ALLERGIC B CELLS ARE CENTRAL TO ESTABLISHING MURINE PEANUT ALLERGY IN AN ADOPTIVE TRANSFER MODEL BUT ARE NOT REQUIRED
FOR MAINTAINING LONG-LIVED SERUM IMMUNOGLOBULIN E LEVELS by
DAPHNE MOUTSOGLOU
B.S., South Dakota State University, 2008
A thesis submitted to the Faculty of the Graduate School of the University of Colorado in partial fulfillment
of the requirements for the degree of Doctor of Philosophy
Immunology Program 2015
This thesis for the Doctor of Philosophy degree by Daphne Moutsoglou
has been approved for the Immunology Program
by
John Cambier, Chair Rafeul Alam Andrew Fontenot Philippa Marrack
Angie Ribera Stephen Dreskin, Advisor
Moutsoglou, Daphne (Ph.D., Immunology)
Allergic B Cells Are Central to Establishing Murine Peanut Allergy in an Adoptive Transfer Model but Are Not Required for Maintaining Long-Lived Serum
Immunoglobulin E Levels
Thesis directed by Professor Stephen Dreskin. ABSTRACT
Peanut allergy, characterized by elevated anti-peanut IgE levels that are typically lifelong, is a growing problem worldwide. The role B cell subsets play in maintaining long-lived serum anti-peanut IgE levels in peanut-allergic humans is unknown. We developed an adoptive transfer model of murine peanut allergy to investigate the role of B cells in establishing this immune response. We also independently treated peanut-allergic mice with anti-CD20 antibody or bortezomib to determine the role B cell subsets play in maintaining peanut allergy. Our results demonstrate that B cells with a memory phenotype are required but are not sufficient for the adoptive transfer of peanut allergy. Purified B220+
cells from peanut-allergic splenocytes and purified CD4+ cells from naïve splenocytes are
the minimal requirements for the adoptive transfer of peanut allergy. Prolonged treatment with anti-CD20 antibody in peanut-allergic mice resulted in significant depletion of B cell subsets but did not affect anti-peanut IgE levels, symptoms, or numbers of IgE antibody secreting cells in the bone marrow. Adoptive transfer of bone marrow cells and splenocytes from donors treated with anti-CD20 antibody abrogated the adoptive transfer of peanut allergy to recipients suggesting that treatment with anti-CD20 antibody depletes B cells with a memory phenotype. To determine a role for long-lived plasma cells in murine peanut
allergy, allergic mice were treated with the proteasome inhibitor, bortezomib. Bortezomib therapy resulted in significant, long-term depletion of serum anti-peanut IgE levels, suggesting a role for long-lived plasma cells in maintaining long-lived IgE levels; however, despite prolonged depletion of serum IgE, symptoms of peanut allergy were not affected. These results suggest a need for the development of non-toxic therapeutics targeted against IgE-secreting cells to treat peanut allergy and other atopic conditions.
The form and content of this abstract are approved. I recommend its publication. Approved: Stephen Dreskin
ACKNOWLEDGEMENTS
I thank my advisor, Stephen Dreskin, for allowing me to pursue my passion for science and research in his laboratory and for his support.
I thank my committee members: Philippa Marrack, John Cambier, Angie Ribera, Rafeul Alam, and Andrew Fontenot for their help in directing my research and their support.
I thank Raul Torres for scientific discussions and for help with my manuscript. I thank Brian Tooker and Lee Newman for their shared resources.
I thank Dan Atkins for allowing me to see patients with peanut allergy and other atopic conditions in his pediatric allergy clinic.
I also thank all of my good friends Layne Dylla, Jonathon Parker, Genevieve Park, Kiran Dyamenahalli, Alexandra Antonioli, Justin Holt, and Caleb Kelly for our adventures in becoming physician scientists.
TABLE OF CONTENTS CHAPTER
I. INTRODUCTION ... 1
Pathophysiology of the Allergic Response ... 1
IgE-Mediated Food Allergy ... 2
Risks factors and protective factors for the development of food allergy and atopic conditions ... 2
Mainstay treatment of food allergy ... 4
Experimental treatments of food allergy ... 4
Peanut allergy versus other food allergies ... 5
Allergens, Are They Special Antigens? ... 6
Effects of common allergens on the host ... 6
Proteomics of peanut proteins. ... 7
Allergy and the Immune System... 8
T cell subsets in the allergic response ... 9
Group 2 innate lymphoid cells ... 10
IgE antibody class switching ... 10
The role of IgE in B cell perpetuation of allergic conditions ... 11
IgE regulation of FcεRI expression on the surface of mast cells and maintenance of IgE memory despite a loss of serum IgE ... 11
Memory B Cells ... 12
Tracking IgE+ Cells ... 12
Plasma Cells ... 14
Depletion of Plasma Cells with Bortezomib ... 15
Persisting antigen model of long-lived plasma cells. ... 18
Polyclonal activation model of long-lived plasma cells. ... 18
Rituximab ... 19
Rituximab mechanism of action ... 19
Studies investigating the effects of anti-CD20 antibody depletion in mice ... 20
Transfer of Human Peanut Allergy through Organ Transplantation ... 21
Murine Models of Peanut Allergy ... 22
IgG- versus IgE-mediated anaphylaxis in murine models of peanut allergy ... 23
Murine anaphylaxis is not dependent on toll-like receptor expression and can be mediated by complement activation ... 24
Rodent Models That Elicit Serum IgE Responses ... 26
Rationale of Thesis ... 27
II. MATERIALS AND METHODS ... 28
Crude Peanut Extract and the 20kD Fraction Containing Ara h 2 and Ara h 6 .... 28
Murine Model of Peanut Allergy ... 28
Adoptive Transfer Experiments ... 29
Anti-CD20 and Isotype Control Antibody and In Vivo Depletion of B Cells ... 30
Adoptive Transfer of Cells from Donors Treated with ... 31
