Nitrogen Fixation Among Marine
Bacterioplankton
Kjärstin H. Boström
Department of Biology & Environmental Science
University of Kalmar
Sweden
2006
AKADEMISK AVHANDLING
som för avläggande av Filosofie Doktorsexamen vid Naturvetenskapliga Fakulteten vid högskolan i Kalmar kommer att offentligt försvaras fredagen den 20 januari 2006
Doctoral thesis 2006 University of Kalmar Faculty of Natural Sciences Dissertation series No. 26 Kjärstin H. Boström
Department of Biology and Environmental Science University of Kalmar, SE 391 82 Kalmar, Sweden
Supervisor:
Dr. Lasse Riemann, Assistant Professor
Department of Biology and Environmental Science University of Kalmar, SE 391 82 Kalmar, Sweden Opponent:
Dr. Grieg Steward, Assistant Professor Department of Oceanography
University of Hawaii, Honolulu, HI 96822, USA
2006 Kjärstin H. Boström
ISBN: 91-89584-52-X, ISSN: 1650-2779, pp. 1-26 Printed by: Högskolans tryckeri, Kalmar
To
Frida
&
Emma
TABLE OF CONTENTS
TABLE OF CONTENTS...4 ABSTRACT...5 SVENSK SAMMANFATTNING ...6 LIST OF PUBLICATIONS...8 INTRODUCTION ...9BACTERIOPLANKTON IN THE MARINE FOOD WEB...9
CULTIVATION AND BACTERIOPLANKTON DIVERSITY...9
BIOGEOCHEMICAL SETTING...11
NITROGEN...12
The nitrogen cycle...12
N2-fixation ...13
Regulation of N2-fixation...14
Methods to measure N2-fixation...15
AIMS OF THESIS...16
THESIS OUTLINE ...17
CONCLUDING REMARKS...21
REFERENCES ...22
ABSTRACT
While bacterioplankton indisputably control vital biogeochemical paths in the cycling of carbon and nutrients in the world’s oceans, our knowledge about the functional and genetic diversity of bacterioplankton communities is negligible. In this thesis, molecular and more traditional microbiological methods were used to study the specific function of N2-fixation
and in a general sense diversity of marine bacterioplankton species.
Most oceans are nitrogen limited and, therefore, adaptive to bacterioplankton capable of N2-fixation. Recent studies have found nifH genes (coding for the nitrogenase enzyme)
related to diverse heterotrophic bacteria in oceanic seawater samples indicating that, along with cyanobacteria, also heterotrophic bacteria benefit from N2-fixation. Here, molecular and
cultivation methods were used to examine diazotrophic bacterioplankton in the Baltic Sea. We successfully isolated heterotrophic N2-fixing bacteria belonging to the γ-proteobacterial
class by means of low-nitrogen plates and semi-solid diazotrophic medium tubes. The isolates required low-O2 conditions for N2-fixation. Using Real-time PCR it was found that
heterotrophic bacterioplankton carrying the nifH gene was abundant (3 x 104 nifH gene
copies L seawater-1) at locations in the Southwest Baltic proper.
With the aim to identify the main N2-fixing organisms in Baltic Proper surface waters, a
clone library of nifH gene transcripts (RNA) was generated. Clone inserts were exclusively related to Aphanizomenon sp. and Nodularia sp. Using quantitative real-time PCR it was found that the nifH gene expression from Nodularia sp. was highly variable between stations in the Baltic Proper but was 10-fold higher during mid summer relative to early summer and fall. A diel study showed a 4-fold increase in Nodularia transcript concentrations at early to mid day relative to rest of the day. Real-time PCR was found to be a powerful and highly sensitive method for measuring gene expression.
Since nucleic acids are a prerequisite for molecular analyses of bacterioplankton dynamics a protocol to extract DNA from seawater samples was developed with the aim to maximize the yield of high-quality DNA. Each step in the protocol was important for the efficiency of extraction. The obtained extraction efficiencies were up to 92% for seawater samples and up to 96% for isolates. The protocol provides a guideline for DNA extraction from seawater samples for other studies.
In a global sampling campaign (9 locations from polar, tropical and temperate regions) we sampled DNA from surface water and constructed 16S rRNA gene libraries to investigate diversity and biogeography of bacterioplankton. Approx. 80% of the sequences found were similar to sequences already deposited in GenBank, indicating that a large fraction of the marine bacterioplankton already has been sampled, which in turn suggests a limited global bacterioplankton diversity.
This thesis have improved our knowledge about the composition and nifH gene expression of the diazotrophic bacterioplankton community in the Baltic Sea and contribute significantly to the discussion on global marine bacterioplankton diversity and biogeography.
SVENSK SAMMANFATTNING
Östersjön är ett av världens största brackvattensystem. Den ekologiska balansen i detta hav är hotad på grund av övergödning. Mycket arbete har därför fokuserats på att reducera utsläppen av näringsämnen, speciellt kväve. Dessa ansträngningar kan dock motverkas av bakterier som har förmåga att omvandla luftens kväve till metaboliskt användbart ammonium (kvävefixering).
På sommaren är Östersjöns primärproduktion begränsad av kväve, med följden att det årligen uppstår massiva blomningar av kvävefixerande bakterier, framför allt cyanobakterier. Dessa är främst Aphanizomenon och Nodularia, men inte endast de fototrofa cyano-bakterierna har förutsättningar att fixera N2. NifH gener (genen som kodar för nitrogenas)
bärs också av heterotrofa bakterioplankton, vilket har visats i studier i främst Atlanten och Stilla havet. Med hjälp av två olika odlingsmetoder lyckades vi isolera heterotrofa kvävefixerande bakterier tillhörande klassen γ-proteobakteria från Östersjön. Svårigheten med att finna dessa bakterier ligger i att de kräver en miljö med mycket låg syrehalt för att kunna fixera kväve.
Resultaten från denna studie ledde oss vidare till att undersöka vilka organismer som uttrycker nifH genen (och då troligen även fixerar kväve) i Östersjön. En av de bakterier som isolerats kunde påvisas med Realtids PCR i ett relativt stort antal (3 x 104 nifH genkopior per
liter) vid en av de ursprungliga provtagningsstationerna. För att söka rätt på de olika organismtyper som uttrycker nifH skapades ett klonbibliotek baserat på mRNA extraherat från havsvatten. Det visade sig då att alla de närmare 100 kloner som sekvenserades tillhörde antingen Aphanizominon eller Nodularia. De heterotrofa bakteriernas nifH genuttryck var troligen i jämförelse med dessa cyanobakterier alltför lågt för att kunna detekteras. Realtids PCR mätningar av Nodularias nifH genuttryck visade på en stor variation mellan de olika provtagningsstationerna samt mellan de olika provtagningstillfällena. Vi fann dock en kraftig ökning under juli med en nedgång igen i augusti. En dygnscykelstudie visade att Nodularia
nifH genuttrycket ökade under förmiddagen med en topp mitt på dagen för att sedan minska
igen. Detta troligen med anledning av att den energikrävande kvävefixeringsprocessen sker under de ljusa timmarna då cellen får energi från fotosyntesen.
