig. 2).
Histological analysis
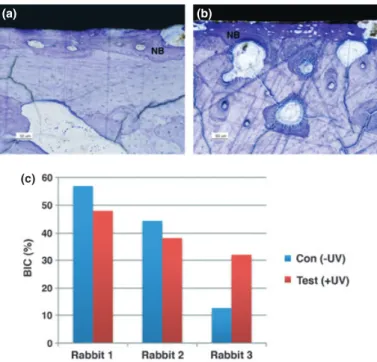
The histological sections presented newly formed trabeculae with deeply stained miner-alized tissue for both control and test groups after 12 weeks of healing, and no signs of
inflammatory responses were visible for both groups (Fig. 3a and b).
The histomorphometric values for the control and test groups for each rabbit are pre-sented in Fig. 3c. Due to the low number of samples, statistical comparison was not possi-ble, and thus for reference, the mean BIC (SD) values for the control and the test groups at 12 weeks were 37.99% (22.78) and 39.35% (8.05), respectively.
Discussion
The objective of this study was to investigate the longer healing properties (12 weeks) of P25-coated discs with and without UV irradia-tion by using histological and genetic analysis techniques.
The histological and histomorphometric observation was in accordance with our hypothesis showing that there were no nota-ble histological or histomorphometric differ-ences. Although the number of samples was only three from each group, and is difficult to come to a statistical conclusion, the histolog-ical observation suggested that osseointegra-tion was achieved successfully for both
groups without any signs of negative
responses. Intriguingly, the gene expression for the test surface (+UV) showed higher val-ues of ALP, RUNX-2 and IL-10 compared with the control surface ( UV).
RUNX-2 is known to be a transcription factor indispensable for osteoblast differentia-tion and funcdifferentia-tions upstream to osterix (Ma et al. 2011), while ALP plays an important regulatory role during matrix mineralization (Suzuki et al. 2006), and both markers are expressed during active bone remodelling. Klein et al. demonstrated that the hydro-philic modified SLA titanium surface showed the highest values in gene expression of ALP and RUNX-2 using human osteoblasts in
48 h of incubation compared with the
smooth and the SLA titanium surfaces (Klein et al. 2010). They concluded that the combi-nation of submicron-scale roughness and sur-face hydrophilicity synergistically promoted osteogenic cell adhesion and maturation that enhanced osseointegration.
In this study, the expression levels of ALP and RUNX-2 were significantly higher for the test surface (+UV) compared with the control surface ( UV)(p= 0.0229 and P= 0.0447, respectively); furthermore, the mean relative expression levels for insulin-like growth factor (IGF), which is known to be the most abundant growth factor in bone
Fig. 2. Gene expressions of selected markers quantified by real-time RT-PCR at 12 weeks of healing time (n= 6). The relative expressions of target genes were normalized with the housekeeping geneb-actin (*P < 0.05).
that has significant effects on bone remod-elling (Canalis et al. 1988; Shinar et al. 1993), presented a strong tendency in favour
for the +UV group (P = 0.0798).
Concur-rently, the mean relative expression levels of the pro-inflammatory cytokine marker, TNF-a, also presented a strong tendency to be
higher for the+UV compared with the UV
(P= 0.0715). It is a fact that non-pathological inflammation is part of the bone remodelling process (Lisignoli et al. 1999), which is in accordance with the histological study from Araujo & Lindhe (2005), who indicated that inflammation occurs at the same time as bone remodelling in the fresh extraction socket (Araujo & Lindhe 2005). Thus, it is strongly
speculated that the +UV group underwent
active bone remodelling at the time of 12 weeks, and this was detected by the gene expression analysis.
