http://www.diva-portal.org
Postprint
This is the accepted version of a paper published in Surface and Interface Analysis. This paper has been peer-reviewed but does not include the final publisher proof-corrections or journal pagination.
Citation for the original published paper (version of record): Eslami, M., Speranza, G., Deflorian, F., Zanella, C. (2020)
Polypyrrole coatings on rheocast aluminum-silicon alloy: A correlation between properties and electrodeposition conditions
Surface and Interface Analysis, 52(1-2): 4-15 https://doi.org/10.1002/sia.6709
Access to the published version may require subscription. N.B. When citing this work, cite the original published paper.
Permanent link to this version:
1
Polypyrrole Coatings on Rheocast
Aluminum-Silicon Alloy: A Correlation Between Properties
and Electrodeposition Conditions
Maryam Eslami *‡, Giorgio Speranza **, Flavio Deflorian * and Caterina Zanella ***
‡ Corresponding author. Email: maryam.eslami@unitn.it
* Department of Industrial Engineering, University of Trento, 38123, Trento, Italy
** Center for Materials and Microsystems, Fondazione Bruno Kessler (FBK), 38123, Trento, Italy
*** Department of Materials and Manufacturing, School of Engineering, Jönköping University, 553 18, Jönköping, Sweden
Abstract
The effect of the electrolyte composition and the deposition potential range on the physical properties and possible corrosion protection effect of polypyrrole coatings on rheocast aluminum- 2.5 % silicon alloy is investigated. Solutions with different concentrations of sodium nitrate and an electron transfer mediator reagent were used for the electrodeposition.
Polypyrrole coating is able to hinder the entrance of electrolyte. Upon the penetration of chloride ions, the coating can induce passivation of the alloy’s surface by its reduction. The thickness of the coating, and its ion-barrier properties, controlled by the electrodeposition conditions, are shown as the important factors influencing the protection efficiency. However, localized drastic galvanic coupling at the polypyrrole/aluminum interface forms blisters, causes failure and limits the possible protection.
Keywords
conductive polymer; polypyrrole; aluminum-silicon alloy; Rheo-HPDC; corrosion protection
1. INTRODUCTION
Polypyrrole is an intrinsically conducting polymer (ICP). It exhibits electroactivity and some level of conductivity 1,2.
The electrochemical interaction of conductive polymer coatings with metallic substrate such as steel or aluminum can be exploited as an effective corrosion protection strategy 1-5. In their
oxidized state, these polymers can act as an oxidizer and in the appropriate conditions promote the passivation of the substrate. This protection mechanism is known as “ennobling/passivation
2
mechanism” 3,6. When reduced, the polymer needs to be reoxidized by an oxidizing agent (e.g
the atmospheric oxygen) to keep the protection activity 2.
David W. DeBerry was the first one to indicate and investigate the potential of a conductive polymer (polyaniline) in the corrosion protection of stainless steel in an acidic solution 7. Since
then, conductive polymers, especially polypyrrole and polyaniline, have been studied for the possible corrosion protection effect on steel 8-13, magnesium 14-17 and aluminum alloys 18-31.
Despite the reported results, the application of these coatings in the presence of large defects and in concentrated sodium chloride solution is still doubtful 3,6. Moreover, the galvanic coupling
of the conductive polymer and metal should be tailored very carefully in order to promote passivation and not accelerated corrosion 32.
The protection of the coating can be improved by modifying the polymer film during the electrochemical polymerization 33. By controlling and altering the doping anions 25,34-43, the film
conductivity and its thickness 2,33, the coating electroactivity can be engineered. Desired fillers
such as titania and graphene oxide can be added to increase the coating barrier properties 44,45.
Doping anions with large volume (e.g. benzene sulfonate, dodecyl sulfate, p-toluene sulfonate) into the conductive polymer film is one approach to increase its ion-barrier properties 35,36,43, while
introducing corrosion inhibitor anions (e.g. molybdate) leads to anti-corrosive coating with inhibition capability. The inhibitor can be released during the reduction of the conductive polymer and migrate to the corroding defect passivating the exposed metal surface 34,39-41.
Electrodeposition of conductive polymers on some metals such as aluminum and magnesium is challenging. Since the concomitant anodic oxidation and dissolution of the substrate during the electrodeposition process can hinder the formation of a homogenous adherent coating. Therefore, care should be taken in selecting the supporting electrolyte and the electrodeposition conditions. The applied potential (or current) needs to be high enough to oxidize the monomer but low enough to limit corrosion of the electrode 5,46. In this case, the application of electron transfer mediators
has been found helpful in reducing the required overpotential 46. The presence of passivating
anions (e.g. nitrate, molybdate, etc.) in the electropolymerization electrolyte has been proven to be beneficial in limiting the dissolution of the aluminum substrate as well 20,47.
Despite the available research on the electrodeposition of polypyrrole coatings on aluminum alloys such as those on AA2024 19,21,25-28,42,48, AA6061 23, AA6082 26,28 and AA7075 44,49, no
research in this field has been carried out on cast aluminum-silicon (Al-Si) alloys.
Binary system of Al-Si is the base for 90% of casting aluminum alloys 50. Their excellent castability,
low melting point, low shrinkage, low coefficient of thermal expansion, proper wear, and good corrosion resistance are of special interest in different applications such as automotive and
3
aerospace components 51. Low silicon content aluminum alloys, produced by new casting
technologies such as Rheo-high pressure die casting (HPDC) with improved thermal conductivity are promising candidates for applications such as heatsink for electronics cooling. Rheo-HPDC technology provides the possibility of producing components with complex geometries (containing thin sections) from alloys with low alloying elements (e.g. Si, Fe, etc.) 52. Reducing the
concentration of these alloying elements results in improved thermal conductivity 53.
Considering the susceptibility of Rheo-HPDC Al-Si alloys to the localized forms of corrosion, especially in sodium chloride solutions 54-56, exploring a new effective and non-toxic coating to
protect them from corrosion (in high concentrated NaCl solution) is valuable.