I de molekylärbiologiska metoderna som används för att få information om identitet och aktivitet hos skilda organismer krävs att DNA och RNA kan extraheras från prover tagna i naturliga vattenmiljöer. Även om antalet bakterier tillsynes är högt, så är mängden DNA och RNA per liter havsvatten relativt låg, därför krävs ett väl fungerande protokoll för denna extraktion. I en inledande studie i denna avhandling optimerades en metod för att utvinna DNA. Ett antal sådana protokoll finns publicerade men dessa har ofta lågt utbyte. Det nya protokollet har hög effektivitet, vilket gör att små provvolymer kan användas (2 ml jämfört med tidigare flera liter) och därmed ökar hanterbarheten. Vi visar i denna studie att varje steg
i DNA-extraktionsprotokollet är viktigt för att ge en hög effektivitet. Detta protokoll kan med fördel användas som vägledning för många olika typer av studier.
På grund av att många havsbakterier inte kan bilda kolonier och alltså inte växa på traditionella medier har det varit svårt att få en klar bild av artrikedomen. Molekylärbiologin har dock gjort det möjligt att identifiera bakterier med hjälp av 16S rRNA genen, en enorm mängd gensekvenser från världens alla hav har inkommit till den gemensamma databanken (GenBank). År 2002 gjordes en studie där man sammanställde informationen i denna databank, för att få en bild av artrikedomen i världshaven. Resultatet av denna studie var att det i världshaven fanns färre bakterietyper än vad många forskare har spekulerat i. I denna avhandlig har vi utfört en studie där vi gjorde en stor global provtagning för att se om denna undersökning överensstämde med den datainformativa. Provtagning från nio lokaliteter gjordes i de tempererade, tropiska och polarhaven. Ett genbibliotek från varje lokal gjordes och kloner sekvenserades. Resultatet visar i likhet med den datainformativa undersökningen på en begränsad artrikedom. 80% av gensekvenserna fanns redan i databanken, vilket tyder på att de flesta arter redan har blivit funna. Dessutom visade det sig att få av bakterierna återfanns på alla ställen och många återfanns endast på ett ställe. Utöver detta visade det sig att det fanns en ökad artrikedom ju närmare ekvatorn man kom, vilket tidigare har visats för större organismer.
Studierna i denna avhandling har ökat förståelsen för hur sammansättningen av det kvävefixerande bakteriesamhället i Östersjön ser ut samt bidragit till diskussionen om den globala artrikedomen bland bakterioplakton och dess utbredning.
LIST OF PUBLICATIONS
This thesis is based on the following papers that will be referred to in the text by roman numerals I-IV.
I Boström, K.H., Simu, K., Hagström, Å., Riemann, L. (2004) Optimization of DNA extraction for quantitative marine bacterioplankton community analysis. Limnology
and Oceanography: Methods 2: 365-373.
I I Boström, K.H., Riemann, L., Kühl, M., Hagström, Å. Isolation and gene quantification of heterotrophic N2-fixing bacterioplankton in the Baltic Sea.
Submitted.
III Boström, K.H., Riemann, L., Zweifel, U.L., Hagström, Å. Distribution of N2
-fixation in the Baltic Sea. Submitted.
IV Pommier, T., Canbäck, B., Riemann, L., Boström, K.H., Simu, K., Lundberg, P., Tunlid, A., Hagström, Å. Global sampling of marine bacterioplankton reveals latitudinal gradients of diversity and limited cosmopolitan distribution. Submitted.
Paper I is reproduced with the permission from the American Society of Limnology and Oceanography, Inc.
INTRODUCTION
Bacterioplankton in the marine food web The oceans cover about 71% of the planet surface and contain both the largest and the smallest organisms on Earth. Thirty-five years ago, free-living minuscule heterotrophic bacteria in the ocean were largely ignored and thought to play a minor ecological role (Khailov & Finenko 1970). However, with the development of new methods for counting bacteria, based on filters allowing efficient size fractionation and fluorescent staining (Zimmermann & Meyer-Reil 1974), it was realized that marine bacterioplankton are highly abundant; on average 1 x 106 ml-1 (Hobbie et
al. 1977, Porter & Feig 1980). Thus, the
biogeochemical importance of heterotrophic bacteria in the sea was established. Yet, their identity was almost completely unknown and has gradually been revealed over the last two decades primarily through molecular based phylogeny (Rappé & Giovannoni 2003).
Cultivation and bacterioplankton diversity
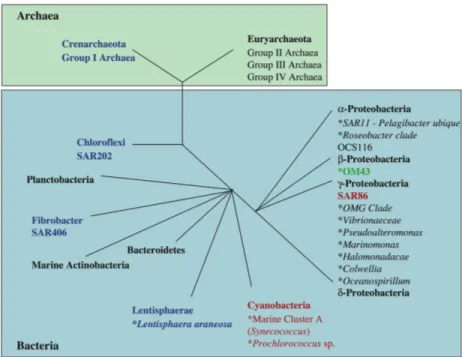
Application of molecular methods in microbial ecology throughout the last decades has produced a tremendous amount of DNA sequences from marine bacteria. The ribosomal 16S RNA gene is the most widely used gene for phylogenetic studies due to its high degree of functional consistency and occurrence in all organisms (Woese 1987). Currently, the known marine bacterial diversity, based on the 16S rDNA sequences available in GenBank, is
comprised of 52 phyla of which 26 still have no representatives in culture (Rappé & Giovannoni 2003; Fig. 1). Among marine bacterioplankton available in culture the class γ-proteobacteria are the most common; including genera such as Vibrionaceae,
Pseudomonas, Pseudoalteromonas, and Shewanella. The second most common class
is the α-proteobacteria with genera such as
Roseobacter and Sphingomonas. In contrast
to the cultured isolates, clone libraries of PCR amplified community DNA from marine waters are dominated by α- rather than γ-proteobacteria (Hagström et al. 2000, Moeseneder et al. 2005, Giovannoni & Rappé 2000).
Currently there are contrasting views on the magnitude of marine bacterioplankton diversity. In a recent shotgun sequencing-based effort to characterize diversity of Sargasso Sea bacterioplankton, Venter et al. (2004) identified 1164 distinct 16S rRNA genes including 148 previously unknown bacterial species. When estimating diversity using models based on depth of coverage a substantial oceanic microbial diversity (up to 47,733 ribotypes) was suggested. In contrast, by evaluating the number of different ribotypes and rate of new entries into GenBank, Hagström et al. (2002) estimated the total number of bacterial ribotypes in the marine plankton to ~1100. In a recent update, this was increased to ~2300 ribotypes (Pommier et al. 2005), which is still fairly low when considering the large depositions in GenBank in the intervening period (e.g. Venter et al. 2004). Since barriers to migration and dispersal are ineffective in the continuous global ocean,
Fig. 1. Schematic illustration of the phylogeny of the major plankton clades. Black letters indicate microbial groups that seem ubiquitous in seawater. Red indicates groups found in the photic zone. Blue indicates groups confined to the mesopelagic and surface waters during polar winters. Green indicates microbial groups associated with coastal ocean ecosystems (redrawn from Giovannoni & Stingl 2005).
there is a strong tendency towards cosmo-politanism of microorganisms supporting the hypothesis of a rather low diversity (Finlay 1998). Thus there is still controversy around the magnitude of the diversity in the bacterial communities in the world’s oceans.