It has been reported that TNF-a as a
pro-inflammatory cytokine induces osteoclas-togenesis (Lam et al. 2000). In the study, the
authors reported that along with TNF-a
upreg-ulation, IL-6, another pro-inflammatory
marker, and TRAP, a marker for osteoclasto-genesis, were also expressed. Intriguingly, in this study, there were no significant differences on the relative expression values of IL-6 and TRAP between the UV and the+UV surfaces (P= 0.4658 and P = 0.4126, respectively). In addition, the relative expression of IL-10, an anti-inflammatory cytokine produced by mac-rophages and lymphocytes, was significantly
higher for the +UV than the UV groups
(P= 0.0479). IL-10 functions in a negative feed-back loop, in which it suppresses the release of inflammatory cytokines and dampens the acute inflammatory response (de Vries 1995). It also has potent inhibitory effects on osteoclas-togenesis (Xu et al. 1995). Carmody et al. (2002) demonstrated that viral IL-10 interfered with the critical steps involved in particle-induced inflammation, osteoclastogenesis and bone loss in vitro and in vivo (Carmody et al. 2002). It is strongly suspected that with the
photocatalytically activated surface, the expression of IL-6 and TRAP was suppressed due to the significantly upregulated expression of the IL-10. Thus, it is speculated that due to the suppression of pro-inflammatory and osteo-clastogenesis markers by the IL-10, bone remodelling activity was undisturbed, which resulted in the significantly high expression of ALP and RUNX-2.
Conclusion
Based on the histological analysis, after a healing period of 12 weeks, there seemed to be no qualitative and quantitative differences in bone formation between the UV-irradiated and non-irradiated groups. However, the genetic analysis using the real-time RT-PCR suggested that for the+UV group, osteogene-sis was significantly enhanced in terms of active remodelling; at the same time, inflam-matory and osteoclastic responses were sup-pressed as a result of significantly increased anti-inflammatory cytokine IL-10. Thus, the initial hypothesis that there would be no
dif-ferences between the two groups after
12 weeks of healing was rejected. As it was believed that the effect of photocatalytically activated surface was restricted only to the initial stages of osseointegration, the results of this study may suggest that the biologi-cally enhancing effect remained even after 12 weeks of healing time.
Acknowledgements:
The authorsgratefully acknowledge Dr. Humberto Osvaldo Schwartz-Filho for the excellent support of surgical section. The current study was supported by grants from Biofilm PhD Student Grant (Malm€o University), Malm€o University, the Swedish Research Council (VR), and the Scandinavia-Japan Sasakawa Foundation.
The authors declare no conflict of interests.
References
Araujo, M.G. & Lindhe, J. (2005) Dimensional ridge alterations following tooth extraction. An experi-mental study in the dog. Journal of Clinical Peri-odontology32: 212–218.
Buser, D., Broggini, N., Wieland, M., Schenk, R.K., Denzer, A.J., Cochran, D.L., Hoffmann, B., Lussi, A. & Steinemann, S.G. (2004) Enhanced bone apposition to a chemically modified SLA tita-nium surface. Journal of Dental Research 83: 529–533.
Canalis, E., McCarthy, T. & Centrella, M. (1988) Growth factors and the regulation of bone remodeling. The Journal of Clinical Investigation81: 277–281. Carlsson, L.V., Alberktsson, T. & Berman, C. (1989)
Bone response to plasma-cleaned titanium implants. International Journal of Oral and Max-illofacial Implants4: 199–204.
Carmody, E.E., Schwarz, E.M., Puzas, J.E., Rosier, R.N. & O’Keefe, R.J. (2002) Viral interleukin-10 gene inhi-bition of inflammation, osteoclastogenesis, and bone
resorption in response to titanium particles. Arthritis and Rheumatism46: 1298–1308.
Donath, K. (1987) Preparation of Histologic Sections by the Cutting-grinding Technique for Hard Tissue and Other Material Not Suitable to be Sectioned by Routine Methods. Norderstedt: Exakt-kulzer-publication.
Fujishima, A. & Honda, K. (1972) Electrochemical photolysis of water at a semiconductor electrode. Nature238: 37–38.
(a)
(c)
(b)
Fig. 3. Light microscope images of (a) the control ( UV) and (b) the test (+UV) discs, 12 weeks after implantation. NB: new bone. Bars represent 50lm. (c) Per rabbit histomorphometric bone-to-implant contact (BIC) comparison for the control and the test discs (n= 3).