This paper presents the investigation on the effect of electrolyte composition and the potential range (in cyclic voltammetry (CV) process) on the electropolymerization and the possible protection effect of polypyrrole coatings on Rheo-HPDC Al-Si alloy in 0.6 M NaCl solution.
2. MATERIALS AND METHODS
2.1. Substrate preparation and electropolymerization
Samples of Rheo-HPDC Al-Si alloy, with the composition of (wt.%) Si: 2.41, Fe: 0.46, Cu: 0.13, Mn: 0.02, Mg: 0.58, Zn: 0.04, and Al: balance, were used. The details of the Rheo-HPDC process have been described in previous research papers on the same alloy 56,57.
Substrates (2 cm × 2 cm) were wet-abraded using 1200 grit SiC abrasive paper and cleaned ultrasonically in acetone for 10 minutes. An etching step of 15 seconds in 1 M NaOH solution at room temperature was also applied prior to the electropolymerization.
The chemical reagents in the solutions included the pyrrole monomer (Py), 4,5-dihydroxy-1,3-benzenedisulfonic acid disodium salt (DHBDS), sodium dodecyl sulfate (SDS) and sodium nitrate (NaNO3) with variable concentrations listed in Table 1.
The electrochemical cell was a 100 ml beaker containing the substrate as the working electrode, a graphite rod as the counter electrode and an Ag/AgCl (3 M KCl) reference electrode. The electropolymerization was performed by CV at the potential rages listed in Table. 1, at 10 mV/s scan rate and for 20 cycles. The CV was selected over galvanostatic or potentiostatic methods as it produces more homogenously deposited coatings 58.
The studied variables in this research include the concentration of DHBDS and NaNO3 and the
upper limit of the potential range. In Table. 1, X refers to the coating deposited from the plain solution, while X-Nit refers to the coating deposited from NaNO3-containing solution. Number two
4
The numbers 0.7, 0.8 and 0.9 are added to indicate the upper limit of the potential range during CV.
2.2. Coating characterization
The corrosion protection of the coatings was evaluated in 0.6 M NaCl solution using open circuit potential (OCP) monitoring and electrochemical impedance spectroscopy (EIS) for 24 and 168 h. For the EIS measurements (performed at the OCP), the frequency varied between 100 kHz and 10 mHz, the amplitude of the sinusoidal potential signal was 10 mV (RMS). Equal measurements were performed on the bare alloy for comparison.
The electrochemical measurements were carried out using a three-electrode cell, containing the sample as the working electrode (with the exposed area of 0.8 cm2), Ag/AgCl (3 M KCl) reference
electrode, and a platinum counter electrode. The electrochemical measurements were performed using a computer-controlled potentiostat (Metrohm Autolab PGSTAT302N). The reproducibility of the electrochemical data was checked by repeating each test three times.
Coatings’ surface morphologies before and after the immersion tests were examined using scanning electron microscopy (SEM/JSM-IT300) under high vacuum conditions using 20 kV beam energy. Energy dispersive X-ray spectroscopy (EDXS) was employed to measure the composition of the polymer coatings.
For the thickness measurement, five cross-sectional SEM images were taken of each coating. The measurement was performed on 15 points using a Java-based image processing program. The chemical composition of the coatings was examined by X-ray photoelectron spectroscopy (XPS). This analysis was performed using an Axis DLD Ultra from Kratos-UK and consisted of the acquisition of a wide spectrum at a pass energy of 160 eV. To analyze the core lines of interest, higher energy resolution was obtained at a pass energy of 20 eV resulting in an energy resolution of ~ 0.3 eV.
3. RESULTS AND DISCUSSION
3.1. Electropolymerization and morphology
Fig. 1 (a) depicts the CV curves (the first cycle) applied to the Al-Si substrate in different solutions and at different potential ranges. It presents the effect of the concentration of DHBDS (commercially known as Tiron, the electron transfer mediator) and NaNO3 (the passivating
5 Fig.1. CV curves (1st cycle) of electropolymerization of the polypyrrole coating on the Al-Si substrate at (a)
0-0.9 VAg/AgCl and (b) 0-0.7 and 0-0.8 VAg/AgCl (scanning rate was 10 mV/s). (Inserted graph in (a) 1st, 5th,
10th, 15th and 20th CV scans in the solution X)
As (approximately) shown in Fig. 1, oxidation of pyrrole and the formation of polypyrrole film start at ~ 0.65 VAg/AgCl. At this inflection point, the current density increases sharply and it reaches its
highest value at the upper limit of the scanning range. The chemistry of the solution does not show any significant effect on the oxidation potential. Besides, in all solutions, changes in the values of current density during twenty scans are negligible (see the inserted graph in Fig. 1 (a) as an example).
Both DHBDS and NaNO3 have an increasing effect on the deposition current density. However,
their action mechanism is quite different.
The mechanism through which nitrate (NO3-) anions increase the deposition current density (and
probably the growth of the polypyrrole film) is passivation of the aluminum substrate. As in their presence, the dissolution of the substrate is alleviated, therefore, a thicker coating can be electrodeposited on the surface 20,47,59,60. According to a similar research on pure aluminum 61, the
contribution of passivation in decreasing the current density is probably dominated by the increase in the current density due to the oxidation of monomer. This can justify the higher current density values recorded for the samples treated in NaNO3-containing solutions.
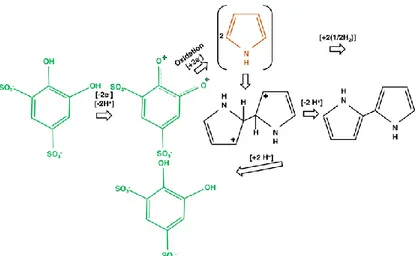
The mechanism of the catalytic action of the electron transfer mediator (DHBDS) is presented in Fig. 2. According to this figure, DHBDS provides extra electrons for the oxidation reaction and therefore it can be utilized to reduce the deposition overpotential of polypyrrole and increase the kinetics of the reaction 46,48,62.
