1
NanoWear of Salivary Films vs. Substratum Wettability
Javier Sotres1*, Torbjörn Pettersson2, Liselott Lindh3, Thomas Arnebrant1
1
Biomedical Science, Faculty of Health and Society Malmoe University, 20506 Malmoe, Sweden
2
Fibre and Polymer Technology, KTH Royal Institute of Technology, 10044 Stockholm, Sweden
3
Prosthetic Dentistry, Faculty of Odontology, Malmoe University, 20506 Malmoe, Sweden
*Corresponding author, e-mail address: javier.sotres@mah.se
Running head: Strength of Salivary Films Abstract word count: 145
Total word count: 2698 Number of figures: 4 Number of references: 30
2
ABSTRACT
The pellicle serves as a multifunctional protective layer providing i.e. lubrication, remineralisation and also acts as diffusion barrier. In addition, as the formation of the pellicle precedes adhesion of microorganisms, it is also important as a conditioning film. We present a novel approach to study the influence of the water wettability of solid surfaces on the strength of adsorbed salivary films. It is based on studying the wear resistance of the films with an atomic force microscope operated in the friction force spectroscopy mode. This methodology provides the strength of the films in terms of the forces needed for breaking and removing them. Our results indicate that these forces are highly dependent of the water wettability of the underlying substrata, decreasing with increasing hydrophobicity. Thus, this study provides valuable information for the design of materials exposed in the oral cavity, e.g. designing materials that will minimize plaque formation and being easy to clean.
INTRODUCTION
Saliva forms a protective film, the acquired pellicle (Dawes et al., 1963), on all surfaces within the oral cavity: teeth, oral mucosa, dentures, crowns, etc. The pellicle has many important functions. It reduces the dissolution rate of enamel and modulates the process of mineral precipitation at its surface (Hannig and Joiner, 2006; Lendenmann et al., 2000). The lubricating properties of the pellicle not only facilitate talking and swallowing, but also protect oral surfaces from abrasive forces (Hannig and Joiner, 2006). The pellicle also forms the innermost layer of dental plaque, i.e. constitutes the substratum to which bacteria adhere in the early stages of plaque formation (Hannig and Joiner, 2006; Lendenmann et al., 2000).
The adsorption of proteins on solid surfaces is a complex process determined by the interplay of surface, protein and surrounding medium properties (Norde, 1986; Norde and Lyklema, 1989). The salivary pellicle constitutes a representative example of these systems with numerous questions waiting to be answered. One of them is how the pellicle is affected by the physicochemical properties of the underlying substratum. We have focused on this aspect, and
3
more specifically on the influence of the water wettability of the substratum. This property, which differs for the different surfaces present intraorally (de Jong et al., 1982; van der Mei et al., 2004), has a high influence on the pellicle. The in vitro rate of adsorption and the adsorbed amount of saliva are dependent on the wettability of the substratum, i.e. higher amounts adsorb on hydrophobic surfaces compared to hydrophilic ones (Arnebrant, 2003). The composition of in vitro formed pellicles is different when formed on different types of substrata with different wettabilities (Svendsen and Lindh, 2009). A correlation has also been established between the wettability of solids and their adhesiveness for in situ formed dental plaque (Glantz, 1969; Teughels et al., 2006), which is attributed to the innermost salivary film (Glantz and Baier, 1986).
Even though the relevance of the adhesiveness of the pellicle has been thoroughly discussed (Busscher and van Der Mei, 1995; Christersson et al., 1989; Glantz, 1969; Hannig and Hannig, 2009), its direct experimental study has been postponed due to the lack of suitable techniques. We have shown that such studies of protein layers could be approached by probing their resistance to wear with an Atomic Force Microscopy (AFM) operated in the Friction Force Spectroscopy (FFS) mode (Sotres et al., 2011a). AFM-based FFS offers clear advantages for studying wear such as its nanometer spatial resolution, which is optimal when studying coatings with thickness in the nanometer range. Moreover, single asperity contacts are simulated due to the nanometer size of AFM tips. We recently applied this methodology to study pellicles formed in vitro from fresh saliva (Sotres et al., 2011b). In these experiments we showed that pellicles formed on model hydrophilic substrata were stronger than those formed on model hydrophobic substrata. The purpose of the present work was to perform a rigorous study with the aim of establishing the dependence of the strength of salivary films on the water wettability of the substrata. To this end, AFM-based FFS was used to study the wear resistance of pellicles formed
from the same batch of saliva, i.e. avoiding compositional differences, on substrata of different wettabilities. Our results reveal a constant and pronounced increase of the strength of the films with increased water wettability of the substratum.