While some bacterial species/groups are widespread, only few are known to have a worldwide distribution. Two of these belong to the cyanobacteria (Synechococcus and
Prochlorococcus; Carr & Mann 1994),
while the most abundant bacteria in the world’s oceans belong to the ubiquitous, and recently cultivated, SAR11 cluster (Giovannoni et al. 1990, Rappé et al. 2002). To cultivate SAR 11, Rappé et al (2002)
used a dilution to extinction approach first introduced by Button et al. (1993), where community bacterioplankton samples are diluted in sterile seawater medium down to a single bacterium and thereafter incubated for several weeks. It turned out that growth by these previously uncultured bacteria is somehow hampered by nutrient-rich conditions and that they propagate independently rather than associated in groups. Simu & Hagström (2004) suggested that the strategy of these bacteria might be to actively prevent colony formation since in a low-nutrient environment, such as the ocean, it might be advantageous to avoid the competition for nutrients implicit in colony
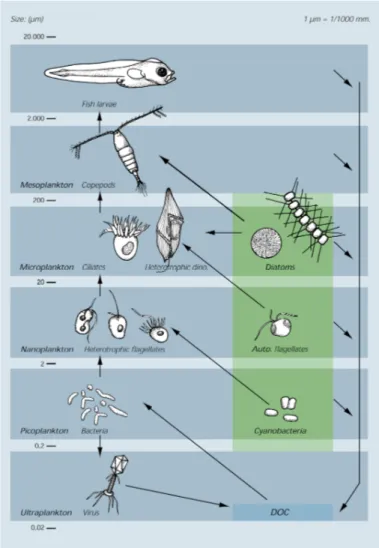
Fig. 2. Conceptual drawing of the marine food web (Nielsen & Hansen 1999). Heterotrophic organisms are shown to the left, photosynthesizing organisms to the right, and mixotrophs in the middle. Most organisms and processes generate dissolved organic carbon (DOC) that are taken up by bacteria.
growth. Several recent studies, applying modified versions of the original dilution-to-extinction technique, have reported the cultivation of many previously uncultured marine bacteria (Connon & Giovannoni 2002, Page et al. 2004, Simu et al. 2005) emphasizing a large future potential for this
cultivation method. Biogeochemical setting
The detection of large numbers of bacterioplankton and estimates of high bacterial production rates (Hagström et al. 1979, Fuhrman & Azam 1982) appearing in
the beginning of the 1980s, implied the importance of bacterioplankton in the marine carbon and nutrient cycling. The formulation of a microbial loop was therefore an important conceptual step in the restructuring of the marine food web compared to the views of the 1970´s (Azam
et al. 1983; Fig. 2). In the resulting
biogeochemical models up to 50% of the carbon produced by planktonic photosynthesis passes through the bacterioplankton community (Cole et al. 1988). However, it should be noted that while nutrient cycling comprise both mineral salts and organic components the current understanding of the circulation of matter in the marine environment is primarily based on the flow of carbon. The obvious reason for the choice of carbon lies in the advent of the radioisotope measurement of primary production (Steemann-Nielsen 1952). With carbon used as a tracking element the flow of for instance nitrogen and phosphorous in the marine environment has largely been calculated as ratios in relation to the carbon flow. However, considering that the primary productivity in most oceans is nitrogen limited (Howarth 1988), it is essential to obtain a detailed understanding of the factors controlling the nitrogen cycle as well identifying the participating organisms.
Nitrogen
The nitrogen cycle
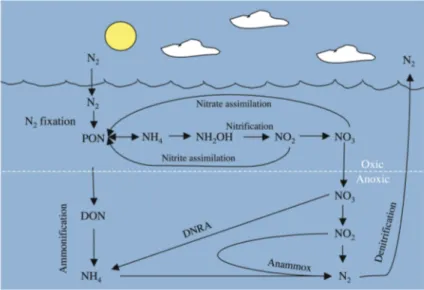
The fundamental steps of the nitrogen cycle involve primarily microbiological transformations between elemental nitrogen
(Arrigo 2005; Fig. 3). As bacteria and fungi mineralize organic compounds in the quest for reduced carbon, ammonium (NH4+) is
released. This ion can be oxidized to nitrite (NO2-, by ammonium oxidizers) and further
to nitrate (NO3-, by nitrite oxidizers) in the
nitrification process. Phytoplankton and heterotrophic bacteria can directly assimilate NO3- and NH4+, with ammonium as the
preferred source of nitrogen due to its reduced state. In the absence of oxygen as the terminal electron acceptor, NO3- can be
reduced in three steps to N2 gas by
denitrification. The anaerobic denitrification was long thought to be the only process removing fixed nitrogen from the environment; however, recently it was discovered that ammonium may be oxidized anaerobically in sediments in the presence of NO2- in a process called anammox. This
pathway is thought to contribute significantly to benthic N2 production
(Thamdrup & Dalsgaard 2002). The loss of nitrogen in the form of N2 gas and to some
extent nitrous oxide (N2O) into the
atmosphere is compensated for by N2
-fixation, the enzyme-mediated process reducing N2 gas to ammonium:
Nitrogenase
N2 + 8H+ + 8− + 16ATP → 2NH3 + H2 + 16ADP + 16Pi
Recent estimates of global ocean N2
-fixation are in the range of 100-200 Tg N yr-1, similar to terrestrial N2-fixation, but
carry large uncertainties (Karl et al. 2002). Since nitrogen often limits marine primary productivity and dissolved nitrogen gas is freely available, N-fixation appears to be
Fig. 3. The marine nitrogen cycle. PON, particulate organic nitrogen, including phytoplankton; DON, dissolved organic nitrogen; DNRA, dissimilatory nitrate reductase to ammonium (redrawn from Arrigo 2005).
a key process for marine productivity (Capone 2000).
N2-fixation
Prokaryotes are the only organisms capable of N2-fixation and, therefore,
favored in the nitrogen limited oceans (Karl
et al. 2002). The key enzyme involved in
N2-fixation is nitrogenase. The high degree
of similarity of protein sequences of nitrogenase among microorganisms suggests an early origin or lateral gene transfer of this gene complex among prokaryotic lineages (Postgate & Eady 1988 cited by Zehr et al. 2003a). This may also explain why diazotrophs (N2-fixers) are found among
such diverse groups of bacteria. These include representatives from:
• Heterotrophic anaerobic (e.g., Clostridium), microaerophilic (e.g., Klebsiella), and aerobic (e.g, Azotobacter) bacteria.