According to Fig. 1 (a), the effect of doubling the concentration of DHBDS (in the absence of nitrate ions) on increasing the current density is more significant compared to that of doubling the concentration of NaNO3.
6
Considering the accelerating effect of DHBDS and to reduce the anodic dissolution of the substrate, electropolymerization of polypyrrole coating using the 2X solution was performed at the smaller potential ranges (lower overpotentials). In this case, the upper potential limit was decreased from 0.9 to 0.8 and 0.7 VAg/AgCl. Moreover, to assist the deposition, 0.05 M NaNO3 was
added to the solution as well. Fig. 1 (b) presents the CV curves for these samples.
As mentioned earlier, the current density is mainly dominated by the coating growth and not by the anodic dissolution of the substrate. Having the same overpotentials, the current densities are fairly similar at the same potential range for the three samples 2X (0.9), 2X (0.8) and 2X (0.7). SEM analysis was utilized to investigate and compare the anodic dissolution of the aluminum substrate during the electrodeposition. SEM images of the substrate surfaces underneath the different coatings are presented in Fig. 3. The coatings were carefully peeled off from the edges of the samples where the adhesion is not perfect so that the condition of the substrate was unchanged. In the case of the samples 2X (0.7) and 2X (0.8) and X-Nit (0.9) (Figs. 3 (b), (c) and (e)), the surface is not much different compared to the untreated sample (Fig. 3 (a)). However, for the sample 2X (0.9) (Fig. 3 (d)) the partial anodic dissolution is obvious, especially at the interface of aluminum grains with the eutectic silicon phase and iron-rich intermetallic particles, making these two latter phases appear more prominent. For the sample treated using NaNO3-containing
solution (with 0.05 M concentration) (Fig. 3 (e)), the anodic dissolution is prevented by the passivating effect of nitrate anions.
According to Fig. 3 (f), the substrate underneath the coating X-2Nit (0.9) has been locally (over) oxidized due to the high concentration of NaNO3.
Fig. 4 illustrates the granular cauliflower surface morphology of the coatings X (0.9), X-Nit (0.9) and X-2Nit (0.9). The anion doping has a noticeable influence on the surface morphology of the polypyrrole coating 63. Based on Figs. 4 (b) and (c), introducing NO
3- into the coating increases
7 Fig. 2. The catalytic effect of DHBDS on the electropolymerization of pyrrole.
Fig. 3. SEM-BSE image of (a) the bare Al-Si alloy and underneath the coating (b) 2X (0.7), (c) 2X (0.8), (d) 2X (0.9), (e) X-Nit (0.9) and (f) X-2Nit (0.9) (the EDXS results are included).
The presence of inorganic doping anions such as NO3- can alter the growth behavior of
polypyrrole coating from a laminar-type (in the presence of organic doping anions) to a fractal-type 64. The presence of a rougher surface (hills and downs) (Figs. 4 (b) and (c)) can indicate a
more complicated 3D growth pattern. In the cross-sectional images (not shown here), however, no difference in the compactness of different coatings was observed. Effect of upper potential limit on the morphology is depicted in Fig. 5. This parameter has a negligible influence on the growth pattern and morphology. However, it slightly increases the size of the polypyrrole globules
8
The chemical composition of the two polypyrrole coatings, X and X-Nit, examined by XPS, is shown in Fig. 6. This figure depicts the high resolution spectra of carbon, nitrogen, and sulfur. The two coatings seem similar in composition, however, a small variation in the nitrogen peak (Fig. 6 (b)), in terms of the intensity, at the high binding energy is observed for the coating X-Nit, which may be related to the incorporation of NO3- anions. The component at the low binding
energy can be assigned to C=NH, while components at ~ 401 and 402 eV can be assigned to C-N+ and C=N+, respectively 65,66. The results of XPS analysis on the polypyrrole coating on the pure
aluminum, reported in an earlier publication, showed no strong proof of the incorporation of nitrate anions into the polymer coating 61. This can be attributed to different phenomena including higher
growth rate of polypyrrole on the Al-Si alloy compared to the pure aluminum (due to the presence of eutectic silicon and iron-rich intermetallic particles), higher thickness of the coating on the Al-Si alloy and the presence of secondary phases influencing the level of nitrate doping into the coating 67. The effect of thickness of polypyrrole coating on the anion doping level has previously
been investigated by other researchers 68.
9 Fig. 5. SEM-SE image of the polypyrrole coatings deposited at different potential ranges.
Fig. 6. High resolution XPS spectra of the polypyrrole coatings on the Al-Si alloy (a) C 1s, (b) N 1s, (c) S 2p.
The thicknesses of the different polypyrrole coatings are summarized in Fig. 7. As it is expected from the deposition CV curves, the thickness increases by the concentration of DHBDS and NaNO3 and increasing the upper limit of the potential range.
10
3.2. Electrochemical properties
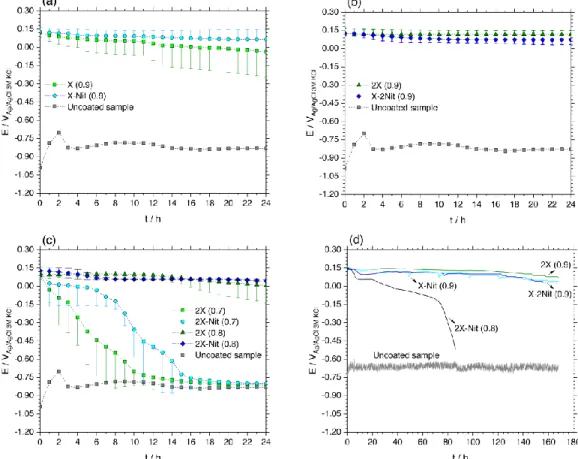
Evolution of the OCP values during 24 h immersion in 0.6 M NaCl for the coatings X (0.9), 2X (0.9), X-Nit (0.9) and X-2Nit (0.9) are reported in Figs. 8 (a) and (b). Each test has been repeated three times to assure the reproducibility of the results.