4
MATERIALS & METHODS
Cleaning and hydrophobization of substrata
Both clean (hydrophilic) and hydrophobized silica surfaces were used in this study. Silica surfaces (Semiconductor Wafer Inc.) were cleaned as described elsewhere (Wahlgren and Arnebrant, 1990). For hydrophobization, clean silica surfaces were immersed in a solution containing dichlorodimethylsilane (Sigma-Aldrich) diluted in trichloroethylene (Sigma-Aldrich). Hereafter this treatment is referred to as methylation. The dichlorodimethylsilane concentration and the immersion time were varied in order to obtain different surface coverage of methyl groups and, therefore, different water wettabilities (specific details are given in Supplemental File S1). After methylation, the surfaces were washed in trichloroethylene, ethanol, and finally stored in ethanol in order to avoid contamination of the substrata. Before sample preparation, substrata were dried under a nitrogen stream. The substrata were not further treated, in order to focus on the interaction of saliva with clean surfaces.
Contact angle measurements
The water wettability of the substrata was characterized through their water contact angle, θc
(Fig. 1a). For this a DSA100 Contact Angle Measuring System (Krüss GmbH) was used. Specific technical details are given in Supplemental File S2.
Sample preparation
Unstimulated saliva from two healthy male donors, 56 and 32 years old respectively, was collected (Dawes, 1974). After collection, saliva was frozen, kept at -20°C, and thawed only just before sample preparation. Immediately after thawing, saliva without further treatment (ca. 100µl) was pipetted onto the substrata, left to adsorb for 1 hour at room temperature, and rinsed with UHQ water to remove loosely bound components (Arnebrant, 2003). Immediately after rinsing, samples were placed and scanned in an AFM fluid cell filled with UHQ water. Ethical approval was obtained from the committee of research ethics at Lund University (LU 518-02).
5
Friction force spectroscopy
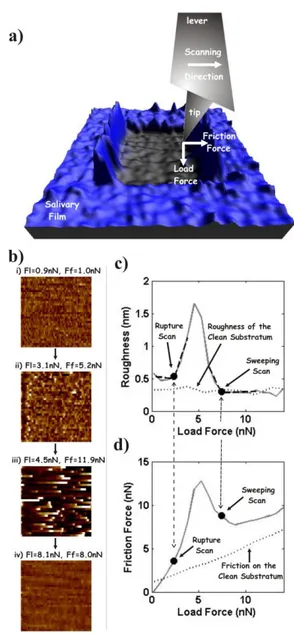
Friction force spectroscopy (FFS) measurements were performed at room temperature with a commercial AFM (MultiMode 8, Bruker AXS) equipped with a fluid cell. An AFM uses a nanometer-sized tip attached at the free end of a cantilever to scan samples. While scanning, the load and friction forces applied by the tip are monitored (Fig. 2a). Standard AFM imaging consists in varying the vertical position of the sample during the scan so that the load on the tip,
FL, remains constant. In this way, the inverse of the sample vertical position corresponds to its
topography. The capability of AFM to simultaneously record topography and friction constitutes the basis for FFS. Shortly (detailed technical and calibration explanation are given in Supplemental File S3 and in (Pettersson et al., 2007)), FFS is based on performing two-dimensional constant-load scans. The topography of the sample and the average value of the tip-sample friction force, FF, are registered for each of these scans. Then the applied load is
increased between scans. Thus, FFS measurements (which can be considered as scratches) are characterized by the variation with the applied load of the topography of the sample (Fig. 2b) and of the tip-sample friction (friction plot, Fig. 2d). The changes in the topography are also represented by plotting its roughness (height standard deviation as specified in Supplemental File S3) against the applied load (roughness plot, Fig. 2c).