Anti-CD20 or Isotype Control Antibody ... 31
Harvest of Blood, Bone Marrow, Spleen, and Peritoneal Cells ... 31
Intravenous Treatment with Bortezomib or Diluent ... 31
Flow Cytometry ... 32
ELISPOT Assays ... 33
Statistical Analyses ... 34
III. ADOPTIVE TRANSFER STUDIES AND IN VIVO DEPLETION OF MEMORY B CELLS WITH ANTI-CD20 ANTIBODY ... 35
Introduction ... 35
Results ... 37
Peanut-allergic splenocytes but not cells from the peanut-allergic bone marrow are required for the adoptive transfer of peanut allergy ... 37
In vitro depletion of either splenic B220+ or CD19+ cells abrogates the adoptive transfer of murine peanut allergy ... 40
B220+ cells from peanut-allergic spleens are not sufficient for the adoptive transfer of peanut allergy ... 43
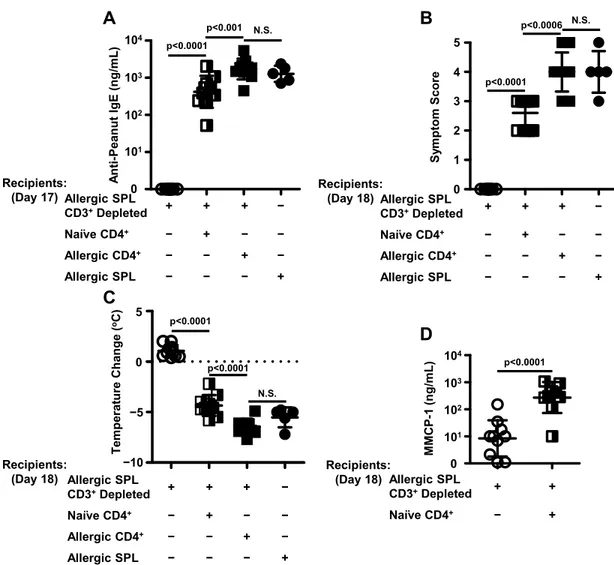
In vitro depletion of CD3+ cells from peanut-allergic splenocytes abrogates the adoptive transfer of peanut allergy, which can be restored by the addition of CD4+ cells purified from naïve splenocytes ... 46
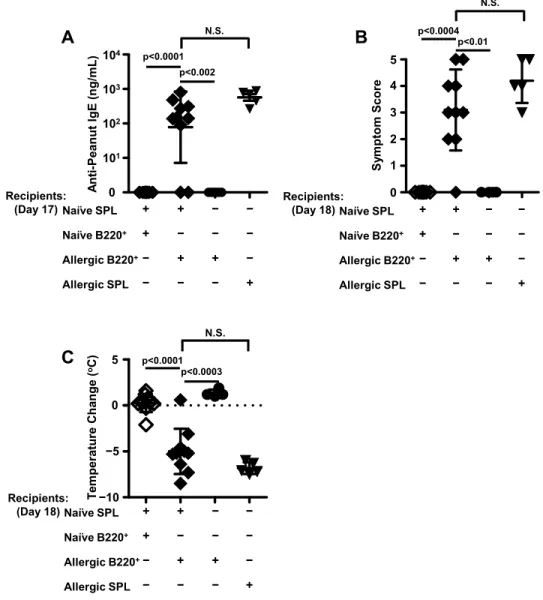
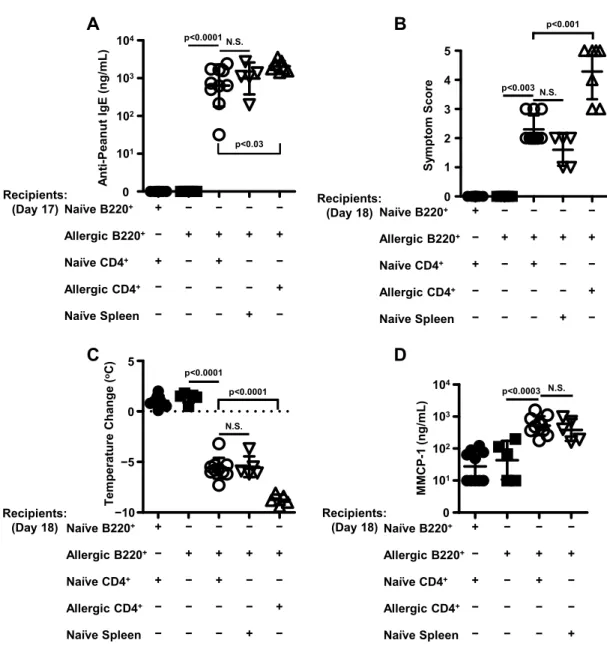
B220+ cells purified from peanut-allergic splenocytes and CD4+ helper T cells purified from naïve splenocytes are the minimal requirements for the adoptive transfer of peanut allergy... 49
CD20+ B cells are not required for maintaining long-lived serum anti-peanut IgE levels ... 54
Prolonged in vivo depletion of B cells with anti-CD20 antibody abrogates the adoptive transfer of peanut allergy ... 64
Summary ... 67
IV. DEPLETION OF LONG-LIVED PLASMA CELLS WITH BORTEZOMIB ... 69
Introduction ... 69
Results ... 70
Prolonged treatment with bortezomib significantly depletes serum immunoglobulin titers ... 71
Prolonged treatment with bortezomib does not affect symptoms
of murine peanut allergy ... 73
Prolonged treatment with bortezomib does not affect antibody secreting cell numbers ... 74
Prolonged treatment with bortezomib does not affect numbers of B cell subsets in the bone marrow and spleen ... 76
Prolonged bortezomib therapy results in significant reductions in anti-peanut IgE titers that persist after prolonged cessation of bortezomib therapy ... 79
Prolonged treatment with bortezomib followed by prolonged cessation of treatment of bortezomib results in permanent decreases in serum anti-peanut IgE titers. ... 81
Summary ... 84
V. OVERARCHING SUMMARY AND DISCUSSION ... 85
In-Depth Discussion... 85
Conclusions ... 92
VI. CLINICAL EXPERIENCES IN PEDIATRIC ALLERGY CLINIC ... 93
LIST OF FIGURES FIGURE
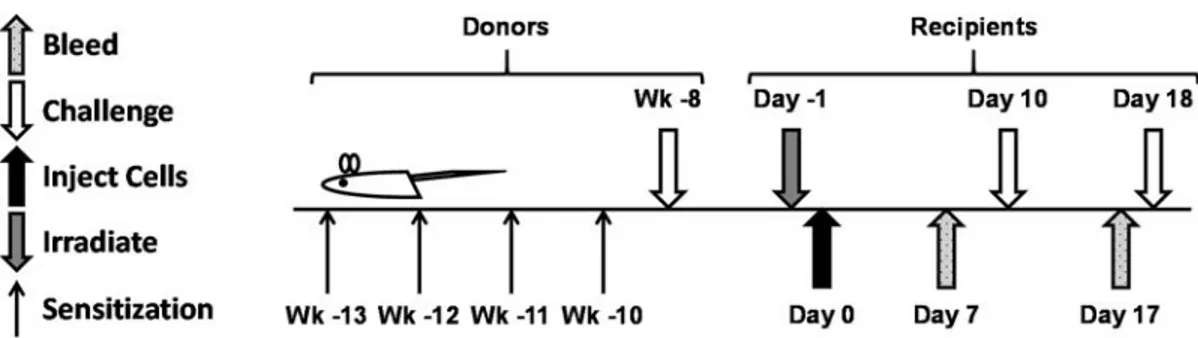
3.1. Timing of sensitization and challenge in donors and timing of irradiation,
adoptive transfer, and challenges in recipients ... 37 3.2. Peanut-allergic splenocytes, not bone marrow cells, are required for the
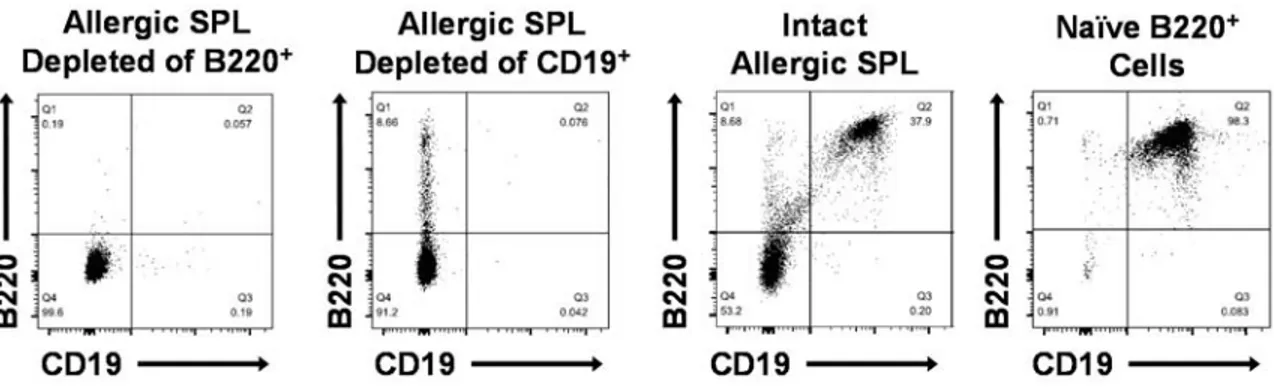
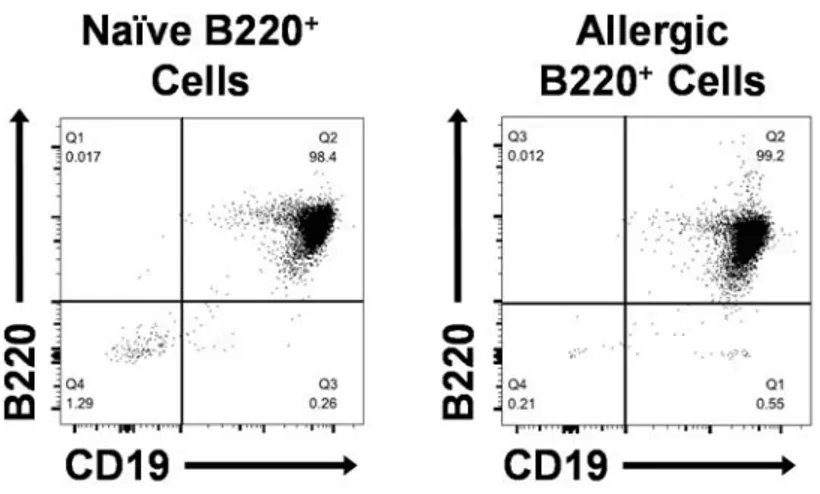
adoptive transfer of peanut allergy ... 39 3.3. Flow cytometric analysis of allergic splenocytes depleted of either B220+
or CD19+ cells, intact (unfractionated) peanut-allergic splenocytes, and
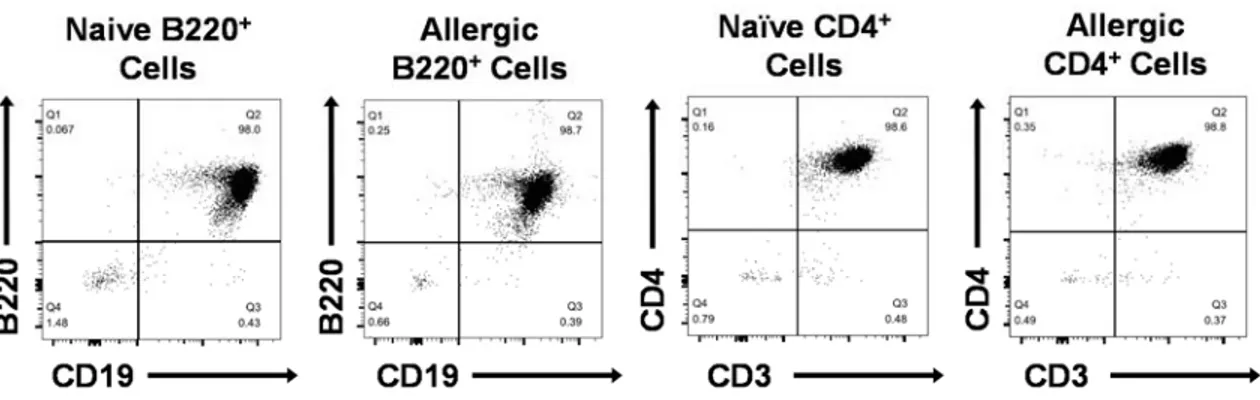
naïve B220+ cells purified from naïve splenocytes ... 41
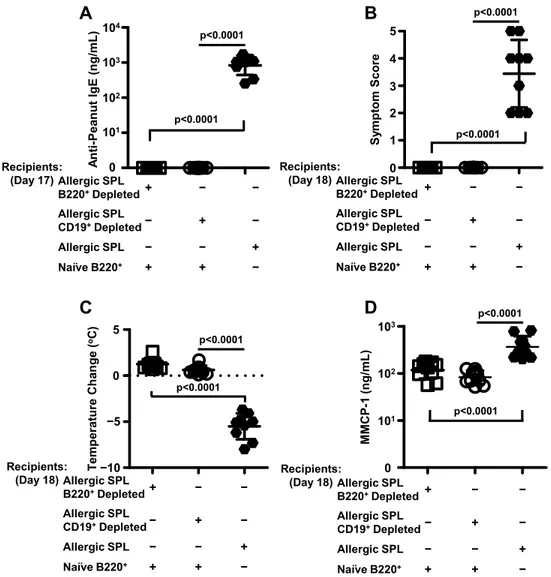
3.4. B cells are required for the adoptive transfer of peanut allergy ... 42 3.5. Flow cytometric analysis of B220+ cells purified from naïve and
peanut-allergic splenocytes ... 44 3.6. B220+ cells from allergic donors are not sufficient for the adoptive transfer
of peanut allergy ... 45 3.7. Flow cytometric analysis of allergic splenocytes depleted of CD3+ cells,
intact allergic splenocytes, and CD4+ cells purified from naïve and allergic
splenocytes ... 46 3.8 CD4+ cells isolated from naïve splenocytes can help allergic splenocytes
depleted of CD3+ cells differentiate into IgE antibody secreting cells ... 48
3.9. Flow cytometric analysis of B220+ cells and CD4+ cells purified from
naïve and peanut-allergic splenocytes ... 49 3.10. B220+ cells from peanut-allergic donor spleens and CD4+ cells isolated
from naïve spleens are the minimal requirements for the adoptive transfer
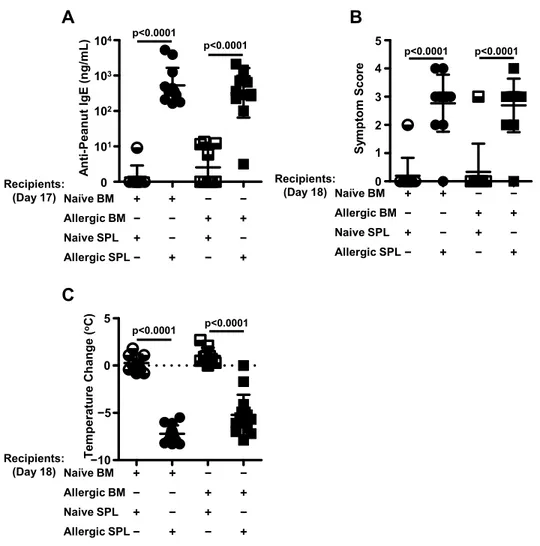
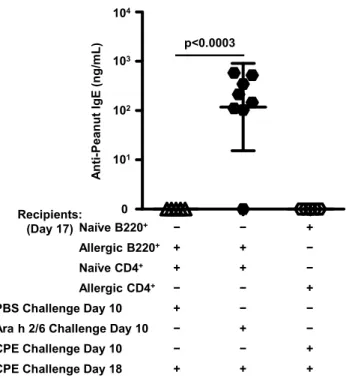
of peanut allergy ... 51 3.11. IgE levels in recipient controls for adoptive transfer experiments on day
17 after one challenge. ... 53 3.12. Symptom scores and temperature drops in recipient controls for adoptive
transfer experiments on day 18 upon the second challenge ... 54 3.13. Temperature changes in mice upon the first challenge on week -21 prior to
treatment with anti-CD20 or isotype control antibody ... 55 3.14. Timing of sensitization, challenge, acquisition of sera, and treatment of
3.15. Cell populations after 18 weeks of treatment with anti-CD20 or isotype
control antibody ... 56 3.16. Prolonged treatment (18 weeks) with anti-CD20 antibody results in
significant depletion of B220+ CD19+ cells in splenocytes but not in bone
marrow cells ... 57 3.17. Prolonged treatment (18 weeks) with anti-CD20 antibody results in
significant depletion of CD19+ cells in the bone marrow that are double
positive for surface and intracellular immunoglobulin kappa light chain ... 58 3.18. Numbers of CD19+ cells in the bone marrow and spleen that are double
positive for surface and intracellular immunoglobulin kappa light chain in
each treated mouse ... 58 3.19. Flow cytometric analysis of bone marrow cells and splenocytes of one
mouse after one dose (3 weeks of treatment) with either anti-CD20
antibody or isotope control antibody ... 59 3.20. Prolonged B cell depletion does not affect anti-peanut IgE levels. ... 60 3.21. Prolonged treatment with anti-CD20 antibody does not affect symptoms or
temperature drops upon challenge ... 60 3.22. Total IgG levels in the sera of mice treated with either anti-CD20 antibody