The OCP values of the coatings are considerably nobler compared to that of the bare Al-Si substrate. However, a decrease with the immersion time is observed for all coatings except the coating 2X (0.9).
The coating X shows the fastest decrease in the OCP values and considering the values of standard deviation, it shows the least reproducibility. These and the previous results (on pure aluminum) show that the addition of NaNO3 to the base solution (X) improves the corrosion
protection efficiency by passivating the aluminum substrate during the electrodeposition process
61.
However, the present results show that doubling the concentration of DHBDS has almost the same effect (at least for the Al-Si alloy and for 24 h immersion) while increasing the concentration of NaNO3 does not result in a significant difference in the OCP values (coating X-Nit (0.9) versus
X-2Nit (0.9)).
In this paper, the failure of the polypyrrole coatings is determined as the point when the OCP value decreases sharply until it reaches a value similar to that of the unprotected substrate. Before the failure, the noble constant values of OCP can indicate to an intact surface (with no interaction or reduction of the polypyrrole) and show the barrier effect of the coating impeding the entrance of electrolyte. By this assumption, the coating 2X (0.9) presents the best barrier properties. The slight decrease in the OCP value can suggest the entrance of electrolyte (chloride ions) probably activating the protection mechanism later in which the polypyrrole coating induces passivation of the surface by its reduction. A sharp decrease in the OCP can suggest that the coating is no longer protecting the surface (neither as a barrier nor an oxidizer).
According to Fig. 8 (c), the coatings electrodeposited at the potential range of 0-0.7 VAg/AgCl are
not able to keep the surface potential noble for 24 h. The coatings electrodeposited at the potential range of 0-0.8 VAg/AgCl present better performance. These results suggest that lower anodic
dissolution of the substrate (during the electrodeposition) does not have a significant influence on the protection effect of the coatings. Considering the results in Fig. 7, it seems that the thickness of the polypyrrole film has a controlling influence on its protection effect (especially the barrier properties). Based on these results the minimum thickness for the polypyrrole coating to be effective (for at least 24 h) can be estimated as 8-10 µm.
11 Fig. 8. OCP values of the polypyrrole coated and bare samples of the Al-Si alloy versus time immersed in
0.6 M NaCl solution (a) and (b) coatings deposited from different solutions (at 0-0.9 VAg/AgCl), (c) coatings
deposited from different solutions and potential ranges for 24 h and (d) for 168 h (In some cases due to overlapping of standard deviation bars, to make it clearer for the reader, the bars are shown only in the
positive or negative direction).
Fig. 8 (d) shows the evolution of OCP values for the coatings, 2X (0.9), X-Nit (0.9), X-2Nit (0.9) and 2X-Nit (0.8), during the long term exposure (168 h). The surface potential of the first three coatings is considerably noble even after seven days of immersion. While the latter coating (2X-Nit (0.8)) completely fails after ~ 72 h. A slight decrease in the OCP values of the coatings 2X (0.9), X-Nit (0.9), X-2Nit (0.9) with the immersion time is observed.
Based on the OCP results, the possible protective effect of the three coatings, 2X (0.9), X-Nit (0.9) and X-2Nit (0.9), seems comparable. Therefore, further electrochemical and microscopic investigations were carried out to distinguish any difference.
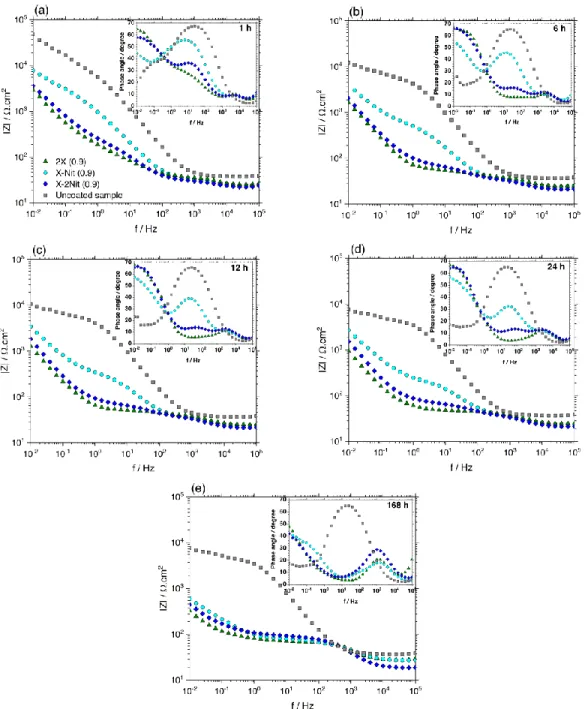
Results of EIS measurements (performed at the OCP) can provide an insight into the possible protection mechanism. The representative EIS spectra of the coatings after 1, 6, 12, 24 and 168 h immersion in 0.6 M NaCl are shown in Fig. 9. The measurement has been repeated three times for each coating to assure the reproducibility.
12
As it can be observed in Fig. 9, the total impedance values of the polypyrrole coatings are significantly lower compared to the Al-Si alloy, which is normal and reported by other researchers for conductive coatings 23,69.
The EIS spectra of the polypyrrole coatings in 0.6 M NaCl present three time constants at the high, medium and low frequency ranges. Their presence, as it can be observed in Fig. 9, is affected by the immersion time. Each of these time constants stands for one electrochemical reaction occurring in the system. The equivalent circuit suggested explaining the electrochemical responses is depicted in Fig. 10. As mentioned in an earlier publication 61 due to the electroactive
nature of polypyrrole proposing a certain equivalent circuit is difficult. Therefore, the suggested circuit is considered only as a possible (and not certain) explanation. Especially since the relative standard error of some of the fitted parameters can be high.
The time constant at the high frequency range (Rga and CPEga) can be related to the galvanic
interaction (coupling) between the polypyrrole coating and aluminum, resulting in passivation (or excessive oxidation) of the substrate. As the previous research shows the presence of this time constant can be affected by chloride concentration 61. It remains in the spectra until the end of the
test at 168 h.