Hayashi, M., Jimbo, R., Lindh, L., Sotres, J., Saw-ase, T., Mustafa, K., Andersson, M. & Wenner-berg, A. (2012) In vitro characterization and osteoblast responses to nanostructured photocat-alytic tio(2) coated surfaces. Acta Biomaterialia 8: 2411–2416.
Hirakawa, Y., Jimbo, R., Shibata, Y., Watanabe, I., Wennerberg, A. & Sawase, T. (2012) Accelerated bone formation on photo-induced hydrophilic titanium implants: an experimental study in the dog mandible. Clinical Oral Implants Research doi: 10.1111/j.1600-0501.2011.02401.x.
Jimbo, R., Coelho, P.G., Vandeweghe, S., Schwartz-Filho, H.O., Hayashi, M., Ono, D., Andersson, M. & Wennerberg, A. (2011a) Histological and three-dimensional evaluation of osseointegration to nanostructured calcium phosphate-coated implants. Acta Biomaterialia7: 4229–4234. Jimbo, R., Ono, D., Hirakawa, Y., Odatsu, T.,
Tanaka, T. & Sawase, T. (2011b) Accelerated photo-induced hydrophilicity promotes osseointe-gration: an animal study. Clinical Implant Den-tistry and Related Research13: 79–85.
Jimbo, R., Sawase, T., Baba, K., Kurogi, T., Shibata, Y. & Atsuta, M. (2008) Enhanced initial cell responses to chemically modified anodized tita-nium. Clinical Implant Dentistry and Related Research10: 55–61.
Jimbo, R., Sawase, T., Shibata, Y., Hirata, K., Hishikawa, Y., Tanaka, Y., Bessho, K., Ikeda, T. & Atsuta, M. (2007) Enhanced osseointegration by the chemotactic activity of plasma fibronectin for cellular fibronectin positive cells. Biomateri-als28: 3469–3477.
Johansson, C. B., Jimbo, R. & Roeser, K. (2011) 3.313– histological analysis. In: Editor-in-Chief: Paul, D., ed. Comprehensive Biomaterials, 215– 233. Oxford: Elsevier.
Karimi, L., Mirjalili, M., Yazdanshenas, M.E. & Nazari, A. (2010) Effect of nano tio(2) on self-cleaning property of cross-linking cotton fabric with succinic acid under UV irradiation. Photo-chemistry and Photobiology86: 1030–1037. Klein, M.O., Bijelic, A., Toyoshima, T., Gotz, H.,
von Koppenfels, R.L., Al-Nawas, B. & Duschner, H. (2010) Long-term response of osteogenic cells on micron and submicron-scale-structured hydrophilic titanium surfaces: sequence of cell proliferation and cell differentiation. Clinical Oral Implants Research21: 642–649.
Lam, J., Takeshita, S., Barker, J.E., Kanagawa, O., Ross, F.P. & Teitelbaum, S.L. (2000) TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of rank ligand. The Journal of Clinical Investigation 106: 1481–1488.
Lisignoli, G., Toneguzzi, S., Pozzi, C., Piacentini, A., Riccio, M., Ferruzzi, A., Gualtieri, G. & Facchini, A. (1999) Proinflammatory cytokines and chemokine production and expression by human osteoblasts isolated from patients with rheumatoid arthritis and osteoarthritis. Journal of Rheumatology26: 791–799.
Ma, H.P., Ming, L.G., Ge, B.F., Zhai, Y.K., Song, P., Xian, C.J. & Chen, K.M. (2011) Icariin is more potent than genistein in promoting osteoblast dif-ferentiation and mineralization in vitro. Journal of Cellular Biochemistry112: 916–923.
Parussulo, A.L., Huila, M.F., Araki, K. & Toma, H.E. (2011) N3-dye-induced visible laser anatase-to-rutile phase transition on mesoporous TiO2 films. Langmuir27: 9094–9099.