or isotype control for 18 weeks ... 61 3.23. Peanut-specific and total IgG antibody secreting cells in the spleen and
bone marrow after 18 weeks of treatment with anti-CD20 or isotype
control antibody. ... 62 3.24. Total IgE antibody secreting cells in the spleen and bone marrow after 18
weeks of treatment with anti-CD20 or isotype control antibody ... 63 3.25. Number of CD138+ cells in the bone marrow and spleen in mice after 18
weeks of treatment with isotype control or anti-CD20 antibody ... 63 3.26. Schematic of sensitization, challenge, and treatment of donors with
anti-CD20 or isotype control antibody followed by adoptive transfer of bone
marrow cells and splenocytes into naïve recipients ... 64 3.27. Anti-peanut IgE levels in donors after 18 weeks of treatment with either
anti-CD20 or isotype control are not significantly different ... 65 3.28. Numbers of various B cell subsets from donors treated with anti-CD20 or
3.29. B cells with a memory phenotype are depleted in peanut-allergic mice
treated with anti-CD20 antibody ... 68 4.1. Timing of sensitization, challenge, treatment with bortezomib or diluent in
peanut-allergic mice, and re-challenge at the conclusion of treatment ... 70 4.2. Anti-peanut IgE levels and body temperature changes upon challenge are
not significantly different between the groups receiving either bortezomib
or diluent prior to treatment. ... 71 4.3. Treatment of peanut-allergic mice with bortezomib significantly reduces
serum anti-peanut IgE levels ... 72 4.4. Bortezomib treatment significantly reduces serum peanut-specific IgG1
and total IgG titers in peanut-allergic mice ... 73 4.5. Symptoms of peanut allergy are not affected by prolonged treatment with
bortezomib ... 74 4.6. Prolonged bortezomib therapy does not affect IgG antibody secreting cell
numbers in the bone marrow and spleens of peanut-allergic mice ... 75 4.7. Prolonged bortezomib therapy does not affect total IgE antibody secreting
cell numbers in the bone marrow and spleens of peanut-allergic mice. ... 76 4.8. Numbers of CD138+ cell subsets in the bone marrow and spleen after
prolonged treatment with bortezomib ... 77 4.9. Prolonged bortezomib therapy does not affect populations of cells in the
bone marrow or spleen ... 78 4.10. Timing of sensitization, challenge, and treatment with bortezomib or
diluent ... 79 4.11. Anti-peanut IgE levels, symptom scores, and body temperature changes
are not significantly different between groups of mice prior to treatment
with bortezomib or diluent on week 0 ... 80 4.12. Treatment with bortezomib for 22 weeks, followed by 14 weeks of
cessation of treatment results in a persistent, significant reduction in serum
anti-peanut IgE titers ... 82 4.13. Symptom scores and body temperature changes in mice treated with
diluent or bortezomib for 36 weeks, or treated with bortezomib for 22
LIST OF ABBREVIATIONS ASC Antibody Secreting Cell
BM Bone Marrow
BSA Bovine Serum Albumin
Bz Bortezomib
C3a Complement Component 3a
C5a Complement Component 5a
CD Cluster Differentiation
Cγ Common Chain Gamma
Cε Common Chain Epsilon
Cµ Common Chain Mu
CPE Crude Peanut Extract
CpG Cytosine-Phosphate-Guanine
Dil Diluent
ELISA Enzyme Linked Immuno Assay ELISPOT Enzyme Linked ImmunoSpot FACS Fluorescence Activated Cell Sorting
Fc Fragment Crystalizable
FcεRI Fragment Crystalizable Epsilon Receptor I FcγRIII Fragment Crystalizable Gamma Receptor III
FCS Fetal Calf Serum
IC Isotype Control
Ig Immunoglobulin
IgE Immunoglobulin E
IgG Immunoglobulin G
IgHa Immunoglobulin Heavy Chain a
IgHb Immunoglobulin Heavy Chain b
iIgκ Intracellular Immunoglobulin Kappa Light Chain IκB Inhibitor of Kappa B
IL Interleukin
ip Intraperitoneal
kD Kilo Dalton
LCMV Lymphocytic Choriomeningitis Virus
LPS Lipopolysaccharide
µg Microgram
mg Milligram
MHCII Major Histocompatibility Complex Class II
mL Milliliter
µm Micrometer
MMCP-1 Mouse Mast Cell Protease-1
NF-κB Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells
ng Nanogram
OVA Ovalbumin
p105 105 Kilo Dalton Precursor Protein PBMC Peripheral Blood Mononuclear Cell PBS Phosphate Buffered Saline
RBC Red Blood Cell
RBL Rat Basophilic Leukemia
RPMI Roswell Park Memorial Institute Medium
SD Standard Deviation
Sγ Switch Gamma
Sε Switch Epsilon
sIgκ Surface Immunoglobulin Kappa Light Chain
Sµ Switch Mu
SPL Splenocytes
STAT-6 Signal Transducer and Activator of Transcription-6 Th1 T Helper Type 1 Cell
Th2 T Helper Type 2 Cell Th5 T Helper Type 5 Cell Th9 T Helper Type 9 Cell Th17 T Helper Type 17 Cell TLR4 Toll Like Receptor 4
CHAPTER I INTRODUCTION
Pathophysiology of the Allergic Response
Allergy is a type I hypersensitivity reaction, characterized by the production of immunoglobulin E (IgE) antibodies against a given antigen, or allergen. IgE antibody formation can occur in response to allergens (which are intrinsically unharmful antigens found in the environment) or due to parasitic infections, for which IgE aids in their clearance. Allergen-specific IgE antibodies can trigger allergic responses when the fragment crystalizable (Fc) region of the IgE antibody binds to the high affinity IgE receptor, Fc epsilon receptor I (FcεRI), on the surface of a mast cell or basophil. Upon crosslinking of two or more IgE antibodies bound to FcεRIs by their specific allergen, compounds such as pre-formed mediators (histamine and tryptase), newly-synthesized lipid mediators, and cytokines are released from mast cells (1). Release of these mediators results in symptoms of allergic reactions that can be relatively mild, including the formation of hives, itching, sneezing, or more severe reactions involving the gastrointestinal, respiratory, and cardiovascular systems, possibly resulting in death.