• Phototrophic bacteria (e.g., Rhodospirillum) including cyanobacteria (filamentous, uni-cellular, heterocystous, and non-hetero-cystous).
• Chemolithoautotrophic bacteria (e.g.,
Thio-bacillus).
• Archaea (e.g., Methanococcus).
The filamentous non-heterocystous cyanobacterium Trichodesmium has been regarded as a main N2-fixer in the open
ocean (Carpenter & Romans 1991, Capone
et al. 1997) together with the
diatom-associated endosymbiotic R i c h e l i a (Carpenter et al. 1999). Recently however, several studies have shown that unicellular
cyanobacteria contribute significantly to oceanic N2-fixation (Zehr et al. 2001, Falcón
et al. 2004, Montoya et al. 2004).
Heterotrophic bacteria presumably able to fix nitrogen (carrying the nitrogenase gene) have also been found in various marine waters (Zehr et al. 1995, Zehr et al. 1998, Short et al. 2004, Jenkins et al. 2004, Church et al. 2005a, Bird et al. 2005). Further, recent studies by Church et al. (2005b) and Zani et al. (2000) documented the expression of nitrogenase by hetero-trophic bacterioplankton. However, whether unicellular cyanobacteria and heterotrophic bacterioplankton significantly contribute with fixed nitrogen to the nitrogen pool is still unclear.
Regulation of N2-fixation
The cellular process of breaking the triple bound in N2 is an energy demanding
process and, therefore, highly regulated on transcriptional, translational, and protein activity levels (Merrick & Edwards 1995). Nitrogenase, the enzyme responsible for N2
-fixation, consists of the proteins dinitrogenase and dinitrogenase reductase. The most extensively examined nitrogenase gene is the n i f H gene coding for dinitrogenase reductase, which is an iron (Fe) containing protein made up of two equal subunits. The nifH gene is relatively conserved and can therefore be used as a phylogenetic marker, although it is not as conserved as the 16S rRNA gene (Zehr et al. 2003a). The current view of the diversity of diazotrophic marine bacterioplankton is mainly based on nifH gene sequences. The other protein, dinitrogenase, harbors the
substrate-binding site where N2 is reduced to
NH4+ in the presence of a Fe-Molybdenum
(Mo) cofactor. Dinitrogenase is composed of two hetero-dimers each consisting of two subunits alpha and beta, encoded by nifK and nifD (Paerl & Zehr 2000). In addition to these structural genes there are several regulatory nif genes in the n i f operon (Collins & Brill 1985, Merrick & Edwards 1995). At least 17 nif genes have been characterized in Klebsiella pneumoniae (Merrick 1983). Some diazotrophs have an additional alternative nitrogenase using vanadium (V) or Fe as cofactor instead of Mo. These different nitrogenases are encoded by separate sets of genes (vnfH/vnfDGK and anfHDGK, respectively; Bageshwar et al. 1998, Joerger et al. 1989). Nitrogenase nifH genes cluster into four basic groups (Zehr et al. 2003a):
Cluster I: The conventional Mo-containing
nifH and some vnfH.
Cluster II: The anfH and nifH from some Archaea.
Cluster III: A diverse group, many that are strictly anaerobes.
Cluster IV: A divergent, loosely coherent group with nifH-like sequences. Nitrogenase is sensitive to oxygen and may be inhibited reversibly (at low oxygen concentrations) or irreversibly (at high oxygen concentrations) (Guerinot & Patriquin 1981, Urdaci et al. 1988). Many N2-fixing bacteria are anaerobic or
facultative anaerobic to protect the oxygen-sensitive nitrogenase; however, some marine cyanobacteria have managed to protect the
nitrogenase in oxygen-free, non-photo-synthesizing cells i.e. heterocysts (Paerl & Zehr 2000). These cells spatially separate the oxygen-producing photosynthesis from the oxygen sensitive N2-fixation.
Cyanobacteria with no heterocysts have found other ways to protect the nitrogenase such as a temporal separation of photosynthesis (day) and N2-fixation (night)
(Bergman et al. 1997, Church et al. 2005b).
Trichodesmium, a multicellular
cyano-bacterium, has an alternative strategy with a diel cycle with highest N2-fixation rate
during the day when also oxygen concentration is highest due to photo-synthesis (Chen et al. 1998, Church et al. 2005b, Bergman et al. 1997, Lin et al. 1998). For protection of the nitrogenase
Trichodesmium has the N2-fixation allocated
to a few cells that do not photosynthesize (Berman-Frank et al. 2001). Fixing nitrogen during the day is advantageous from an energetic point of view as energy for the energy demanding N2-fixation process is
provided from photosynthesis. An alternative way to protect the nitrogenase is to maintain a low intracellular oxygen concentration through a high respiration rate (Bergman et al. 1997), which has also been documented in e.g. the well-studied aerobic soil bacterium Azotobacter vinelandii (Post
et al. 1983).
Methods to measure N2-fixation
N2-fixation may be measured using
either direct or indirect methods. The stable isotope assay is an example of a direct method whereby the incorporation of N2,
artificially enriched with 15N
2, into biomass
is measured (Paerl 1998). While this method is sensitive and nonselective, it is rather time consuming, and requires elaborate equipment such as a mass spectrometer. The acetylene reduction assay technique is a more widely used indirect method, which makes use of the capability of nitrogenase not only to break the triple bond in N2 gas
but also the triple bond in acetylene. Adding acetylene to a sample to measure the resulting ethylene production with a gas chromatograph is an old and robust method, though with a limited sensitivity (Capone 1993, Moisander et al. 2003). Due to the limited sensitivity measurements are often performed on concentrated plankton tows, which exclude unicellular organisms. Though, the sensitivity of the acetylene reduction method has lately been significantly improved (Staal et al. 2001), the use of concentrated samples seems still a requirement. In light of the recent findings of genetic potential among unicellular cyanobacteria as well as heterotrophic bacteria, this size exclusion may have generated serious underestimates of N2
-fixation.
While these methods have yielded important information about N-flux and provided our existing estimates of N2
-fixation, they provide no insights into the identity of the organisms behind the process since they are applied on whole plankton communities. Hence, our current knowledge about species specific contribution to global marine N2-fixation is limited.
AIMS OF THESIS
Following the introduction of molecular biology techniques into microbial ecology nifH soon emerged as an exciting possibility to assay at the same time expression and identity of a functional gene. To determine the spatial and temporal scales at which cyanobacteria and heterotrophic bacteria actively fix nitrogen and accurately quantify their importance relative to anthropogenic inputs of nitrogen, stood out as a major challenge for my thesis. A main priority was to gain insight into the diversity of organisms fixing nitrogen, but also to cultivate them, and establish a coupling between laboratory-based analyses and in situ function.
Though, still, knowledge on bacterioplankton diversity remained a prerequisite for a thorough understanding of marine biogeochemical cycling of nitrogen and other elements essential to life in the ocean. Subjects such as diversity and biogeography of marine bacterioplankton in general could not be excluded from discussions on the specific organisms responsible for N2-fixation.