The time constant at the medium frequency range (Rf and CPEf) can be representing the
electrochemical behavior of the polypyrrole coating itself. This can include the reduction reaction of the polymer (which induces the passivation of the aluminum alloy substrate), the conductivity of the coating or the anion exchange with the electrolyte. The reduction of polypyrrole is accompanied by the release of the dopant anions and its re-oxidation by the uptake of anions including chloride to maintain the polymer electroneutrality 70. The entry of electrolyte during the
immersion can decrease the coating resistance and increase its capacity 71,72. This time constant
shows the most significant change during the test. The semi-circle related to this time constant suppresses and finally disappears through the immersion time (Fig. 9 (a) versus Fig. 9 (e)). The evolution of this time constant through the immersion time is different for the coating 2X (0.9) compared to the coatings X-Nit (0.9) and X-2Nit (0.9).
The time constant at the low frequency range (CPEdl and Rct) can probably be related to the
reduction of oxygen at the polypyrrole/electrolyte interface 19,71. Only as an example, the results
of fitting of the representative EIS spectra of the coatings 2X and X-2Nit after 12 and 24 h immersion in 0.6 M NaCl are presented in Table. 2.
The EIS results and the proposed circuit can suggest that the protection mechanism is passivation of the aluminum induced by the reduction of the conductive polymer.
13
As observed in Table. 2, the difference between the two coating mainly lies in the values of Rf,
which are higher for the coating 2X compared to the coating X-2Nit. This resistance might be associated with the anion exchange with the electrolyte or the entry of electrolyte during the immersion and the higher values for the coating 2X suggest slower or lower exchange or electrolyte entrance. Since, as mentioned before, the entry of solution can decrease the resistance of the coating 70.
Further microscopy examination is required for final judgment on the differences in the protection efficiencies of the two coatings.
Fig. 11 depicts the surface of the coatings, 2X (0.9) and X-2Nit (0.9), after 24 h and 168 h immersion in 0.6 M NaCl solution. Due to the similarity to the coating X-2Nit (0.9), SEM images of the coating X-Nit (0.9) are not presented. After 24 h (Figs. 11 (a) and (b)), the presence of some blisters is evident on both coatings. These blisters are considerably smaller on the coating 2X (0.9) compared to the coating X-2Nit (0.9).
At the end of 168 h, these blisters spread (Figs. 11 (c) and (d)). The coating X-2Nit (0.9) is cracked on top of a blister, while the coating 2X (0.9) is still quite adhesive to the substrate.
This coating (X-2Nit (0.9)) was peeled off and the substrate underneath was examined by SEM (Figs. 11 (e) and (f)). A considerable fraction of the surface is protected from any corrosion including the localized corrosion (e.g. pitting and localized micro-galvanic corrosion), showing the barrier properties of the coating and probably the passivation protection mechanism. However, some thick oxide layers are present underneath a few blisters, which indicate the localized corrosion of the substrate as a result of drastic galvanic coupling at polypyrrole/aluminum interface.
The growth of these oxide layers leads to the formation of blisters and eventually failure of the coating. These oxide layers are considerably thicker and therefore recognizable from those formed during the electropolymerization (Fig. 3 (f)).
The exposed surface of the bare Al-Si alloy after 24 h and 168 h is presented in Figs. 11 (g) and (h) for comparison. In the absence of any coating, the surface suffers from localized attacks at the interface of the aluminum matrix with the iron-rich intermetallic particles and the eutectic silicon phase due to the micro-galvanic corrosion mechanism. It is heavily covered by the porous corrosion products at the end of the 168-hour immersion test (Fig. 11 (g)).
14 Fig. 9. EIS spectra of the polypyrrole coated and bare samples of the Al-Si alloy after (a) 1 h, (b) 6 h, (c)
12 h, (d) 24 h and (e) 168 h immersion in 0.6 M NaCl solution.
Fig. 10. Equivalent circuit for fitting the EIS responses of polypyrrole coatings electrodeposited on the Al-Si alloy.
15 Fig. 11. SEM-SE image of the polypyrrole coating (a) and (c) 2X (0.9); (b) and (d) X-2Nit (0.9) after 24 h
and 168 h immersion in 0.6 M NaCl solution, respectively. (e) and (f) SEM-BSE image of substrate underneath the coating X-2Nit (0.9) after 168 h immersion in 0.6 M NaCl solution. (g) and (h) SEM-BSE
image of the surface of bare Al-Si substrate after 24 h and 168 h immersion in 0.6 M NaCl solution, respectively.
16
The SEM exanimation shows that due to less severe blistering, probably as a result of better barrier properties, a longer/more efficient corrosion protection can be expected for the coating 2X (0.9) compared to the coatings X-Nit (0.9) and X-2Nit (0.9).
Increasing the concentration of NaNO3 does not result in a coating with significantly improved
corrosion protection properties however doubling the concentration of DHBDS (Tiron) does. This improvement can be related to the incorporation of large DHB2- anions into the polymer, which
decreases exchange with chloride ions and increases the barrier properties.
4. CONCLUSIONS
The effect of the electrolyte composition and the potential range on the electropolymerization and electrochemical and physical properties of the polypyrrole coatings was investigated on Rheo-HPDC Al- 2.5% Si alloy. Following main conclusions were drawn:
Increasing the concentration of NaNO3 and DHBDS in the electropolymerization solution
increases the thickness of the coating.
Decreasing the upper potential limit reduces the anodic dissolution of aluminum during the electrodeposition.
Polypyrrole coatings are able to relatively protect the Al-Si substrate in 0.6 M NaCl solution for considerably long immersion times.
Based on the EIS results, passivation protection mechanism (induced by the reduction of polypyrrole) can be suggested.
The thickness of the coating and its ion-barrier are the most important factors affecting possible corrosion protection efficiency.
Polypyrrole coating deposited from a solution with the higher concentration of DHBDS provides higher ion-barrier properties and therefore an improved corrosion protection.
Severe localized galvanic coupling at the polypyrrole/aluminum interface results in blistering and the failure of the coating but it can be reduced by changing the electrolyte composition.