Allergens found in the environment include, but are not limited to: foods, pollens, drugs, insect venoms, molds, and animal danders. Sensitization, or the production of IgE, to allergens occurs when allergens are presented by antigen-presenting cells in an atopically-skewed immunological environment to B and T cells resulting in the production of allergen-specific IgE by plasma cells (specialized cells that secrete antibodies). However, the presence of allergen-specific IgE (signifying sensitization) does not guarantee allergic reactivity and symptoms.
IgE-Mediated Food Allergy
IgE-mediated food allergies are a growing problem in industrialized countries, including the United States. The most common IgE-mediated food allergies in the United States include allergies to milk, wheat, egg, fish, shellfish, soy, peanut, and tree nuts (not limited to almond, cashew, pistachio, and walnut). IgE-mediated food allergies typically present in the pediatric population, affecting 4-6% of children and 2% of adults in the United States (2) with 2% of the pediatric population allergic to peanut (3). Milk, egg, wheat, and soy allergies are typically outgrown, while peanut, tree nut, fish, and shellfish allergies are more likely to persist into adulthood. Patients with peanut allergy are instructed to avoid consuming peanuts; however, accidental ingestion of peanut protein and reactions to small doses still remain a persistent problem (4) resulting in severe reactions and psychosocial harm significantly affecting the quality of life for many individuals (5).
Risks factors and protective factors for the development of food allergy and atopic conditions. Emerging studies have identified many possible risks associated with acquiring atopic conditions. These involve complex gene-environment interactions. Many identified risk factors have not been conclusively defined, and many conflicting studies exist. The greatest predictors of atopic disease in offspring include a family history of atopic conditions (such as asthma and allergies) in the mother or father (6). Other risk factors include the male sex, presence of elevated cord serum IgE, passive smoke inhalation in children, and formula feeding prior to three months of age (6). Factors that correlate negatively with the development of atopic conditions include (but are not limited to) increased food diversity within the first year of life (7), exposure to farming environments (8), and a larger number of shared siblings (8).
Exposure to allergens through various routes can either be protective or causative for the development of atopic conditions. For example, the lung is thought to be a sensitizing environment for pollen and dander, and the skin is thought to be a sensitizing environment for food allergens. Alternatively, the gut is thought to be a tolerizing environment for food allergens; in contrast, a “leaky gut” is thought to be a sensitizing environment for the development of food allergies. The effect these local routes have on the development of food allergies depends on the local immune environment that the food allergens and immune system first interact.
Not only does the location of allergen-immune system interaction affect the development of food allergies, but the timing of introduction of foods also plays an important role, with introduction of foods at an earlier age yielding a more protective effect. A recent study found that in infants that are “high risk” for the development of food allergy, early introduction of peanut into the diet can prevent onset of peanut allergy (9). In this study, infants aged 4-11 months who had severe atopic dermatitis or egg allergy with sensitization to peanut (determined by skin-prick testing) were randomized into two groups. One group avoided consuming peanuts until 60 months of age, and one group regularly consumed peanuts under physician supervision (9). The group that consumed peanuts had a significant decrease in the onset of peanut allergy by 60 months of age (9), indicating that early introduction of peanut in the diet is protective in preventing the onset of peanut allergy. Further studies will need to determine if this protection lasts, and if continued weekly consumption of peanuts is required.
Mainstay treatment of food allergy. The current mainstay treatment for food allergy involves complete avoidance and abstinence in ingesting the particular food for which one is allergic. Many patients are advised to carry epinephrine and antihistamines with them at all times in case of an accidental ingestion. If ingestion of the offending food and allergic symptoms occur, the patient is advised to use epinephrine when two or more organ systems are involved (for example, hives and vomiting, or difficulty in breathing with facial swelling). Patients are also advised to seek emergency care immediately after epinephrine use.
Anaphylactic reactions can also be biphasic, re-occurring after the initial anaphylactic reaction even with epinephrine use and complete resolution of symptoms (10). Thus, the possibility of a biphasic anaphylactic reaction occurring, further complicates anaphylaxis requiring possible hospitalization and/or close monitoring after an episode, not only making these reactions dangerous and traumatic, but also costly.
Experimental treatments of food allergy. Current experimental therapies to treat food allergy include desensitization to induce tolerance to a given allergen so that the food may again be consumed without reactions. These experimental therapies include sublingual, subcutaneous, oral, and epicutaneous immunotherapy. These are thought to desensitize patients or induce immune tolerance through increasing T regulatory cell populations that can suppress T effector cell activation and secretion of cytokines resulting in decreased secretion of IgE from B cells (3). In addition, T regulatory cells can directly suppress mast cell function through OX40-OX40 ligand interactions resulting in increased cyclic adenosine monophosphate levels within mast cells and decreased calcium influx,
decreasing mast cell activation in patients with food allergy, and raising the threshold for the amount of allergen that can be ingested without a reaction (11).
Sublingual immunotherapy involves placing a small drop of allergen extract under the tongue from three times a week to daily, with therapy continuing for three to five years. Subcutaneous immunotherapy, commonly known as allergy shots, involves injecting allergen extracts into the subcutaneous tissue. Subcutaneous immunotherapy is not widely used to treat food allergy but can be helpful to treat allergies to pollens and danders. Peanut-allergic patients treated with oral immunotherapy consume small amounts of peanut allowing the allergen to interact with the immune-tolerizing environment of the gut. Oral immunotherapy slowly results in an increase in the amount of allergen that can be tolerated without triggering a reaction. Epicutaneous immunotherapy involves giving the allergen through a patch placed on the skin.
These experimental immunotherapies are promising; however, problems persist. Many patients are excluded from receiving them due to a high likelihood of reactivity or due to severe reactions that may occur during therapy. Patients are also required to continue daily home maintenance dosing to maintain desensitization or tolerance. If daily home maintenance dosing is not continued, patients may lose tolerance and may revert to becoming allergic.
Peanut allergy versus other food allergies. Unlike food allergies to milk, egg, wheat, and soy, which are typically outgrown, peanut allergy is lifelong for 80% of children (12). Peanut allergy also accounts for 60% of food-allergen-induced anaphylactic deaths (13), making it a dangerous food allergy. Children with milk and egg allergy may more easily consume baked forms of egg and milk, and the continued consumption of these baked
forms increases desensitization and tolerance. However, for most patients with peanut allergy, strict avoidance is advised, and strict adherence to refrain from consuming foods that may be cross-contaminated is also required. It has been estimated that accidental ingestion of peanut can be as high as 14% in school-children (14), indicating that avoidance is not a viable long-term treatment option.
Allergens, Are They Special Antigens?
Effects of common allergens on the host. Many allergens have intrinsic enzymatic activity that may or may not have direct effects on the host (such as causing increased permeability at epithelial or mucosal barriers) further increasing their allergenicity. Der p 1, a dust mite allergen, has been found to have 30% sequence homology with papain (15), a cysteine protease with intrinsic enzymatic activity found in the papaya. Another dust mite allergen, Der p 3, has serine proteinase activity and also has 40-50% homology with invertebrate and vertebrate trypsins (16). Der p 1 has been found to increase permeability of bronchial epithelium (17), contributing to its immunogenicity. Der p 1 also cleaves CD23, the low affinity Fc receptor for IgE (18). Cleavage of CD23 and its decreased expression on the surface of B cells is thought to enhance IgE responses, since binding of IgE to CD23 can result in feedback inhibition of immune cells (18).
