Gas Emission from Landfills
An overview of issues and research needs
Survey
Catherine Fischer,
ECONS SA, Environmental engineering
Christian Maurice, Luleå University of Technology
Division of Landfill Science & Technology
Anders Lagerkvist, Luleå University of Technology
Division of Landfill Science & Technology
October 1999
AFR-REPORT 264 AFN, Naturvårdsverket
Swedish Environmental Protection Agency 106 48 Stockholm, Sweden
ISSN 1102-6944 ISRN AFR-R-264-SE Stockholm 1999
1. Introduction and Summary...1
2. Landfill and waste types ...3
2.1 Conclusions ...7
3. Compounds emitted by landfills into the air...8
3.1 Carbon dioxide (CO2) ...9
3.2 Methane (CH4) ...10
3.3 Hydrogen (H2)...11
3.4 Non methane organic compounds (NMOC's) ...12
3.5 Hydrogen sulphide (H2S) and organosulphur compounds ...16
3.6 Ammonia and nitrogen compounds ...17
3.7 Carbon monoxide (CO) ...18
3.8 Mercury (Hg) ...19
3.9 Silicon (Si) compounds...20
3.10 Particulate emissions and aerosols ...20
3.11 Conclusions ...21
4. Landfill processes and gas emissions ...25
4.1 Gas balance and processes governing emissions...25
4.1.1 LFG generation... 26
4.1.2 Gas abstraction ... 28
4.1.3 Emissions ... 29
4.2 Methods for measuring gas emissions ...30
4.2.1 Chambers techniques ... 31
4.2.2 Subsurface vertical concentration gradients... 32
4.2.3 Tracer techniques and FTIR spectroscopy... 34
4.2.4 Isotopic fractionation... 36
4.2.5 Remote LFG emission measurements... 36
4.3 Modelling emissions ...37
4.3.1 Methane ... 37
4.3.2 Carbon dioxide ... 40
4.3.3 Non methane organic compounds... 41
4.4 Conclusions ...42
4.4.1 Landfill processes and gas generation... 42
4.4.2 Gas abstraction ... 43
4.4.3 Emission measurements: present and future needs ... 44
4.4.4 Optimising emission control... 45
4.4.5 Improving national estimates of methane emissions from landfills... 45
5. Concluding remarks and recommendations - towards sustainable waste disposal ...47
1. Introduction and Summary
This report presents a brief overview of issues related to the management of solid wastes at landfills and the resulting impact on emissions to the air. It does not seek to give an extensive review of the field, but only to outline important issues and to indicate relevant research areas.
The discussion has been divided along three themes:
• the landfills and waste types,
• the (known) emissions,
• gas formation and emissions.
For each of the above topics conclusions and recommendations are presented at the end of the individual chapters.
The last chapter presents a synthesis of the previous chapters and also includes recommendations about what measures should be taken. In summary, the authors claim that:
• Gaseous emissions from waste management (including landfilling) are environmentally significant on a global scale today and will acquire more importance in the future.
• Gaseous emissions are also important for the working environment and for people living near landfills and other waste treatment facilities.
• The main problems associated with gas emissions from wastes are: - human health hazards,
- contribution to greenhouse effect, - odour problems,
- explosion and fire hazards.
• Recent trends in changes of material flows to landfills increase the complexity of the issue and hence the need for research.
• The areas where further R & D is needed include:
- characterisation of materials and their potential emissions,
- emission control (both active gas abstraction and passive treatments), - emission prediction and evaluation tools,
- impacts of emissions on the working environment.
• In addition to research funding, there are also other measures which could contribute in a positive way to the development of knowledge and the technical level of operations within the field, such as:
- supporting networking through meetings and travel grants,
- refining and making more accessible the statistics on landfilling and waste treatment in general,
2. Landfill and waste types
There are about 300 active landfills receiving more than 50 tons of waste per year in Sweden (excluding some landfills owned by the industry). In 1994, a total of 6 Mt were landfilled (Table 2.1). The amounts of wastes landfilled have been declining somewhat over the last decade and seem to have stabilised now. A further decrease may be expected after the
introduction of the landfill tax in the year 2000, but an increase in conjunction with increased construction activity cannot be excluded either.
Table 2.1 Total amount (kt yr-1) of landfilled waste in 1994 (Modified after RVF 1996 and http:www.rvf.se)
Waste category 1994 1995 1996 1997
Household and similar 1380 1200 1110 1150
Yard and garden 80 60 70 50
Construction and demolition 900 950 885 700
Energy extraction 660 680 700 675
Municipal sewage treatment 610 540 470 455
Industrial sewage treatment 190 190 205 210
Industrial, general 1060 1120 1050 970
Industrial, sector specific 490 470 435 405
Mineral recycling industry 10 1 2
Special 90 120 130 133
Other 620
The main generic waste categories entering municipal landfills which may be expected to cause gas emissions are household wastes, ashes, construction wastes and sludges. Of these wastes, the household (and similar commercial) wastes are expected to generate the highest amounts of landfill gas, strongly dominated by the main components, methane and carbon dioxide. The other gas-generating waste categories could possibly release a higher proportion of various trace components. It should also not be forgotten that ashes and other alkaline wastes will absorb atmospheric carbon dioxide, and thus serve as a sink. Similarly the
methane emissions result in the establishment of active methane oxidising microorganisms in landfill covers, which may then also serve as a sink for atmospheric methane (Bogner et al., 1997b).
An increasing activity at landfills is the intermediate storage and processing of recyclable materials and composting of yard and garden wastes. About a million tonnes of recyclable materials were placed in intermediate storage at municipal landfills in 1997 and more than 230 kt of wastes were composted at 118 landfills in 1997 (http://www.rvf.se). Emissions to air from the intermediate storage, sorting and composting activities are likely but little
documented. The main emissions that could be anticipated to emanate from these practices are carbon dioxide, methane, hydrogen sulphide and nitrous compounds, but some wastes may also emit more hazardous organic compounds.
In recent years a "producer responsibility" principle has been enacted for packages and some other materials. At the moment whether to also include electronic wastes in this category is still under consideration. The management of these materials are organised by so-called "material companies" who purchase services from various entrepreneurs for the actual
collection and handling of the materials, some of which is done at landfills. It is likely that the management of these recyclables will generate new opportunities for emissions to the
atmosphere, but there is no data available to support or denounce this assumption.
A number of sector specific industrial wastes may cause gas emissions. One known major source of emissions are different organic wastes, e.g. from the paper industry. These waste are often in the form of sludges or relatively finely graded materials which are not easily degassed using conventional LFG systems. There are also degradable organic compounds in some other industrial wastes such as ashes and various filter wastes from air cleaning processes. Some industrial wastes also give off gas by chemical reactions upon wetting, for example lime and coke dust, carbide wastes, silica iron slag, some filter dusts, and spent potlining from
aluminium production (Bergman, 1996). A large and increasing part of the material being landfilled is waste from various recycling operations. Some of it is deposited in municipal landfills and some in special landfills, for example car-scrapping wastes. The potential for atmospheric emissions from these wastes varies with the materials processed and should be assessed individually for each site. No comprehensive data is available today.