The specific aims of the present thesis were:
To develop and fine-tune methods to identify marine
bacteria at the species level with particular emphasis on
N
2-fixers
To identify the main N
2-fixing organisms in the Baltic
Sea and in particular to determine the importance of
heterotrophic bacteria for planktonic N
2-fixation
To use molecular expression to examine spatial and
temporal variability of nitrogenase activity for single
bacterial species
To increase the understanding of bacterioplankton
diversity and distribution in the global ocean.
THESIS OUTLINE
Molecular-based methods have become an integral part of microbial ecology. Most of these methods rely on nucleic acids extracted from environmental samples. The relatively low number of organisms along with their limited DNA/RNA contents poses a serious problem for marine microbial ecologist when extracting nucleic acids from seawater. Today a variety of relatively inefficient protocols for extraction of bacterioplankton DNA/RNA are in use. In Paper I, we optimized and developed a protocol to extract DNA from seawater samples and obtained extraction efficiencies of up to 92% for seawater samples and up to 96% for isolates. In most previous protocols, several liters of seawater had to be filtered to recover satisfactory DNA amounts; however, the high efficiency of this protocol allows for 2 ml samples to be centrifuged and extracted. Each step in the protocol was important for the efficiency of extraction: e.g., a prolonged proteinase K incubation increased the extraction efficiency two fold, ethanol-precipitation may cause a DNA loss of ≈30%, and the addition of a co-precipitant (tRNA) may increase the recovery six fold.
With the optimized protocol we sought to maximize the yield of DNA while maintaining a quality suitable for real-time PCR. Combined with an internal DNA standard, the protocol offers quantitative and efficient DNA extraction, which is a prerequisite for quantitative methods in aquatic microbial ecology.
A major goal in aquatic microbial ecology is to link the identity and function for the predominant bacterioplankton species. One approach is to cultivate bacterioplankton and try to establish a coupling between laboratory-based analyses and in situ function. In Paper II, we used this approach and combined cultivation and
in situ dynamics in an effort to examine the
role of heterotrophic bacteria in pelagic N2
-fixation in the Baltic Sea Proper.
The ecological balance of the Baltic Sea is severely threatened by eutrophication. Therefore, costly measures are taken to reduce the output of in particular nitrogen. However, N2-fixation has been estimated to
provide a yearly nitrogen input in the same order as the entire riverine load to the Baltic Sea (Larsson et al. 2001), and is therefore a process that may overthrow the efforts to reduce anthropogenic nitrogen input. A detailed understanding of N2-fixation and
the organisms involved in the process is therefore of particular interest in the Baltic Sea area. In summer, when primary production in the Baltic Proper is limited by nitrogen, massive blooms of diazotrophic heterocyst-forming cyanobacteria appear and so far, N2-fixation in the Baltic has been
solely attributed to filamentous cyano-bacterial activity. However, since heterotrophic bacteria with a genetic potential for N2-fixation recently have been
found in several oceans around the world, a major goal of this thesis was to investigate whether heterotrophic bacteria play a role for pelagic N2-fixation in the Baltic Sea.
At the onset of this thesis work, the published and widely acclaimed nested nifH PCR approach was intended as the primary method to detect and identify organisms with genetic potential to fix nitrogen. However, despite much effort and extensive precautions to avoid PCR contamination, this approach often yielded variable and incoherent results. Consistent with our spurious results, Zehr et al. (2003b) showed that PCR reagents might contain trace amounts of nifH-containing genomic DNA producing unwanted amplification products in nested PCR; hence, emphasizing that this was a general problem to the research field. This insight partly shifted the focus of the study towards the development of new methods for isolating diazotrophic bacterioplankton.
One reason for the historical underestimation of bacterial abundance is the inability to cultivate most marine bacteria on nutrient agar plates. The discrepancy between colony forming units and the several orders of magnitude higher number of bacterioplankton countable by epifluorescence microscopy has been termed “the great plate count anomaly” (Staley 1985). This discrepancy is still not fully resolved. Comparisons between sequences from cellular clones and environmental clone libraries obtained from the same seawater sample suggest that many of the most abundant bacterioplankton species are not readily culturable on conventional agar plates (Suzuki et al. 1997, Eilers et al. 2000). In the Baltic Sea however, culturable bacteria make up a significant fraction of
bacterioplankton (Pinhassi et al. 1997). Recently, Simu et al. (2005) showed the ratio of colony-forming to non-colony-forming bacteria being 10-20 fold higher in the brackish, eutrophic Baltic Sea relative to the North Sea. Thus, the prospects of isolating N2-fixing heterotrophic bacteria
with the relatively simple means of agar plates and agar tubes seemed feasible in the Baltic environment.
In Paper II, we successfully isolated N2
-fixing heterotrophic bacteria from the Baltic Sea Proper by using low nitrogen agar plates and semi-solid diazotrophic medium tubes (SSDM). The isolates, which were related to γ-proteobacteria, carried the nifH gene and were capable of N2-fixation as measured by
acetylene reduction. By means of microelectrode measurements, it was shown that they required microaerophilic to anaerobic conditions for active N2-fixation.
To determine the in situ abundance of one of the isolates (BAL281), a specific primer-probe set was designed for quantitative real-time PCR. Community DNA was extracted using the protocol developed in Paper I. BAL281 nifH gene copies were found in two of eleven locations along a transect in the Baltic Proper with relatively high abundance (3±0.4 x 104 nifH gene copies l-1)
only in the sample from which the bacterium was originally isolated.
The paper shows that N2-fixing
heterotrophic bacteria may be isolated from the Baltic Sea and that these are at least locally abundant in situ. This finding highlights the potential importance of
heterotrophic bacteria for N2-fixation in the
Baltic Sea and emphasizes the need for further research to quantify their importance. The study illustrates how the combination of extensive cultivation efforts and molecular quantification of specific nitrogenase genes can provide insights to the role and ecology of N2-fixing heterotrophic bacterioplankton
in the sea.
While the results of Paper II showed that some heterotrophic bacteria in the Baltic Sea have the genetic potential for N2
-fixation, as well as the capability to fix nitrogen, Paper III focused on the in situ expression of the nifH gene. A large clone library (84 clones) was constructed from
nifH transcripts in a sample integrating 4
surface waters from the Baltic Proper during the summer season. The clone library consisted exclusively of nifH transcripts from the heterocystous cyanobacteria
Nodularia sp. and Aphanizomenon sp. The nifH gene of an Aphanizomenon sp. isolate
was sequenced in this study. No genes related to heterotrophic bacteria were found, suggesting that they were non-existing or more likely too few to be detected in the clone library. Hence, indicating that the contribution of heterotrophic bacteria to N2
-fixation was of limited importance at the time of sampling.
To determine the temporal and spatial variability of N2-fixation, a specific
primer-probe set targeting the Nodularia sp. nifH gene cluster was designed. Real-time PCR showed that n i f H gene expression by
Nodularia was highly variable between
stations but was one order of magnitude higher during mid summer relative to early summer and fall. The patchy distribution of expressed Nodularia nifH genes suggests that high-resolution sampling is required to assess N2-fixation in the Baltic Sea.