5. ACKNOWLEDGMENTS
COMPtech AB (Sweden) is gratefully acknowledged for the production of the components and the technical support.
17
6. REFERENCES
1. Spinks GM, Dominis AJ, Wallace GG, Tallman DE. Electroactive conducting polymers for corrosion control. J Solid State Electrochem. 2002;6(2):85-100.
2. Deshpande PP, Sazou D. Corrosion Protection of Metals by Intrinsically Conducting Polymers.
Taylor & Francis Group; 2016.
3. Rohwerder M, Michalik A. Conducting polymers for corrosion protection: What makes the difference between failure and success? Electrochim Acta. 2007;53(3):1300-1313.
4. Deshpande PP, Jadhav NG, Gelling VJ, Sazou D. Conducting polymers for corrosion protection: a review. J Coat Technol Res. 2014;11(4):473-494.
5. de Leon A, Advincula RC. Chapter 11 - Conducting Polymers with Superhydrophobic Effects as Anticorrosion Coating. In: Intelligent Coatings for Corrosion Control. Boston: Butterworth-Heinemann; 2015:409-430.
6. Michalik A, Rohwerder M. Conducting Polymers for Corrosion Protection: A Critical View. zpch.
2005;219(11):1547–1559.
7. DeBerry DW. Modification of the Electrochemical and Corrosion Behavior of Stainless Steels with an Electroactive Coating J Electrochem Soc. 1985;132(5):1022-1026.
8. Iroh JO, Su W. Corrosion performance of polypyrrole coating applied to low carbon steel by an electrochemical process. Electrochim Acta. 2000;46:15–24.
9. Koene L, Hamer WJ, de Wit JHW. Electrochemical behaviour of poly(pyrrole) coatings on steel. J Appl Electrochem. 2006;36(5):545-556.
10. Sazou D, Kourouzidou M, Pavlidou E. Potentiodynamic and potentiostatic deposition of polyaniline on stainless steel: Electrochemical and structural studies for a potential application to corrosion control. Electrochim Acta. 2007;52(13):4385-4397.
11. González MB, Saidman SB. Electrodeposition of polypyrrole on 316L stainless steel for corrosion prevention. Corros Sci. 2011;53: 276–282.
12. González MB, Saidman SB. Corrosion protection properties of polypyrrole electropolymerized onto steel in the presence of salicylate. Prog Org Coat. 2012;75:178–183.
13. El Jaouhari A, Laabd M, Bazzaoui EA, et al. Electrochemical and spectroscopical studies of polypyrrole synthesized on carbon steel from aqueous medium. Synth Met. 2015;209:11-18. 14. Turhan MC, Weiser M, Jha H, Virtanen S. Optimization of electrochemical polymerization
parameters of polypyrrole on Mg–Al alloy (AZ91D) electrodes and corrosion performance.
Electrochim Acta. 2011;56(15):5347-5354.
15. Turhan MC, Weiser M, Killian MS, Leitner B, Virtanen S. Electrochemical polymerization and characterization of polypyrrole on Mg–Al alloy (AZ91D). Synth Met. 2011;161(3):360-364.
16. Grubač Z, Rončević IŠ, Metikoš-Huković M. Corrosion properties of the Mg alloy coated with polypyrrole films. Corros Sci. 2016;102:310-316.
18 17. Ascencio M, Pekguleryuz M, Omanovic S. Corrosion behaviour of polypyrrole-coated WE43 Mg
alloy in a modified simulated body fluid solution. Corros Sci. 2018;133:261-275.
18. Saidman SB, Bessone JB. Electrochemical preparation and characterisation of polypyrrole on aluminium in aqueous solution. J Electroanal Chem. 2002;521(1):87-94.
19. He J, Tallman DE, Bierwagen GP. Conjugated Polymers for Corrosion Control: Scanning Vibrating Electrode Studies of Polypyrrole-Aluminum Alloy Interactions. J Electrochem Soc.
2004;151(12):B644-B651.
20. Lehr IL, Saidman SB. Characterisation and corrosion protection properties of polypyrrole electropolymerised onto aluminium in the presence of molybdate and nitrate. Electrochim Acta.
2006;51(16):3249-3255.
21. Arenas MA, Bajos LG, de Damborenea JJ, Ocón P. Synthesis and electrochemical evaluation of polypyrrole coatings electrodeposited onto AA-2024 alloy. Prog Org Coat. 2008;62(1):79-86. 22. Yan M, Tallman DE, Bierwagen GP. Role of oxygen in the galvanic interaction between polypyrrole
and aluminum alloy. Electrochim Acta. 2008;54(2):220-227.
23. Martins NCT, Moura e Silva T, Montemor MF, Fernandes JCS, Ferreira MGS. Electrodeposition and characterization of polypyrrole films on aluminium alloy 6061-T6. Electrochim Acta.
2008;53(14):4754-4763.
24. Yan MC, Tallman DE, Rasmussen SC, Bierwagen GP. Corrosion Control Coatings for Aluminum Alloys Based on Neutral and n-Doped Conjugated Polymers. J Electrochem Soc. 2009;156(10): C360-C366.
25. Balaskas AC, Kartsonakis IA, Kordas G, Cabral AM, Morais PJ. Influence of the doping agent on the corrosion protection properties of polypyrrole grown on aluminium alloy 2024-T3. Prog Org Coat. 2011;71(2):181-187.
26. Rizzi M, Trueba M, Trasatti SP. Polypyrrole films on Al alloys: The role of structural changes on protection performance. Synth Met. 2011;161(1):23-31.
27. Volpi E, Trueba M, Trasatti SP. Electrochemical investigation of conformational rearrangements of polypyrrole deposited on Al alloys. Prog Org Coat. 2012;74(2):376-384.
28. Volpi E, Trueba M, Trasatti SP, Trasatti S. Effect of polypyrrole conformational rearrangement on Al alloys corrosion protection. J Electroanal Chem. 2013;688:289-297.
29. Castagno KRL, Dalmoro V, Azambuja DS. Characterization and corrosion of polypyrrole/sodium dodecylbenzene sulfonate electropolymerised on aluminum alloy 1100. Mater Chem Phys.