In addition to enzymatic activity, many food allergens remain un-degraded in the presence of gastric juices (19) resulting in increased stability allowing allergenic proteins to be intact after passing through the digestive system and permitting them to absorbed systemically. This allows them to persist for longer periods at mucosal surfaces and increases the chances for allergens to interact with the immune system in a potentially non-tolerizing environment.
Proteomics of peanut proteins. Many allergenic proteins also have functions that aid in plant physiology and defense. Interestingly, Ara h 2, a major allergen in the peanut, functions as a trypsin inhibitor to protect degradation of Ara h 1, another allergen of the peanut (20). Additionally, roasting peanuts has also been shown to enhance the ability of Ara h 2 to act as a trypsin inhibitor (20).
In addition to the intrinsic enzymatic activity of allergens, post-translational modification of protein allergens can also enhance their allergenic activity. One such example is glycosylation, which may increase the stability of allergens, increase IgE binding capacity, and increase uptake of the allergen by antigen-presenting cells (21, 22). Ara h 1, an allergen in the peanut, has two sites that can become glycosylated (23), and the effects of advanced-glycation end products occurring via the Maillard reaction during roasting have been documented to occur in Ara h 1 and Ara h 3, but not in Ara h 2 (24). Advanced-glycation end products of allergens can increase antigen-presenting cell uptake of allergens and presentation in an inflammatory manner (24).
The peanut, Arachis hypogaea, is a legume that contains many allergic proteins belonging to families and superfamilies of proteins shared between legumes and other plants (25). Within the peanut, three major groups of proteins contain most of the important allergens, known as major allergens. These include the cupin superfamily, the prolamin superfamily, and defense proteins important for plant survival to stressors (25).
The prolamin superfamily contains the 2S albumin seed storage proteins and non-specific lipid transfer proteins (25). The cupin superfamily includes a group of proteins known as vicilins (Ara h 1), legumins (Ara h 3), and also seed storage proteins (25).
Many allergens found within the peanut are very stable and resist digestion. One example is Ara h 1, which contains B cell epitopes located in areas resistant to protease digestion (26). Ara h 2 and Ara h 6 belong to the conglutin protein family, a relative of the 2S albumin family (27). Much of the literature has focused on defining Ara h 1, Ara h 2, and Ara h 3 as major allergens of the peanut. However, new evidence has pointed to the two proteins, Ara h 2 and Ara h 6, both related to seed storage proteins, as being the major allergens of the peanut. An in vitro tool to identify the allergen effector activity of peanut proteins uses a rat basophilic leukemia cell line (RBL SX-38) that expresses human FcεRI allowing for binding of IgE from sera of peanut-allergic patients. Specific proteins (such as Ara h 2 and Ara h 6) from the peanut can be added to these cells (which have bound polyclonal human IgE antibodies in culture), inducing degranulation from RBL SX-38 cells. When the 20 kilodalton (kD) fraction (containing Ara h 2 and Ara h 6) purified using gel filtration of crude peanut extract (CPE) was added to RBL SX-38 cells incubated with peanut-allergic sera, it was found that these two proteins account for 80% of the effector activity found in CPE (28). Ara h 2 and Ara h 6 can also be used to desensitize peanut-allergic mice (29) indicating that these two proteins are major peanut allergens in humans.
Allergy and the Immune System
Allergies are triggered by cross-linking of IgE on sensitized mast cells or basophils by allergen, resulting in the release of allergic mediators that cause allergic symptoms. Therefore, studying B cell subsets, and how various cells of the immune system interact with and instruct these cells to secrete IgE should be a major focus of allergy research. There are a variety of cells, such as various subsets of T cells and group 2 innate lymphoid
cells (ILC2) that can either suppress or promote allergic responses and instruction of B cells to secrete IgE.
T cell subsets in the allergic response. T helper type 1 (Th1) and T helper type 2 (Th2) cell populations and their regulation of the allergic immune response have been well characterized. Th1 cells express CD4, are activated by interleukin (IL)-12 and IL-1, secrete interferon-γ, and activate the immune response against bacteria. Th1 cells inhibit the allergic response and secrete antagonistic cytokines that inhibit cells that promote atopic responses.
Th2 cells express CD4, and in contrast to Th1 cells, they are activated by IL-4. Th2 cells secrete IL-4, IL-5, IL-9, IL-10, and IL-13. IL-10 inhibits Th1 cell activation and function, and IL-4 is responsible for B cell antibody class-switching from IgG1 to IgE.
T helper type 5 (Th5) cells are CD4+ cells that, in the presence of IL-33 and antigen,
become IL-5 secreting but not IL-4 secreting cells. IL-5 has been found to promote immunoglobulin secretion from B cells, promote eosinophil responses, and also promote the development of asthma and allergic rhinitis (30).
T helper type 9 (Th9) cells are CD4-expressing cells that preferentially produce IL-9. This interleukin is important for clearing parasitic infections and for the development of allergic conditions (such as asthma, for which it is a candidate gene) (31).
T helper type 17 (Th17) cells form in the presence of transforming growth factor β, IL-1β, IL-6, and IL-23 (32). Th17 cells predominantly secrete IL-17A, IL-17F, and IL-22 resulting in neutrophil recruitment. These cytokines contribute to neutrophil-dominated reactive airway disease in asthmatic patients (33) and also contribute to the development of atopic dermatitis (32).
T follicular helper cells are antigen-experienced cells found within B cell follicles of secondary lymphoid organs (spleen and lymph nodes) that are important for the development of antibody responses. T follicular helper cells express B cell lymphoma 6 and can secrete IL-4 and IL-21 to help germinal center B cells differentiate into antibody secreting cells (ASCs) (34). Important B cell responses, such as somatic hypermutation, affinity maturation, and antibody class-switch recombination occur within the germinal center (34). A critical cytokine secreted by T follicular helper cells that induces B cell proliferation and antibody isotype switching is IL-21 (34).
Group 2 innate lymphoid cells. Another important cell type that drives allergic inflammatory responses are ILC2. ILC2 secrete IL-5, IL-6, IL-9, and IL-13 to drive allergic responses (35). Innate lymphoid cells are lymphoid in origin but are also innate cells of the immune system that do not respond in an antigen-specific manner. In a model of allergic hypersensitivity using ovalbumin (OVA) and house dust mite antigen, ILC2-deficient mice did not develop allergic airway reactivity when OVA and house dust mite antigen were given through intranasal challenge (36). When these antigens were given systemically, mice developed allergic responses, suggesting that ILC2 play a role in allergy development with local administration of allergen and are not required for the development of systemic responses to allergen in the mouse (36).
IgE antibody class switching. IL-4 induces IgE class switching by activating a transcription factor known as signal transducer and activator of transcription-6 (STAT-6). IL-4 also induces B cell proliferation, and up-regulation of CD40 and major histocompatibility complex II (MHCII). After IL-4 signaling, STAT-6 promotes common chain gamma (Cγ) and common chain epsilon (Cε) germline transcription (37). A second
signal from CD40-CD40 ligand interaction helps with formation of the germinal center and is also required for IgE production (38). Analysis of DNA switch circles for IgE class switching has shown that class-switching to IgE occurs from the common chain µ (Cµ) to the Cγ, and from the Cγ to the Cε (through the detection of switch epsilon switch gamma (SεSγ) DNA switch circles), and that IgE class-switching could also be direct from the Cµ to the Cε (through the detection of switch epsilon switch mu (SεSµ) DNA switch circles) (39). However, mice that are deficient in production for IgG1 are unable to produce high-affinity IgE antibodies (40), indicating that direct switching from the Cµ to the Cε may not be ideal to allow for both affinity maturation and somatic hypermutation to occur.
The role of IgE in B cell perpetuation of allergic conditions. IgE in the serum has been shown to facilitate peripheral blood mononuclear cell (PBMC) proliferation through a proposed mechanism of IgE facilitated antigen presentation (41). PBMCs from peanut-allergic donors incubated with serum depleted of IgE have decreased proliferation to peanut protein compared to PBMCs from peanut-allergic donors incubated with intact serum (41). IgE facilitated antigen presentation is an antigen-specific (not polyclonal) response. It is thought that IgE facilitated antigen presentation can increase antigen presentation to T cells from either dendritic cells or B cells.
IgE regulation of FcεRI expression on the surface of mast cells and maintenance of IgE memory despite a loss of serum IgE. To determine the longevity of IgE serum responses, the role of IgE in FcεRI expression on the surface of mast cells in B cell deficient (C57BL/6-µm-/-) mice was studied (42). Trinitrophenyl-specific IgE was passively
transferred into B cell deficient recipients (42). Six days after passive transfer, serum IgE levels became undetectable, and treatment of recipients on days 14, 28, and 42 (after
passive transfer of serum) with anti-IgE monoclonal antibody or with trinitrophenyl-bovine serum albumin (BSA) induced body temperature drops indicating that IgE-dependent cross-linking on mast cells resulted in anaphylaxis (42). These results suggest that mast cells, via FcεRI binding to available IgE, can maintain IgE memory and allergic symptoms, despite an eventual loss in detectable serum IgE. This study also found that IgE memory in the absence of serum IgE is shorter in B cell deficient than in wild-type mice (42). They postulate that this is because in wild-type mice, basal levels of polyclonal IgE stabilize FcεRI on the surface of mast cells, increasing the amount of time that IgE of a given specificity can be bound to mast cells, even in the absence of detectable antigen-specific IgE in the serum (42).