Landfill gas (LFG) was abstracted at 59 of the active landfills in 1997. At 34 of these landfills special cells were used for biodegradable materials, in order to improve the collection
efficiency. A total of about 437 GWh of energy from LFG was used from the 59 active and some closed landfills equipped with gas abstraction systems. In addition LFG corresponding to about 9 GWh of heat was flared (http:www.rvf.se). Using a conversion factor of 10 kWh m
-3
of methane gas, the total amount of energy released would correspond to an abstraction of about 45 Mm3 yr-1 of methane and a similar amount of carbon dioxide. In effect, a greater amount is probably collected since all use and flaring might not be accounted for, e.g. internal use. The gas is in any case mainly abstracted from landfilled household waste, from which it
has been estimated that about 100-150 Mm3 of methane is formed per year (SNV, 1993, 1994). This would imply an abstraction efficiency of 30-50% of the potential, which seems to be a bit on the optimistic side, in view of the low average specific yield of the existing plants. In 1995, the average yield of Nordic LFG plants was less than 4 m3 of LFG per tonne of waste in place and year, which is about 20-25% of what was abstracted at Swedish test cells
(Lagerkvist, 1997; Lagerkvist, 1997). All of the numbers cited are of course approximations. It is in particular difficult to assess the quality of the emission potential estimates published by the Swedish Environmental Protection Agency (SNV), since no method description and base data are given.
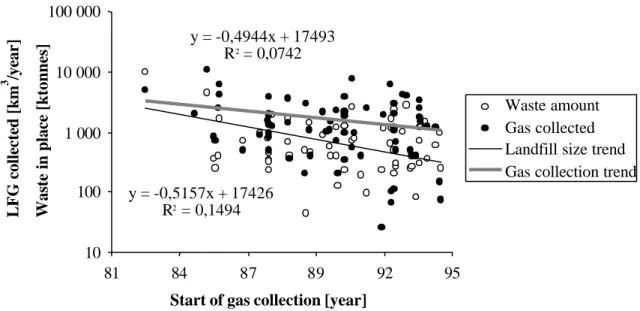
The landfills vary much in size and in technical level. The 25 largest municipal landfills receive about half the total amount of waste whereas the 175 smallest only receive about 10 percent (http:www.rvf.se). Since all of the major landfills already have gas collection systems installed, the average size of additional landfills where gas is abstracted tends to decline with each passing year, as illustrated by Figure 2.1. One could imagine that it would be more difficult to design and operate smaller landfills for minimal emissions. Based on the assumed dependency between the landfill size and gas collection efficiency, one could expect a still lower gas collection efficiency in LFG plants established in recent years. However the correlation between size and efficiency is very weak, as illustrated in Figure 2.2.
y = -0,5157x + 17426 R2 = 0,1494 y = -0,4944x + 17493 R2 = 0,0742 10 100 1 000 10 000 100 000 81 84 87 89 92 95
Start of gas collection [year]
Waste in place [ ktonnes] LFG collected [km 3 /year] Waste amount Gas collected Landfill size trend Gas collection trend
Figure 2.1 LFG plants established before 1995 where gas was used in 1994. Amount of waste being degassed and volume of LFG collected (from Lagerkvist 1997).
y = 0,7571x + 1036,4 R2 = 0,3085 0 2000 4000 6000 8000 10000 12000 0 2 000 4 000 6 000 8 000 10 000 12 000
Waste in place [ktonnes]
LFG collected [km
3 /year]
Figure 2.2 LFG plants established before 1995 where gas was used in 1994. Volume of LFG collected as a function of the amount of waste being degassed (from Lagerkvist 1997).
This indicates that fairly small plants can have a high efficiency and thus that the scope for development and improvement of the gas collection technology is substantial.
In view of the present trends and regulations (for example the new European Landfill Directive) one can predict a further decrease in the landfilling of untreated household waste and similar biodegradable wastes which will eventually result in a less gas being generated from such wastes. One must however remember the present potential, and substantial gas emissions are to be expected from existing landfills also 20 years into the future. Since investment decisions are based on expectations, there is a risk that some LFG collection systems that could have a fairly good collection efficiency will never be built. There is also a risk that the technical advances made over the last decades with regard to biocells and gas collection technology may remain under-exploited.
One can also predict that an increased landfilling of mechanical-biological pre-treated wastes and recycling wastes will take place. The amounts of materials which are handled in treatment and recycling operations and kept in intermediate storage for these purposes is also likely to increase, as are the possible emissions from these materials. The impact of this cannot be evaluated on the basis of the data available today. There are good grounds to improve the information about these wastes, since on the one hand a number of potentially very hazardous compounds may be emitted from some wastes, and on the other there is a great exposure and human health risk, in addition to the potential environmental impact.
Another class of landfills which may come into existence in the near future is the in situ stabilised ones. In order to stabilise landfilled waste, and contaminated soils, in situ aeration is employed today and this practice has been shown to have a reduce the potential of both gas and leachate emissions. In comparison to landfill mining combined with mechanical-biological or thermal treatment, the method is cost effective (Heyer & Stegmann, 1999) and may be expected to find increased application over the next decades. The treatment has in itself of course an emission potential, which will differ both qualitatively and quantitatively from that of an anaerobic landfill. There will also be some residual emissions generated after treatment. Since the method has been only sparingly documented at the moment, one cannot rule out the possibility that hazardous compounds may be generated/released, when specific types of wastes/contaminants are treated in this manner, so there is a need for further evaluation and testing of protocols.
Other major waste fractions, such as ashes and sludges, will probably remain much the same, while construction and demolition wastes will fluctuate with the economic situation as before. For all these wastes there is a lack of basic process data from landfills, and of a basis for emission estimates. For industrial waste landfills there is a need for evaluations on a case by case basis.
2.1 Conclusions
Landfills are an important element of waste management, both today and in the future. The complexity of landfills is increasing and there are many aspects which may affect emissions to air which are poorly documented today. A number of "new" waste types and waste processing or storing practices need to be characterised. much could be gained through a better implementation of the existing knowledge on gas generation and abstraction. There is also a need to develop better practices with regard to operation and emission control, in particular to better adapt the techniques to different waste materials.
3. Compounds emitted by landfills into the air
These emissions constitute one of the major environmental concerns regarding landfills. Gaseous compounds are produced following biochemical reactions, such as the methane and carbon dioxide generated in MSW landfills from the anaerobic degradation of the organic fraction of the waste, or they may be released by direct volatilization from sources already present in the waste. Particulates, in particular dust, and aerosols are other significant atmospheric emissions from landfills.
The gaseous compounds emitted from landfills have various impacts on their surroundings and act on different scales, as illustrated by Figure 3.1. In addition to having impacts over a large spatial scale, gaseous emissions also act on different time scales. Compared to most other processes used in waste treatment, those occurring inside the landfill and the emissions they generate extend over a very long period of time after the waste has been disposed: from tens to hundreds of years. Not only is the period of significant emissions long, but the
compounds emitted will themselves have effects and life-spans of varying duration. Odours and dust, for example, are mainly transient phenomena, while some of the anthropogenic trace compounds in LFG may persist and accumulate in organisms or natural ecosystems over very long periods of time. Methane constitutes both a very short term and acute explosion hazard and has a much more far-reaching and long-term effect on global warming. In the following paragraphs, we will review some of these compounds, their origins, characteristics, impacts and relevance. Distance (km) 1 10 10 000 Landfill Damage to vegetation Fire, explosion Health Odours Greenhouse effect Odours, dust After Kjeldsen, 1996
Local Regional Global
Groundwater pollution
3.1 Carbon dioxide (CO
2)
Carbon dioxide is the main gaseous form of carbon. It arises as one of the waste products of the biodegradation of organic compounds, both aerobically and anaerobically. Alongside methane, carbon dioxide is one of the two principle components of the gas generated in MSW landfills, where it typically makes up between 20 and 50% of the gas.
Waste from energy abstraction from MSW (MSW incinerator residues) contain between 2 and 4 weight-% of organic carbon for bottom ash and 1 wt% for air pollution control (APC) residues. Microbial processes may therefore occur and degrade the organic compounds either aerobically or anaerobically, with a resulting production of CO2. Heyer and Stegmann (1997)
measured a small release of CO2 in simulated deposits of bottom ash, while Belevi and
coworkers (1993) observed microbial degradation of the organic carbon of incinerator
residues during incubation experiments. CO2 may also evolve from various kinds of industrial
wastes.