2011;130(1):721-726.
30. Mrad M, Amor YB, Dhouibi L, Montemor F. Electrochemical study of polyaniline coating electropolymerized onto AA2024-T3 aluminium alloy: Physical properties and anticorrosion performance. Synth Met. 2017;234:145-153.
19 31. Bandeira RM, van Drunen J, Garcia AC, Tremiliosi-Filho G. Influence of the thickness and roughness of polyaniline coatings on corrosion protection of AA7075 aluminum alloy. Electrochim Acta. 2017;240:215-224.
32. Rohwerder M, Duc LM, Michalik A. In situ investigation of corrosion localised at the buried interface between metal and conducting polymer based composite coatings. Electrochim Acta.
2009;54(25):6075-6081.
33. Vernitskaya TV, Efimov ON. Polypyrrole: a conducting polymer; its synthesis, properties and applications. Russ Chem Rev. 1997;66(5):443 – 457.
34. Paliwoda-Porebska G, Stratmann M, Rohwerder M, et al. On the development of polypyrrole coatings with self-healing properties for iron corrosion protection. Corros Sci. 2005;47(12):3216-3233.
35. Hien NTL, Garcia B, Pailleret A, Deslouis C. Role of doping ions in the corrosion protection of iron by polypyrrole films. Electrochim Acta. 2005;50(7):1747-1755.
36. Branzoi V, Pilan L, Golgovici F, Branzoi F. Electrochemical Activity and Corrosion Protection Properties of Doped Polypyrrole Electrodeposited at Pure Aluminium Electrode. Mol Cryst Liq Cryst. 2006;446:305–318.
37. Atobe M, Tsuji H, Asami R, Fuchigami T. A study on doping–undoping properties of polypyrrole films electropolymerized under ultrasonication. J Electrochem Soc. 2006;153(1):D10-D13.
38. Zor S, Kandemirli F, Yakar E, Arslan T. Electrochemical synthesis of polypyrrole on aluminium in different anions and corrosion protection of aluminium. Prot Met Phys Chem Surf. 2010;46(1):110-116.
39. Kowalski D, Ueda M, Ohtsuka T. Self-healing ion-permselective conducting polymer coating. J Mat Chem. 2010;20(36):7630-7633.
40. Mrad M, Dhouibi L, Montemor MF, Triki E. Effect of doping by corrosion inhibitors on the morphological properties and the performance against corrosion of polypyrrole electrodeposited on AA6061-T6. Prog Org Coat. 2011;72(3):511-516.
41. Ryu H, Sheng N, Ohtsuka T, Fujita S, Kajiyama H. Polypyrrole film on 55% Al–Zn-coated steel for corrosion prevention. Corros Sci. 2012;56:67-77.
42. Jung HS, Kim KS, Kwak MK, Ko JH. Effect of polystyrenesulphonate and electrolyte concentration for electrical properties of polypyrrole film on aluminium alloy using conductive AFM. Corr Eng Sci Technol. 2014;49(7):608-613.
43. Vera R, Schrebler R, Grez P, Romero H. The corrosion-inhibiting effect of polypyrrole films doped with p-toluene-sulfonate, benzene-sulfonate or dodecyl-sulfate anions, as coating on stainless steel in NaCl aqueous solutions. Prog Org Coat. 2014;77(4):853-858.
44. Doğru Mert B. Corrosion protection of aluminum by electrochemically synthesized composite organic coating. Corros Sci. 2016;103:88-94.
20 45. Li M, Ji X, Cui L, Liu J. In situ preparation of graphene/polypyrrole nanocomposite via electrochemical co-deposition methodology for anti-corrosion application. J Mat Sci.
2017;52(20):12251-12265.
46. Tallman DE, Vang C, Wallace GG, Bierwagen GP. Direct Electrodeposition of Polypyrrole on Aluminum and Aluminum Alloy by Electron Transfer Mediation. J Electrochem Soc.
2002;149(3):C173-C179.
47. Saidman SB, Quinzani OV. Characterisation of polypyrrole electrosynthesised on aluminium.
Electrochim Acta. 2004;50(1):127-134.
48. Tallman DE, Dewald MP, Vang CK, Wallace GG, Bierwagen GP. Electrodeposition of conducting polymers on active metals by electron transfer mediation. Curr Appl Phys. 2004;4(2):137-140. 49. Mert BD, Solmaz R, Kardaş G, Yazıcı B. Copper/polypyrrole multilayer coating for 7075 aluminum
alloy protection. Prog Org Coat. 2011;72(4):748-754.
50. Zolotorevsky VS, Belov NA, Glazoff MV. Casting aluminum alloys. Vol 12: Elsevier Amsterdam; 2007.
51. Faraji M, Katgerman L. Distribution of trace elements in a modified and grain refined aluminium– silicon hypoeutectic alloy. Micron. 2010;41(6):554-559.
52. Payandeh M, Belov I, Jarfors AEW, Wessén M. Effect of Material Inhomogeneity on Thermal Performance of a Rheocast Aluminum Heatsink for Electronics Cooling. J Mater Eng Perform.
2016;25(6):2116-2127.
53. Shin J-S, Ko S-H, Kim K-T. Development and characterization of low-silicon cast aluminum alloys for thermal dissipation. J Alloys Compd. 2015;644:673-686.
54. Arrabal R, Mingo B, Pardo A, Mohedano M, Matykina E, Rodríguez I. Pitting corrosion of rheocast A356 aluminium alloy in 3.5 wt.% NaCl solution. Corros Sci. 2013;73:342–355.
55. Mingo B, Arrabal R, Pardo A, Matykina E, Skeldon P. 3D study of intermetallics and their effect on the corrosion morphology of rheocast aluminium alloy. Mater Charact. 2016; 112:122–128. 56. Eslami M, Payandeh M, Deflorian F, Jarfors A, Zanella C. Effect of Segregation and Surface
Condition on Corrosion of Rheo-HPDC Al–Si Alloys. Metals. 2018;8(4):209.