Memory B Cells
Memory B cells are antigen-experienced B cells which, upon reactivation to cognate antigen, can rapidly undergo class-switch recombination, affinity maturation, and differentiation into plasmablasts that secrete antibody. These responses occur in the germinal center and are T cell-dependent. Within the germinal center, complement receptor 2 on the surface of follicular dendritic cells provides antigen-antibody complexes allowing for the prolonged survival of memory B cells (43). Memory B cells serve as a broad repertoire of antigen-experienced cells that can rapidly differentiate into ASCs upon re-exposure to cognate antigen.
Tracking IgE+ Cells
In recent years, the ways in which IgE+ memory cells form within the germinal
center and differentiate to become plasma cells has been investigated through the development of B and T cell monoclonal mice (38) as well as IgE+ B cell fluorescent mice
(44-46). A study conducted by Erazo et al. found that IgE+ cell development is initiated
within the germinal center, but largely occurs outside of germinal centers, and that these cells express a plasma cell phenotype early on in their development (47). They found that IgE+ cells first form through an IgG1+ intermediate memory cell (in which somatic
hypermutation and affinity maturation occur within the germinal center) that can rapidly class-switch to differentiate into an IgE+ cell expressing more of a plasma cell phenotype
indicating that there likely is not a pool of classical IgE+ memory cells (47). Direct
switching from Sµ to Sε has been shown to form “failure-to-thrive” IgE germinal center cells that undergo high rates of apoptosis, and that indirect switching from Sγ1 to Sε forms IgE+ cells with a plasma cell phenotype (44). Using monoclonal mice that express B cell
receptors specific for hemagglutinin and T cell receptors specific for OVA, staining for hemagglutinin-specific B cell receptors showed that IgE+ germinal center cells expressed
lower numbers of B cell receptor per cell than IgE+ plasma cells and IgG1+ germinal center
cells express (44). IgE+ germinal center cells were also found to have decreased proximal
signaling through their B cell receptors upon phosphatase inhibition (44). Using an IgE-GFP reporter mouse, it was found that direct switching generates IgE+ cells that have more
of a germinal center phenotype, and that indirect switching through Sγ generates IgE+ cells
with a plasma cell phenotype that express antibody with a higher affinity (44). IgE+ cells
that undergo sequential switching to form an IgE+ cell with a plasma cell phenotype must
first exit the germinal center (44).
Using an independent mouse model expressing a fluorescent GFP-like marker linked to IgE expression, Yang et al. found that IgE+ cells differentiated into germinal
(46). In their model, IgE+ B cells exited the germinal center early and differentiated into
short-lived plasma cells that underwent decreased affinity maturation compared to IgG1+
B cells (46). Yang et al. also found that IgE+ plasma cells can be divided into two groups:
IgE+ early and IgE+ late plasma cells (46). IgE+ early plasma cells do not develop from an
IgE+ germinal center intermediate, whereas IgE+ late plasma cells can develop from a
germinal center intermediate (46). These results contrast from the work by Erazo et al. that showed that the formation of IgE+ plasma cells occurs from an IgG1+ germinal center cell
that does not have an IgE+ germinal center cell intermediate (47). In either case, both
groups support a model of IgE+ plasma cells that are short-lived and lack affinity
maturation compared to IgG+ plasma cells.
Plasma Cells
Plasma cells are important for maintaining serological memory. These are specialized cells that function to secrete antibody of a given isotype and antigen specificity. Newly formed ASCs, known as CD138+ plasmablasts, still retain their ability to divide and
still express many surface markers such as MHCII and surface antibody (allowing them to internalize and present antigen to T cells). Long-lived CD138+ plasma cells are thought to
be quiescent and to downregulate surface marker expression of B220, CD19, CD20, MHCII, and surface immunoglobulin expression. In one study using mice immunized with OVA and alum, OVA-specific long-lived plasma cells were found to not undergo cell division (DNA replication) for at least 90 days, as evidenced by analysis of bromodeoxyuridine incorporation (48).
Depletion of Plasma Cells with Bortezomib
Plasma cells express increased amounts of organelles such as the rough endoplasmic reticulum and golgi apparatus to allow for secretion of large amounts of immunoglobulin. Within plasma cells, proteasomes function to degrade ubiquitinated, misfolded proteins (49) and to also degrade proteins that regulate cell-cycle control (50). Proteasomes are important for plasma cell function and survival. Inhibition of proteasomes (by agents such as bortezomib) can be used to treat cancers of plasma cells (such as multiple myeloma).
Bortezomib is a modified dipetidyl boronic acid that binds to the active sites of the proteasome, functioning as a reversible inhibitor of chymotryptic-like activity of the proteasome (51). When studied in vitro, multiple myeloma cells were found to be 1,000 times more sensitive to the effects of bortezomib-induced cell death than normal plasma cells (52). In multiple myeloma cells, nuclear factor kappa-light-chain-enhancer of B cells (NF-κB) expression is required for survival. Degradation of the inhibitor protein, inhibitor kappa B (IκB), by the proteasome is required for NF-κB expression. The proteasome also proteolytically cleaves the inactive subunit, 105 kD precursor protein (p105) into subunit 50 kD precursor protein (p50) that binds to 65 kD precursor protein (p65) to result in the transcription of NF-κB (53, 54). Multiple myeloma cells are killed by bortezomib due to inhibition of the proteasome, which is required for formation of p50 and also the degradation of IκB. Thus, expression of NF-κB is suppressed by bortezomib in multiple myeloma cells, and decreased NF-κB expression in multiple myeloma cells results in a decrease in survival of these cells. However in normal plasma cells, bortezomib functions to inhibit the proteasome from degrading misfolded proteins. Excess accumulation of
unfolded proteins results in activation of the unfolded protein response that eventually induces apoptosis.
Bortezomib is currently used to treat multiple myeloma and relapsed mantle cell lymphoma; however in recent years, studies using this compound to treat autoimmune disorders such as refractory systemic lupus erythematosus (55), inflammation associated with uveitis (56), Sjorgen’s syndrome (57), early onset myasthenia gravis (58), and autoimmune hemolytic anemia (59) have shown promise.
Bortezomib’s efficacy in treating atopic conditions such as atopic dermatitis and asthma has been investigated recently in mouse models. One study examining the efficacy of bortezomib to treat murine atopic dermatitis found that although bortezomib decreased serum IgE levels, depleted allergen-specific ASCs measured by Enzyme Linked ImmunoSpot (ELISPOT) and plasma cells measured by fluorescent activated cell sorting (FACS) analysis, and decreased numbers of CD4+ and CD8+ cells in atopic dermatitis
lesions, these lesions still persisted in mice despite treatment (60).
In a study investigating the efficacy of bortezomib to treat OVA-induced airway hyper-reactivity, mice were sensitized with intraperitoneal (ip) OVA adsorbed in alum and were challenged repeatedly with aerosolized OVA. These mice were treated with bortezomib for 6 weeks and showed a significant reduction in serum anti-OVA IgE levels without a reduction in anti-OVA IgG1 or anti-OVA total IgG levels (61). Despite the decrease in serum anti-OVA IgE levels, airway hyper-responsiveness and numbers of plasma cells were not decreased by bortezomib treatment (61).
Proposed Models for Long-Lived Plasma Cells
In some cases, humoral immunity against a given antigen derived through infection or some types of vaccination has been shown to be lifelong. For example, in individuals that were infected during the 1918 influenza pandemic, serum neutralizing antibody to hemagglutinin from the 1918 virus was detected in 32 out of 32 tested individuals nearly 90 years after infection with the virus (62). This long-lived seroreactivity suggests that long-lived plasma cells could be responsible for maintaining long-lived humoral immunity. It was also observed that 7 out of 8 individuals infected with the 1918 Spanish flu also had B cells in peripheral blood that could produce neutralizing antibodies to the virus (62). These results suggest that memory B cells could also be long-lived. Therefore, it is unknown if memory B cells are long-lived and constantly form plasma cells throughout the life of the individual, or if, instead, plasma cells formed during immune responses are long-lived.
Studies by Slifka et al. in 1998 aimed to answer the question of whether plasma cells could be long-lived. Slifka et al. studied these cells in mice infected with lymphocytic choriomeningitis virus (LCMV) and investigated the effects of irradiation (which should deplete memory B cells) on plasma cell longevity. Mice, 60 days post-infection with LCMV, were irradiated with 6 gray of total body irradiation (63). When splenocytes (SPL) were adoptively transferred from irradiated mice, recipients were unable to produce LCMV-specific antibody after LCMV challenge; however, recipients of intact spleens from non-irradiated donors 60 days post-infection with LCMV were able to establish LCMV-specific antibodies titers after one challenge (63). In a separate experiment, Slifka
heavy chain a (IgHa) allotype that were 60 days post-LCMV infection and irradiated them,
transplanting them with cells from a naïve congenic strain that secreted antibodies of the immunoglobulin heavy chain b (IgHb) allotype (therefore they could monitor if resulting
antibody came from the recipient, or if donor cells would be primed with LCMV indicating an antigen depot) (63). They found that the recipients continued to secrete LCMV-specific antibody of the IgHa allotype for 8 months and did not secrete any LCMV-specific antibody
of the IgHb allotype (indicating no donor cell priming with residual LCMV) (63). These
results suggest that virus-specific plasma cells can maintain antibody for long periods (8 months) without a memory B cell pool, and that plasma cells have a half-life of approximately 138 days (63).