Atmospheric CO2 is a limiting factor in photosynthesis and is essential to plants. However,
when present at high concentrations in soils, it can result in asphyxia due to oxygen
displacement or even be directly toxic to plants. The normal CO2 concentration in soils varies
between 0.04 and 2%. An elevated concentration of CO2 in a soil, a situation typical of
landfill cover soils, is directly toxic to the plant roots, even if there is enough oxygen available. An indirect effect of carbon dioxide could be a lowering of the soil pH and the consequent changes in soil composition. Normal development can occur till 5%, though tolerance may vary between plant species (Maurice, 1998).
CO2 is classified as an intermediary between toxic and non toxic substances. It acts by
displacing oxygen in the respiratory system. Its ambient concentration lies around 250 - 350 ppm. The Threshold Limit Value (Nivågränsvärde - NGV) for CO2 is 0.5%, with a Short
Term Exposure Limit of 1%. At 3%, breathing becomes laboured and headache or drowsiness develop. Above 5%, it represents an acute danger for life.
Carbon dioxide must therefore be considered a serious safety threat in landfill environments. Several deaths due to CO2 asphyxia have occurred on or near landfills in recent years, in
underground or closed environments such as drains or culverts, where landfill gas had accumulated. In LFG treatises this hazard is generally treated in far less detail than the explosion and fire hazards due to methane (see e.g. Christensen, et al. eds, 1996; Gendebien et al., 1992). It is interesting to note however, that a ten times higher dilution is required to lower the CO2 concentration in pure biogas to below the NGV (0.5%), than is required so as
not to exceed the lower explosivity limit (LEL) for methane (5%). Requirements for buildings situated on our near landfill sites often do not include monitoring of CO2 levels in the critical
areas, but only of methane. It is however a well-known fact that methane can be rapidly oxidised to CO2 in soils. Absence of monitoring for CO2 will result in the LFG migration
going undetected, though it may still constitute a hazard (Damiani & Gandolla, 1992; Gandolla et al., 1997).
3.2 Methane (CH
4)
Methane is the second main component of MSW landfill gas generated during the anaerobic degradation of the waste, where its concentration typically ranges between 30 and 60% of the gas under stable methanogenic conditions. Methane emissions from MSW landfills last for decades. They can be reduced by so-called mechanical-biological pretreatment of MSW which reduces the organic content of the waste before it is landfilled. Conversely, the start of methanogenesis and thus methane emissions may be retarded due to excessive accumulation of acids, insufficient or excessive moisture. It is also possible to speed up the initiation of the methane generation stage and increase the methane production rate by various operational measures, which help avoid the initial accumulation of acids or improve the water balance and distribution. In this way the duration of emissions may be reduced, but the total amount of emissions is not thought to be affected (Lagerkvist et al., 1999).
Methane may also result from the anaerobic degradation of the organic carbon fraction in slag and ash or industrial wastes, such as sludges from the paper industry.
Methane is an important greenhouse gas. Despite its relatively low atmospheric concentration compared to carbon dioxide (currently 1.75 ppm as opposed to 358 ppm), the higher infrared absorption potential of methane makes its global warming potential some 27 times higher than that of CO2 (mole for mole). The present global methane emissions are estimated at
about 550 Tg yr-1 and the net annual increase at 40 Tg yr-1 (Dlugokencky & Masarie, 1998). The total yearly methane emissions seem to have remained relatively constant since 1984, though the distribution of emissions may be changing and the emission rates from individual sources are not well enough defined to quantify trends in them, since the sources, both natural and anthropogenic, are all diffuse and thus difficult to quantify precisely (Conrad, 1996). Wetlands are the main natural source of methane, accounting for some 20% of the total emissions, about 110 ± 26 Tg yr-1 (Boeckx & Van Cleemput, 1996). In total, human activities presently account world-wide for some 70% of the emissions, with landfills as the third most important anthropogenic source. Various estimates have put the contribution of landfill CH4
emissions at about 30-70 Tg of CH4 yr-1, an amount comparable to the current imbalance
between sources and sinks (Boeckx & Van Cleemput, 1996).
Methane is generally not considered toxic to plants or other organisms. The major effect of methane in soils is believed to be due to methane oxidation which depletes the oxygen
present, increases the carbon dioxide levels and may also raise the soil temperature. This may lead to plant death by enhanced asphyxiation due to a lower solubility of gases inside the plant cells or by drying up the soil.
The main hazard of methane for people and property are due to its flammable and explosive properties. The flammability range for methane in air at atmospheric pressure and ambient temperature is 5 to 15% and the safety concentration limit for confined environments where people live or work is 1% in air (20% of the Lower Explosivity Limit). Flammable mixtures will be explosive when contained in a small volume from which the products of combustion cannot readily escape. The potential of methane for flammability or explosion will also be influenced by the other components in the mixture. Thus no flammable mixtures exist when the diluent is carbon dioxide or nitrogen and the oxygen level falls below 12.8%. The
flammability range is lower than the normal concentrations of methane in pure landfill gas. However, during migration, landfill gas will mix with air and become depleted in methane, through dilution and/or oxidation, and therefore fall within the flammability range. This is typically the case when methane migrates into a building through cracks in the foundations or through service mains. Dozens of reports of accidents involving the explosion or ignition of methane from landfill gas have been reported in recent years (Christensen, et al. eds, 1996; Gendebien et al., 1992).
3.3 Hydrogen (H
2)
Hydrogen is a non poisonous, odourless and colourless, but highly inflammable gas. In MSW landfills, it is produced both by the fermentative and the acetogenic bacteria and consumed by the methanogens. As this last group of microorganisms are the slowest to develop in the metabolic succession of anaerobic degradation, molecular hydrogen may accumulate during the initial stages of waste degradation and be transiently present at high levels (up to 20%) in the gas phase of young landfills, well above its Lower Explosivity Limit of 4%.
Hydrogen is also produced in ash and slag deposits: elemental aluminium, which is the fifth most abundant element in bottom ash, reacts with water to form aluminium hydroxyde and gaseous hydrogen (Chandler et al., 1997). Other metallic components with valence states of zero, such as zinc or copper, could also promote similar redox reactions resulting in hydrogen evolution. Heyer and Stegmann (1997) observed hydrogen evolution in simulated bottom ash deposits, with a maximum of 15%-vol right after deposition, followed by a steady decrease to zero over the next 40 days. No quantitative data on hydrogen evolution is however available. A variety of industrial wastes may have a potential of hydrogen formation on wetting.
No reports exist of accidents involving hydrogen on landfills. It is relatively easily oxidized in soils by micro-organisms and this has been documented experimentally in landfill top covers (Nozhevnikova et al., 1993).
3.4 Non methane organic compounds (NMOC's)
The gas from MSW landfills usually contains a variety of trace organics which may make up to 1%vol of the gas. The many published research studies have generally detected between 100 and 200 different compounds at each single site investigated (see Gendebien et al., 1992, for a review). These trace components are either the sub-products of the biological and chemical processes occurring in the waste, and consist for example of oxidised carbon compounds: alcohols, ketones, esters, organic acids, furans and sulphur compounds. Or they may be of anthropogenic origin (such as halogenated hydrocarbons) and have been deposited together with the wastes. They will undergo various processes in the waste body and cover soil of the landfill, such as volatilisation, adsorption or biodegradation, which will govern their fate inside the landfill and their release into the atmosphere.
The concentrations of these compounds are extremely variable from one site to another and it is difficult to give typical values for specific landfill types. The nature of the wastes deposited and the rates and mechanisms of degradation occurring will have a decisive influence on the types of compounds and concentrations detected, as will the atmospheric conditions and the sampling and analytical procedures used. It is also often unclear whether the samples analyzed were recovered from deep in the landfill or at the effective emission sites on the landfill surface.