RESULTS
Silica and methylated silica were used as substrata for the salivary films. Controlling the methylation process, different concentrations of methyl groups and, therefore, different wettabilities were achieved. The water contact angle, θc, was used as the indicator of the water
wettability (Bonn et al., 2009), higher values corresponding to more hydrophobic substrata. Six different batches of surfaces with different wettabilities were employed. These batches are referred to as groups I to VI (Fig. 1b). Group I corresponds to clean hydrophilic silica surfaces (θc=3.4±0.6°), while groups II to VI were numbered according to their increasing hydrophobicity
6
A representative example of a FFS measurement in UHQ water on a salivary film (formed on methylated silica, group VI), is shown in Fig. 2. Figure 2b shows the topography of representative scans of the FFS measurement. The changes in the topography induced by the applied load are also illustrated by plotting the roughness of the complete set of scans (roughness plot, Fig. 2c). The tip-sample friction force is also shown for all the scans (friction plot, Fig. 2d). For the lower applied loads, the topography shows a planar surface characterized by a low and homogeneous roughness. This is indicative of non-destructive sliding along the intact salivary film. When the load is further increased the film eventually breaks as shown by a sudden increase in roughness. The scan for which this occurs is referred to as “Rupture Scan”. A planar topography is again visualized when the load is increased even further, the roughness reaching a value similar to that measured on a similar substratum with no saliva adsorbed (from now on called clean substratum, dotted line in Fig. 2c). The scan from where this is observed is referred to as “Sweeping Scan”. From this scan friction increases with load at the same rate measured when probing a clean substratum (dotted line in Fig. 2d). These observations indicate that the film is removed and the underlying substratum is visualized. In this work we did not focus on the net force difference observed between the friction measured in this regime for the salivary film and for a clean substratum. Nevertheless, it is worth to mention that this is indicative of the lateral diffusion of some of the components of the salivary film (Sotres et al., 2011b). In this work, the forces for which the Rupture and Sweeping Scans occur were chosen as indicators of the strength of the salivary films.
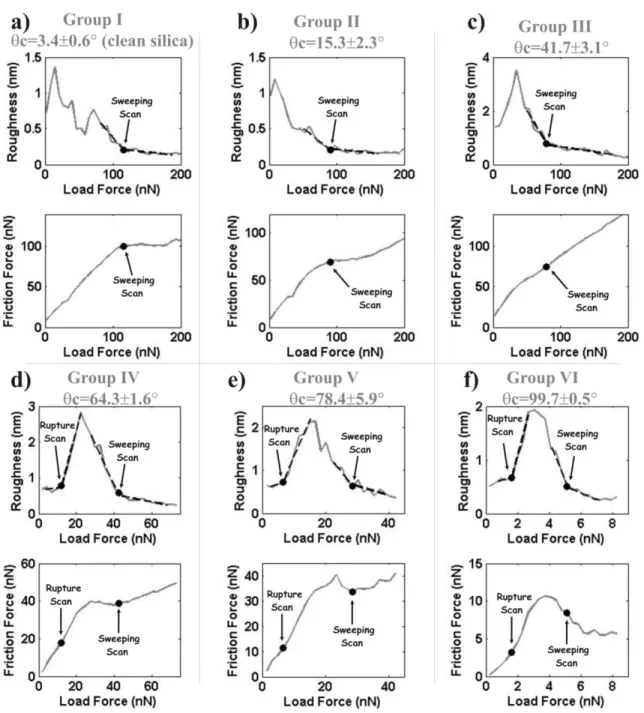
Figure 3 shows six sets of roughness and friction plots performed on films formed on all the studied types of substrata using the same batch of saliva from a single donor. Sweeping Scans are identified for all the cases whereas Rupture Scans are identified only for films formed on more hydrophobic substrata. Nevertheless, the clear identification of film removal implies that rupture does occur. It may be hypothesized that, as a consequence of films being rougher in this case, rupture is no longer reflected as a drastic increase in roughness (Sotres et al., 2011b). Despite this, it is clear from Fig. 3 that Rupture and Sweeping Scans occur at higher load and higher friction forces for films formed on substrata with higher water wettability.