Persisting antigen model of long-lived plasma cells. A second model for long-lived plasma cells postulates that persisting antigen drives memory B cell differentiation into plasma cells throughout the life of the individual. Persisting antigen could be in the form of latent virus or in some form of antigen depot, such as antigen-antibody complexes on the surface of follicular dendritic cells (64). These generated plasmablasts and plasma cells are thought to be short-lived. In this model, due to antigen persistence, it is possible that memory B cells would continue to undergo somatic hypermutation.
Polyclonal activation model of lived plasma cells. A third model for long-lived plasma cells proposes that a pool of memory B cells constantly differentiates into plasma cells in an antigen-independent manner involving toll-like receptor (TLR) stimulation, cytokine stimulation, or bystander T cell help involving CD40-CD40 ligand interactions (65). In this model, memory B cell activation occurs in a polyclonal manner. In a study conducted by Bernasconi et al., sorted, human CD27+ cells were labeled with
carboxyfluorescein succinimidyl ester and were stimulated in vitro with various treatments (65). Treatment with cytosine-phosphate-guanine (CpG) plus IL-15 induced memory B cell proliferation, whereas treatment with either alone, did not (65). Memory B cell treatment with anti-human light chain immunoglobulin, alone, did not induce proliferation of memory B cells, but when anti-human light chain immunoglobulin was given in combination with CpG and IL-15, it resulted in greater proliferation (65). These results show that B cell receptor stimulation, alone, does not result in activation of memory B cells, and that potentially another stimulus, such as T cell or cytokine help is required.
Rituximab
Rituximab is a chimeric monoclonal antibody directed against CD20. The variable regions are murine in origin, and the constant region of the IgG1 antibody is human (66). Rituximab was developed to treat B cell lymphomas, including non-Hodgkin’s lymphoma, follicular mantle cell lymphoma, and diffuse large B cell lymphoma (67). Rituximab is also used to treat a variety of autoimmune diseases including rheumatoid arthritis (68), relapsing-remitting multiple sclerosis (69), systemic lupus erythematosus (70), and autoimmune anemias including thrombotic thrombocytopenic purpura, autoimmune hemolytic anemia, and idiopathic thrombocytopenic purpura (71). Recently it has been utilized to treat antibody-mediated rejection in kidney transplant patients (72).
Rituximab mechanism of action. Rituximab binds to the extracellular domain of CD20 (73), expressed on the surface of malignant and non-malignant B cells. CD20 begins its expression on B cells starting at the late pre-B cell stage and spans expression through the mature B cell and plasmablast stage (74). CD20 expression is down-regulated during
plasma cell differentiation (75). The function of CD20 is not completely known, but in the mouse, it has been shown to function in calcium signaling (74).
Rituximab mediates cell death through a number of different mechanisms: antibody-dependent cellular cytotoxicity (76), complement-mediated lysis (77), induction of apoptosis via signaling induced by anti-CD20 binding (78), and other effects induced by the ligation of CD20. Some of these ligation effects include decreased B cell activation, proliferation, differentiation, as well as a decrease in the secretion of immunoglobulin from the B cell (79-82).
The Fc region of rituximab activates antibody-dependent cellular cytotoxicity and can also fix complement. Killing mediated through antibody-dependent cellular cytotoxicity occurs by binding of CD16 on the surface of natural killer cells to the Fc region of CD20. Upon binding, CD20 enters lipid rafts inducing formation of a cap where bound anti-CD20 antibody is polarized to one side of the B cell (76). This polarization allows for more efficient killing of the targeted B cell by natural killer cells (76).
Studies investigating the effects of anti-CD20 antibody depletion in mice. Studies of humoral responses in mice treated with anti-CD20 antibody have found that B cell depletion does not affect established basal immunoglobulin titers but does affect the adaptive immune response in generating new antibody titers (83). B cell depletion with anti-CD20 antibody has also been shown to effectively deplete memory B cells, germinal center cells, follicular cells, marginal zone cells, and B1 B cells, but not ASCs (83).
Another study using transgenic mice for the expression of human CD20 and B cells specific for 4-hydroxy-3-nitrophenyl were immunized and allowed to rest for 15-19 weeks to generate a memory B cell pool (84). Following this, immunized mice were treated with
anti-CD20 antibody, and ASC decay was measured for 16 weeks (84). During immunization, mice were treated with bromodeoxyuridine. This uridine analog is taken up into cells that are rapidly dividing. When bromodeoxyuridine is no longer supplied in the drinking water, cells that continue to divide lose bromodeoxyuridine. Therefore, if plasma cells were formed during immunization, and they are long-lived, they should be positive for bromodeoxyuridine inclusion. In this study, prolonged depletion of memory B cells did not affect ASCs in the bone marrow (BM) or SPL (84).
Transfer of Human Peanut Allergy through Organ Transplantation
Food allergies, including peanut allergy, have been reported to be transferred from allergic donors to recipients who were tolerant prior to transplant. Some cases involved transfer of peanut allergy through organ transplantation of the liver (85-87). A few cases involving liver transplantation from peanut-allergic donors resulted in transient peanut allergy in recipients (85), while one recipient was reported to have peanut allergy lasting 48 weeks post-transplant (87). The transfer of peanut allergy from allergic donors to tolerant recipients has also occurred through lung transplantation (88-90), through a combined liver and kidney transplantation (91), and through a combined kidney and pancreas transplantation (92).
Documentation of the transfer of food allergies and other atopic conditions, including peanut allergy, through BM transplantation from related (93-95) and non-related (95, 96) donor-recipient pairs has been reported but has not been well-characterized partially due to a loss of patient follow-up. Polyclonal IgE levels have also been found to be increased in patients following BM transplantation due to viral infection or due to acute graft-versus-host disease (97). It is unknown if these reported cases occurred due to a
transfer of cellular components (IgE-sensitized mast cells, T cells, B cells, allergen-primed dendritic cells, or hematopoietic stem cells) or due to transplant conditioning with immunosuppressive agents. Immunosuppressive agents used for transplant conditioning can break tolerance by inhibiting Th1 responses, including T regulatory cell responses, without inhibiting Th2 cells, allowing sensitization or breakthrough of IgE production to occur more easily.
Murine Models of Peanut Allergy
Mice can be sensitized (produce IgE) to peanut protein and can also react with symptoms upon challenge to peanut protein. During challenge, allergic mice exhibit symptoms of peanut allergy and also become hypothermic. Symptoms are scored as follows: 0-no symptoms; 1-increased scratching/rubbing of the snout and face and/or ear canal digging; 2-increased respiratory rate, slightly decreased activity, diarrhea, pilar erecti; 3-severely decreased activity, severe difficulty in breathing; 4-tremor, convulsion, no response to prodding; and 5-death. Body temperatures are typically taken prior to challenge and then again during scoring, which can occur from 30 to 60 minutes post-challenge.
The method used to sensitize and challenge mice varies. For example, peanut proteins can be adsorbed onto alum and given through ip injection for sensitization, or peanut can be delivered intragastrically through oral gavage using cholera toxin as the adjuvant. Another model uses the combination of peanut protein with staphylococcal enterotoxin B as the adjuvant delivered intragastrically. A model that introduces peanut extract epicutaneously without adjuvant has also been developed in the BALB/c strain (98).
The route used for challenge can also vary. Some models use ip injection to deliver peanut protein during challenge. The amount of peanut protein needed to induce anaphylaxis through ip challenge is small, usually ranging from 200 to 1000 micrograms (µg) versus the intragastric model which requires much larger amounts of peanut protein, up to 200 milligrams (mg) (99). The intragastric method of challenge also typically requires adjuvants such as non-steroidal anti-inflammatory agents or ethanol that aid in increasing gastric permeability, allowing peanut proteins to affect the mouse systemically. Mice can also be challenged with peanut intravenously (100), which also requires lower doses of peanut protein than the intragastric challenge method.
Various strains can be sensitized to peanut including the C3H/HeOuJ, the C3H/HeJ, the C57BL/6, and the BALB/c strain. The C3H/HeJ strain is the most widely used strain for the murine model of peanut allergy. This strain has a defect in TLR4 signaling due to a mutation in the toll-IL-1 receptor domain that prevents downstream signaling (101). Sensitization to peanut protein in mice is not dependent on defective TLR4 signaling, since C3H/HeOuJ mice, which are on the C3H background but do not have a TLR4 mutation, can also be sensitized. Wild-type C57BL/6 and BALB/c strains can also be sensitized to peanut proteins.
IgG- versus IgE-mediated anaphylaxis in murine models of peanut allergy. While anaphylaxis in the C3H/HeJ strain is thought to largely be IgE-mediated, anaphylaxis in C57BL/6 and BALB/c strains has been shown, in part, to be IgG-mediated (102). Release of platelet activating factor through IgG cross-linking of Fc gamma receptor three (FcγRIII) on macrophages, basophils, and neutrophils is thought to mediate this response (102-104). IgG-mediated anaphylaxis in mice occurs through the release of platelet
activating factor, whereas IgE-mediated anaphylaxis occurs through mast cell release of histamine and mouse mast cell protease-1 (MMCP-1) (99, 105-107).