The distribution of these compounds between the solid, liquid and gas phases in the landfill may also vary widely from one compound to another. Their presence in the gas phase will depend on their polarity: more polar compounds will tend to solubilize in the leachate, while the more apolar ones will be found in a higher proportion in the gas phase. However, most NMOC's are sorbed for more than 98% to the solid phase, from which they will only be removed very slowly. Exceptions are vinyl chloride and some CFC's that one can expect to be 90% removed in less than five years (if one excludes any de novo synthesis from degradation of higher chlorination compounds).
The majority of the organic carbon in bottom ash is not characterised at this point in time, but it is thought to be mainly composed of unburned municipal solid waste (Chandler et al., 1997). Compared to the information available on the inorganic characteristics of bottom ash, the information on trace organics is very limited. Trace organics identified in bottom ash and which might be of potential concern for human health include polychlorinated dibenzo-p-dioxins (PCDD's), polychlorinated dibenzo-p-furans (PCDF's) and polyaromatic
hydrocarbons (PAH's). The formation and partitioning of these compounds during incineration will greatly depend on the concentration of compounds or precursors in the waste, the type of incinerator and operating conditions. Improved process technology and control has resulted in the last years in a decrease of the presence of these compounds both in the flue gas and in the solid residues of incineration. Dioxins, furans and PAH's are semi-volatile organic compounds, characterized by a boiling point above 100°C, so their
subsequent atmospheric emission from ash and slag deposits will in all probability be very slow. The heating induced by exothermic reactions occurring in such deposits, such as the
formation of metal hydroxydes (see paragraph 0), may however increase the atmospheric emissions of these compounds. No or only very little data is available.
Trace compounds generated during refuse degradation are generally themselves easily
biodegradable and are associated with high degradation activities, i.e. younger landfills. They do not generally constitute a health or environmental hazard. Along with the sulphur
compounds (see Table 3.1 and paragraph 3.5) they may however contribute to the foul smell of the gas.
Aromatic hydrocarbons, owing to their widespread utilisation, are widely detected in landfill gas (see Table 3.1). Compared to the emissions from other sources, such as traffic or chemical industries, emissions of aromatic hydrocarbons from landfills are certainly negligible with regard to their environmental effect. Nevertheless, some of these components, and in particular benzene, a proven carcinogen, may have adverse effects on landfill workers and should therefore be monitored. Biochemical degradation of aromatic hydrocarbons is generally very limited under methanogenic conditions, so they will mostly persist in the landfill.
Chlorinated hydrocarbons and other halogenated hydrocarbons will occur in MSW landfill gas due to contamination of household waste with solvents, propellants from spray cans and cleansing agents (see Table 3.1). They may also originate from wastes with a high plastic content, such as car-scrapping residues, which are known to increase the levels of adsorbable organically bound halogens (AOX's) in the landfill gas and leachate. The toxicity of the
majority of these substances is rather low, but their persistence in the environment is generally very long, so their concentration in LFG has been extensively investigated (see Rettenberger & Stegmann, 1996, for a review). Biochemical degradation of halogenated hydrocarbons generates readily volatile non-polar substances, which will appear in the landfill gas. In some cases, it is the product of degradation of the original contaminants which present the greatest toxic risk. This is in particular the case for tricholoroethylene and the carcinogenic vinyl chloride, which result from the anaerobic biodegradation of tetrachloroethylene.
Contamination of landfill gas with halogenated hydrocarbons may also cause corrosion problems in gas motors, due to acid formation upon combustion of the gas.
Table 3.1 shows that several anthropogenic NMOC's have been detected in landfill gas at concentrations exceeding the exposure limits. It should be noted however that most of the values cited for these compounds correspond to undiluted biogas, while the actual exposure levels are probably lower. Very few studies on the impacts of these compounds on the health of landfill workers have been carried out to date (see Loth, 1998 for a review).
Toxic or environmentally significant NMOC's are also emitted during landfill fires. A Finnish study (Ruokojärvi et al., 1997) showed that between 1990 and 1992, some 380 fires occurred annually at Finnish landfill sites, for a total of some 630 landfills. In the course of this study
two landfill fires, one experimental and one spontaneous, were also monitored and air samples collected. Unsurprisingly, PCDD and PCDF concentrations exceeded by some 4 orders of magnitude the limit value of 0.1 ng(I-TE) m-3 for emissions from waste incineration plants (see Table 3.1). It also appeared that the maximum acceptable daily intake values for these compounds would be exceeded for the fire-fighters and landfill staff, if pressurised breathing equipment was not used. The PCB levels measured during the two fires which were
monitored did not exceed the NGV levels, and were related to the type of waste, since the fire involving industrial waste showed the highest levels of PCB's (Table 3.1). PAH's were also measured in the air near the fire (Table 3.1). A study conducted on Swedish landfills showed that the amounts of dioxins emitted annually from landfill fires might exceed by 3 to 4 times the amount emitted by the existing Swedish waste incineration plants (Pettersson et al., 1996).
Some reports exist on emissions of PCDD's, PCDF´s and PAH's after combustion of LFG in flares, muffle furnaces or engines. PAH's result from incomplete combustion and are therefore formed primarily in case of operational problems. For dioxins and furans, de novo synthesis could also occur in zones of lower temperature of the combustion systems (> 250°C), but there is as yet little evidence to support this.
Little is known about the mitigation of LFG trace compounds in landfill cover soils. Methane oxidizing bacteria are known to be able to co-metabolize halogenated hydrocarbons, in particular tricholoroethylene (Hanson & Hanson, 1996). Preliminary field measurements on US and Danish landfills (Bogner et al., 1997, Kjeldsen et al., 1996) indicate that
biodegradation or adsorption do occur in the cover soil and could substantially contribute to the mitigation of such emissions. Some of the trace components of LFG could also have an effect on the vegetation of the cover soil. Ethylene in particular, which has been detected in LFG at concentrations up to 180 ppm, interferes with the hormonal system regulating development and may have an adverse effect on plant growth (Maurice, 1998).
Table 3.1. Concentrations detected in MSW landfill gas for some aromatic and chlorinated hydrocarbons and sulphur compounds, and Swedish threshold limit values for exposure. A1, carcinogen, A2, induces mutations (Compiled from various sources: Christensen et al. eds, 1996; Gendebien et al., 1992; Ruokojärvi, et al., 1997; AFS, 1996). Substance Range reported (mg m-3) NGVa (mg m-3) KTVb (mg m-3) Comment Aromatic compounds Benzene 0.03 - 15 1.5 9 A1 Toluene 0.2 - 615 200 400 Ethylbenzene 0.5 - 236 200 450 Xylene 0.2 - 383 200 450 Propylbenzene 1.5 - 173 - -PAH's 24 810 - 1670c - -Chlorinated hydrocarbons Dichlorodifluoromethane (CFC 12) 4 - 700 2 500 4 000 Trichlorofluoromethane (CFC 11) 0.4 - 500 3 000 4 500 Chlorodifluoromethane (CFC 22) 2 - 276 1 800 2 500 Dichloromethane 0 - 684 20 40 A1 Trichloroethylene 0 - 312 50 140 A1 Tetrachloroethylene 0.1 - 250 70 170 A1 Vinyl chloride 0 - 264 2.5 13 A1 Dichloroethylene 0 - 294 4 20 A1 Chloroform 0 - 50 10 25 A1
PCDD's / F's (pg(I-TE) m-3) not detected 51 - 427c 5 pg(TE-nord.) kg-1 100d PCB's (ng m-3) 0.2 11 - 590c 10 000 30 000 A1 Sulphur compounds Hydrogen sulphide 0 - 20 000 14 20 Methyl mercaptan 0.1 - 430 1 ppm -Ethyl mercaptan 0 - 120 1 ppm -Carbon disulphide <0.5 - 22 16 25 A2 a
Nivågränsvärde: threshold limit value per working day
b
Korttidsgränsvärde: threshold limit value (time-weighted average) for short exposure time
c
Baseline concentration in landfill air and concentration during a fire (Ruokojärvi et al., 1997)
d
Limit value for emission from waste incinerators in many European countries
e
NGV for sum of dimethyl disulphide, ethyl mercaptan and methyl mercaptan
The analysis of anthropogenic trace components in LFG gas may also be used for source identification in cases of suspected gas migration, to distinguish landfill-derived gas from other sources. In a study on a landfill gas plume emanating from a U.K. landfill, Ward and his co-workers (1996) detected 79 different volatile organic compounds, similar to those found in other landfills. The concentration profiles of some of the halogenated compounds suggested that they had been flushed out of the landfill during its early stages and persisted in the plume, since their concentrations outside the landfill were higher than the maximum levels detected on the landfill, even exceeding the NGV in the case of vinyl chloride.