57. Eslami M, Fedel M, Speranza G, Deflorian F, Andersson N-E, Zanella C. Study of selective deposition mechanism of cerium-based conversion coating on Rheo-HPDC aluminium-silicon alloys. Electrochim Acta. 2017;255:449-462.
58. Saidman SB. The effect of pH on the electrochemical polymerisation of pyrrole on aluminium. J Electroanal Chem. 2002;534(1):39-45.
59. Pyun S-I, Moon S-M. The inhibition mechanism of pitting corrosion of pure aluminum by nitrate and sulfate ions in neutral chloride solution. J Solid State Electrochem. 1999;3(6):331-336.
60. Grari O, Dhouibi L, Lallemand F, Lallemand S, Triki E. Effects of nitrate ions on the electrochemical synthesis and behavior of polypyrrole films. Prog Org Coat. 2014;77(11):1867-1873.
21 61. Eslami M, Speranza G, Fedel M, et al. Electropolymerization and possible corrosion protection effect of polypyrrole coatings on AA1050 (UNS A91050) in NaCl solutions. CORROSION.
2019;75(7):745-755.
62. Levine K. Nanocomposite PPy Coatings for Al Alloys Corrosion Protection. In: Mittal V, ed. Polymer Nanocomposite Coatings. CRC Press; 2013.
63. Eftekhari A, Kazemzad M, Keyanpour-Rad M. Significant Effect of Dopant Size on Nanoscale Fractal Structure of Polypyrrole Film. Polym J. 2006;38:781.
64. K. SR, Amit K, Khushboo A, et al. DC electrical conduction and morphological behavior of counter anion-governed genesis of electrochemically synthesized polypyrrole films. J Polym Sci, Part B: Polym Phys. 2012;50(5):347-360.
65. Malitesta C, Losito I, Sabbatini L, Zambonin PG. New findings on polypyrrole chemical structure by XPS coupled to chemical derivatization labelling. J Electron Spectrosc Relat Phenom.
1995;76:629-634.
66. Tabačiarová J, Mičušík M, Fedorko P, Omastová M. Study of polypyrrole aging by XPS, FTIR and conductivity measurements. Polym Degrad Stab. 2015;120:392-401.
67. Eslami M. Surface Treatments to Protect Conventional and Rheo-High Pressure Die Cast Al-Si Alloys from Corrosion. Trento, Italy: Industrial Engineering, University of Trento; 2019.
68. Idla K, Talo A, Niemi HE-M, Forsén O, Yläsaari S. An XPS and AFM study of polypyrrole coating on mild steel. Surf Interface Anal. 1997;25(11):837-854.
69. Bazzaoui M, Martins JI, Costa SC, Bazzaoui EA, Reis TC, Martins L. Sweet aqueous solution for electrochemical synthesis of polypyrrole: Part 1-A. On non-ferrous metals. Electrochim Acta.
2006;51(12):2417-2426.
70. Hosseini MG, Raghibi-Boroujeni M, Ahadzadeh I, Najjar R, Seyed Dorraji MS. Effect of polypyrrole– montmorillonite nanocomposites powder addition on corrosion performance of epoxy coatings on Al 5000. Prog Org Coat. 2009;66(3):321-327.
71. Scully JR. Electrochemical impedance of organic‐ c oated steel: correlation of impedance parameters with long ‐ term coating deterioration. J Electrochem Soc. 1989;136(4):979-990. 72. Mansfeld F. Use of electrochemical impedance spectroscopy for the study of corrosion protection
22
TABALES
Table. 1. Details of the electropolymerization conditions (the scanning rate and the number of cycles are 10 mV/s and 20, respectively)
Coating Potential range
(VAg/AgCl) Solution
X (0.9) 0-0.9 0.1 M Py, 0.05 M DHBDS, 0.005 M SDS 2X (0.9) 0-0.9 0.1 M Py, 0.1 M DHBDS, 0.005 M SDS 2X (0.8) 0-0.8 0.1 M Py, 0.1 M DHBDS, 0.005 M SDS 2X (0.7) 0-0.7 0.1 M Py, 0.1 M DHBDS, 0.005 M SDS
X-Nit (0.9) 0-0.9 0.1 M Py, 0.05 M DHBDS, 0.005 M SDS, 0.05 M NaNO3
X-2Nit (0.9) 0-0.9 0.1 M Py, 0.05 M DHBDS, 0.005 M SDS, 0.1 M NaNO3
2X-Nit (0.8) 0-0.8 0.1 M Py, 0.1 M DHBDS, 0.005 M SDS, 0.05 M NaNO3
23 Table. 2. Summary of results derived from fitting the EIS responses of 2X and X-2Nit polypyrrole coatings in 0.6 M NaCl solution
(A) Relative standard error
(B) Chi-squared: the sum of the squares of the residuals
Parameter Rga (Ω.cm2) Qga Rf (Ω.cm2) Qf Rct (Ω.cm2) Qdl 2*(B) n Y (S.sn.cm-2) n Y (S.sn.cm-2) n Y (S.sn.cm-2) Coating 2X 12 h 27.81±10%(A) 0.38±10% 8.16×10-4±23% 784.90±32% 0.91±2% 6.31×10-3±3% 1.47×104±73% 0.89±10% 3.35×10-3±12% 1.60×10-4 24 h 22.39±5% 0.54±6% 2.52×10-4±25% 192.30±46% 0.90±5% 6.64×10-3±10% 1.67×104±69% 0.81±6% 4.14×10-3±20% 1.46×10-4 Coating X-2Nit 12 h 47.78±11% 0.56±9% 1.31×10-3±17% 10.29±26% 0.84±9% 2.73×10-5±66% 4.43×104±78% 0.84±1% 5.67×10-3±1% 2.46×10-4 24 h 48.03±9% 0.55±8% 1.13×10-3±13% 10.18±25% 0.84±8% 2.76×10-6±58% 1.31×104±22% 0.85±1% 6.85×10-3±1% 1.64×10-4