Murine anaphylaxis is not dependent on toll-like receptor expression and can be mediated by complement activation. Anaphylaxis in mice is not dependent on activation of TLRs. When TLR2, TLR4, and MyD88 deficient mice are sensitized to peanut protein and are challenged, these mice did not have any defects in mounting an anaphylactic response and were able to react with drops in body temperatures similarly to wild-type mice (100).
Lipopolysaccharide (LPS) found within the peanut also does not contribute significantly to anaphylaxis. Wild-type C57BL/6 mice, sensitized with CPE and challenged with pure LPS, experienced temperature drops, but their temperature drops were significantly less than those experienced in CPE-sensitized mice challenged with CPE (100). There were also no significant differences in temperature drops when sensitized mice were challenged with LPS-depleted CPE compared to mice that were challenged with CPE containing LPS (100).
Complement has also been shown to play a role in anaphylaxis to CPE in the BALB/c strain. This occurs due to lectins found in CPE. The complement cascade is initiated through binding of lectins in the peanut to mannose binding lectin that results in activation of C3 convertase (108).
To determine the role of complement-mediated anaphylaxis due to CPE, mice that were un-sensitized (naïve) to CPE were used. To determine a role for complement component 3a (C3a) in anaphylaxis to CPE, naïve C3a-deficient mice were treated with propranolol and IL-4 to decrease the threshold of vasoactive mediators required for
anaphylaxis to occur. Upon challenge with CPE, these mice did not drop their body temperatures as significantly as naïve wild-type mice treated in the same manner (100). Furthermore, wild-type mice pretreated with a C3a receptor antagonist did not develop drops in body temperature; however, wild-type mice that did not receive pretreatment with a C3a receptor antagonist developed temperature drops upon challenge (100), indicating that CPE activates C3a to promote complement-mediated anaphylaxis, resulting in the release of platelet activating factor. These results indicate that complement-mediated anaphylaxis does not result in release of mediators from mast cells and occurs independently of peanut-specific IgE. This was further evidenced by the fact that anti-histamines given to a separate group of mice did not prevent temperature drops, showing that the anaphylaxis was not mast-cell mediated (100). These results show that peanut extract can activate complement-mediated anaphylaxis through C3a in BALB/c mice.
A role for C3a-mediated anaphylaxis in the BALB/c strain has been shown to occur; however, when investigated, activation of complement by CPE through the complement component 5a (C5a) pathway does not occur (100). In one experiment, naïve C5a-receptor deficient mice on the BALB/c background were pretreated with propranolol and IL-4 and were challenged intravenously with CPE. Upon challenge, C5a-receptor deficient mice did not have significantly different drops in body temperature compared to wild-type mice that were pretreated and challenged in the same manner, suggesting that C5a activation does not play a role in complement-mediated anaphylaxis (100). Furthermore, when naïve, wild-type BALB/c mice were pretreated with propranolol and IL-4 and were also pretreated with a C5a receptor antagonist, there were no significant differences in temperature drops upon challenge with intravenous CPE compared to naïve wild-type BALB/c mice pretreated and
challenged in the same manner, further suggesting that C5a activation does not occur in peanut-mediated anaphylaxis (100).
Rodent Models That Elicit Serum IgE Responses
IgE production in mice can be induced through a number of methods. These include infecting mice with parasites, giving mice a particular protein antigen adsorbed in alum or another type of adjuvant such as cholera toxin, or administering a lectin such as ricin.
Recently, many studies tracking IgE+ cells have utilized inoculation with the
nematode, Nippostrongylus brasiliensis (a gastrointestinal parasite for rodents), to produce robust serum IgE levels. Depending on the strain of rodent and the method used for sensitization, serum IgE responses can either be short- or long-lived. Polyclonal IgE and antigen-specific IgE levels in N. brasiliensis-inoculated mice typically last for four weeks and resolve after clearance of the parasite (45, 109, 110). Thus, serum IgE generated by N.
brasiliensis is relatively short-lived. Another nematode parasite, Heligmosomoides polygyrus, which is not cleared by immune systems of rodents, persists and results in
long-lived serum IgE levels (111).
Several other methods of sensitization, such as using proteins adsorbed in adjuvants such as alum, given with Bacillus pertussis, or given with ricin (a lectin) have resulted in long-lived serum IgE responses in mice (112-115) with serum IgE levels persisting even after irradiation (116, 117). Another study that sensitized mice with a combination of OVA and alum followed by challenges with aerosolized OVA in an asthma model, detected long-lived ASCs, including IgE-specific ASCs, at several sites: BM, spleen, and lung following aerosolized challenges with OVA (118). Therefore, when studying IgE+ memory B cells
and plasma cells, both models, with short-lived and also long-lived serum IgE levels should be utilized.
Rationale of Thesis
In peanut-allergic humans, anti-peanut IgE levels can be maintained for an entire lifetime, even in the absence of antigenic stimulation (most peanut-allergic people avoid consuming peanut to prevent life-threatening reactions). Thus, we hypothesized that long-lived plasma cells were responsible for maintaining anti-peanut IgE levels. We determined the role of B cells in establishing murine peanut allergy through adoptive transfer studies and also studied the role of B cells in maintaining long-lived serum anti-peanut IgE levels through depletion studies, using anti-CD20 antibody to deplete memory B cells and bortezomib to deplete plasma cells.
CHAPTER II
MATERIALS AND METHODS
Crude Peanut Extract and the 20kD Fraction Containing Ara h 2 and Ara h 6 Whole, raw peanuts were ground manually with a mortar and pestle in liquid nitrogen and were defatted for 6 hours in diethyl ether using a Soxhlet extractor. After drying, defatted flour was resuspended 1:5 (weight/volume) in 4oC Tris-buffered saline
(150 mM sodium chloride, 50 mM Tris, pH 7.4) overnight with ethylenediaminetetraacetic
acid, acid-free protease inhibitors (Roche, one tablet added per 50 mL). This CPE was
centrifuged to remove insoluble material, dialyzed in phosphate buffered saline (PBS), sterile filtered with a 0.22 µm filter, and frozen in aliquots at -80oC. Prior to freezing, the
concentration of CPE was determined using BSA as the standard per the Pierce BCA Kit (Pierce). To isolate the potent Ara h 2 and Ara h 6 proteins devoid of lectins, aliquots of CPE were thawed, spun at 10,000 X g for 10 minutes at 4°C, filtered with a 0.22 µm filter, and chromatographed on a HiLoad 26/60 Superdex 75 Prep-Grade column (GE Healthcare) at 2 ml per minute. A fraction of CPE, known as the 20 kD fraction, containing predominantly (~97%) the 2S albumins Ara h 2 and Ara h 6 without evidence of peanut lectins, was eluted at 15-25 kD (28).
Murine Model of Peanut Allergy
Female, C3H/HeJ mice from Jackson Laboratories were housed in specific pathogen-free conditions and were maintained on a peanut- and soy-free diet immediately after weaning (three weeks of age). Procedures conducted with mice were in accordance with and approved by the University of Colorado Denver Institutional Animal Care and Use Committee. Mice were sensitized starting at five weeks of age with 1 mg of CPE plus
20 µg of cholera toxin (List Biological Laboratories, Inc.) given intragastrically by oral gavage once per week for four weeks. Sensitized mice were challenged at 11 weeks of age (two weeks after the last dose was given for sensitization) with 250-350 µg of CPE through
ip injection. Any mice not reacting upon the first challenge were challenged a second time
at 12 weeks of age. Prior to ip injection with CPE, body temperatures were recorded using a RET-3 temperature probe (Physitemp Instruments, Inc.). Thirty minutes post-injection, mice were graded for symptoms and body temperatures were again measured. Mice were scored for symptoms as follows: 0-no symptoms; 1-increased scratching/rubbing of snout, ear-canal digging; 2-puffiness around the eyes, diarrhea, pilar erecti, decreased activity, increased respiratory rate; 3-severe difficulty in breathing, severe decrease in activity; 4-no response to prodding, tremor and convulsion; 5-death (119).
Adoptive Transfer Experiments
All naïve recipients (8 weeks of age) were maintained on a peanut- and soy-free diet starting at three weeks of age and were placed on antibiotic water (enrofloxacin, 0.09 mg/mL) two days prior to lethal irradiation. A split dose of 8 gray (X-ray source, with a four hour wait in-between doses) was administered to recipients one day prior to reconstitution with cells. Cells were delivered through retro-orbital injection in recipients under isoflurane anesthesia. Each mouse received 6x106 cells from the BM of naïve donors
(most experiments) or from the BM of peanut-allergic donors (select experiments). Peanut-allergic donors received their last dose of CPE during challenge 8 weeks prior to sacrifice for cell isolation. Donor BM was not lysed of red blood cells (RBCs). In groups given unfractionated (intact) splenocytes (SPL), RBCs were lysed (ACK Lysing Buffer, Quality Biological, Inc.). Pan B cells (B220+) and CD4+ cells were negatively selected from SPL