3.5 Hydrogen sulphide (H
2S) and organosulphur
compounds
Organosulphur compounds, i.e. mercaptans and carbon or methyl sulphides, are important contributors to the foul smell of MSW landfill gas (see Table 3.1). They most probably arise from the degradation of proteins, which typically form some 6% of food wastes. After an initial peak in concentration just after waste deposition, when the most easily degradable substrates are being consumed, their level in LFG has been shown to decrease rapidly (Gendebien et al., 1992), to levels around 0.1 mg m-3 (3 ppm).
Generally, the levels of sulphur compounds are lower in LFG than in other biogases, below 100 mg m-3, since the latter often contain a higher initial proteic fraction. In MSW landfills, volatile sulphur levels may be further decreased by formation of insoluble metal sulphides. However, if wastes containing even small amounts of organic carbon, or another reductant such as iron, are mixed with sulphate-containing waste, typically gypsum from C & D wastes, sulphide production can become important, in the range of 0.1 to 6 g m-3, even up to 20 g m-3 or 59%vol (Rettenberger, 1996).
Sulphur is abundant in bottom ash and has been found in concentrations ranging from 1 000 to 5 000 mg kg-1 (Chandler et al., 1997). Most of the sulphur is in the form of sulphate. Sulphur, in the form or sulphate and sulphite, is one of the major constituents of APC residues, with concentrations exceeding 10 g kg-1 (Chandler et al., 1997). Since bottom ash and APC residues also contains small amounts of organic carbon, it is not possible to exclude microbial H2S generation from slag and ash deposits.
Hydrogen sulphide is highly toxic and affects the nervous system. It also has a repugnant odour and is highly flammable. Its odour threshold is comprised between 5 and 40 ppm. Above 50 ppm it paralyses the olfactory system, which makes it a particularly pernicious intoxicant. Concentrations above 400 ppm affect the nervous system and above 700 ppm there is risk of death by respiratory failure. The NGV is 14 mg m-3 (10 ppm) and the KTV is 20 mg m-3 (15 ppm).
The most toxic of organosulphur compounds is methyl mercaptan, which affects the central nervous system. The NGV for total organic sulphides (methyl mercaptan, ethyl mercaptan and dimethyl disulphide) is 1 ppm. Concentrations well above this threshold, up to 430 mg m-3, have been measured in landfill gas (Rettenberger & Stegmann, 1996).
H2S and organosulphur compounds may well represent the most critical group of LFG
components encountered to date, due to their toxicity, odour potential and corrosiveness. When in contact with water, H2S gives rise to sulphuric acid, which may corrode gas
utilization facilities. It may also result in high SO2 emissions after LFG combustion. H2S may
be removed from LFG before its utilization by various processes such as oxidative washing, adsorption catalysis, chemical reaction and oxidation or passage through a biofilter or bioscrubber (Rettenberger, 1996).
3.6 Ammonia and nitrogen compounds
Ammonia and organic compounds containing nitrogen mainly methylated amines may also be found in MSW landfill gas. Like the organically bound sulphur they arise from the
(Christensen et al., 1996), while little data is available for organically bound nitrogen. The NVG for ammonia is 18 mg m-3 (25 ppm), and its odour threshold is 1 ppm. Landfill workers could thus be exposed to above-limit levels, though the very low odour threshold of ammonia can be considered a security factor. Ammonia may also be released during leachate treatment, for example during aeration. Ammonia stripping is in effect still widely used as a way of reducing the nitrogen load of the leachate. The amount of ammonia stripped will increase with the pH of the leachate since the NH4+↔ NH3 + H+ equilibrium will be shifted towards NH3
under alkaline conditions (pKa = 9.3), which is the case for leachates from methanogenic
landfills.
Some types of industrial wastes have a potential for higher ammonia emissions. For example, Bergman (1996) observed up to about 20%-vol of ammonia in the headspace of physical landfill simulators with spent potlining from aluminium production. The gas was generated on wetting of the wastes. Wastes generated at elevated temperatures and in contact with air may be expected to contain various volatile nitrogen compounds such as cyanides and ammonia.
Oxidised forms of nitrogen are unwanted products of the combustion of landfill gas and are largely influenced by the combustion technology and by parameters such as temperature, reaction time and oxygen supply. NOx emissions are generated at temperatures above 1 000°C
and reach their maximum at 2 000°C.
Nitrous oxide (N2O) and nitric oxide (NO) may also be produced in soils during the microbial
oxidation of ammonium. N2O emissions in particular are environmentally relevant as it is a
greenhouse gas with a global warming potential 300 times that of carbon dioxide. Of the Swedish contribution to the greenhouse effect, about 9% comes from N2O. Observations on
Swedish landfills (Börjesson & Svensson, 1993) have shown that N2O emissions are
influenced by the methane content of the soil, with concentrations above 5% stimulating N2O
formation. However, the microbiological background of this process is still poorly understood and deserves further study (see also chapter 0).
3.7 Carbon monoxide (CO)
Carbon monoxide has been found in municipal solid waste LFG at concentrations between 0 and 3%vol, with normal levels being around 0.001%vol (Gendebien et al., 1992). If higher concentrations are present, this may be a sign of oxygen-starved burning of the refuse. During an experimental landfill fire in Finland, Ettala et al. (Ettala et al., 1996) measured up to 1600 ppm of CO inside the burning waste mass. The levels were highest during the extinguishing of the fire. CO may also be emitted during combustion of LFG, as a result of incomplete combustion. It is a highly toxic gas which binds to hemoglobin, thus causing respiratory failure and death above 5 000 ppm. Its NGV is 40 mg m-3 (35 ppm), and KTV 120 mg m-3 (120 ppm). Carbon monoxide can be oxidised by bacteria to CO2 and this has been shown to
3.8 Mercury (Hg)
Municipal solid waste has been identified as a potentially significant source of mercury. MSW contains mercury due to the disposal of a variety of products which contain this metal. Mercury is found in varying amounts in batteries, fluorescent and high intensity light bulbs, thermometers, thermostats, light switches, with batteries as the most important contributor. A reduction of the contamination of MSW with mercury essentially depends on upstream measures, such as the reduction of the mercury content of batteries, which is the case in Sweden, and increased separate collection of household hazardous wastes.
During incineration, most of the mercury passes into the gas phase and from there into the APC residues, which concentrate 70 to 80% of the mercury input (Chandler et al., 1997). Swedish incinerator slag contains an average of 0.20 mg kg-1 (Chandler et al., 1997), which is a rather low value, only about a factor 10 higher than the average world-wide soil
concentration.