7
The roughness and friction plots of Figs. 2c and 2d slightly differ from those shown in Fig. 3f, despite being obtained on saliva adsorbed on similar substrata. The fact is that these measurements were performed on films formed with saliva from different donors. Thus, it is needed to investigate if the θc-dependence observed in Fig. 3 holds when probing films formed
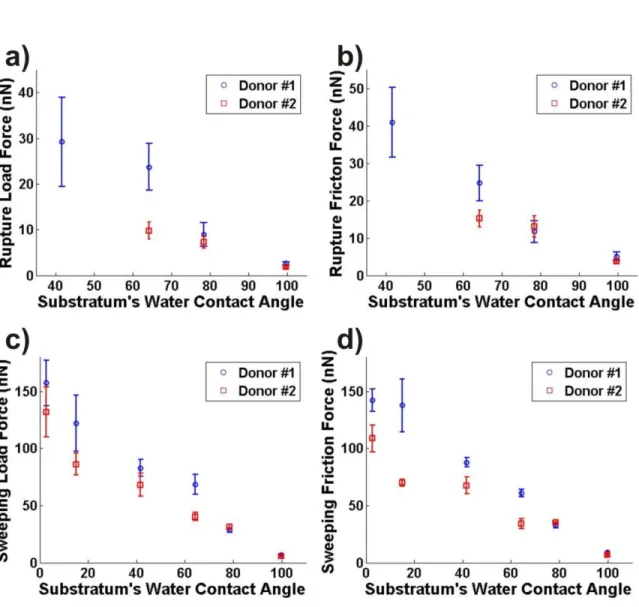
from saliva from different donors. This was verified by probing films from two donors on all the groups of substrata used in this work. In Fig. 4 the load and friction forces corresponding to the Rupture and Sweeping Scans measured on those systems are plotted against the θc value of the
corresponding substrata. Each force is plotted as a mean value with standard deviation calculated from a set of three to five different measurements on different areas of the samples. Despite of the intraindividual and interindividual differences shown by these measurements, their results validate that the overall strength of salivary films increases with the increased water wettability of the substratum.
DISCUSSION
The aim of this work was to study the dependence of the strength of salivary films with the water wettability of their substrata. As substrata we used silica surfaces with different coverage of methyl groups. Both silica and methylated silica have comparable surface charge densities (Malmsten et al., 1998). Moreover, AFM imaging showed that methylation did not induce significant changes in the roughness of the surfaces (Supplemental File S4). Thus, the employment of silica with different coverage of methyl groups was validated as, mainly, they only differed in their water wettability.
The water wettability of a surface is determined by its surface free energy (Bonn et al., 2009). A characterization in terms of the critical surface tension would have been more robust because of being a liquid-independent quantity (Baier and Glantz, 1978; Fox and Zisman, 1950). However, we decided to employ water wettability (or more specifically, the static water contact angle θc) to
characterize the substrataas i) it is widely used to characterize oral surfaces (de Jong et al., 1982; van der Mei et al., 2004), andii) it is specifically relevant in this case as saliva is in its majority
8
ambient gas (air) varied between measurements, θc could be regarded as direct indicator of the
surface free energy of the substrata. Higher θc values corresponded to substrata with lower
surface free energies (more hydrophobic).
A critical point of this work is the employment of pellicles formed in vitro from thawed saliva. Studying pellicles formed in situ might have given additional information as they differ from those formed in vitro (Carlén et al., 1998; Carlén et al., 2003). Moreover, freezing and thawing also alter saliva (Francis et al., 2000). Despite this, we used thawed saliva since it allowed testing saliva from a single donation (batch), i.e. at the same compositional conditions, on the different substrata, minimizing preparation and storage induced differences. In this way differences between the strength of the pellicles could be unequivocally associated to differences between the wettability of their substrata. If either pellicles formed in situ or in vitro from fresh saliva were studied, saliva would have to be collected prior to each experiment (different batches), leading to compositional differences between the pellicles formed on each substratum, the effect of this on their strength being unknown. Therefore this work is a reasonable starting point for establishing the methodology and for studying the influence of the water wettability of substrata. Nevertheless studies on pellicles formed from fresh saliva, or even in situ, should follow.