As exemplified in Papers II and III, the combination of cultivation, to examine physiology and metabolism of single bacterial species, and monitoring of in situ dynamics, is one way to elucidate the functional role of bacterioplankton species in the sea. Another option is to examine the overall diversity of bacterioplankton and the large-scale processes that regulate global species distribution. The establishment of such patterns may help reveal the ecology of certain bacterial species or groups. An example of this was provided by Hagström
et al. (2002) in their efforts to distill the
information available in GenBank. To access this vast amount of information, Hagström et al. (2002) retrieved all marine bacterioplankton 16S rDNA sequences, merged fragments of these, and sorted out unique contigs (ribotypes) based on ≥ 97% similarity for identical ribotypes. In total, they found only 1117 different ribotypes, which is in contrast to the great biodiversity previously anticipated for marine bacterio-plankton. This in silico finding of a limited diversity prompted the study presented in Paper IV, in which we sought to get a global view of bacterioplankton diversity in
situ. In this single study, covering samples
from 9 locations in polar, tropical and temperate oceanic regions, we found 562 distinct ribotypes, which is about half of the
diversity found by Hagström et al. (2002) and ~1/4 of the diversity found by Pommier
et al. (2005) in an updated search in
GenBank. Of the 562 ribotype sequences, ~80% were similar to sequences in GenBank indicating that a large fraction of the marine bacterioplankton has already been sampled. The results show that only few ribotypes are cosmopolitan and that the number of endemic ribotypes is high. In addition, the study suggests a latitudinal gradient of increasing number of ribotypes with decreasing latitude, which is a pattern known from macroorganisms. This study provides a unique view of global marine microbial diversity and suggests a limited number of 16S rDNA ribotypes in the global bacterioplankton community.
CONCLUDING REMARKS
The results in this thesis have been obtained by traditional microbiological methods combined with novel high-technology molecular-based techniques, such as real-time PCR and extensive cloning/sequencing. The presented conclusions demonstrate that the applied integration of methods provides powerful means of approaching a mechanistic understanding of aspects of bacterioplankton function.
The thesis contributes some information of direct value to our ultimate understanding of the biogeochemical role of bacterioplankton in marine waters. However indirectly, the obtained knowledge on methodology, bacterial N2-fixation, and global bacterioplankton
diversity constitute a valuable platform for future scientific progress. In a near-future context, I find that the main challenges will be:
I. To optimize the SSDM tube isolation method used in Paper II to exclusively select for N2-fixers. This would allow for extended analysis of the genetic and
functional diversity of marine heterotrophic N2-fixing bacteria.
II. To develop nifH gene expression analyses, as used in Paper III, into a means of quantifying N2-fixation. This goal, which would allow distinguishing the
relative importance of heterotrophic bacteria and cyanobacteria in planktonic N2-fixation, would have to be preceded by an establishment of the relationship
between nifH gene expression and actual N2-fixation. For instance, a tentative
calibration of nifH gene expression relative to N2-fixation measured by the 15N
2 assay would be a first step. Absolute quantification of nifH gene
expression would also require knowledge of RNA extraction efficiency and cDNA synthesis efficiency (e.g. by using RNA standards). III To investigate the large-scale distribution of functional genes such as nifH. The use of 16S rRNA gene information in studies of bacterial distribution has generated new knowledge about bacterioplankton biogeography (Paper IV). A similar analysis focusing on functional genes could constitute a rewarding task. A more general future challenge will be to establish the relationship between 16S rDNA homology and genomic variability. Today, 16S rDNA is widely used to delineate species and to constrain bacterial population in situ (Paper IV is an example). Though, whether bacteria with a specific function (ecotypes) may be resolved by 16S rDNA identity is, to my knowledge, an open question.
REFERENCES
ARRIGO, K.R. 2005. Marine microorganisms and global nutrient cycles. Nature 437: 349-355. AZAM, F. and others 1983. The ecological role of
water-column microbes in the sea. Mar. Ecol. Prog. Ser. 10: 257-263.
BAGESHWAR, U.K., R. RAINA, N.R. CHOUDHURY, and H.K. DAS. 1998. Analysis of upstream activation of the vnfH promotor of
Azotobacter vinelandii. Can. J. Microbiol. 44:
405-415.
BERGMAN, B., J.R. GALLON, A.N. RAI, and L.J. S T A L . 1997. N2 fixation by non-hetocystous
cyanobacteria. FEMS Microbiol. Rev. 19: 139-185. BERMAN-FRANK, I. and others 2001. Segregation of
nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science 294: 1534-1537.
BIRD, C., J.M. MARTINEZ, A.G. O'DONNELL, and M. WYMAN. 2005. Spatial distribution and transcriptional activity of an uncultured clade of planktonic diazotrophic γ-Proteobacteria in the Arabian Sea. Appl. Environ. Microbiol. 71: 2079-2085.
BUTTON, D.K., F. SCHUT, P. QUANG, R. M A R T I N , and B.R. ROBERTSON. 1993. Viability and isolation of marine bacteria by dilution culture: Theory, procedures, and initial results. Appl. Environ. Microbiol. 59: 881-891. CAPONE, D.G. 1993. Determination of nitrogenase
activity in aquatic samples using the acetylene reduction procedure. In Handbook of methods in aquatic microbial ecology. Kemp, P.F., Sherr, B.F., and Cole, J.J. (eds). NY: Lewis Publishers, pp. 621-631.
---. 2000. The marine microbial nitrogen cycle. In Microbial ecology of the oceans. Kirchman, D.L. (eds), Wiley-Liss. pp. 455-494.
CAPONE, D.G., J.P. ZEHR, H.W. PAERL, B. BERGMAN, and E.J. CARPENTER. 1997.
Trichodesmium, a globally significant marine
cyanobacterium. Science 276: 1221-1229. CARPENTER, E.J., and K. ROMANS. 1991. Major
role of the cyanobacterium Trichodesmium in nutrient cycling in the North Atlantic Ocean. Science 254: 1356-1358.
CARPENTER, E.J., J.P. MONTOYA, J. BURNS, M.R. MULHOLLAND, A. SUBRAMANIAM, and D.G. CAPONE. 1999. Extensive bloom of a N2-fixing diatom/cyanobacterial association in the
tropical Atlantic Ocean. Mar. Ecol. Prog. Ser. 185: 273-283.
CARR, N.G., and N.H. MANN. 1994. The oceanic cyanobacterial picoplankton. In The molecular biology of cyanobacteria. Bryant D.A. (eds), Kluwer academic publishers. pp. 27-48.
CHEN, Y.-B., B. DOMINIC, M.T. MELLON, a n d J.P. ZEHR. 1998. Circadian rhythm of nitrogenase gene expression in the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp. strain IMS 101. J. Bacteriol. 180: 3598-3605. CHURCH, M.J., B.D. JENKINS, D.M. KARL, and
J.P. ZEHR. 2005a. Vertical distributions of nirogen-fixing phylotypes at stn ALOHA in the ologotrophic North Pacific Ocean. Aquat. Microb. Ecol. 38: 3-14.