Rupp, F., Haupt, M., Klostermann, H., Kim, H.S., Eichler, M., Peetsch, A., Scheideler, L., Doering, C., Oehr, C., Wendel, H.P., Sinn, S., Decker, E., von Ohle, C. & Geis-Gerstorfer, J. (2010) Multi-functional nature of UV-irradiated nanocrystal-line anatase thin films for biomedical applications. Acta Biomaterialia6: 4566–4577. Sawase, T., Jimbo, R., Wennerberg, A., Suketa, N.,
Tanaka, Y. & Atsuta, M. (2007) A novel charac-teristic of porous titanium oxide implants. Clini-cal Oral Implants Research18: 680–685. Sawase, T., Jimbo, R., Baba, K., Shibata, Y., Ikeda,
T. & Atsuta, M. (2008) Photo-induced hydrophi-licity enhances initial cell behavior and early bone apposition. Clinical Oral Implants Research 19: 491–496.
Schmittgen, T.D. & Livak, K.J. (2008) Analyzing real-time PCR data by the comparative c(t) method. Nature Protocols3: 1101–1108. Schwarz, F., Wieland, M., Schwartz, Z., Zhao, G.,
Rupp, F., Geis-Gerstorfer, J., Schedle, A., Broggini, N., Bornstein, M.M., Buser, D., Ferguson, S.J., Bec-ker, J., Boyan, B.D. & Cochran, D.L. (2009) Poten-tial of chemically modified hydrophilic surface characteristics to support tissue integration of tita-nium dental implants. Journal of biomedical materials research. Part B, Applied biomaterials 88: 544–557.
Shinar, D.M., Endo, N., Halperin, D., Rodan, G.A. & Weinreb, M. (1993) Differential expression of insulin-like growth factor-I (IGF-I) and IGF-II messenger ribonucleic acid in growing rat bone. Endocrinology132: 1158–1167.
Suzuki, A., Ghayor, C., Guicheux, J., Magne, D., Quillard, S., Kakita, A., Ono, Y., Miura, Y., Oiso, Y., Itoh, M. & Caverzasio, J. (2006) Enhanced expression of the inorganic phosphate transporter pit-1 is involved in BMP-2-induced matrix mineralization in osteoblast-like cells. Journal of Bone and Mineral Research 21: 674–683.
Svanborg, L.M., Hoffman, M., Andersson, M., Cur-rie, F., Kjellin, P. & Wennerberg, A. (2011) The effect of hydroxyapatite nanocrystals on early bone formation surrounding dental implants. International Journal of Oral and Maxillofacial Surgery40: 308–315.
de Vries, J.E. (1995) Immunosuppressive and anti-inflammatory properties of interleukin 10. Annals of Medicine27: 537–541.
Wang, R., Hashimoto, K., Fujishima, A., Chikuni, M., Kojima, E., Kitamura, A., Shimohigoshi, M. & Watanabe, T. (1997) Nature388: 431–432. Wennerberg, A. & Albrektsson, T. (2009)
Struc-tural influence from calcium phosphate coatings and its possible effect on enhanced bone inte-gration. Acta Odontologica Scandinavica 67: 333–340.
Xu, L.X., Kukita, T., Kukita, A., Otsuka, T., Niho, Y. & Iijima, T. (1995) Interleukin-10 selectively inhibits osteoclastogenesis by inhibiting differen-tiation of osteoclast progenitors into preosteo-clast-like cells in rat bone marrow culture system. Journal of Cellular Physiology 165: 624–629.
Zhang, F., Huang, Y., Li, X. & Zhao, S. (2011) Sur-face modification and its effect on attachment, spreading, and proliferation of human gingival fibroblasts. International Journal of Oral and Maxillofacial Implants26: 1183–1192.
Zhou, W., Pan, K., Qu, Y., Sun, F., Tian, C., Ren, Z., Tian, G. & Fu, H. (2010) Photodegradation of organic contamination in wastewaters by bonding TiO2/single-walled carbon nanotube composites with enhanced photocatalytic activity. Chemo-sphere81: 555–561.