AFM-based FFS was used to determine the load and friction forces needed to break, i.e. forces applied during the Rupture Scan, and to remove, i.e. forces applied during the Sweeping Scan, salivary films formed on the different substrata. Our results show that there is a drastic increase of these forces, i.e. of the overall strength of the films, with increasing water wettability of the substrata. From the technical/methodological side, the possibility of performing these measurements, and the robustness of the results obtained, show that AFM-based FFS is a powerful technique for performing in vitro tests for the saliva-compatibility of materials.
Because of the pellicle constituting its innermost layer, the data provided is especially relevant for understanding mechanical removal processes of dental plaque. In line with early investigations (Glantz, 1969) this study suggests that, when subjected to mechanical forces, plaque formed on substrata of low wettability would be more prone to separate from the
9
substrata than to exhibit disruption and rupture processes. In contrast, on substrata with a high wettability it would be the other way around.
It is not straightforward to explain the mechanism underlying our results. We are currently investigating whether one origin could be differences in the composition of the pellicles. Despite this, it is interesting that our data support the predictions of the “theta surface” theory for the biocompatibility of materials (Baier, 2006). This theory states that weakest adhesion of biological material occurs on substrata with critical surface tension similar to that of completely methylated surfaces, i.e. 22-24 mN/m (Zisman, 1964). It also states that, from this point, adhesion will increase with the critical surface tension of the substrata, i.e. with their hydrophilicity, this being also supported by our results. The experimental verification of this theory supports its applicability to the a priori design of non-adhesive surfaces in contact with saliva.
ACKNOWLEDGMENTS
This study was supported by Malmö University. Thomas Arnebrant acknowledges the Gustaf Th. Ohlsson foundation, and Liselott Lindh acknowledges the Swedish Laryng Foundation for financial support.
REFERENCES
Arnebrant T (2003). Protein Adsorption in the Oral Environment. In: Biopolymers at Interfaces, Second Edition. M Malmsten editor. New York: Marcel Dekker, pp. 811-855.
Baier R (2006). Surface behaviour of biomaterials: The theta surface for biocompatibility J.
Mater. Sci. Mater. Med. 17:1057-1062.
Baier RE, Glantz PO (1978). Characterization of oral in vivo films formed on different types of solid surfaces. Acta Odontol. Scand. 36:289-301.
Bonn D, Eggers J, Indekeu J, Meunier J, Rolley E (2009). Wetting and spreading. Rev. Mod.
10
Busscher HJ, van Der Mei RBHC (1995). Initial microbial adhesion is a determinant for the strength of biofilm adhesion. FEMS Microbiol. Lett. 128:229-234.
Carlén A, Börjesson AC, Nikdel K, Olsson J (1998). Composition of Pellicles Formed in vivo on Tooth Surfaces in Different Parts of the Dentition, and in vitro on Hydroxyapatite. Caries Res. 32:447-455.
Carlén A, Rüdiger SG, Loggner I, Olsson J (2003). Bacteria-binding plasma proteins in pellicles formed on hydroxyapatite in vitro and on teeth in vivo. Oral Microbiol. Immunol. 18:203-207. Christersson CE, Dunford RG, Glantz P-OJ, Baier RE (1989). Effect of critical surface tension on retention of oral microorganisms. Eur. J. Oral Sci. 97:247-256.
Dawes C, N. JG, H. TC (1963). The Nomenclature of the Integuments of the Enamel Surface of Teeth. Br. Dent. J. 115:65-68.
Dawes C (1974). Rhythms in salivary flow rate and composition. Int J Chronobiol. 2:253-279. de Jong HP, van Pelt AWJ, Arends J (1982). Contact Angle Measurements on Human Enamel - An in vitro Study of Influence of Pellicle and Storage Period. J. Dent. Res. 61:11-13.
Fox HW, Zisman WA (1950). The spreading of liquids on low energy surfaces. I. polytetrafluoroethylene. J. Colloid Sci. 5:514-531.
Francis CA, Hector MP, Proctor GB (2000). Precipitation of specific proteins by freeze-thawing of human saliva. Arch. Oral. Biol. 45:601-606.
Glantz P-O (1969). On Wettability and Adhesiveness. Odontol. Revy 20:1-132.