CHURCH, M.J., C.M. SHORT, B.D. JENKINS, D.M. KARL, and J.P. ZEHR. 2005b. Temporal patterns of nitrogenase gene (nifH) expression in the oligotrophic North Pacific Ocean. Appl. Environ. Microbiol. 71: 5362-5370.
COLE, J.J., S. FINDLAY, and M.L. PACE. 1988. Bacterial production in fresh and saltwater ecosystems: a cross-system overview. Mar. Ecol. Prog. Ser. 43: 1-10.
COLLINS, J.J., and W.J. BRILL. 1985. Control of
Klebsiella pneumoniae nif mRNA synthesis. J.
Bacteriol. 162: 1186-1190.
CONNON, S.A., and S.J. GIOVANNONI. 2002. High-throughput methods for culturing microorganisms in very-low-nutrient media yield diverse new marine isolates. Appl. Environ. Microbiol. 68: 3878-3885.
EILERS, H., J. PERNTHALER, F.O. GLÖCKNER, and R. AMANN. 2000. Culturability and in situ abundance of pelagic bacteria from the North Sea. Appl. Environ. Microbiol. 66: 3044-3051. FALCÓN, L.I., E.J. CARPENTER, F. CIPRIANO, B.
BERGMAN, and D.G. CAPONE. 2004. N2
fixation by unicellular bacterioplankton from the Atlantic and Pacific Oceans: Phylogeny and in situ rates. Appl. Environ. Microbiol. 70: 765-770. FINLAY, B.J. 1998. The global diversity of protozoa
and other small species. Int. J. Parasitol. 28: 29-48. FUHRMAN, J.A., and F. AZAM. 1982. Thymidine
incorporation as a measure of heterotrophic bacterioplankton production in marine surface waters: Evaluation and field results. Mar. Biol. 66: 109-120.
GIOVANNONI, S.J., T.B. BRITSCHGI, C.L. M O Y E R , a n d K.G. FIELD. 1990. Genetic diversity in Sargasso Sea bacterioplankton. Nature 345: 60-63.
GIOVANNONI, S., and M. RAPPÉ. 2000. Evolution, diversity and molecular ecology of marine prokaryots. In Microbial ecology of the oceans. Kirchman, D.L. (eds). Wiley-Liss. pp. 47-84. GIOVANNONI, S.J., a n d U. STINGL. 2005.
Molecular diversity and ecology of microbial plankton. Nature 437: 343-348.
GUERINOT, M.L., and D. G. PATRIQUIN. 1981. N2-fixing vibrios isolated from the gastrointestinal
tract of sea urchins. Can. J. Microbiol. 27: 311-317.
HAGSTRÖM, Å., U. LARSSON, P. HÖRSTEDT, and S. NORMARK. 1979. Frequency of dividing cells, a new approach to determination of bacterial growth rates in aquatic environments. Appl. Environ. Microbiol. 37: 805-812.
HAGSTRÖM, Å., J. PINHASSI, and U.L. ZWEIFEL. 2000. Biogeographical diversity among marine bacterioplankton. Aquat. Microb. Ecol. 21: 231-244.
HAGSTRÖM, Å. and others 2002. Use of 16S ribosomal DNA for delineation of marine bacterioplankton species. Appl. Environ. Microbiol. 68: 3628-3633.
HOBBIE, J.E., R.J. DALEY, and S. JASPER. 1977. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 33: 1225-1228.
HOWARTH, R.W. 1988. Nutrient limitation of net primary production in marine ecosystems. Annu. Rev. Ecol. 19: 89-110.
JENKINS, B.D., G.F. STEWARD, S.M. SHORT, B.B. WARD, and J.P. ZEHR. 2004. Fingerprinting diazotroph communities in the Chesapeake Bay by using a DNA macroarray. Appl. Environ. Microbiol. 70: 1767-1776.
JOERGER, R.D., M.R. JACOBSON, R. PREMAKUMAR, E.D. WOLFINGER, and P.E. B I S H O P . 1989. Nucleotide sequence and mutational analysis of the structural genes (anfHDGK) for the second alternative nitrogenase from Azotobacter vinelandii. J. Bacteriol. 171: 1075-1086.
KARL, D. and others 2002. Dinitrogen fixation in the world's oceans. Biogeochem. 57/58: 47-98. KHAILOV, K.M., and Z.Z. FINENKO. 1970.
Organic macromolecular compounds dissolved in sea-water and their inclusion into food chains. In Marine food chains. Steele, J.H. (eds). Oliver & Boyd, Edingburgh. pp. 6-18.
LARSSON, U., S. HAJDU, J. WALVE, and R. ELMGREN. 2001. Baltic Sea nitrogen fixation estimated from the summer increase in upper mixed layer. Limnol. Oceanogr. 64: 811-820. LIN, S., S. HENZE, P. LUNDGREN, a n d B.
BERGMAN. 1998. Whole-cell immunolocali-zation of nitrogenase in marine diazotrophic cyanobacteria, Trichodesmium spp. Appl. Environ. Microbiol. 64: 3052-3058.
MERRICK, M. J. 1983. Nitrogen control of the nif regulon in Klebsiella pneumoniae: involvement of the ntrA gene and analogies between ntrC and nifA. The EMBO Journal 2: 39-44.
MERRICK, M. J., and R.A. EDWARDS. 1995. Nitrogen control in bacteria. Microbiol. Rev. 59: 604-622.
MOESENEDER, M.M., J.M. ARRIETA, and G.J. HERNDL. 2005. A comparison of DNA- and RNA-based clone libraries from the same marine bacterioplankton community. FEMS Microbiol. Ecol. 51: 341-352.
MOISANDER, P.H., T.F. STEPPE, N.S. HALL, J. KUPARINEN, and H.W. PAERL. 2003. Variability in nitrogen and phosphorus limitation for Baltic Sea phytoplankton during nitrogen-fixing cyanobacterial blooms. Mar. Ecol. Prog. Ser. 262: 81-95.
MONTOYA, J.P., C.M. HOLL, J.P. ZEHR, A. HANSEN, T.A. VILLAREAL, a n d D . G . CAPONE. 2004. High rates of N2 fixation by
unicellular diazotrophs in the oligotrophic Pacific Ocean. Nature 430: 1027-1031.
NIELSEN, T.G., and P.J. HANSEN. 1999. (In Danish) “Dyreplankton i danske farvande” report from The Danish Environmental Research Centre.
PAERL, H. W. 1998. Microbially mediated nitrogen cycling. In Techniques in microbial ecology. Burlage R.S., R. Atlas, .D. Stahl, G. Geesey and G. Sayler (eds). Oxford University Press. pp. 3-30.