Once released to the environment, mercury is distributed to the earth's surface including soils, wetlands, lakes, and oceans. It can then undergo chemical transformations, predominantly oxidation-reduction and methylation-demethylation. Biological processes play an important part in these transformations. Methylation, the addition of (-CH3) to ionized mercury has been
documented in water, sediments and soil. It is a microbial process and is in particular affected by dissolved oxygen, sulphur, organic matter or clay, and by the pH. More methylated
mercury is formed in oxygen-limited environments and sulphate-dependant bacteria are thought to be involved in the process. These conditions are characteristic of MSW landfills, so it is probable that methylation may also occur there. Clay particles and organic matter bind the mercury, thus decreasing its bioavailibilty for methylation. It is in its methylated form that mercury bioaccumulates in higher organisms. Methyl mercury is toxic and fairly soluble in water. Dimethyl mercury is much less soluble, so it is less toxic. In anaerobic environments mercury may also be converted into the insoluble mercury sulphide.
Most information available on mercury in landfills concerns the solid and liquid phases. Elemental mercury has however been detected (10-24 ng m-3) in the air above Swedish landfills (Wallin, 1989). The concentrations were positively correlated to the air temperature. These levels, though significant in a wider environmental perspective, are 3 orders of
magnitude lower than the Swedish NGV for mercury (0.03 mg m-3) and seem to indicate that mercury does not constitute an occupational health hazards in landfills.
Other elements may also be methylated, such as for example arsenic and platinum. At present there is no information available about such compounds in landfill gas.
3.9 Silicon (Si) compounds
Siloxanes, or silicones, are commercially produced compounds containing carbon, silicon, oxygen and hydrogen, and must be distinguished from inorganic silicon which contains no carbon, but only SiO2 units. When combusted however, the product will be inorganic,
regardless of its original form and the impact is believed to be the same.
The increasing utilization of landfill gas in reciprocating engines or turbines for energy recovery has sparked awareness of technical problems due to corrosion and fouling from residues generated by the combustion of trace impurities in the gas. The discovery of high levels of silicon in such deposits, combined with the volatility of specific siloxanes, has prompted investigators to name siloxanes from discarded commercial and consumer products as the primary source of these deposits and a major contributor to reduced engine lifetime (Niemann et al., 1997). This has lead several engine manufacturers to add elemental Si limits on their equipment.
The identification of the sources of Si deposits in gas utilization equipment is complicated by several factors. Firstly, a large variety of siloxane compounds exist, due to their polymeric nature. Not only the low molecular weight volatile siloxanes are entrained, but high molecular weight siloxanes have been shown to aerosolize and thus be transported in gas streams. This variety complicates their quantification in the gas stream. Poly (siloxanes) are solvent soluble, but their degradation products, silanols, which known to occur in soils and may well also be present in landfills, are water soluble and may enter the LFG both by direct volatilization or through water entrainement. Secondly, naturally occurring silicon compounds are very common in landfill environments. Though they are exclusively non volatile mineral species, they may be transported with the gas as particulate matter or as solutes in water droplets.
Elemental characterization of combustion deposits in four US landfills and of Si compounds in the LFG gas and gas condensate (Niemann et al., 1997) has shown that engine operational history (i.e. valve maintenance) is not only correlated to siloxane levels in the gas, but may also be influenced by other factors, such as the oil additives and the presence of acidic compounds.
3.10 Particulate emissions and aerosols
The emissions of dust and aerosols on landfill sites is of concern, due to their possible impacts on workers health. Some studies of dust levels on landfills (reviewed in Loth, 1998) showed that atmospheric concentrations above exposure limits may easily be reached, in particular inside the cabins of the compactors or of the loaders handling wastes or cover soil. The increase of waste sorting and pre-treatment activities carried out at landfill sites may also influence these emissions and consequently the exposure of the workers, though almost no data is at present available and this should be the object of further study.
3.11 Conclusions
The diversity of gas-phase compounds emitted by landfills is considerable. The impacts of the quantitatively most important emissions from landfills, methane and carbon dioxide, are well recognised today. Much research concerning these compound focuses on questions related to the global budgets of these two greenhouse gases. These aspects will be further discussed in chapter 0.
Generally speaking, information about their present levels of emission in landfill gas is lacking for most compounds outside methane. Furthermore, quantitative and qualitative predictions of their emissions are rendered difficult by the considerable uncertainties surrounding waste generation, in particular those aspects linked to public awareness and environmentally-friendly behaviour, i.e. the choice of "green-label" products, or the separation of toxic household wastes from the urban waste stream.
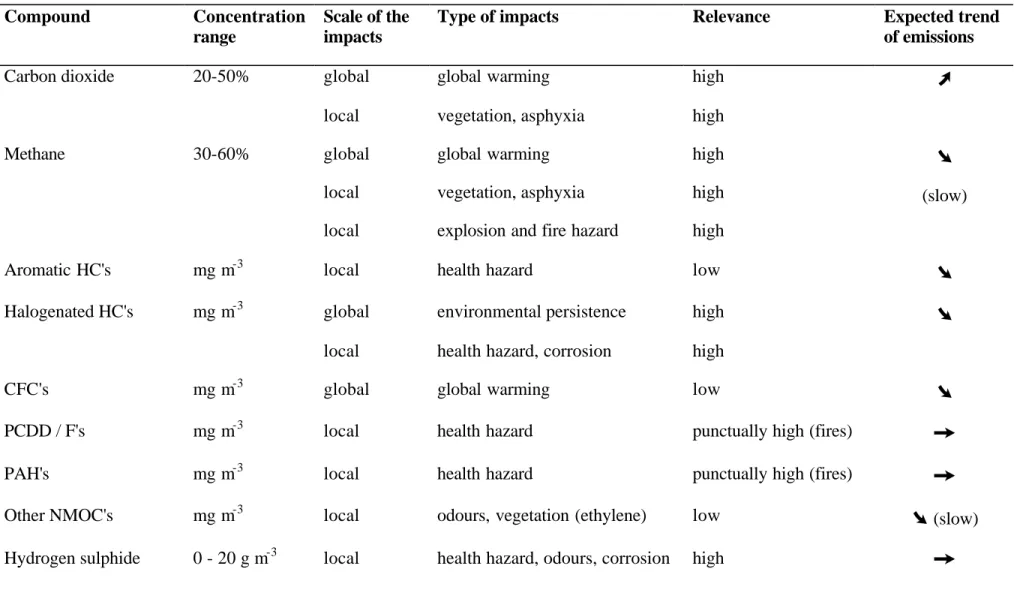
Present trends in legislation are also to incite the producers of the goods to offer more sustainable products, the elimination of which poses less or no threat to the environment. Therefore, in the long term, one should be able to expect a decrease of harmful emissions from landfills. However, many of the substances or compounds in question, in particular the NMOC's, are characterised by their persistence, and will continue to be emitted at low levels long after their elimination from the consumer goods. Table 3.2 summarises the impacts of the compounds discussed in paragraphs 0 to 0 and suggests possible future trends.
Some of these emissions will however simply be displaced towards the waste sorting and pre-treatment stages which are becoming more common. Green-waste pre-treatment facilities and waste sorting plants are known to release large amounts of NMOC's and nitrous and sulphur compounds, which are often malodorous, as well as dust, spores, microorganisms and
aerosols, which may adversely affect the health of the people employed there. As for landfills sites they are increasingly diversifying their waste treatment activities, and serving more and more as "multifunctional platforms", where different waste fractions are sorted, sent to the recycling industry, or pre-processed before landfilling.
These changes in waste management practice have not always, by far, been accompanied by an assessment of the atmospheric emissions and their potential ecological or occupational health impacts. Studies of the health impact of gas-phase emissions from landfills are at present extremely few (Loth, 1998), and mainly concern hazardous waste sites and therefore principally address the exposure to anthropogenic organic compounds or heavy metals. Sulphur compounds, despite their odour potential, high toxicity and the very low threshold limit values allowed for them, have not been studied. Some assessments have been made of the health effects and odour emissions from waste sorting and composting plants. Global comparative occupational health studies of various alternative treatment schemes are however non existent.