Glantz P-O, Baier RE (1986). Recent Studies on Nonspecific Aspects of Intraoral Adhesion. The
Journal of Adhesion 20:227-244.
Hannig C, Hannig M (2009). The oral cavity—a key system to understand substratum-dependent bioadhesion on solid surfaces in man. Clin. Oral Investig. 13:123-139.
Hannig M, Joiner A (2006). The Structure, Function and Properties of the Acquired Pellicle.
Monogr. Oral Sci. 19:29-64.
Jenkins GN (1978). Saliva. In: The physiology and biochemistry of the mouth. Oxford: Blackwell Scientific Publications, pp. 284-359.
11
Lendenmann U, Grogan J, Oppenheim FG (2000). Saliva and Dental Pellicle - A Review. Adv.
Dent. Res. 14:22-28.
Malmsten M, Burns N, Veide A (1998). Electrostatic and Hydrophobic Effects of Oligopeptide Insertions on Protein Adsorption. J. Colloid Interface Sci. 204:104-111.
Norde W (1986). Adsorption of proteins from solution at the solid-liquid interface. Adv. Colloid
Interface Sci. 25:267-340.
Norde W, Lyklema J (1989). Protein adsorption and bacterial adhesion to solid surfaces: A colloid-chemical approach. Colloids Surf. 38:1-13.
Pettersson T, Nordgren N, Rutland MW, Feiler A (2007). Comparison of different methods to calibrate torsional spring constant and photodetector for atomic force microscopy friction measurements in air and liquid. Rev. Sci. Instrum. 78:093702.
Sotres J, Barrantes A, Arnebrant T (2011a). Friction Force Spectroscopy as a Tool to Study the Strength and Lateral Diffusion of Protein Layers. Langmuir 27:9439–9448.
Sotres J, Lindh L, Arnebrant T (2011b). Friction Force Spectroscopy as a Tool to Study the Strength and Structure of Salivary Films. Langmuir 27:13692-13700.
Svendsen IE, Lindh L (2009). The composition of enamel salivary films is different from the ones formed on dental materials. Biofouling 25:255-261.
Teughels W, Van Assche N, Sliepen I, Quirynen M (2006). Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Imp. Res. 17:68-81.
van der Mei HC, White DJ, Busscher HJ (2004). On the wettability of soft tissues in the human oral cavity. Arch. Oral Biol. 49:671-673.
Wahlgren M, Arnebrant T (1990). Adsorption of b-Lactoglobulin onto Silica, Methylated Silica, and Polysulfone. J. Colloid Interface Sci. 136:259-265.
Zisman WA (1964). Relation of the Equilibrium Contact Angle to Liquid and Solid Constitution. In: Contact Angle, Wettability, and Adhesion. RF Gould editor. Washington DC: American Chemical Society, pp. 1-51.
12
FIGURES Figure 1
Figure 1. a) Illustration of contact angle measurement of a UHQ water drop placed on a surface. b) Images of UHQ
water drops captured immediately after placement on the different substrata used in this work. The corresponding group number and the mean and standard deviation values for the measured contact angles are shown for each type of substrata.
13
Figure 2
Figure 2. a) Illustration of an AFM tip scratching a salivary film. b) Representative images of the changes in the
topography induced by increased applied load during the scratch of a salivary film formed on methylated silica (group VI, θc, of 99.7±0.5°). The load force, FL, and the average friction force, FF, exerted during the acquisition of each of the images is also shown. Scan area: 2µm x 2µm. Color scale goes from 0nm (black) to 6nm (white). c) Corresponding roughness and d) friction plots. The determined Rupture and Sweeping Scans are highlighted in both plots. For reference, roughness and friction measured on a clean substratum are also shown as dotted lines in c) and
14
Figure 3
Figure 3. Friction and roughness plots obtained on salivary films formed on each of the substrata studied in this
15
Figure 4
Figure 4. Load and friction forces needed to break adsorbed salivary films (a) and b) respectively), and to
completely remove them (c) and d) respectively) plotted against the θc value of the corresponding substratum (for corresponding groups see Fig. 1). Note that the Rupture Scan for films adsorbed on substrata from the group III batch (those with a θc value of 41.7±3.1°) could only be detected for those formed with saliva from one of the donors (donor #1).