PAERL, H.W., and J.P. ZEHR. 2000. Marine nitrogen fixation. In Microbial ecology of the oceans. Kirchman, D.L. (eds). Wiley-Liss. pp. 387-426. PAGE, K.A., S.A. CONNON, a n d S . J .
GIOVANNONI. 2004. Representative freshwater bacterioplankton isolated from Crater Lake, Oregon. Appl. Environ. Microbiol. 70: 6542-6550. PINHASSI, J., U.L ZWEIFEL, and Å. HAGSTRÖM.
1997. Dominant marine bacterioplankton species found among colony-forming bacteria. Appl. Environ. Microbiol. 63: 3359-3366.
POMMIER, T., J. PINHASSI, and Å. HAGSTRÖM. 2005. Biogeographic analysis of ribosomal RNA clusters from marine bacterioplankton. Aquat. Microb. Ecol. 41: 79-89.
PORTER, K.G., and Y.S. FEIG. 1980. The use of DAPI for identifying and counting aquatic microflora. Limnol. Oceanogr. 25: 943-948. POST, E., D. KLEINER, and J. OELZE. 1983. Whole
cell respiration and nitrogenase activities in
Azotobacter vinelandii growing in oxygen
controlled continuous culture. Arch. Microbiol. 134: 68-72.
RAPPÉ, M.S., S.A. CONNON, K.L. VERGIN, and S.J. GIOVANNONI. 2002. Cultivation of the ubiquitous SAR11 marine bacterioplankton clade. Nature 418: 630-633.
RAPPÉ, M.S., and S.J. GIOVANNONI. 2003. The uncultured microbial majority. Annu. Rev. Microbiol. 57: 369-394.
SHORT, S.M., B.D. JENKINS, and J.P. ZEHR. 2004. Spatial and temporal distribution of two diazotrophic bacteria in the Chesapeake Bay. Appl. Environ. Microbiol. 70: 2186-2192.
SIMU, K., and Å. HAGSTRÖM. 2004. Oligotrophic bacterioplankton with a novel single-cell life strategy. Appl. Environ. Microbiol. 70: 2445-2451. SIMU, K., K. HOLMFELDT, U.L. ZWEIFEL, and Å.
of colony-forming and single-cell marine bacterioplankton. Appl. Environ. Microbiol. 71: 4793-4800.
STAAL, M., S. LINTEL-HEKKERT, F. HARREN, a n d L. STAL. 2001. Nitrogenase activity in cyanobacteria measured by the acetylene reduction assay: a comparison between batch incubation and on-line monitoring. Environ. Microbiol. 3: 343-351.
STALEY, J.T. 1985. Measurement of in situ activities of nonphotosynthetic microorganisms in aquatic and terrestrial habitats. Ann. Rev. Microbiol. 39: 321-349.
STEEMANN-NIELSEN, E. 1952. The use of radioactive carbon 14C for measuring organic
production in the sea. J. Cons. Perm. Int. Explor. Mer. 18:117-140.
SUZUKI, M.T. and others 1997. Bacterial diversity among small-subunit rRNA gene clones and cellular isolates from the same seawater sample. Appl. Environ. Microbiol. 63: 983-989.
THAMDRUP, B., and T. DALSGAARD. 2002. Production of N2 through anaerobic ammonium
oxidation coupled to nitrate reduction in marine sediments. Appl. Environ. Microbiol. 68: 1312-1318.
URDACI, M.C., L.J. STAL, and M. MARCHAND. 1988. Occurrence of nitrogen fixation among
Vibrio spp. Arch. Microbiol. 150: 224-229.
VENTER, C.J. and others 2004. Environmental genome shotgun sequencing of the Sargasso Sea. Sience 304: 66-74.
WOESE, C.R. 1987. Bacterial evolution. Microbiol. Rev. 51: 221-271.
ZANI, S., M.T. MELLON, J.L. COLLIER, and J.P. ZEHR. 2000. Expression of nifH genes in natural microbial assemblages in Lake George, New York, detected by reverse transcriptase PCR. Appl. Environ. Microbiol. 66: 3119-3124.
ZEHR, J.P., M. MELLON, S. BRAUN, W. LITAKER, T. STEPPE, and H.W. PAERL. 1995. Diversity of heterotrophic nitrogen fixation genes in a marine cyanobacterial mat. Appl. Environ. Microbiol. 61: 2527-2532.
ZEHR, J.P., M.T. MELLON, and S. ZANI. 1998. New nitrogen-fixing microorganisms detected in oligotrophic oceans by amplification of nitrogenase (nifH) genes. Appl. Environ. Microbiol. 64: 3444-3450.
ZEHR, J.P. and others 2001. Unicellular cyanobacteria fix N2 in the subtropical North Pacific Ocean.
Nature 412: 635-638.
ZEHR, J.P., B.D. JENKINS, S.M. SHORT, and G.F. STEWARD. 2003a. Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Environ. Microbiol. 5: 539-554. ZEHR, J.P., L.L. CRUMBLISS, M.J. CHURCH, E.O.
OMOREGIE, and B.D. JENKINS. 2 0 0 3 b . Nitrogenase genes in PCR and RT-PCR reagents: implications for studies of diversity of functional genes. BioTechniques 35: 996-1001.
ZIMMERMANN, R., and L.A. MEYER-REIL. 1974. A new method for fluorescence staining of bacterial populations on membrane filters. Kieler Meresforsch. 30: 24-27.
ACKNOWLEDGEMENTS
This thesis would not have been completed without the help and support from numerous people. Thank you all!!!
In particular, I would like to thank:
My supervisor Lasse Riemann, who guided this work and helped whenever I was in need. Your encouragement, enthusiasm and energy have been invaluable!
My co-supervisor Åke Hagström, for giving me the opportunity to work in the microbiology group. Your always positive attitude and support kept me going, when times were tough.
My first supervisor Ulla Li Zweifel, who was my supervisor in the initial stages of my study until she changed job, for introducing me to this field of research.
Colleagues in the ÅH-group: Karin Simu for sharing her large lab-experience and for being such a good friend, and Cissi, Sabina, Karin, Laura and Jarone for all the fun chats, not always about work…
Heléne Werthwein, for not giving up with the endless isolation experiments. Cecilia Petersen, for helping me out with some of the Gotland cruises. Anders, Conny and Sven, I am especially grateful for your help fixing “things”. Gunilla Åkesson-Nilsson for all help with the acetylene reduction assay, even if the
results didn’t become as superb as expected.
The “Norrgård people” for letting me use some of your equipment and for nice chats in the coffee room every now and then.
The crew at M/S Tjelvar for help and company at the many sampling trips between Oskarshamn and Visby.
Friends, for being around talking about something else than science. Sofia and Pelle, I hope the wine discussions will continue.
My family, for always being there for me.
My wonderful girls Frida and Emma, I love you!
My wonderful “extra” girls Jessika and Josefin, for contributing to the joyful atmosphere in our home, love you!
My Mats, I know you have had a hard time not knowing whether to support me to go on with my Ph.D. studies, or to recommend me to quit.
You made the right choice! Tack Mats, jag älskar dig!