Similarly, few assessments have been made of the odours that may be generated by the expansion of such practices. Though their actual hazard potential is very low, foul-smelling emissions are possibly among the most deleterious in terms of image of a waste-treatment plant and their avoidance figures among the operators top priorities. Some assessments should therefore be made of the impacts that the new waste handling practices may have in terms of odours, as well as of measures that may be taken to prevent their development.
The trends that are now apparent in most European countries towards increasing separation of the putrescible fraction of waste and its treatment by composting or anaerobic digestion, as well as towards generalised incineration of waste fractions with high calorific value, will in the long-term, reduce the amounts of methane generated and emitted, but not those of carbon dioxide, which will rather increase, due to the increased use of aerobic processes.
The biochemical processes occurring in future landfills will probably change markedly over the next 20 years. Landfills are expected to receive proportionally more waste fractions which are not biologically active. However, emissions can occur from non biodegradable residues, such as AOX from car-scrapping wastes. Some consideration in particular should be given to the emissions from incinerator residues landfills, about which very little is known at present. The same applies for residues from waste sorting activities, such as for example scrapped tyres. In view of the reduction of the putrescible fraction of wastes going to landfills, while no or little change is expected for C & D wastes, the conditions favouring the emissions of hydrogen sulphide should also be investigated in more detail.
Despite these important changes in the composition of landfilled waste, the amounts of biogas emitted will still continue to be high for many years after the landfilling of large amounts of biodegradable waste has ceased and will in part also simply be transferred to anaerobic digestion plants. Investigations on alternative uses for this combustible gas, that are both ecologically and economically sustainable, especially for small landfills, should be encouraged.
With the economic sustainability of such plants in mind, the trace contaminants inducing corrosion and wearing in the gas utilisation facilities deserve further investigation. This is in particular the case for silicon compounds, for which the analytical characterisation and quantification in landfill gas is still problematic, so little is as yet known of their effective impact. A better knowledge as to their origins and fate, and the development of technical means of eliminating them from the gas are certainly to be hoped for.
Table 3.2. Impacts, relevance and trends of gas-phase emissions from landfills
Compound Concentration range
Scale of the impacts
Type of impacts Relevance Expected trend of emissions
Carbon dioxide 20-50% global
local global warming vegetation, asphyxia high high
Ú
Methane 30-60% global local local global warming vegetation, asphyxia explosion and fire hazardhigh high high
Ø
(slow)
Aromatic HC's mg m-3 local health hazard low
Ø
Halogenated HC's mg m-3 global
local
environmental persistence health hazard, corrosion
high high
Ø
CFC's mg m-3 global global warming low
Ø
PCDD / F's mg m-3 local health hazard punctually high (fires)
Ù
PAH's mg m-3 local health hazard punctually high (fires)
Ù
Other NMOC's mg m-3 local odours, vegetation (ethylene) low
Ø
(slow)Organosulphides mg m-3 local health hazard, odours, corrosion
high low
Ù
Carbon monoxide 0 - 3% local asphyxia, explosion low
Ø
Hydrogen 0 - 20% local explosion low
Ù
Mercury 10 - 24 ng m-3 a global
local
dispersion and bioaccumulation health hazard
unknown unknown
Ø
Si compounds mg m-3 local wearing of equipment unknown
Ú
Ammonia mg m-3 global
local
NOx formation odours, health hazard
low low
Ø
Nitrous oxide mg m-3 global global warming unknown possibly
Ú
Nitric oxide mg m-3 global acid deposition low
Ø
Dust / aerosols mg m-3 local health hazard high for workers possibly
Ú
a
4. Landfill processes and gas emissions
4.1 Gas balance and processes governing emissions
All gases formed within a landfill will eventually leave it, driven either by differential pressure or by concentration gradients. Gas transport processes include both
spontaneous gas exchange over the landfill perimeter and active gas abstraction as indicated in Figure 4.1. In a properly managed landfill, lateral gas migration should be equal to zero, but this is not the case in reality. If it is different from zero, it could also be included in the "emission" term, since in the long run, the gas migrating in the sub-soil will also be emitted to the atmosphere, with or without conversion (i.e. oxidation). Transport by leachate and storage inside the landfill should also be included in this balance and could be of some relevance, especially for gases with a high solubility in water, such as carbon dioxide.
Methane generated = methane abstracted + methane emitted +
methane migrated + methane oxidized + difference in methane storage Carbon dioxide generated = carbon dioxide abstracted + carbon dioxide from methane oxidation +
carbon dioxide from methane combustion + carbon dioxide migrated + carbon dioxide in leachate + difference in carbon dioxide storage
Carbon dioxide and methane emission Carbon dioxide emission Microbial methane oxidation LFG recovery or combustion
Carbon dioxide and methane migration LFG generation
Methane and
carbon dioxide storage
Carbon dioxide in leachate Modified from Bogner & Scott, 1997
Carbon dioxide emission
Figure 4.1 Methane and carbon dioxide balance of a MSW landfill
Due to its importance as a greenhouse gas, methane has been by far the most studied atmospheric emission from landfills. Therefore, most of the methods, findings and open research questions we will be discussing in the following paragraphs concern methane emissions. However in the future, the emphasis may well shift somewhat to other compounds, as changes in waste composition and management practices will result in notable changes in the nature and level of landfill atmospheric emissions in the coming decades.
4.1.1 LFG generation
Gas formation in landfills depends on the wastes landfilled, the environmental conditions at and in the landfill and on landfill technology. LFG composition is the result of degradation processes in the landfill, as well as of evaporation of volatile substances and exchange of gaseous compounds between the landfill and the
atmosphere. The composition of the gas will also in itself affect landfill processes, and thus the formation of gas. Oxygen intrusion, for example, may trigger aerobic
degradation in parts of the landfill, and thus increase CO2 production with respect to
methane, but may also enhance the volatilisation of some compounds due to the elevated temperatures reached by aerobic degradation processes.
When predicting landfill gas formation, the focus is usually put only on biogas formation as a result of the anaerobic degradation of the biodegradable fraction of the waste, neglecting the fraction resulting from other processes. A potential gas generation can be calculated from the composition of the substrate. Empirical data is used or anaerobic degradation tests are carried out. The rate and ultimate yield of LFG is highly variable from site to site. A typical yield may be in the range of 200-300 m3 t-1 of fresh
MSW and the range of methane ge neration may range from below 1 m3 up to about 40
m3 per tonne and year (Lawson et al. 1992).
The models used for predicting LFG generation are based on the waste composition, i.e. its carbon content, degradability and the kinetics of degradation. Thus, other processes, such as volatilisation, are not taken into consideration. The most widely used model in Europe is probably Tabasaran's expression (Tabasaran 1976), a relationship originally deve loped for the anaerobic digestion of sewage:
Ga = Ge (1 – e – ka), (Eq. 3.1)
Ga: accumulated gas generation until year a [Nm3 t-1]
Ge: gas formation potential [Nm3 t-1]
k: degradation constant = ln2/t½ [time unit-1]
a: time [number of time units]
Ge = 1.868 C0 (0.014T + 0.28)
C0: content of degradable carbon in the waste [kg t-1]
T: temperature [°C]
The temperature correction in the Ge determination is derived from the fact that the
portion of substrate which is used for cell synthesis may vary with temperature. Since the "sludge retention time" of landfills is on a completely different scale than that of an anaerobic digester, the correction is irrelevant for landfills.
In the USA, the Scoll Canyon model occupies a similar position to Tabasaran's model in Europe. This is also a first order decay model and it does not have the questionable temperature correction. For the same situation, both models give identical results. The USEPA used the Scoll Canyon model for the LANDGEM program which is published on the net (http://www.epa.gov/oar/oaqps/). This model can also be used to calculate emissions of a number of trace components, but based on fixed concentrations in the LFG, i.e. as a function of biogas generation.
A first order decay is the usual assumption in LFG generation modelling, but zero order models and higher order models have also been used. According to van Oonk et al. (1994), precision will increase with the order of the model. Furthermore, an additional refinement can be applied to models irrespective of their order, in the form of different degradation constants for different waste materials, so-called multi-phase modelling. However, waste composition data grouped according to degradability is then needed to allocate the different time constants. Contrary to van Oonk's results, a validation of gas generation models of zero, first and second order using operational data from 17 american landfills indicated that only small or no improvements could be gained by using higher order models (Augenstein et al. 1996).
If one considers the fact that reaching a similar degree of degradation takes decades in landfills and only days in anaerobic digestors (Bogner & Lagerkvist 1997), it seems likely that other factors than substrate availability may be equally important for gas
generation in landfills. Today, there is an information gap needing to be bridged about the impact of various factors on the degradation of landfilled waste. Although a number of factors are known to have an impact, the knowledge is in part only qualitative. In particular, very few studies have been undertaken where the incoming wastes have been characterised and the landfill conditions monitored with sufficient precision. The data available from model experiments is richer, but its interpretation is not always easy. One problem with model experiments is their temporal limitation. Since there will be a lag time for many biological reactions, some processes may stay undetected during such experiments.
Over the last 30 years, numerous studies aiming towards a better understanding and control of landfill processes have been undertaken. Over the last decade some larger-scale studies have focused on different methods to enhance LFG generation, while maintaining a good control of landfill gas emissions (Ecke & Lagerkvist 1997). Usual methods for improving gas yields include water addition, seeding (with sludge), heating and the addition of pH buffers and nutrients.
The first measure in order to achieve a better control of landfill processes is to control and select the waste materials entering the landfill or landfill part, since no techniques for enhancement of degradation or other control measures, are universally applicable to all waste materials. To allow for the separation of materials with different properties, a landfill needs to be divided into cells. Another good reason for this, is that it permits to reach filling height faster, and therefore also to seal the top faster.
Experiences of cellfills are relatively limited today, but it is already clear that landfilling in cells in itself promotes a better control of the processes occurring in the landfilled waste and above all, a more efficient capture of the gas formed. Simply through the disposal of degradable wastes in specially designed landfill cells, called biofills, the recovery of landfill gas can be increased 2 to 10-fold in comparison to conventional mixed landfills (Lagerkvist 1997). Other advantages of the biofill technique are lowered emissions of greenhouse gases, increased energy efficiency and an easier reclamation of the digestate in the future. The results of the co-ordinated Swedish LFG program (Lagerkvist 1997) indicated that methane emissions could be kept down to less than 10% of the methane formation. Cellfilling also leads to improved routines and better organised landfills, accomp anied by volume savings and reduction of problems due to birds, wind littering, noise and visual disturbances.
4.1.2 Gas abstraction
Gas abstraction systems were originally developed from groundwater abstraction technology. The adaptation of this technology to landfill conditions and for landfill gas abstraction has been the object of much testing by waste companies and engineering consultants, but these investigations have rarely been systematically and properly documented. The know-how exists however, and it may be time for a compilation and
critical evaluation of present practices and opinions. For example, there is a widespread assumption that gas abstraction will affect gas generation, but the mechanisms by which this would occur are not clear. It has already been shown that the abstraction of LFG can be substantially improved by better structured landfills and by increasing the active surface of the gas collectors (Lagerkvist 1997), but there also exists a potential for improving the economy of gas abstraction through better designs and materials, especially with regard to maintenance.
Today gas is abstracted from landfills by distributing a partial vacuum from one or several sources through a grid of collection points. For a deep and large landfill with a thick cover, this is generally a workable solution, but there is a need to improve techniques for sites which are more sensitive to wind and other weather impacts. A better understanding of gas movements inside landfills would be useful for improving the design of collection systems. Instead of drawing on the experience of groundwater abstraction from uniform sediments, it might be possible to use models derived from the study of fractured bedrock for example, which may be more representative of the
situation inside a landfill.
There is also a need to develop the automatic monitoring and control of landfill gas abstraction systems. There are a number of questions touching on the control of gas abstraction which are poorly elucidated today, stretching from which analytes should be used as inputs in a control system, over to which techniques could be employed to handle the mass of analytical data which is generated by such systems and exploit it for real-time data utilisation. Some promising results have been reported from the use of real-time multivariate analysis (Birkeland 1996) and this needs further research and development. Landfill gas monitoring is also coupled to the general problem of landfill information management – an issue which comes repeatedly to the fore, e.g. when a new environmental impact from landfills is discovered or suspected. Gas generation and emissions are in effect correlated to many, if not all, other aspects of landfill planning, operation and control, be it the waste types, amounts and handling, the leachate quality and management, the landfill topography and design. Doubtless much could be
improved in the quality of the collected data and in its successive exploitation, by the development of geographic information systems (GIS) for landfill management, where data from various sources and of different types could be connected and analysed .
4.1.3 Emissions
Emissions vary widely over time, both diurnally and seasonally. Several environmental factors may influence both the gas quality and its flux, e.g. changes in barometric pressure, rainfall and temperature. In addition, during the transport of the gas through the soil cover many processes may occur, which will modify the composition of the gas phase, such as the adsorption and oxidation of organic compounds or their solubilization in the liquid phase.
An important conversion process is the microbial oxidation of methane. Like any process involving microorganisms, methane oxidation is sensitive to factors such as temperature, moisture and nutrient availibility. The soil type and structure has also been shown to influence methane oxidation and certain compounds are known to act as inhibitors. Ammonium and nitrogen fertilisation for example have been found to affect methane uptake, both in landfill and other environments (Conrad, 1996), and their effect has been much investigated. In the case of landfill environments, the trace components of the gas itself may actually also interact with methane oxidation processes and more attention should be given to this aspect, which is specific of landfills. Bogner et al. (1997a) observed that the levels of several NMOC´s were reduced during the transport of landfill gas through the cover soil, though the processes involved, whether microbial of physico-chemical, were not elucidated. Similarly, Kjeldsen et al. (1996) observed degradation of aromatic hydrocarbons and chlorinated aliphatics in landfill soils. Degradation rates were higher in the presence of methane, and, in the case of trichloroethylene, degradation only occurred when methane was present.
Methane oxidation in soils has been studied extensively in the laboratory, using mainly soil incubation experiments (batch tests) or soil microcosms flushed by a continuous gas flow (see Humer, 1997, for a review), but field-scale experiments are still few. In one case, data obtained from laboratory investigations was combined with field
measurements of CH4 emissions, to quantitatively estimate oxidation at a series of
locations on a landfill and then extrapolate the data to the whole landfill (Czepiel et al., 1996). Quantification of methane oxidation at field scale poses special challenges in the landfill setting. One important aspect to consider is the integration between active gas abstraction systems and methane oxidation in the cover soil, in particular the adaptative capacity of microbial population to variations in the methane flow. Landfills
environments have been shown to harbour methane oxidizing populations adapted to different levels of methane and oxygen and to also, in some cases, act as sinks for atmospheric methane (Bogner et al., 1997b). It is of interest that such soils harbour large and adaptable enough populations of microorganisms to take care of eventual emission peaks related to disturbances in the LFG collection system. The dynamics of methane oxidizing populations, as well as the conditions allowing for their maximum efficiency in landfill environments need therefore to be further studied.
Understanding the processes occurring depends first of all on the possibility of adequately measuring the amount and nature of the emissions. As we shall see in the following paragraph this is in itself not a simple problem, due to the complex dynamics and spatial variability of gas generation and movement in landfills.
4.2 Methods for measuring gas emissions
Gases move through the subsurface under the influence of both concentration and pressure gradients, that is both diffusion and convection. Usually one process is